Abstract
Many aspects of the life cycle of torquetenoviruses (TTVs) are essentially unexplored. In particular, it is still a matter of speculation which cell type(s) replicates the viruses and maintains the generally high viral loads found in the blood of infected hosts. In this study, we sequentially measured the TTV loads in the plasma of four TTV-positive leukemia patients who were strongly myelosuppressed and then transplanted with haploidentical hematopoietic stem cells. The findings provide clear quantitative evidence for an extremely important role of hematopoietic cells in the maintenance of TTV viremia.
Torquetenoviruses (TTVs) are small naked DNA viruses distinguished by a circular single-stranded DNA genome of only 3.8 kb, classified within the newly established family Anelloviridae (7). TTVs have been found in several animal species but do not appear capable of interspecies transmission. Due to their extensive genetic heterogeneity, human TTVs have been operatively subdivided into 5 genogroups and more than 40 genotypes (4). A remarkable feature of these TTVs is their presence in the plasma of nearly all people, regardless of geographical origin, age, and health status, raising many questions about their life cycle and possible pathological implications (2, 5). Plasma loads of TTVs vary extensively in both healthy and diseased individuals, usually ranging between 103 and 107 DNA copies per ml of plasma. However, some patients, including those with selected inflammatory or neoplastic disorders, transplant recipients, and human immunodeficiency virus-infected individuals, have a tendency to carry especially high burdens of TTVs (1, 6, 13, 22-24).
By studying the dynamics of TTV viremia in individuals treated with alpha interferon for hepatitis C, the kinetics of virus replication was found to be quite high, with numbers of virions released into plasma and cleared from it daily on the same order of magnitude as other chronic plasma viremia-inducing viruses, such as the hepatitis B, hepatitis C, and human immunodeficiency viruses (16). Yet, due to considerable difficulties encountered in propagating TTVs in culture and in distinguishing the virions passively adsorbed onto the cells from the ones replicating inside cells, the tissue or tissues where these large numbers of TTV virions originate have yet to be established. Given that the amino acid compositions of the capsid protein believed to mediate viral adsorption to cells are quite diverse in different TTVs (2, 3, 9), it is also possible that permissive cells vary depending on the TTV considered. Relevant studies are limited. Short-term cultures of phytohemagglutinin-stimulated peripheral lymphocytes, but not resting lymphocytes were found to permit a measurable level of TTV replication (15, 18), indicative of at least a moderate degree of lymphotropism. On the other hand, the detection of replicative forms of TTV DNA in several tissues, including bone marrow, peripheral blood mononuclear cells, and liver, has suggested that TTVs might be polytropic in nature (2, 21).
In 1999, Kanda et al. (10), researching TTV plasma of bone marrow transplant recipients with a qualitative PCR, noticed that 5 out of 6 previously positive patients tested negative in a sampling collected during the myelosuppressed period and became positive again after graft reconstitution, leading them to suggest that TTV might replicate mainly in hematopoietic cells. In the present study, we further developed this observation by measuring the TTV load in sequential plasma samples obtained from four TTV-positive leukemia patients undergoing hematopoietic stem cell transplantation. This procedure basically consists of a myeloablative conditioning regimen (chemotherapy plus radiotherapy) followed by reinfusion of a positively selected CD34+ stem cell population. The findings are of interest because, in addition to confirming the decrease of TTV load observed by Kanda et al., they shed light on the kinetics of the effect, thus providing a better insight onto the role of hematopoietic cells in the maintenance of TTV viremia and on the life cycle of TTV in general.
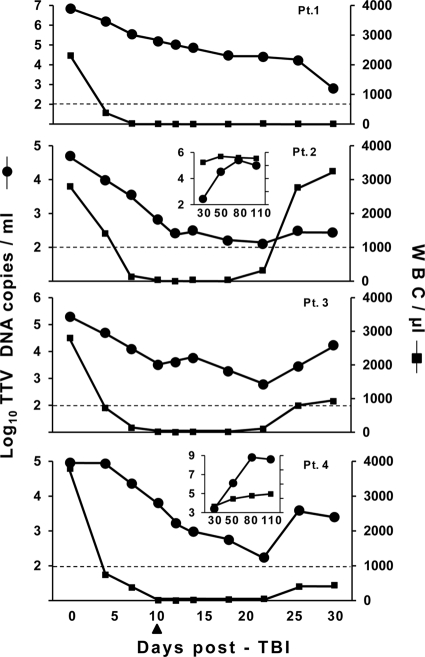
Table 1 summarizes the main characteristics of the patients selected for the study. They were treated with 10 Gy total-body irradiation (TBI) on day 0 and received 5 mg/kg/day thiotepa on days 2 and 3, 40 mg/m2/day fludarabine on days 3 to 7, and 1.2 mg/kg/day antithymocyte globulin on days 4 to 8, and then, on day 10, they received the indicated numbers of positively selected CD34+ hematopoietic stem cells from HLA-haploidentical donors. Peripheral blood samples were collected for TTV studies immediately before TBI and at selected times for the next 30 days, and plasma was stored in aliquots at −80°C until DNA extraction. The assay used for TTV quantification was a previously described highly sensitive TaqMan real-time PCR having the potential to detect and quantitate all hitherto recognized genetic forms of the virus (15, 16). All samples from each patient were assayed in a single run and in triplicate, and at least two independent DNA extractions for each sample were examined. The DNA extracts obtained at time zero were also typed with a previously described panel of five distinct PCR assays (12), each specific for one of the genogroups into which TTVs are subdivided. At the start of the study, the patients had viral loads ranging from 4.7 to 6.8 log copies per ml of plasma and harbored between 1 and 3 TTV genogroups (Table 1). In particular, all carried genogroup 1, which is highly represented in our area (12), and two carried one or two further genogroups. Consistent with previous findings (12), the patient who harbored three genogroups was the one with the highest viral load. As shown by Fig. 1, in all four patients, TBI was followed by a steady decline of TTV viremia that continued for at least 22 days and progressively brought the virus to levels very close to the detection limit of the detection/quantitation method used, corresponding to values ranging between 0.003 (patient 3) and 0.00009 (patient 1) of the loads present prior to TBI. However, in no instance did the viral loads go below the limit of sensitivity of the assay (2 × 102 TTV DNA copies per ml of plasma). Although the size of the study does not permit firm conclusions on this aspect, it is noteworthy that the extent of decline was unrelated to the type and number of infecting TTV genogroup(s) originally present in the patients.
TABLE 1.
Relevant parameters of the patients enrolled
| Patient | Age in yr (sex) | Clinical diagnosisa | No. of CD34 cells grafted (106 cells/kg) | Survival (days) | TTV in plasma |
|||
|---|---|---|---|---|---|---|---|---|
| Pre-TBI |
Post-TBI |
|||||||
| No. of copies/ml | Genogroup(s) | No. of copies/mlb | Genogroup(s) | |||||
| 1 | 54 (male) | T-ALL | 23.60 | 30 | 6.8 | 1, 3, 5 | NDc | ND |
| 2 | 47 (female) | ALL | 9.41 | 174 | 4.7 | 1, 4 | 5.4 (day 80) | 1, 3, 4, 5 |
| 3 | 41 (female) | B-ALL | 11.70 | 111 | 5.3 | 1 | 4.2 (day 30) | 3 |
| 4 | 58 (female) | AML | 5.90 | 267 | 5.0 | 1 | 7.0 (day 110) | 1, 3, 4, 5 |
T-ALL, T-cell acute lymphoblastic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; AML, acute myeloid leukemia.
The day post-TBI when TTV loads and genogroups were determined is shown in parentheses.
ND, not determined.
FIG. 1.
Plasma TTV loads and WBC counts in the peripheral blood of the 4 patients (Pt. 1 to 4) enrolled in the study. The arrow indicates the day the patients were infused with CD34+ hematopoietic stem cells from HLA-haploidentical donors. The horizontal broken line represents the lower limit of sensitivity of the TTV detection method used.
The viral loads observed during the phase of maximum decline (days 0 to 12) were then exploited to investigate the dynamics of TTV infection in the patients by using the mathematical model originally developed by Neumann et al. (20). The results of this analysis are shown in Table 2. The mean clearance rate of circulating TTVs was 3.8 days−1. The half-life of plasma TTVs ranged between 3.6 and 4.8 h, with a mean of 4.3 h, which is a little shorter than previously calculated in patients treated with alpha interferon (16), possibly due to the fact that TBI may have led to a more complete block of viral replication. Overall, however, these values coupled with the calculated numbers of virions produced per day (Table 2) are a further demonstration that TTV infection is highly dynamic.
TABLE 2.
TTV dynamics in the patients enrolled
| Patient | Viral parameter |
||
|---|---|---|---|
| Clearance rate (c [days−1]) | Virion half-life (days)a | Minimal input and clearance of plasma virions/dayb | |
| 1 | 3.8 | 0.18 | 7.8 × 1010 |
| 2 | 4.5 | 0.15 | 6.7 × 109 |
| 3 | 3.7 | 0.19 | 2.1 × 109 |
| 4 | 3.5 | 0.20 | 9.6 × 108 |
| Mean ± SE | 3.8 ± 0.2 | 0.18 ± 0.01 | 2.0 × 1010 ± 1.0 × 1010 |
Calculated by the equation ln (2)/c.
Daily production of plasma virions was calculated from c multiplied by the pre-TBI viremia load value and by extracellular body fluid volume, which was arbitrarily set at 3.0 × 103 ml.
One patient died of multiorgan failure a few h after the 30-day sampling point without noticeable changes in either TTV viremia and white blood cell (WBC) counts. The other patients, starting from day 26, showed a generally moderate but consistent increase of TTV viremia, so that by the end of the 30-day observation period their viral loads were still somewhat to considerably lower than at baseline (Fig. 1). Interestingly, the increase paralleled the reappearance of WBCs in peripheral blood, a clear indicator of substantial engraftment. For two patients, we could also examine plasma samples collected at days 50, 80, and 110. As shown by the inserts in Fig. 1, at these times both patients exhibited plasma TTV loads higher than at baseline, indicating that TTV shedding into plasma had resumed and was as abundant as or even more abundant than that at the start of the study. Interestingly, the spectrum of TTV genogroups detected in plasma at this time differed substantially from pre-TBI (Table 1), indicating that the patients were now replicating newly acquired TTVs, most likely transmitted by the graft or blood component transfusions required to sustain the procedure.
Collectively, these findings provide solid quantitative evidence that hematopoietic stem cells represent by far the most important, if not the only source of the generally high TTV burdens found in the blood of infected individuals. The alternative explanation that hematopoietic cells or cytokines produced by them might stimulate other cells to replicate TTV seems less likely. Not only did plasma TTV loads fall dramatically during the myelosuppressed period, but also graft reconstitution was accompanied by a parallel return to high TTV loads. That TTVs have a preference for a highly cycling cell compartment is consistent with the well-established notion that single-stranded DNA viruses, including parvoviruses and circoviruses, have a marked preference for or replicate exclusively in DNA-synthesizing cells (14). The minimal levels of viremia that persisted during myelosuppression might suggest that some TTV replication takes place as well outside the hematopoietic compartment. However, since posttransplant the viral genogroups harbored by the patients were at least partly different from the ones harbored pretransplant, it is also possible that such low viral loads were generated by the hematopoietic cells infused into the patients.
The viruses that lack an external lipid envelope are usually cytolytic for the cells in which they replicate. Future studies should therefore focus on clarifying which specific cell type or types within the hematopoietic cell compartment support TTV replication. A preferential replication within the lymphoid cell lineage might explain some of the immunomodulating properties attributed to the TTVs (6, 14, 17), while a preference for the erythroid lineage might explain the cases of aplastic anemia that have been associated with TTV infection (8, 11, 19). On the other hand, the circumstance that the great majority of TTV infections do not emerge clinically is most likely explained by the large regenerative potential of the hematopoietic compartment.
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Bando, M., M. Takahashi, S. Ohno, T. Hosono, M. Hironaka, H. Okamoto, and Y. Sugiyama. 2008. Torque teno virus DNA titre elevated in idiopathic pulmonary fibrosis with primary lung cancer. Respirology 13:263-269. [DOI] [PubMed] [Google Scholar]
- 2.Bendinelli, M., M. Pistello, F. Maggi, C. Fornai, G. Freer, and M. L. Vatteroni. 2001. Molecular properties, biology and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clin. Microbiol. Rev. 14:98-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagini, P. 2004. Human circoviruses. Vet. Microbiol. 98:95-101. [DOI] [PubMed] [Google Scholar]
- 4.Biagini, P. 2009. Classification of TTV and related viruses (anelloviruses). Curr. Top. Microbiol. Immunol. 331:21-33. [DOI] [PubMed] [Google Scholar]
- 5.De Villiers, E. M., and H. Zur Hausen. 2009. TT viruses: the still elusive human pathogens. Curr. Top. Microbiol. Immunol. 331:1-230. [PubMed] [Google Scholar]
- 6.Focosi, D., F. Maggi, M. Albani, L. Macera, V. Ricci, S. Gragnani, S. Di Beo, M. Ghimenti, G. Antonelli, M. Bendinelli, M. Pistello, L. Ceccherini-Nelli, and M. Petrini. 2010. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J. Clin. Virol. 47:189-192. [DOI] [PubMed] [Google Scholar]
- 7.ICTV. 2009. Current virus taxonomy. International Committee on Taxonomy of Viruses. http://www.ictvonline.org/virusTaxonomy.asp.
- 8.Ishimura, M., S. Ohga, M. Ichiyama, K. Kusuhara, H. Takada, T. Hara, M. Takahashi, and H. Okamoto. 9 December 2009, posting date. Hepatitis-associated aplastic anemia during a primary infection of genotype 1a torque teno virus. Eur. J. Pediatr. [Epub ahead of print.] doi: 10.1007/s00431-009-1116-8. [DOI] [PubMed]
- 9.Kakkola, L., K. Hedman, J. Qiu, D. Pintel, and M. Soderlund-Venermo. 2009. Replication of and protein synthesis by TT viruses. Curr. Top. Microbiol. Immunol. 331:53-64. [DOI] [PubMed] [Google Scholar]
- 10.Kanda, Y., Y. Tanaka, M. Kami, T. Saito, T. Asai, K. Izutsu, K. Yuji, S. Ogawa, H. Honda, K. Mitani, S. Chiba, Y. Yazaki, and H. Hirai. 1999. TT virus in bone marrow transplant recipients. Blood 93:2485-2490. [PubMed] [Google Scholar]
- 11.Kikuchi, K., H. Miyakawa, K. Abe, M. Kako, K. Katayama, S. Fukushi, and S. Mishiro. 2000. Indirect evidence of TTV replication in bone marrow cells, but not in hepatocytes, of a subacute hepatitis/aplastic anemia patient. J. Med. Virol. 61:165-170. [PubMed] [Google Scholar]
- 12.Maggi, F., E. Andreoli, L. Lanini, C. Fornai, M. L. Vatteroni, M. Pistello, and M. Bendinelli. 2005. Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J. Clin. Microbiol. 43:4807-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggi, F., E. Andreoli, L. Riente, S. Meschi, J. Rocchi, A. Delle Sedie, M. L. Vatteroni, L. Ceccherini-Nelli, S. Specter, and M. Bendinelli. 2007. Torquetenovirus (TTV) in patients with arthritis. Rheumatology 46:885-886. [DOI] [PubMed] [Google Scholar]
- 14.Maggi, F., and M. Bendinelli. 2009. Immunobiology of the torque teno viruses and other anelloviruses. Curr. Top. Microbiol. Immunol. 331:65-90. [DOI] [PubMed] [Google Scholar]
- 15.Maggi, F., C. Fornai, L. Zaccaro, A. Morrica, M. L. Vatteroni, P. Isola, S. Marchi, A. Ricchiuti, M. Pistello, and M. Bendinelli. 2001. TT virus (TTV) loads associated with different peripheral blood cell types and evidence for TTV replication in activated mononuclear cells. J. Med. Virol. 64:190-194. [DOI] [PubMed] [Google Scholar]
- 16.Maggi, F., M. Pistello, M. L. Vatteroni, S. Presciuttini, S. Marchi, P. Isola, C. Fornai, S. Fagnani, E. Andreoli, G. Antonelli, and M. Bendinelli. 2001. Dynamics of persistent TT virus infection, as estimated in patients treated with interferon-alpha for concomitant hepatitis C. J. Virol. 75:11999-12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi, F., V. Ricci, M. Bendinelli, L. Ceccherini-Nelli, D. Focosi, F. Papineschi, M. Petrini, E. Paumgardhen, and M. Ghimenti. 2008. Changes in CD8+57+ T lymphocyte expansions after autologous hematopoietic stem cell transplantation correlate with changes in torquetenovirus viremia. Transplantation 85:1867-1868. [DOI] [PubMed] [Google Scholar]
- 18.Mariscal, L. F., J. M. López-Alcorocho, E. Rodríguez-Iñigo, N. Ortiz-Movilla, S. de Lucas, J. Bartolomé, and V. Carreño. 2002. TT virus replicates in stimulated but not in nonstimulated peripheral blood mononuclear cells. Virology 301:121-129. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto, M., H. Takahashi, I. Sakata, and Y. Adachi. 2000. Hepatitis-associated aplastic anemia and transfusion-transmitted virus infection. Intern. Med. 39:1068-1070. [DOI] [PubMed] [Google Scholar]
- 20.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelsen. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto, H. 2009. History of discoveries and pathogenicity of TT viruses. Curr. Top. Microbiol. Immunol. 331:1-20. [DOI] [PubMed] [Google Scholar]
- 22.Tokita, H., S. Murai, H. Kamitsukasa, M. Yagura, H. Harada, M. Takahashi, and H. Okamoto. 2002. High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J. Med. Virol. 67:501-509. [DOI] [PubMed] [Google Scholar]
- 23.Touinssi, M., P. Gallian, P. Biagini, H. Attoui, B. Vialettes, Y. Berland, C. Tamalet, C. Dhiver, I. Ravaux, P. De Micco, and X. De Lamballerie. 2001. TT virus infection: prevalence of elevated viraemia and arguments for the immune control of viral load. J. Clin. Virol. 21:135-141. [DOI] [PubMed] [Google Scholar]
- 24.Zhong, S., W. Yeo, M. W. Tang, X. R. Lin, F. Mo, W. M. Ho, P. Hui, and P. J. Johnson. 2001. Gross elevation of TT virus genome load in the peripheral blood mononuclear cells of cancer patients. Ann. N. Y. Acad. Sci. 945:84-92. [DOI] [PubMed] [Google Scholar]