Abstract
The herpes simplex virus Vhs endonuclease degrades host and viral mRNAs. Isolated Vhs cuts any RNA at many sites. Yet, within cells, it targets mRNAs and cuts at preferred sites, including regions of translation initiation. Previous studies have shown that Vhs binds the translation factors eIF4A and eIF4H. Here, we show that Vhs binds the cap-binding complex eIF4F. Association with eIF4F correlated with the ability of Vhs to bind eIF4A but not eIF4H. All Vhs proteins that degrade mRNAs associated with eIF4F. However, simply tethering an active endonuclease to eIF4F is not sufficient to degrade mRNAs. Binding to eIF4H may also be required.
The virion host shutoff (Vhs) (UL41) endonuclease is a component of herpes simplex (HSV) virions (2, 4, 26, 27, 32, 42) that, during lytic infections, accelerates the turnover of many host and viral mRNAs (24). At early times, copies of Vhs from infecting virions degrade many constitutively expressed host mRNAs, with a resultant decrease in translation of the proteins that they encode (9, 11, 31, 38). In addition, Vhs ensures the rapid turnover of most, if not all, viral mRNAs (13, 18, 19, 25, 38), thereby helping to determine viral mRNA levels and facilitating ordered expression of different classes of viral genes. During animal infections, Vhs plays a key role in inhibiting the interferon-mediated antiviral response as well as other components of innate and adaptive immune responses (1, 17, 20-22, 33, 34, 37, 41). As such, it is an important determinant of HSV virulence.
Isolated Vhs has broad substrate specificity. A glutathione S-transferase-Vhs fusion protein cleaves single-stranded RNA to the 3′ side of C and U residues (40). Similarly, a purified complex of recombinant Vhs and cellular eIF4H does not distinguish mRNAs from nonmessenger RNAs and cuts target RNAs at many sites (4). In contrast, within infected cells, Vhs is targeted to mRNAs (12, 18, 19, 31, 36, 38) and cuts mRNAs at preferred sites, including, for some, regions of translation initiation (2, 3, 10). A potential targeting mechanism is suggested by the observation that Vhs binds the cellular translation initiation factors eIF4H and eIF4A (7, 8), which play key roles in cap-dependent ribosome scanning. eIF4A is an ATP-dependent RNA helicase that, along with eIF4E and eIF4G, forms the cap-binding complex eIF4F (28, 35). eIF4H binds to and stimulates the helicase activity of eIF4A (29). Vhs and eIF4A can be coimmunoprecipitated, and to date, every mutant or wild-type Vhs polypeptide that degrades mRNA retains the ability to bind eIF4A (8). Binding eIF4H appears to be required for Vhs cleavage since (i) Vhs mutations, which abolish the interaction, abrogate its ability to degrade housekeeping mRNAs (7, 8) and (ii) small interfering RNA-mediated depletion of eIF4H prior to infection abrogates Vhs degradation (30).
The data suggest a model in which Vhs is targeted to mRNAs and regions of translation initiation by associating with eIF4F. However, the fact that Vhs binds eIF4A and eIF4H does not necessarily imply that it is targeted through eIF4F. Within cells, eIF4A exists in a free form and as a component of eIF4F (23, 39), and it is unknown to which form or forms Vhs binds. Similarly, it is unclear whether a molecule of eIF4H that has bound Vhs can still bind eIF4A. Therefore, it is crucial to determine whether Vhs associates with eIF4F cap-binding complexes isolated by binding to 7-methyl GTP-Sepharose 4B beads.
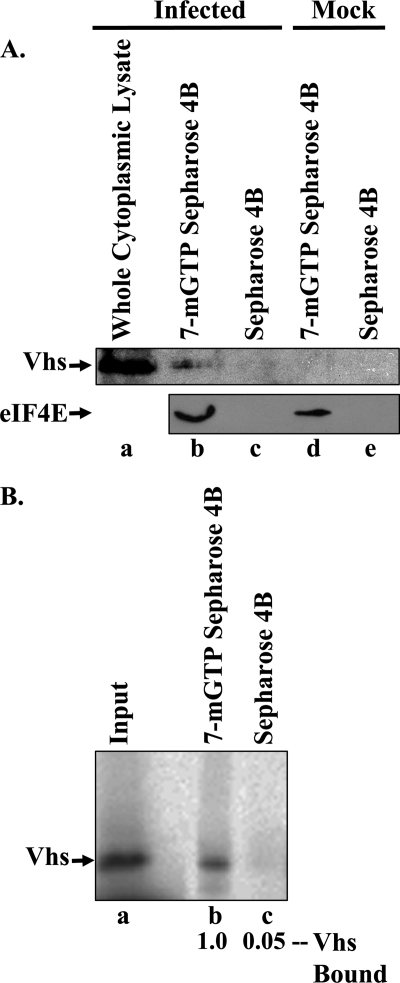
To this end, eIF4F complexes were prepared from HeLa cells 10 h after infection with 10 PFU/cell of wild-type HSV-1 (strain KOS) or mock infection. Briefly, cells were lysed by resuspension in 1 ml per 2 × 107 cells of binding buffer (20 mM Tris, pH 7.5, 100 mM KCl, 0.2 mM EDTA) containing 0.5% (vol/vol) NP-40 (6). Nuclei were pelleted, and 1-ml aliquots of the cytoplasmic supernatant were incubated for 1 h at 4°C with 300 μl (settled volume) of Sepharose 4B (Sigma). After the beads were pelleted, 150-μl aliquots of the supernatant were incubated for 1 h with 20 μl (settled volume) of either 7-methyl GTP-Sepharose 4B (Applied Biosystems) or Sepharose 4B. The beads were pelleted and washed three times with binding buffer containing 1 mM GTP. Bound proteins were eluted by boiling in a small volume of SDS sample buffer (50 mM Tris, pH 7.0, 2% [wt/vol] SDS, 10% [vol/vol] glycerol, 5% [vol/vol] β-mercaptoethanol) and analyzed by SDS-PAGE and Western blotting using polyclonal antiserum against a Vhs-LacZ fusion protein (26) or eIF4A2 (Novus Biologicals) or monoclonal antibody against eIF4E (BD Biosciences) or eIF4G (Cell Signaling Technologies). Vhs was isolated with material that bound 7-methyl GTP-Sepharose 4B (see Fig. 1A, 2A, and 3), as were the eIF4F components eIF4E (Fig. 1A, 2A, and 3), eIF4G (Fig. 2A and 3), and eIF4A (Fig. 3). However, neither Vhs nor any of the eIF4F components bound detectably to Sepharose 4B. A similar result was observed for in vitro-synthesized Vhs. In this case, [35S]methionine-labeled Vhs was synthesized by in vitro transcription and translation using a TNT T7 coupled transcription/translation kit (Promega) and detected by autoradiography. As in infected cells, in vitro-translated Vhs associated with material that bound 7-methyl GTP-Sepharose 4B but not Sepharose 4B (Fig. 1B).
FIG. 1.
Vhs associates with the eIF4F cap-binding complex. (A) Vhs associates with eIF4F in vivo. Cytoplasmic extracts were prepared from HeLa cells 10 h after infection with 10 PFU/cell of wild-type HSV-1 (strain KOS) (lanes a to c) or mock infection (lanes d and e) and incubated for 1 h with 7-methyl GTP-Sepharose 4B (lanes b and d) or Sepharose 4B (lanes c and e). Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting for the presence of Vhs or eIF4E, as indicated to the left of lane a. Lane a contains an aliquot of the whole cytoplasmic extract from infected cells equal to 3% of the amount of extract that was incubated with 7-methyl GTP-Sepharose 4B and Sepharose 4B in lanes b and c. (B) In vitro-translated Vhs associates with eIF4F. [35S]methionine-labeled Vhs was produced by coupled in vitro transcription and translation in rabbit reticulocyte lysates and incubated for 1 h with 7-methyl GTP-Sepharose 4B or Sepharose 4B. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and autoradiography. The relative amounts of Vhs that bound 7-methyl GTP-Sepharose 4B and Sepharose 4B were quantified using a Storm Model 840 PhosphorImager (Molecular Dynamics, Inc.) and are shown below lanes b and c, respectively. An aliquot of the input material is shown in lane a.
FIG. 2.
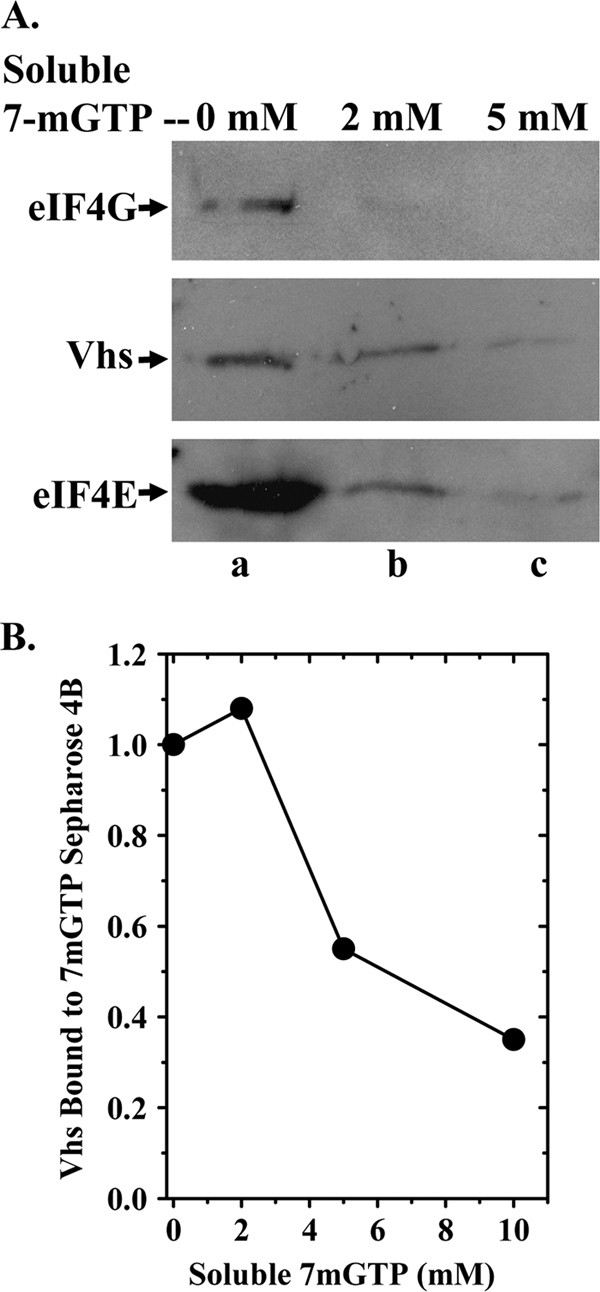
Soluble 7-methyl GTP competes with 7-methyl GTP-Sepharose 4B for binding eIF4F and associated Vhs. (A) Cytoplasmic extracts were prepared from HeLa cells 10 h after infection with 10 PFU/cell of wild-type HSV-1 (strain KOS) and incubated for 1 h with 7-methyl GTP-Sepharose 4B as described for Fig. 1, except that the reaction mixtures contained 0 mM, 2 mM, or 5 mM 7-methyl GTP as indicated above the lanes. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting for Vhs, eIF4E, or eIF4G, as indicated to the left of lane a. (B) [35S]methionine-labeled Vhs was produced by coupled in vitro transcription and translation and assayed for binding to 7-methyl GTP-Sepharose 4B as described for Fig. 1B, except that the binding reaction mixtures contained 0 mM, 2 mM, 5 mM, or 10 mM soluble 7-methyl GTP. Bound proteins were eluted by boiling in SDS sample buffer and analyzed by SDS-PAGE and autoradiography. The relative amounts of Vhs that bound the resin in the presence of various concentrations of 7-methyl GTP were quantified using a Storm Model 840 PhosphorImager (Molecular Dynamics, Inc.) and are plotted in the figure.
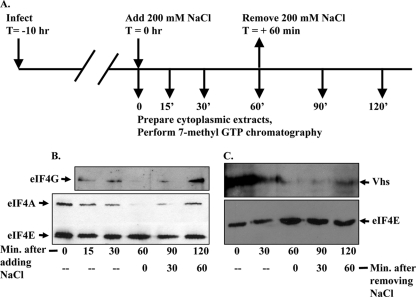
FIG. 3.
Hypertonic shock causes rapid and reversible disruption of eIF4F and reversible dissociation of Vhs from the material that binds 7-methyl GTP-Sepharose 4B. (A) Timeline of the experiment. Ten hours after infection with 10 PFU/cell of wild-type HSV-1 (strain KOS), HeLa cells were subjected to hypertonic shock by replacing the medium with medium that had been supplemented with 200 mM NaCl in addition to that which is present in normal medium. Cells were harvested and lysed, and cytoplasmic extracts were prepared at the time of initiation of the shock or at various subsequent times. For some cultures, the medium was removed 60 min after initiation of the shock and replaced with normal isotonic medium. Cytoplasmic extracts were prepared at various times after reversal of the shock. Binding assays were performed to analyze proteins that bound 7-methyl GTP-Sepharose 4B. (B) Bound proteins were eluted from the beads by boiling in SDS sample buffer and analyzed by SDS-PAGE and Western blotting to detect eIF4E, eIF4G, and eIF4A. (C) Aliquots from the same samples analyzed in panel B were resolved by SDS-PAGE and Western blotting to detect eIF4E and Vhs.
To check binding specificity, we determined whether soluble 7-methyl GTP would compete with 7-methyl GTP-Sepharose 4B for binding to eIF4F and reduce the amounts of Vhs and eIF4F components that bound the resin. Binding assays were performed as described for Fig. 1, except cytoplasmic extracts or rabbit reticulocyte lysates were supplemented with various concentrations of 7-methyl GTP 10 min prior to addition of the resin. Inclusion of a soluble cap analog caused a dose-dependent reduction in the amounts of eIF4E and eIF4G that were recovered with the bound material (Fig. 2A). 7-Methyl GTP significantly reduced the amounts of in vivo (Fig. 2A)- and in vitro (Fig. 2B)-synthesized Vhs associated with material that bound the resin, indicating that the association was due to binding of Vhs to eIF4F and not directly to the beads.
We next examined whether the putative Vhs-eIF4F complex exhibited characteristics expected of eIF4F in intact cells. Specifically, we examined whether hypertonic shock, an event that disrupts eIF4F, abolished the association of Vhs with material that bound 7-methyl GTP-Sepharose and whether the subsequent return of the cells to isotonic medium, with the resultant reassembly of eIF4F, restored the binding of Vhs to the beads. This is an attractive system because the disruption of eIF4F by hypertonic shock is both rapid and reversible (15, 16). Briefly, HeLa cells were infected with 10 PFU/cell of wild-type HSV-1 (Fig. 3A). Ten hours later, the medium was replaced with medium supplemented with 200 mM NaCl in addition to that which is present in normal medium. Cells were harvested and lysed, and cytoplasmic extracts were prepared immediately or at various times thereafter. For some cultures, the medium was removed after 60 min and replaced with normal isotonic medium. Cytoplasmic extracts were prepared at various times after reversal of the shock. Binding assays were performed to analyze proteins that bound 7-methyl GTP-Sepharose. As expected, hypertonic shock disrupted eIF4F, as evidenced by the loss of eIF4A and eIF4G from material that bound 7-methyl GTP-Sepharose (Fig. 3B). This disruption was detectable within 15 min and complete by 60 min after exposure of the cells to hypertonic medium. eIF4E is the cap-binding component of eIF4F and continued to bind the resin, even during hypertonic stress. Restoration of isotonic conditions led to reassembly of much, although not all, of the eIF4F complex within 60 min. Of most significance, hypertonic shock caused the rapid disappearance of Vhs from material that bound the resin, followed by reassociation of some Vhs with 7-methyl GTP binding material within 60 min after reversal of the shock (Fig. 3C). The results indicate that Vhs is an authentic eIF4F-interacting protein and that this interaction does not result from Vhs binding eIF4E.
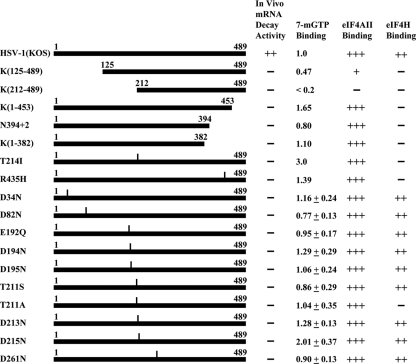
Next, we examined the effect of various Vhs mutations on its association with eIF4F. Mutant and wild-type Vhs polypeptides were synthesized by in vitro transcription and translation, and equal amounts were assayed for eIF4F binding as described for Fig. 1B. The results are tabulated in Fig. 4 to show the relative amounts of wild-type and mutant proteins that bound eIF4F, as well as their binding to eIF4A and eIF4H, and their ability to degrade housekeeping mRNAs, as determined in earlier studies (5, 7, 8). The data allow several conclusions. First, truncation of the protein from the C terminus to 382 amino acids had no detectable effect on its binding to eIF4F (Fig. 4, line 6). In contrast, deletion of the first 211 amino acids reduced the interaction to an undetectable level (Fig. 4, line 3). Thus, sequences within the first 211 amino acids of Vhs are required for eIF4F binding, and the first 382 amino acids are sufficient. Second, binding to eIF4H is not required for the association of Vhs with eIF4F since several mutations abolished Vhs binding to eIF4H but did not affect its interaction with eIF4F. Third, the association of Vhs with eIF4F correlated with its ability to bind eIF4A, since every polypeptide that associated with eIF4F bound eIF4A, and mutations that reduced or abolished eIF4A binding also reduced or abolished the eIF4F interaction. However, the data should be interpreted cautiously since, to date, every mutation we have identified that reduces eIF4A binding also affects binding to eIF4H, suggesting that these mutant proteins may contain structural changes that affect multiple functions. Finally, while the data are consistent with the idea that eIF4F binding is required for Vhs degradation of mRNAs that are translated by cap-dependent scanning, simple tethering of an active Vhs endonuclease to the cap through eIF4F cannot be sufficient for Vhs-mediated decay. The T214I mutant retains endonuclease activity (14) and binds eIF4A and eIF4F yet does not degrade housekeeping mRNAs. However, it lacks the ability to bind eIF4H. These and previous data (5, 7, 8) suggest that binding to both eIF4H and eIF4F may be required for Vhs degradation of scanned mRNAs. Clearly, much remains to be learned about the mechanisms of Vhs targeting.
FIG. 4.
Effect of Vhs mutations on binding of the protein to eIF4F, eIF4A, and eIF4H and its in vivo mRNA degradation activity. The 489-amino-acid Vhs polypeptide encoded by wild-type HSV-1 (strain KOS) is represented by a solid rectangle in line 1, and the structures of various deletion and point mutants are shown in lines 2 through 18. For deletion mutants, the Vhs residues included in the mutant proteins are indicated. For each point mutant, the location of the altered residue is indicated by a vertical line above the bar representing the protein, and the nature of the substitution is indicated by the name of the mutant. For example, in the T214I mutant, threonine at position 214 is replaced by isoleucine. Mutant and wild-type Vhs polypeptides were synthesized by in vitro transcription and translation, and equal amounts were assayed for eIF4F binding as described for Fig. 1B. The results are tabulated in the third column from the right to show the relative amounts of wild-type and mutant proteins that bound eIF4F in parallel assays in the same experiment. The relative binding of mutant and wild-type proteins to eIF4A and eIF4H and their relative in vivo mRNA degradative activities were determined in previous studies (4, 7, 8) and are summarized here for comparison to binding of Vhs polypeptides to eIF4F. +++ indicates binding or mRNA degradation activity similar to that of the wild-type protein, + indicates binding that is greatly reduced relative to that of the wild-type protein, and − indicates no detectable binding or degradative activity.
Acknowledgments
We thank Lora Shiflett, Jouliana Sadek, Deepali Agarwal, and our other colleagues at the University of Missouri—Kansas City for many helpful discussions.
This work was supported by Public Health Service grant R01 AI-21501 to G.S.R. from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Duerst, R. J., and L. A. Morrison. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322:158-167. [DOI] [PubMed] [Google Scholar]
- 2.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 73:7153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgadi, M. M., and J. R. Smiley. 1999. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J. Virol. 73:9222-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everly, D. N., Jr., and G. S. Read. 1999. Site-directed mutagenesis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): analysis of functional differences between HSV type 1 (HSV-1) and HSV-2 alleles. J. Virol. 73:9117-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigenblum, D., and R. J. Schneider. 1993. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J. Virol. 67:3027-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, P., D. N. Everly, Jr., and G. S. Read. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenwick, M. L., and M. M. McMenamin. 1984. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 65:1225-1228. [DOI] [PubMed] [Google Scholar]
- 10.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 11.Khodarev, N. N., S. J. Advani, N. Gupta, B. Roizman, and R. R. Weichselbaum. 1999. Accumulation of specific RNAs encoding transcriptional factors and stress response proteins against a background of severe depletion of cellular RNAs in cells infected with herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 96:12062-12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krikorian, C. R., and G. S. Read. 1991. In vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J. Virol. 65:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. U. S. A. 84:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, P., H. A. Saffran, and J. R. Smiley. 2001. The vhs1 mutant form of herpes simplex virus virion host shutoff protein retains significant internal ribosome entry site-directed RNA cleavage activity. J. Virol. 75:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maroney, P. A., Y. Yu, J. Fisher, and T. W. Nilsen. 2006. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat. Struct. Mol. Biol. 13:1102-1107. [DOI] [PubMed] [Google Scholar]
- 16.Morley, S. J., and S. Naegele. 2002. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J. Biol. Chem. 277:32855-32859. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61:604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasieka, T. J., C. Cilloniz, B. Lu, T. H. Teal, S. C. Proll, M. G. Katze, and D. A. Leib. 2009. Host responses to wild-type and attenuated herpes simplex virus infection in the absence of Stat1. J. Virol. 83:2075-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasieka, T. J., B. Lu, S. D. Crosby, K. M. Wylie, L. A. Morrison, D. E. Alexander, V. D. Menachery, and D. A. Leib. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasieka, T. J., B. Lu, and D. A. Leib. 2008. Enhanced pathogenesis of an attenuated herpes simplex virus for mice lacking Stat1. J. Virol. 82:6052-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pause, A., N. Methot, Y. Svitkin, W. C. Merrick, and N. Sonenberg. 1994. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 13:1205-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Read, G. S. 1997. Control of mRNA stability during herpes simplex virus infections, p. 311-321. In J. B. Harford and D. R. Morris (ed.), mRNA metabolism and post-transcriptional gene regulation. Wiley-Liss, Inc., New York, NY.
- 25.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read, G. S., and M. Patterson. 2007. Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions. J. Virol. 81:1148-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers, G. W., Jr., A. A. Komar, and W. C. Merrick. 2002. eIF4A: the godfather of the DEAD box helicases. Prog. Nucleic Acid Res. Mol. Biol. 72:307-331. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, G. W., Jr., N. J. Richter, W. F. Lima, and W. C. Merrick. 2001. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J. Biol. Chem. 276:30914-30922. [DOI] [PubMed] [Google Scholar]
- 30.Sarma, N., D. Agarwal, L. A. Shiflett, and G. S. Read. 2008. Small interfering RNAs that deplete the cellular translation factor eIF4H impede mRNA degradation by the virion host shutoff protein of herpes simplex virus. J. Virol. 82:6600-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schek, N., and S. L. Bachenheimer. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 33.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonenberg, N., and T. E. Dever. 2003. Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 13:56-63. [DOI] [PubMed] [Google Scholar]
- 36.Sorenson, C. M., P. A. Hart, and J. Ross. 1991. Analysis of herpes simplex virus-induced mRNA destabilizing activity using an in vitro mRNA decay system. Nucleic Acids Res. 19:4459-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 61:2198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svitkin, Y. V., A. Pause, A. Haghighat, S. Pyronnet, G. Witherell, G. J. Belsham, and N. Sonenberg. 2001. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7:382-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taddeo, B., and B. Roizman. 2006. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 80:9341-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wylie, K. M., J. E. Schrimpf, and L. A. Morrison. 2009. Increased eIF2alpha phosphorylation attenuates replication of herpes simplex virus 2 vhs mutants in mouse embryonic fibroblasts and correlates with reduced accumulation of the PKR antagonist ICP34.5. J. Virol. 83:9151-9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelus, B. D., R. S. Stewart, and J. Ross. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 70:2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]