Abstract
Infection with human immunodeficiency virus type 1 (HIV-1) causes an inexorable depletion of CD4+ T cells. The loss of these cells is particularly pronounced in the mucosal immune system during acute infection, and the data suggest that direct viral cytopathicity is a major factor. Cell cycle arrest caused by the HIV-1 accessory protein Vpr is strongly correlated with virus-induced cell death, and phosphorylation of Vpr serine 79 (S79) is required to activate G2/M cell cycle blockade. However, the kinase responsible for phosphorylating Vpr remains unknown. Our bioinformatic analyses revealed that S79 is part of a putative phosphorylation site recognized by protein kinase A (PKA). We show here that PKA interacts with Vpr and directly phosphorylates S79. Inhibition of PKA activity during HIV-1 infection abrogates Vpr cell cycle arrest. These findings provide new insight into the signaling event that activates Vpr cell cycle arrest, ultimately leading to the death of infected T cells.
AIDS results from the dramatic loss of CD4+ T lymphocytes following human immunodeficiency virus type 1 (HIV-1) infection. Recent studies with HIV-infected individuals and the rhesus macaque model of simian immunodeficiency virus (SIV) documented a massive loss of memory CD4+ T cells that occurs during the acute phase of infection, primarily in the gastrointestinal tract (9, 20, 53, 79). While the death of bystander cells has been proposed to explain the massive loss of CD4+ T cells during HIV-1/SIV infection (54), mainly infected cells were lost during this short period (10 to 14 days postinfection), suggesting direct viral infection is the cause of cell death (53). Although a cytotoxic-T-lymphocyte response could contribute to some elimination of infected cells, a strong cellular-mediated immune response to HIV-1/SIV is detectable only late in the depletion time period (43, 70). However, we and others have shown that in vitro infection of CD4+ T lymphocytes with HIV-1 leads to direct viral cytopathicity by necrosis (7, 11, 46). Understanding the mechanism of HIV-1-induced cell death could elucidate the mechanism of T-cell depletion, especially during the early destruction of the mucosal immune system.
The HIV-1 accessory viral protein R (Vpr) contributes importantly to HIV-1-induced cell death (8, 69, 75, 82). The pathological significance of Vpr in vivo is illustrated by the observation that the deletion of vpr and vpx, the SIV homologues of HIV-1 vpr, retarded viral replication and disease progression in rhesus macaques (26). Vpr is also a preferred target of the CD8+ T-cell response in HIV-1-infected individuals (3). Vpr has many well-characterized functions in HIV-1-infected CD4+ T cells (48). Among these are the induction of cell cycle arrest in the G2/M phase (33, 40, 67, 68), increasing transcriptional activity from the HIV-1 long-terminal-repeat (LTR) promoter (21, 80), reducing the mutation rate during reverse transcription (13, 52), and facilitating infection of nondividing cells (18, 34). Vpr-induced cell cycle arrest in the G2/M phase is highly correlated with its ability to cause cell death (8, 69, 75, 82). In fact, halting cell proliferation regardless of the phase of the cell cycle is critical in HIV-1 viral cytopathicity (8). Therefore, defining the mechanism of Vpr cell cycle arrest may provide therapeutic targets to reduce Vpr-mediated cell death during HIV-1 infection.
Vpr in infected cells and virions is phosphorylated on multiple serine residues (57, 83). Previous mutational analysis revealed that serine 79 (S79), but not S28, S94, or S96, was crucial for Vpr cell cycle arrest. Moreover, substituting alanine for the arginine adjacent to S79 (R80A) reduced both cell cycle arrest and phosphorylation of Vpr (83). Although controversial, the phosphorylation of serine 79 may also be important in the ability of Vpr to facilitate macrophage infection (2, 83). Although previous work was able to determine the functional impact of S79 phosphorylation, the kinase responsible for this modification was never determined. Identifying the kinase could provide new insight into the signaling event that activates Vpr G2/M blockade.
Protein kinase A (PKA) exists as a heterotetrameric holoenzyme complex in the inactive state, with two catalytic subunits bound to a dimer of regulatory subunits (10, 44, 78). Cyclic AMP (cAMP) binding to the regulatory subunits triggers a conformational change that releases the catalytic subunits, which constitute the active kinase (1, 10). cAMP signaling is known to be increased during HIV-1 infection, leading to increased PKA activity (36, 59, 60). A number of functions for PKA during HIV infection have been reported; for example, elevated cAMP leads to a PKA-dependent increase in viral replication (60). In addition, PKA has also been shown to phosphorylate the viral proteins p24 capsid and Nef (12, 49). Importantly, active PKA catalytic subunits are specifically recruited into HIV-1 virions, and the phosphorylation of the viral capsid protein was suggested to be critical in the HIV-1 uncoating process early in the infection cycle (12). The phosphorylation of Nef by PKA caused enhancement of infectivity in resting lymphocytes (49).
We applied in silico bioinformatic analyses to explore candidate kinase(s) that could phosphorylate serine 79 of Vpr, and PKA was indicated as a strong candidate. Indeed, we show that PKA directly interacts with Vpr during HIV-1 infection and phosphorylates S79 in an in vitro kinase assay. Furthermore, we demonstrate that Vpr cell cycle arrest is remarkably reduced by inhibiting PKA kinase activity. Thus, the cAMP/PKA pathway facilitates activation of Vpr cell cycle arrest, and likely the subsequent death of the host cell. These findings highlight a new key role for PKA during HIV-1 infection.
MATERIALS AND METHODS
Cells.
Jurkat T cells were maintained in RPMI 1640 (Lonza) supplemented with 10% fetal calf serum, 100 U of penicillin-streptomycin/ml, 2.4 mM l-glutamine, and 50 μM β-mercaptoethanol. The Jurkat 1.9 cell line, a CD4hi subclone of the parental JAK3 cell line, was used for all Jurkat experiments (7). HEK293T (293T) cells were maintained in RPMI 1640 (Lonza) supplemented as described above. PKA inhibitors added to cell cultures include: myristoylated PKA inhibitor 14-22 amide peptide (M14-22; EMD Biosciences), H-89 (EMD Biosciences), KT5720 (Alexis Biochemicals), Rp-cAMPS (Santa Cruz Biotechnology) and Rp-8-Br-cAMPS (Santa Cruz Biotechnology). The vehicle control for M14-22, Rp-cAMPS, and Rp-8-Br-cAMPS was water, and dimethyl sulfoxide (DMSO) was the vehicle control for H-89 and KT5720.
HIV virus stock and infections.
HIV viral plasmids were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program unless otherwise indicated. HIV-1 viral stocks of NL4-3n-GFP (pNLnEGFP-Kp; a gift from H. Akari [25]) were produced in 293T cells as described previously (7). Briefly, env mutants of pNL4-3n-GFP (pNL4-3e-n-GFP) were transfected with pLVSV-G (to pseudotype the virus) into 293T cells using ExGen 500 according to the manufacturer's instructions (Fermentas). Mutant derivatives of pNL4-3e-n-GFP used include a vif mutant (pNL4-3e-n-GFP f- [69]), a vpr mutant (pNL4-3e-n-GFP r- [69]), a vpr/vif double mutant (pNL4-3e-n-GFP fr- [69]), and a vpr substitution mutant R80A (8). Virus titers were determined by a functional multiplicity of infection (MOI) method based on the Poisson distribution as previously described (7). Virion delivery of Vpr (Vprv) has been previously described (8, 63, 76). Briefly, VSV-G-pseudotyped virus was prepared as described above using a reverse transcriptase mutant (D186N, RTm; a gift from E. Freed, National Cancer Institute, NIH) of pNL4-3e-n-GFP. The vpr gene of this construct was deleted (amino acids 22 to 86), and an expression construct for WT or mutant Vpr was cotransfected. MOIs from 0.75 to 3 were used to infect Jurkat T cells in either 12-well (7 × 105 cells/well) or 24-well (3.5 × 105 cells/well) plates in the presence of Polybrene (5 μg/ml; Sigma-Aldrich), and virus was adsorbed for 30 min at 37°C in 5% CO2. The infection plates were centrifuged for 30 min at 800 × g at room temperature.
Transfection.
Jurkat cells were transiently transfected with expression plasmids by electroporation using an Electro-Cell manipulator (BTX) apparatus. Typically, 4 × 106 cells resuspended in 0.4 ml of supplemented RPMI and 10 to 30 μg of DNA were electroporated in a 4-mm gap cuvette (Bio-Rad Laboratories) at 260 V and 1,060 μF. The transfected cells were allowed to recover in supplemented RPMI for 2 to 3 days. A plasmid expressing a fluorescent protein (pEGFP-N1 or pDsRed2-N1; Clontech) was cotransfected at a ratio of 1:5 to the expression plasmid (e.g., 2 μg of pEGFP-N1 with 10 μg of pcDNA3-hVpr) to mark the transfected cells. A transfection efficiency of 70 to 80% was regularly achieved as measured by flow cytometric analysis of green fluorescent protein (GFP) expression at 48 h posttransfection.
Assays for cell cycle and viral expression.
To measure DNA content, cells were stained with 10 μM the cell-permeable DNA dye DRAQ5 at room temperature for 5 min according to the manufacturer's instructions (Axxora). Alternatively, the cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min, washed once in PBS, and incubated for 30 min to overnight in 70% ethanol at −20°C. The cells were washed again in PBS and stained with DNA staining solution (5 μg of propidium iodide [PI]/ml, 50 μg of RNase/ml, and 0.45 mg of sodium citrate/ml in PBS) for 30 min at room temperature. Stained cells were examined on a FACSCalibur flow cytometer (Becton Dickinson). HIV-1 proviral expression in infected cells was measured by GFP fluorescence. Cells stained with DRAQ5 were measured on a FACSCalibur flow cytometer for a constant period of time (30 s) per sample, whereas a constant number of cells stained with DNA staining solution were measured. All fluorescence-activated cell sorting data was analyzed by using FlowJo software (Tree Star, Inc.). Statistical analysis was performed by using Prism software (GraphPad Software, Inc.).
Bioinformatic analysis with pkaPS software.
The primary sequence of NL4-3 Vpr (GenBank accession no. AAK08485.1) was analyzed by the pkaPS program (58). A positive score for a serine or threonine indicates a strong prediction that PKA will phosphorylate this site. A score from −0.5 to 0 indicates a low probability for phosphorylation by PKA. Scores lower than −0.5 indicate a strong prediction that PKA will not phosphorylate the residue.
Expression plasmids.
For virion delivery experiments, a human codon-optimized Vpr expression plasmid (hVpr, described previously [8]) was used. The S79A and R80A mutations were introduced by using a PfuUltra II (Stratagene) site-directed mutagenesis protocol. For fluorescence resonance energy transfer (FRET) experiments, the pEYFP-Vpr and pECFP-Vpr constructs were previously described (8). The coding sequence of the human PKA catalytic subunit, PRKACA (Origene; SC125607), was subcloned into pECFP-N1 (Clontech), yielding PKA with a C-terminal cyan fluorescent protein (CFP) tag (PKA-CFP), pEYFP-C1 (Clontech), yielding PKA with an N-terminal yellow fluorescent protein YFP tag (YFP-PKA), and pDsRed2-N1 (Clonetech), yielding PKA with a C-terminal DsRed protein tag (DsRed-PKA). For endogenous protein kinase inhibitor (PKI) experiments, the coding sequence of the human protein kinase (cAMP-dependent, catalytic) inhibitor alpha (PKIA) (Origene; TC109614) was subcloned into p3xFLAG-CMV-7.1 (p3xFLAG; Sigma-Aldrich), yielding PKI with an N-terminal FLAG tag (FLAG-PKI).
Immunoprecipitation and immunoblotting.
Infected Jurkat T cells were lysed in modified Laemmli buffer (60 mM Tris-HCl [pH 7.5], 10% glycerol, and 2% sodium dodecyl sulfate [SDS]) for 30 min at 4°C, along with treatment with 1 U of DNase (Benzonase nuclease; Novagen)/μl. All lysis buffers contained Complete protease inhibitor cocktail (Roche). Protein concentration in the lysates was estimated by using a bicinchoninic acid assay (Pierce), and equal masses of protein (typically 50 to 100 μg) were loaded onto a 4 to 20% Tris-glycine SDS gel (Invitrogen) for SDS-polyacrylamide gel electrophoresis (PAGE). Protein was transferred to nitrocellulose by using a semidry transfer apparatus (Bio-Rad).
A Nonidet NP-40-based immunoprecipitation buffer (0.2% NP-40, 150 mM NaCl, and 10 mM Tris-HCl [pH 7.5]) was used for immunoprecipitation experiments. A phosphatase inhibitor cocktail set I (EMD Biosciences) was added to all immunoprecipitation buffers. Cells were lysed for 30 min, the nuclei were removed by centrifugation at 10,000 × g for 10 min, and the protein concentration was normalized by using a bicinchoninic acid assay (Pierce). The lysate was precleared with protein G-agarose (Roche) for 30 min and immunoprecipitated with 3 μg of antibody for 3 h to overnight. All steps were performed at 4°C with gentle mixing. After the preclearing step, an aliquot of the lysate was saved as an input sample. Immunoprecipitation pellets were washed three times with lysis buffer, and the protein was eluted with 2× SDS loading buffer and separated by SDS-PAGE as described above.
Nitrocellulose blots were blocked in 5% nonfat milk in 0.1% PBS-Tween 20 (PBS-T) and probed with primary antibody for 1 h at room temperature or overnight at 4°C, followed by secondary antibody conjugated to horseradish peroxidase (HRP) diluted 1:5,000. Antibodies were diluted in 5% nonfat milk in 0.1% PBS-T. After antibody incubation, blots were washed three times in PBS-T for 5 min. For Western blotting of phosphorylated proteins, gels were transferred to methanol-treated polyvinylidene chloride membranes, retreated with methanol, and dried for 30 min. Blots were blocked, and antibodies were diluted in 5% bovine serum albumin in 0.1% PBS-T. The bands were imaged by using enhanced chemiluminescent or SuperSignal West Dura substrates (Pierce). The primary antibodies used included β-actin (Sigma-Aldrich; A1978), DsRed (Clontech; 632496), GFP (Roche; catalog no. 11814460001), FLAG-M2 (Sigma-Aldrich; F1804-200UG), p24-capsid (AIDS Research and Reference Reagent Program; catalog no. 6457), PKA catalytic subunit (Santa Cruz; sc-903), phospho-PKA substrate (P-PKA, Cell Signaling Technology; catalog no. 9624), protein G (antibody directly conjugated to HRP; Thermo Scientific; PA1-26858), and Vpr (kindly provided by K. Strebel and B. Sun). The Vpr antiserum provided by K. Strebel was used in the experiments shown in Fig. 3 and 5. The Vpr antiserum provided by B. Sun was used in the experiments shown in Fig. 1, 4, 6, and 7. Densitometry was performed by using ImageJ software (NIH).
FIG. 3.
Vpr interacts with PKA. (A) Western blots of 293T cells transfected with plasmids encoding GFP alone or GFP-Vpr and DsRed-PKA, immunoprecipitated (IP) with antibodies against GFP or an isotype control (IgG) and then immunoblotted for DsRed (top) and GFP (middle). A blot of the immunoprecipitation antibody light chain is shown to control for equivalent amounts of immunoprecipitated antibodies (bottom). (B) (Top panels) Western blots of Jurkat cells infected with NL4-3e-n-GFP encoding either WT or R80A mutant Vpr, immunoprecipitated with antibodies against PKAc and then immunoblotted for PKAc (top) and Vpr (bottom). The virus encoding WT Vpr was titrated (lo, MOI = 1; hi, MOI = 1.5) to match the Vpr protein control to that of the R80A Vpr virus (MOI = 1.5). Uninfected, mock-treated cells were used as a negative control. (Bottom panels) Western blots of Jurkat cells infected with NL4-3e-n-GFP encoding WT Vpr, immunoprecipitated with either antibodies against PKAc or no antibody, and then immunoblotted for PKAc (top), protein G (middle), and Vpr (bottom). Uninfected, mock-treated cells were used as a negative control. The asterisk denotes the heavy chain of the antibodies used to immunoprecipitate PKAc. (C) 293T cells were transfected with pECFP-N1 and pEYFP-N1-derived constructs, expressing enhanced CFP and enhanced YFP, respectively. FC-FRET analysis was performed 24 h posttransfection on live unfixed CFP/YFP double-positive cells (or CFP-positive cells in the CFP-only controls) as indicated by the gate in the density plots on the left. The control YFP Vpr and CFP cotransfected cells serve as the baseline (shaded) for the FRET signal of the other transfections (bold line) in each histogram. The MFI of the FRET signal is indicated for each sample. (D) 293T cells were transfected with pECFP-N1- and pEYFP-C1-derived constructs. FRET analysis by flow cytometry was performed 24 h posttransfection on CFP/YFP double-positive cells, as indicated by the gate in the density plots on the left. The CFP-Vpr and YFP cotransfected cells serve as the baseline (shaded) for the FRET signal of the other transfections (bold line) in each histogram. The MFI of the FRET signal is indicated for each sample.
FIG. 5.
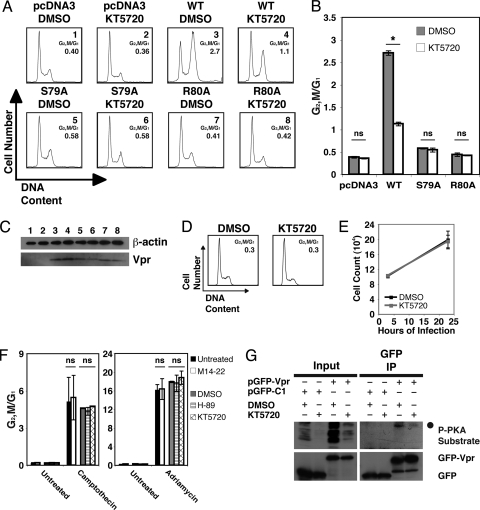
Inhibition of PKA kinase activity abrogates virion-delivered Vpr cell cycle arrest. (A) Vpr was delivered by nonreplicative virions (Vprv) into Jurkat cells and the samples were treated with M14-22 (30 or 100 μM) at the time of infection. Untreated WT Vprv was titrated (lo, low; md, medium; hi, high) to provide a matched Vpr protein control for the M14-22-treated samples. Cells were also infected with virions containing no Vpr (ΔVpr), virions containing mutant Vpr (S79A), or uninfected (Mock). Histograms of cell cycle analysis at 24 h postinfection show DNA content of propidium iodide-stained cells by flow cytometry. (B) G2/M and G1 populations were modeled by using the Dean-Jett-Fox cell cycle model, and the ratio was plotted for the cells corresponding to the numbered samples in panel A. (C) Western blot of the correspondingly numbered samples in panel A displays Vpr (bottom) or β-actin (top). The asterisk denotes a cellular protein cross-reactive with the Vpr antibody that serves as a secondary loading control. (D) Jurkat cells were mock infected and either left untreated or treated with M14-22 (100 μM). Histograms of cell cycle analysis at 26 h postinfection show DNA content of DRAQ5-stained cells by flow cytometry. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was determined. (E) Total viable cell counts at 2 and 26 h postinfection for the mock-infected samples in panel C measured by constant time flow cytometry (data are represented as mean ± the standard deviation [SD] of duplicates and are representative of more than five experiments). Linear regression analysis of the data was performed and plotted as a line with the equation. (F) WT Vpr was delivered (Vprv) into Jurkat cells and treated with either the DMSO vehicle or H-89 (10 or 15 μM) at the time of infection. Virions devoid of Vpr (ΔVpr), mutant Vprv (S79A) delivery, and mock infection were included as controls. Histograms of cell cycle analysis at 22 h postinfection show DNA content of propidium iodide-stained cells by flow cytometry. (G) G2/M and G1 populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted for the cells corresponding to the numbered samples in panel F. (H) Western blot indicates Vpr protein delivery for DMSO- or H-89-treated Jurkat cells in panel F (bottom). β-Actin is shown as a protein loading control (top). The asterisk in the bottom blot denotes a cross-reactive cellular protein that is used as a secondary loading control.
FIG. 1.
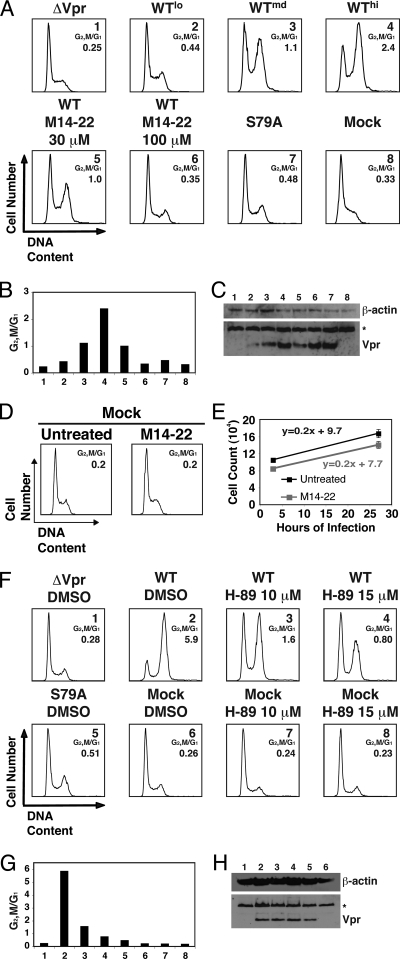
Serine 79 and arginine 80 are critical residues for Vpr cell cycle arrest in T cells. (A) Schematic of the NL4-3 HIV-1 molecular clones used. The clone NL4-3e-n-GFP lacks a functional env gene, due to a frameshift mutation, and the nef gene was replaced with EGFP. The D186N reverse transcriptase, Vpr Δ22-88 mutant (RTm, Vpr Δ22-88), is a nonreplicative clone that lacks endogenous Vpr and is used to deliver transcomplemented functional Vpr via virions into target cells without viral integration or replication. (B) WT or mutant Vpr proteins (denoted by the single-letter amino acid changes) were delivered (Vprv) into Jurkat cells. Virions containing WT Vpr were titrated (lo, low; md, medium; hi, high) so that a matched Vpr protein control could be compared to the mutants. Histograms of cell cycle analysis at 42 h postinfection show DNA content of DRAQ5-stained cells by flow cytometry. The ratio of G2/M to G1 (as in panel C) in each infection is shown. (C) G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted for the cells corresponding to the same numbered samples in panel B. (D) Western blot of the Jurkat cells in panel B for WT and mutant virion-delivered Vpr (bottom). β-Actin is shown as a protein loading control (top). (E) Jurkat cells were cotransfected with pcDNA3-hVpr plasmids expressing WT or mutant Vpr (or the empty vector control) and pEGFP-N1 as a transfection marker at a ratio of 5:1. Histograms of cell cycle analysis at 61 h posttransfection show DNA content of GFP+, DRAQ5-stained cells by flow cytometry. (F) G1 and G2/M populations were modeled and plotted as in panel C for the cells corresponding to the same numbered samples in panel E. (G) Western blot of the Jurkat cells in panel E for WT or mutant Vpr (bottom) with β-actin shown as a protein loading control (top).
FIG. 4.
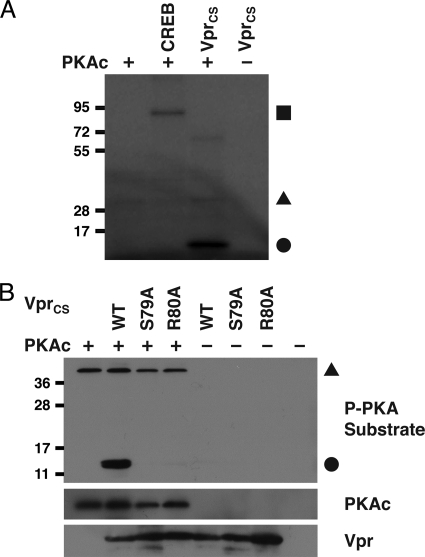
PKA phosphorylates serine 79 of Vpr. (A) An in vitro kinase assay was performed by incubating 50 ng of recombinant PKA catalytic subunit (PKAc) with 1 μg of either recombinant CREB or chemically synthesized full-length WT Vpr (Vprcs) and 0.5 μCi of [γ-32P]ATP. Autoradiography detected phosphorylated CREB (▪), phosphorylated Vpr (•), and autophosphorylated PKA (▴). (B) Western blots of a nonradioactive in vitro kinase assay performed by incubating 100 ng of recombinant PKAc with 1 μg of WT and mutant (S79A and R80A) Vprcs. Phosphorylated Vpr (•) and autophosphorylated PKA (▴) was detected by using an anti-phospho (P) PKA substrate antibody (top). Total protein levels of PKAc (middle) and Vpr (bottom) are shown, and the positions of size markers (relative migration in kilodaltons) are indicated at the left of each blot.
FIG. 6.
Cell cycle arrest induced by transfected Vpr is reduced by inhibition of PKA activity. (A) Jurkat cells were cotransfected with pcDNA3-hVpr plasmids expressing WT or mutant Vpr (or the empty vector control) and pEGFP-N1 as a transfection marker at a ratio of 5:1. At 24 h posttransfection, the cells were replated and treated with either DMSO or KT5720 (1 μM). Histograms of cell cycle analysis at 36 h posttreatment show DNA content of GFP+, DRAQ5-stained cells by flow cytometry. (B) G2/M and G1 populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted for the cells corresponding to the samples in panel A (data are represented as mean ± the SD of duplicates). The asterisk denotes P < 0.001 as analyzed by one-way analysis of variance (ANOVA) with multiple-comparison tests. ns, Not significant. (C) Western blot of the Jurkat cells in panel A indicates protein levels for WT or mutant Vpr (bottom). β-Actin is shown as a protein loading control (top). (D) Jurkat cells were treated with either DMSO or KT-5720 (1 μM). Histograms of cell cycle analysis at 23 h posttreatment show DNA content of DRAQ5-stained cells by flow cytometry. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was determined. (E) Total viable cell counts at 3 and 23 h posttreatment for the samples in panel F measured by constant time flow cytometry (data are represented as mean ± the SD of duplicates and are representative of more than five experiments). (F) Jurkat cells were either untreated or treated with camptothecin (left) or adriamycin (right) and then either left untreated or secondarily treated with M14-22 (100 μM), DMSO, H-89 (15 μM), or KT-5720 (1 μM). Histograms of cell cycle analysis at 48 h (camptothecin) or 24 h (adriamycin) posttreatment show DNA content of DRAQ5-stained cells by flow cytometry. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was determined and plotted (data are represented as mean ± the SD of duplicates and are representative of three experiments). Statistical analysis showed no significant difference (ns) between PKA inhibitors versus the vehicle treated controls by two-way ANOVA. (G) Western blots of 293T cells transfected with plasmids encoding either GFP or GFP-Vpr and treated with either DMSO or KT5720 (10 μM), immunoprecipitated with antibodies against GFP, and then immunoblotted for phosphorylated PKA substrates (top) and GFP (bottom). The P-PKA substrate antibody reveals phosphorylated Vpr (•) in the GFP immunoprecipitation (IP).
FIG. 7.
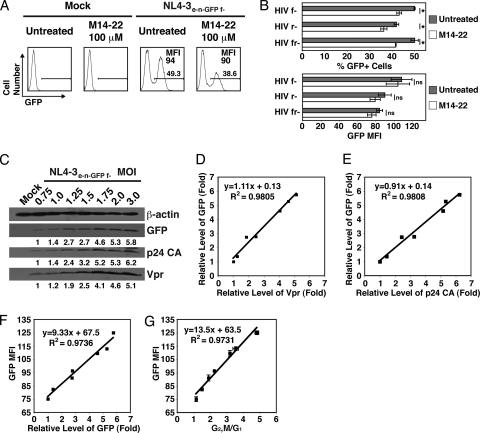
M14-22 treatment reduces HIV-1 infection, and GFP expression from the virus correlates with overall viral expression. (A) Jurkat cells were either mock infected or infected with NL4-3e-n-GFP f- encoding WT Vpr at an MOI of 2 and either not treated or treated with M14-22 (100 μM). Histograms of GFP expression at 26 h postinfection show the percentage of infected cells (GFP+ gate) and the GFP MFI of infected cells. (B) Jurkat cells were infected with NL4-3e-n-GFP f- (HIV f-) encoding WT Vpr and null for Vif, infected with NL4-3e-n-GFP r- (HIV r-) encoding WT Vif and null for Vpr, or infected with NL4-3e-n-GFP fr- (HIV fr-) that is null for both Vif and Vpr at an MOI of 2 and either not treated or treated with M14-22 (100 μM). The percentage of infected GFP+ cells (top) and the GFP MFI in the infected cells (bottom) were plotted for the infected samples (data are represented as mean ± the SD of triplicates from three separate experiments). The asterisk denotes P < 0.01 as analyzed by one-way ANOVA with multiple comparison tests. ns, Not significant. (C) Jurkat cells were infected with NL4-3e-n-GFP f- encoding WT Vpr at the indicated MOIs. Western blot of Jurkat cells indicates expression levels for Vpr, HIV-1 p24 capsid (CA), and GFP at 27 h postinfection. β-Actin is shown as a protein loading control (top). Densitometry of all bands was performed, and the intensity of each viral protein band was normalized to β-actin. The fold change in viral protein expression is shown under the Western blot for each. (D) The fold change of Vpr based on Western blot analysis in panel C was plotted against that of GFP based on the Western blot analysis in panel C. Linear regression analysis of the data was performed and plotted as a line with the equation and R2 value. (E) HIV-1 p24 CA was plotted against GFP and analyzed as in panel D. (F) Flow cytometry to assess GFP expression was performed at 26 h postinfection. The fold change of GFP was plotted against the GFP MFI for each MOI in panel C. Linear regression analysis of the data was performed as in panel D. (G) Flow cytometry to assess GFP MFI and DNA content using DRAQ5 was performed at 26 h postinfection. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted against the GFP MFI for each MOI (data are represented as mean ± the SD of duplicates and are representative of more than five experiments). Linear regression analysis of the data was performed as in panel D.
FC-FRET.
293T cells were transiently transfected by using ExGen 500 (Fermentas) as described above with a 1:1 ratio of CFP and YFP plasmids. The plasmids used in these experiments included pECFP-N1, pEYFP-C1, a fusion of CFP and YFP (pECFP-EYFP), pECFP-PKA, pEYFP-PKA, pEYFP-Vpr, and pECFP-Vpr. The cells were harvested at 24 h posttransfection, and FRET was detected by flow cytometry as previously described (8, 73). Only the CFP+ YFP+ double-positive cells were analyzed for FRET. An increase in mean fluorescence intensity (MFI) indicates real-time physical interaction between proteins in living cells.
In vitro PKA assay.
PKA phosphorylation was assessed in vitro by a PKA assay kit (Millipore) by using purified PKA (50 ng) and substrate (1 μg) in the reaction buffer (40 mM morpholinepropanesulfonic acid [pH 7.0], 17 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 125 μM ATP, 6 mM β-glycerol phosphate, 200 μM sodium orthovanadate). For autoradiography, 0.5 μCi of [γ-32P]ATP (Perkin-Elmer) was added. The reaction was stopped after 30 min at 37°C by adding equal volumes of 2× SDS sample buffer. Vpr phosphorylation was analyzed by autoradiography after SDS-PAGE. Nonradioactive kinase assays were analyzed by Western blotting with a P-PKA substrate-specific antibody. The substrates of in vitro kinase assays were recombinant cAMP response element binding protein (CREB; Active Motif) and Vprcs (WT, S79A, R80A; chemically synthesized by the Research Technologies Branch of National Institute of Allergy and Infectious Diseases [NIAID], NIH).
RESULTS
Mutations of serine 79 and arginine 80 of HIV-1 Vpr to alanine abrogate G2/M cell cycle arrest in Jurkat T cells.
The previous study examining the link between Vpr phosphorylation and cell cycle arrest was performed with HEK293, a human embryonic kidney cell line (83). Therefore, we first validated these findings in the Jurkat CD4+ T-cell leukemia line, a more relevant cellular context for HIV infection (Fig. 1). We examined Vpr cell cycle arrest using virion delivery of Vpr (Vprv, nonreplicative virions were produced using a reverse transcriptase mutant of HIV-1 and Vpr was transcomplemented using separate expression plasmids, as previously described [8]) (Fig. 1A). When Vpr was delivered via HIV-1 virions, we found that both Vpr S79A and R80A mutations reduced cell cycle arrest in Jurkat T cells, which is consistent with previous findings (Fig. 1B and C) (83). Western blot analysis confirmed equivalent delivery and stability of wild type (medium dose; WTmd [3]) and mutant (S79A [5] and R80A [6]) Vpr in the target cells (Fig. 1D). We also compared endogenously expressed WT and mutant Vpr by ectopic expression using transient transfection of Jurkat cells. Similar to the results obtained by virion delivery, transfected S79A and R80A Vpr mutants exhibited reduced G2/M arrest (Fig. 1E and F). Similar levels of expression of the WT and mutant Vpr proteins were confirmed by Western blot analysis (Fig. 1G). These data indicate that phosphorylation of S79 plays a critical role in Vpr-mediated G2/M cell cycle arrest in Jurkat T cells.
Serine 79 is part of a putative phosphorylation site recognized by the cAMP-dependent PKA.
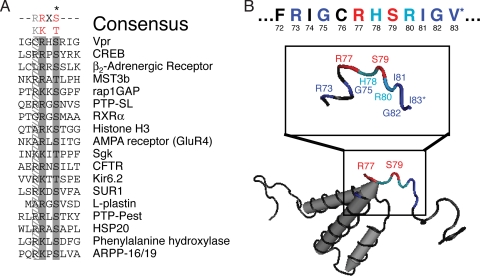
To uncover the kinase that modifies the critical phosphorylation site (S79) in Vpr, we initially used the eukaryotic linear motif (ELM) server to carry out an unbiased interrogation of the primary sequence of Vpr from the molecular clone NL4-3 (GenBank accession no. AAK08485.1) (65). This analysis indicated that serine 79 was a putative PKA phosphorylation site, demarcated by the tripeptide sequence [R/K]X[S/T] (with “X” representing any amino acid), often with an additional arginine at the −3 position (65, 72) (Fig. 2A and B, displayed in red). Because this motif is short with low entropy, we independently confirmed this with pkaPS, a PKA-exclusive phosphorylation prediction program that uses a substrate/protein binding model to more accurately predict PKA phosphorylation sites (58). The motif surrounding serine 79 received a positive score (0.95) from pkaPS, indicating a high likelihood that it is a PKA target, while all other serines and threonines in Vpr received negative scores (−0.75 to −3.89). Although the −2 arginine/lysine is thought to be critical for phosphorylation, PKA sites also contain several key subsidiary amino acids that are present in the putative Vpr target site (Fig. 2B, red for critical residues, dark blue for major, and light blue for minor contributing amino acids) (72). At the −6 position relative to the serine, a positively charged residue is often preferred because it would interact with glutamic acid 203 in the active site of PKA (Fig. 2B, R73 in dark blue) (56, 72). Small amino acids show an increased frequency at position −4 in verified physiological substrates (Fig. 2B, G75 in dark blue) (72). Arginine, and to a lesser extent histidine, are preferred at the −1 residue (Fig. 2B, H78 in light blue) (74). The residue immediately after the phosphorylated serine is weighted toward amino acids preferred in β-strands (Fig. 2B, R80 in light blue) (58). Positions +2 to +4 exhibit a preference for either hydrophobic or small residues (Fig. 2B, I81, G82, and V83 in dark blue) (72). Hence, these bioinformatics analyses provided strong presumptive evidence that Vpr S79 is a substrate of PKA.
FIG. 2.
Serine 79 of Vpr is within a putative PKA phosphorylation motif. (A) Alignment of PKA target sites in human physiological substrates of PKA (adapted from Shabb [72]). The asterisk (*) indicates the phosphorylated serine or threonine. Gray shading indicates conserved residues defining the simple consensus PKA recognition motif (top). (B) Diagram of the nuclear magnetic resonance structure of HIV-1 Vpr (PDB 1M8L), indicating the location of serine 79 (bottom). Serine 79 and the surrounding residues of the extended PKA phosphorylation preferred motif are shown in the inset with the primary sequence of amino acids in Vpr from HIV-1 NL4-3 (top). The two residues forming the putative simple phosphorylation site are indicated in red. Other important residues are colored light and dark blue for minor and major contributing amino acids, respectively. *I83 (middle) in the structure is a valine (top) in the NL4-3 Vpr protein sequence used in the present study (GenBank accession no. AAK08485.1).
Vpr directly interacts with PKA.
To biochemically validate the possibility that Vpr S79 is a target of PKA, we next examined whether Vpr could associate with PKA by using immunoprecipitation (39). We first cotransfected 293T cells with a DsRed-tagged PKA (DsRed-PKA) expression plasmid and either a plasmid expressing GFP alone or GFP-tagged Vpr (GFP-Vpr). Immunoprecipitation of GFP revealed that DsRed-PKA bound GFP-Vpr (Fig. 3A). To investigate the interaction between Vpr and PKA under more physiologically relevant conditions, Jurkat T cells were productively infected with HIV-1 NL4-3e-n-GFPWT expressing wild-type (WT) Vpr (Fig. 1A) at both a low and a high MOI. Vpr was found associated with the PKA catalytic subunit (PKAc) from infected cells in proportion to input Vpr levels (Fig. 3B, top and bottom panels). Mutation of R80 blocks phosphorylation on serine 79 in vivo (83), leading us to hypothesize that it contributes to the recognition of Vpr S79 by PKA (Fig. 2B, R80 in light blue). Indeed, we found that significantly less Vpr R80A mutant binds to PKAc compared to the WT Vpr (Fig. 3B, top panels, compare input and immunoprecipitation [IP] WT lo versus R80A for matched protein control). Control experiments showed that essentially no Vpr nonspecifically precipitated with protein G beads alone (Fig. 3B, bottom panels).
We next confirmed Vpr-PKA interactions in live human 293T cells by using flow cytometric fluorescence resonance energy transfer (FC-FRET) analysis (73). We have previously used this method to observe Vpr dimerization in live cells in real time (8). Significant FRET was detected in the cells transfected with the positive control CFP-YFP fusion protein (Fig. 3C, a FRET MFI of 6,652 versus an MFI of 120 for the YFP-Vpr and CFP control). Cells coexpressing CFP-Vpr and YFP-Vpr also exhibited positive FRET signals as previously observed, indicating self-association (8) (Fig. 3C). More importantly, when a PKA-CFP construct was cotransfected with YFP-Vpr, we observed a significant shift in the FRET MFI, indicating that the two proteins were directly interacting (Fig. 3C). Because flow cytometry is carried out without any fixation or external stains, these data demonstrate the interaction in real time in living cells. Cells expressing CFP-Vpr or PKACFP alone showed no FRET signal above background, as expected in the absence of the acceptor fluorophore (Fig. 3C). Significant FRET was also detected between YFP-PKA and CFP-Vpr (Fig. 3D). Since both forms of PKA exhibited an ∼4-fold increase of FRET signal above the relevant baseline when interacting with tagged Vpr, the location (N or C terminus) or nature (donor or acceptor) of the fluorescent protein fused to PKA does not affect the in vivo interaction with Vpr. This interaction with Vpr could explain the selective packaging of the PKAc into HIV-1 virions (12).
PKA directly phosphorylates serine 79 of Vpr.
We next examined whether PKA could directly phosphorylate Vpr using an in vitro kinase assay. As a positive control, we first showed that recombinant PKA was able to phosphorylate CREB, a well-characterized PKA substrate (Fig. 4 A) (27, 30, 32, 72). In order to be sure that Vpr was devoid of any preexisting phosphoamino acids, we chemically synthesized the full-length WT Vpr protein (Vprcs). We found that Vprcs was directly phosphorylated by recombinant PKA in a radioactive in vitro kinase assay (Fig. 4A), as well as in a nonradioactive assay utilizing Western blot analysis of phosphorylated PKA substrates (Fig. 4B). Chemically synthesized mutant versions of Vpr, harboring either S79A or R80A, failed to be phosphorylated (Fig. 4B). Although S79, S94, and S96 are all phosphorylated in HIV-1-infected cells (83), these results show that PKA specifically phosphorylates serine 79 of Vpr, which requires the adjacent arginine 80, presumably to conformationally favor the recognition.
PKA activity is necessary for virion-delivered Vpr cell cycle arrest.
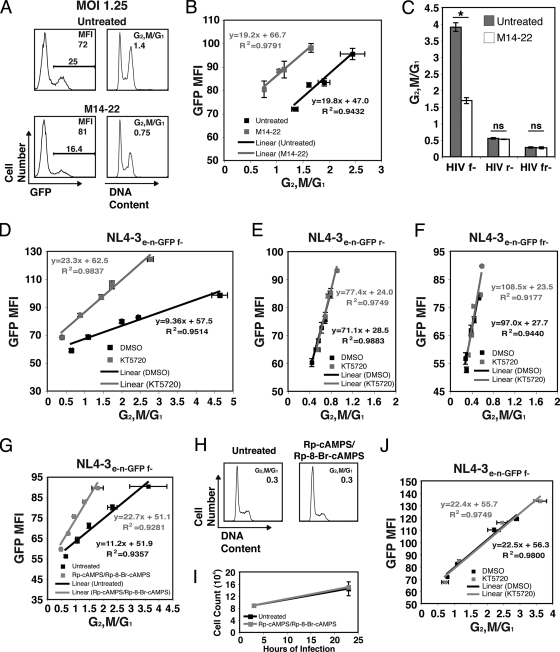
Because PKA phosphorylates S79 of Vpr, we next examined whether PKA function was critical for Vpr cell cycle arrest using multiple PKA inhibitors to chemically “knock out” PKA activity (39). We first tested whether inhibiting PKA activity affects G2/M arrest caused by Vprv. Jurkat cells were treated with the myristoylated 14-22 amide peptide (M14-22) PKA inhibitor at the time of virion delivery. M14-22 is a cell-permeable, nonphosphorylatable peptide pseudosubstrate that interferes with PKA signaling by competing with bona fide substrates for the active kinase (28). Because GFP is not expressed when Vpr is delivered by nonreplicative virions, we tested a range of WT Vprv doses in target cells to normalize the untreated and M14-22-treated samples (Fig. 5A to C). We found that M14-22 dramatically reduced G2/M cell cycle arrest in a dose-dependent manner under these conditions (Fig. 5A and B). The 30 μM sample had a small effect on the cell cycle profile compared to the WTmd sample, which was best matched for levels of Vpr (Fig. 5A to C, compare samples 3 and 5). However, 100 μM M14-22 completely abrogated the Vpr cell cycle arrest, such that the cell cycle profile was indistinguishable from cells receiving no Vpr (ΔVpr) or Vpr S79A (Fig. 5A to C, compare samples 1, 4, 6, and 7). Mock-infected cells either untreated or treated with M14-22 (100 μM) exhibited similar cell cycle profiles and growth kinetics (Fig. 5D and E). We next tested H-89, a selective inhibitor of PKA that blocks ATP binding in the active site of PKA (15). H-89 also reduced virion-delivered Vpr cell cycle arrest in a dose-dependent manner (Fig. 5F to H). However, mock-infected cells exhibited a similar cell cycle profile regardless of DMSO or H-89 treatment (Fig. 5F and G). Thus, we found that two functionally distinct PKA inhibitors implicate PKA activity in Vprv-induced cell cycle arrest.
Transfected Vpr-induced cell cycle arrest is reduced by PKA inhibition.
Transfection of Jurkat cells with WT and mutant Vpr yielded results similar to those seen for virion delivery of Vpr (Fig. 1). We therefore tried inhibiting PKA activity during transient transfection of Vpr using KT5720, another compound that selectively competes with ATP rather than the substrate for the active site of PKA (41). Treatment of WT Vpr-expressing cells with KT5720 significantly reduced Vpr-induced G2/M arrest (Fig. 6A and B). Western blot analysis confirmed equivalent expression of Vpr between the DMSO- and KT5720-treated samples (Fig. 6C). PKA inhibition had no affect on cells expressing mutant Vpr (Fig. 6A to C). Similar to M14-22 and H-89, inhibiting PKA in Jurkat T cells with KT5720 had no effect on the cell cycle profile or growth (Fig. 6D and E). In order to control for any nonspecific effects of PKA inhibition on cell cycle arrest, we tested the ability of M14-22, H-89, and KT5720 to reduce cell cycle arrest induced by camptothecin or adriamycin, which activate the ATR/ATM DNA damage checkpoints (24, 35). As Fig. 6F shows, inhibiting PKA activity had no effect on camptothecin- or adriamycin-induced G2 arrest. Using transient transfection of GFP-Vpr, we could assess the consequence of PKA inhibition on Vpr phosphorylation. Either GFP or GFP-Vpr was expressed in 293T cells that were treated with either DMSO or KT5720. Because GFP was expressed at much higher levels than GFP-Vpr (data not shown), the lysates from the GFP cells were diluted 4-fold, so that the protein levels of GFP and GFP-Vpr would be equivalent before immunoprecipitation. Treatment of 293T cells with KT5720 reduced the overall phosphorylation by PKA as assessed by the P-PKA substrate antibody (Fig. 6G, input). An immunoprecipitation of GFP yielded a phosphorylated band corresponding to the size of GFP-Vpr in the DMSO sample that was significantly reduced in the KT5720-treated cells (Fig. 6G). Western blot analysis indicated that equivalent amounts of GFP and GFP-Vpr were precipitated from DMSO- and KT5720-treated cells (Fig. 6G, IP GFP blot). These data indicate that the inhibition of PKA activity specifically reduces Vpr-induced cell cycle arrest and Vpr S79 phosphorylation during transient transfection.
Inhibition of PKA activity reduced infectivity but did not affect the proviral expression in infected cells.
Since active PKAc incorporated into the HIV-1 virion is apparently important for infectivity (12), we tested whether the addition of a PKA inhibitor at the time of infection reduced infectivity. We found that M14-22 modestly reduced the level of infection (% GFP+ population) when using a single infectious dose of HIV-1 f- (NL4-3e-n-GFP f-) (Fig. 7A and B). HIV-1 strains lacking Vpr (HIV r-, NL4-3e-n-GFP r-; HIV fr-, NL4-3e-n-GFP fr-) also exhibited a modest but significantly reduced infectivity, which agrees with the previous finding that phosphorylation of p24 capsid by PKA contributes to infectivity (12) (Fig. 7B). During productive infection with these viral strains, GFP can be used as a reporter viral protein that is expressed from the integrated provirus (Fig. 1A). Therefore, the GFP MFI accurately reflects the level of proviral expression during HIV-1 infection. While the percentage of GFP+ (infected) cells is reduced by M14-22, the expression of GFP, based on the MFI, was not affected by inhibiting PKA (Fig. 7A and B). To further validate this measurement of viral expression, we infected Jurkat T cells with NL4-3e-n-GFP f- using a range of MOIs. The amount of GFP expressed during infection increased with greater MOIs, similar to p24 capsid (CA) and Vpr, based on densitometry analysis of the Western blots (Fig. 7C). Furthermore, statistical analysis of GFP, p24 CA, and Vpr expression revealed a strong linear correlation between these proteins (Fig. 7D and E). The level of fluorescence for GFP (GFP MFI) was precisely correlated with the amount of GFP protein expressed, confirming GFP as a valid measure of overall proviral expression (Fig. 7F). In addition, GFP MFI was also strongly correlated with the level of Vpr cell cycle arrest (Fig. 7G). These results confirm that GFP MFI could be used as an indirect measure of the per cell level of Vpr in infected cells, strengthening the conclusion that PKA inhibition does not greatly affect viral expression.
Inhibiting PKA activity during HIV-1 infection reduced Vpr cell cycle arrest.
During productive infection, Vpr is initially delivered into the cell from the virion and subsequently endogenously expressed at much higher levels from the integrated provirus. We therefore tested M14-22 during productive infection with a vif mutant strain (NL4-3e-n-GFP f-). Vif is important because it can independently induce G2/M arrest (69). Jurkat cells were infected with increasing MOIs and treated with 100 μM M14-22. Since M14-22 reduced infectivity of the population without affecting the expression of viral proteins (Fig. 7A and B and Fig. 8A, left), we compared the cell cycle arrest of treated cells with that of the untreated cells by comparing equivalent levels of proviral expression (GFP MFI) (Fig. 8A and B). We observed that GFP MFI was linearly correlated with increasing Vpr cell cycle arrest (Fig. 7G and 8B). While M14-22-treated cells also exhibited a strong correlation between GFP MFI and Vpr-induced G2/M arrest, cell cycle blockade was markedly reduced without a change in the level of proviral expression (Fig. 8B). The equivalent slope of the two lines suggests that M14-22 competes with Vpr for active PKA. We also tested the effect of M14-22 treatment on HIV-1 strains lacking Vpr (HIV r-, NL4-3e-n-GFP r-; HIV fr-, NL4-3e-n-GFP fr-). Vif-induced cell cycle arrest, although known to be modest at this time point, was not affected by PKA inhibition (Fig. 8C, HIV r-). Similar to mock-infected cells (Fig. 5D), the cell cycle profile of cells infected with a nonarresting virus was not changed by M14-22 treatment (Fig. 8C, HIV fr-). The GFP MFI of the untreated and M14-22 samples were equivalent (data not shown).
FIG. 8.
Inhibiting PKA activity reduces Vpr cell cycle arrest during HIV-1 infection. (A) Jurkat cells were infected with NL4-3e-n-GFP f- encoding WT Vpr at MOIs of 1.25, 1.5, 1.75, and 2 and either left untreated or treated with 100 μM M14-22. Histograms of GFP expression at 23 h postinfection with an MOI of 1.25 show the percentage of infected cells (GFP+ gate) and the GFP MFI in infected cells (left) by flow cytometry. Histograms of cell cycle analysis at 23 h postinfection show the DNA content of infected (GFP+), DRAQ5-stained cells by flow cytometry (right). (B) The ratios of G2/M to G1 populations were modeled from the infection in panel A by using the Watson Pragmatic cell cycle model, and the ratio was plotted against the GFP MFI for each MOI (right) (data are represented as mean ± the SD of duplicates and are representative of more than five experiments). Linear regression analysis of the data was performed and plotted as a line with the equation and R2 value listed. Statistical analysis showed that the slopes were not statistically different (P = 0.892), but the x axis intercepts differed significantly (P < 0.00017), reflecting the competitive inhibitory effect of M14-22 on G2/M cell cycle arrest. (C) Jurkat cells were infected with NL4-3e-n-GFP f- (HIV f-) encoding WT Vpr and null for Vif, infected with NL4-3e-n-GFP r- (HIV r-) encoding WT Vif and null for Vpr, or infected with NL4-3e-n-GFP fr- (HIV fr-) that is null for both Vif and Vpr at an MOI of 2 and either untreated or treated with M14-22 (100 μM). Flow cytometry to assess GFP MFI and DNA content using DRAQ5 was performed at 26 h postinfection. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted (data are represented as mean ± the SD of triplicates and are representative of three experiments). The asterisk denotes P < 0.001 as analyzed by one-way ANOVA with multiple comparison tests. ns, Not significant. (D) Jurkat cells were infected with NL4-3e-n-GFP f- encoding WT Vpr at MOIs of 0.75, 1.25, 1.75, 2, and 3 and treated with either DMSO (vehicle control) or KT5720 (1 μM). Flow cytometric assessment of GFP and DNA was performed as in panels A and B, and the G2/M and G1 ratios were analyzed and plotted as in panel B (data are represented as mean ± the SD of duplicates and are representative of more than five experiments). Statistical analysis showed that the slopes were statistically different (P < 0.0001), reflecting the noncompetitive inhibitory effect of KT5720 on G2/M cell cycle arrest. (E) Jurkat cells were infected with NL4-3e-n-GFP r- encoding WT Vif and null for Vpr as in panel D. Flow cytometric analysis was performed, and the data were plotted as in panel D (data are represented as mean ± the SD of duplicates and are representative of three experiments). Statistical analysis showed no significant differences between the slopes (P = 0.457) or the x-axis intercepts (P = 0.951). (F) Jurkat cells were infected with NL4-3e-n-GFP fr- that is null for both Vif and Vpr as in panel D. Flow cytometric analysis was performed, and the data were plotted as in panel D (data are represented as mean ± the SD of duplicates and are representative of three experiments). Statistical analysis showed no significant differences between the slopes (P = 0.617) or the x-axis intercepts (P = 0.605). (G) Jurkat cells were infected with NL4-3e-n-GFP f- encoding WT Vpr at MOIs of 0.75, 1.25, 1.75, 2, and 3 and either left untreated or treated with the combination of Rp-cAMPS (500 μM) and Rp-8-Br-cAMPS (500 μM). Flow cytometric assessment of GFP and DNA performed as in panel D, and G2/M and G1 ratios were analyzed and plotted as in panel D (data are represented as mean ± the SD of duplicates and are representative of three experiments). Statistical analysis showed that the slopes were statistically different (P < 0.0003), reflecting the noncompetitive inhibitory effect of cAMP analogs on G2/M cell cycle arrest. (H) Jurkat cells were either left untreated or treated with the combination of Rp-cAMPS (500 μM) and Rp-8-Br-cAMPS (500 μM). Histograms of cell cycle analysis at 23 h posttreatment show DNA content of DRAQ5-stained cells by flow cytometry. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was determined. (I) Total viable cell counts at 3 and 23 h posttreatment for the samples in panel H measured by constant time flow cytometry (data are represented as mean ± the SD of duplicates and are representative of three experiments). (J) Jurkat cells were infected with NL4-3e-n-GFP f- encoding WT Vpr produced from 293T cells treated with either DMSO or KT5720 (1 μM) at MOIs of 0.75, 1.25, 2.5, and 3. Flow cytometric assessment of GFP and DNA was performed as in panel D, and the G2/M and G1 ratios were determined and plotted as in panel D (data are represented as mean ± the SD of duplicates and are representative of three experiments). Statistical analysis showed no significant differences between the slopes (P = 0.982) or the x-axis intercepts (P = 0.748).
To confirm the results with M14-22 inhibition of PKA, we next tested KT5720. Treatment of HIV-1-infected cells with KT5720 reduced Vpr cell cycle arrest at equivalent viral expression levels (GFP MFI) (Fig. 8D). The divergent slopes of the linear correlations suggest noncompetitive inhibition of PKA in relation to Vpr. Similar to M14-22, treatment of cells infected with Vpr-null strains of HIV with KT5720 had no effect on the cell cycle profile (Fig. 8E and F). M14-22, H-89, and KT5720 all directly act on the catalytic subunit of PKA. However, PKA activity can be inhibited without direct inhibition of the kinase. Analogs of cAMP are effective in inhibiting the release of PKAc from PKA regulatory subunits (16). The combination of Rp-8-Br-cAMPS and Rp-cAMPS selectively binds and inhibits type I and II PKA regulatory subunits, respectively, without an effect on the exchange protein directly activated by cAMP (Epac) (16, 64). Treatment with Rp-cAMPS and Rp-8-Br-cAMPS reduced Vpr G2/M arrest at similar viral expression levels during HIV-1 infection (Fig. 8G). This combination treatment did not affect the cell cycle or growth of Jurkat cells (Fig. 8H and I). Because only the catalytic subunits of PKA are incorporated into HIV-1 virions (12), the finding that cAMP analogs could inhibit Vpr cell cycle arrest suggests that the majority of Vpr S79 phosphorylation occurs in the target cell. To further address this issue, we treated 293T cells with either DMSO or KT5720 during virus production to inhibit the PKAc that is incorporated into virions. When these viruses were used to infect untreated Jurkat cells, there was no effect on cell cycle arrest (Fig. 8J), indicating that inhibiting PKA in the producer cell has no effect and that PKAc packaged with the virus likely does not phosphorylate Vpr to any significant degree.
Since the major source of kinase for Vpr phosphorylation is located in the target cell, we inferred that we could inhibit PKA activity without the use of drug inhibitors. Jurkat cells were transiently transfected with a FLAG-tagged full-length protein kinase inhibitor protein (FLAG-PKI), which is a highly selective and potent inhibitor of PKA (61). PKI is the endogenous origin of the synthetic peptide M14-22 (14). Infection of cells expressing FLAG-PKI with HIV-1 resulted in reduced cell cycle arrest with equivalent viral expression compared to cells transfected with an empty FLAG expression plasmid (Fig. 9A). Western blot analysis confirmed the expression of FLAG-PKI (Fig. 9B). As with all methods of inhibiting PKA activity, the overexpression of PKI had no effect on the cell cycle profile or growth of Jurkat cells (Fig. 9C and D). Thus, PKA activity in the newly infected target cell is apparently critical for Vpr-induced G2/M arrest during HIV-1 infection.
FIG. 9.
Endogenous expression of protein kinase inhibitor (PKI) reduces Vpr cell cycle arrest during HIV-1 infection. (A) Jurkat cells were cotransfected with a p3xFLAG-PKI expression plasmid (or the empty vector control) and pDsRed-N1as a transfection marker at a ratio of 3:1. The transfected cells were infected with NL4-3e-n-GFP f- encoding WT Vpr at MOIs of 1.25, 1.75, 2, and 2.5 at 38 h posttransfection. Flow cytometry to assess GFP MFI and DNA content using DRAQ5 was performed at 23 h postinfection. The ratios of G2/M and G1 populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was plotted against the GFP MFI for each MOI (data are represented as mean ± the SD of duplicates and are representative of three experiments). Linear regression analysis of the data was performed and plotted as a line with the equation and R2 value listed. Statistical analysis showed that the slopes were not statistically different (P = 0.149), but the x axis intercepts differed significantly (P < 0.0001) reflecting the competitive inhibitory effect of PKI on G2/M cell cycle arrest. (B) Western blot of the Jurkat cells in panel A indicates expression of PKI by an immunoblot for FLAG (bottom). β-Actin is shown as a protein loading control (top). (C) Jurkat cells transfected as in panel A and left untreated. Histograms of cell cycle analysis at 23 h posttreatment show DNA content of DRAQ5-stained cells by flow cytometry. G1 and G2/M populations were modeled by using the Watson Pragmatic cell cycle model, and the ratio was determined. (D) Total viable cell counts at 3 and 23 h posttreatment for the samples in panel C measured by constant time flow cytometry (data are represented as mean ± the SD of duplicates and are representative of three experiments).
DISCUSSION
In this report, we have shown that PKA phosphorylation of Vpr at serine 79 is necessary for its ability to cause G2/M cell cycle arrest. We found that Vpr is phosphorylated by PKA using the molecular clone of HIV-1, NL4-3, which contains an arginine at the −2 position relative to serine 79. However, that arginine is only found in roughly 35 to 40% of the Vpr sequences annotated in the Pfam database, with a significant proportion of the remaining sequences containing a glutamine at that position (22). Because the change to glutamine may reduce Vpr phosphorylation by PKA (58), it is notable, although contested, that the Vpr R77Q mutation has been associated with lower cell death in vitro and slower disease progression in vivo (51, 55, 66). Other groups have shown no effect of the R77Q mutation on disease progression in vivo and in vitro functions (17, 23, 38). The question of whether the R77Q mutation of Vpr affects S79 phosphorylation by PKA (some confirmed substrates of PKA encode a glutamine at the −2 position [72]), as well as Vpr function, will require further examination. Our results suggest the possibility that abrogating PKA-mediated phosphorylation of Vpr may make HIV-1 less lethal to T lymphocytes. However, we have previously shown the R80A mutation of Vpr, which abrogates the G2/M arrest, uncovers an ability to arrest cells in G1, which proved to be just as lethal during HIV-1 infection (8). There are also caveats to concluding that all of the effects of the R80A mutation are due to reduced phosphorylation. Arginine is a large positively charged amino acid, and the change to alanine, a small nonpolar residue, may affect the overall structure of the protein and not just the ability of PKA to recognize S79. There appear to be multiple molecular mechanisms entrained to HIV-1 infection that contribute to cell cycle blockade and death (8).
Cartier et al. showed that PKA is important in HIV-1 infection, via catalytic subunits packaged into virions (12). That study did not explain how PKAc is selectively incorporated into virions. Because Vpr and PKA are interacting in infected cells, perhaps Vpr acts to bring PKAc into budding virions through its interaction with the p6 region of the Gag p55 precursor (4, 42, 45, 50, 62). A portion of Vpr in the virus is phosphorylated (ca. 5%) (57). However, that study did not define which residue was modified. Our data indicate that the majority of Vpr S79 phosphorylation occurs within the newly infected target cell. Perhaps the Vpr in the virion is phosphorylated on serine 94 and/or 96 but not 79, or the 5% of Vpr that is phosphorylated is not enough to functionally impact the cell cycle of the target cell. Phosphorylation of Vpr S79 has also been implicated in HIV-1 infection of macrophages (2). Thus, it would be intriguing to test whether PKA plays a role in mediating infectivity in this target cell type. The role of Vpr S79 phosphorylation is unclear, though, since Zhou and Ratner tested the infection of macrophages and found no involvement of the S79A mutation in infectivity and replication of the virus (83).
In a previous study, we demonstrated that Vpr nucleates a large protein complex on the 14-3-3θ protein, but the phosphorylation-deficient R80A and S79A mutants do not (6). Specifically, the R80A and S79A Vpr mutants do not increase the binding of the Cdk1/CyclinB1 kinase complex to 14-3-3θ. Since the inactivation of this kinase complex has been attributed to Vpr, perhaps this Vpr-induced 14-3-3θ complex is the site of inactivation. We imagine that PKA phosphorylation may be required to establish a docking site for members of the complex, such as Cdk1 and CyclinB1, that are critical for cell cycle arrest. A number of recent studies propose an interaction between Vpr and an E3 ubiquitin ligase complex containing VprBP/DCAF1, DDB1, Cul4A, and Roc1 as a means of establishing a G2/M cell cycle arrest (5, 19, 37, 47, 71, 77, 81). The proposed mechanism suggests that Vpr brings a substrate critical for cell cycle progression into the E3 ligase complex, facilitating ubiquitination of this substrate protein and subsequent degradation by the proteasome. The S79A and/or R80A Vpr mutants could still interact with the E3 ligase complex, so this association is apparently not dependent on PKA phosphorylation (5, 19, 71). However, as proposed above, perhaps phosphorylated S79 on Vpr serves as a binding site for the critical substrate of the E3 ligase. One possible protein that the Vpr/E3 ligase complex degrades is the UNG2 uracil-DNA glycosylase, but the effect of either S79A or R80A mutation on the degradation of UNG2 has not been examined (71). Thus, it will be interesting to define the role of PKA in the molecular complexes orchestrated by Vpr.
The cAMP/PKA signal pathway is important during the virus life cycle of HIV-1. HIV-1 infection increases cAMP levels in T cells and therefore activates the PKA pathway (36, 59, 60). PKA phosphorylates two serines in the N terminus of the HIV-1 protein Nef, leading to an increase in viral infectivity and replication in resting peripheral blood mononuclear cells (49). The replication of the virus during active infection is also a cAMP/PKA-dependent process (60). Because Vpr increases viral expression by arresting infected cells in G2/M (29, 31), it is likely that increased PKA activity during HIV-1 infection leads to higher virus replication by stimulating Vpr G2/M cell cycle arrest. However, the virus must maintain a balance between increased virus production and the toxicity of cell cycle arrest. Perhaps the PKA pathway regulates this balance between cell cycle arrest and viability to provide for an optimal level of replication. Hence, the stimulation of the cAMP/PKA signaling cascade may therefore be an important cellular pathway usurped by HIV-1 to increase the spread of the virus throughout the body. Blocking the phosphorylation of Vpr by PKA, or the interaction between the two, may represent strategies for therapeutic intervention especially during early HIV-1 infection.
Acknowledgments
We thank Anthony Fauci for generously providing the BL-3 laboratory where this study was performed; David Stephany for FC-FRET assistance; Nicholas Beckloff for bioinformatics assistance; Jan Lukszo for peptide synthesis; Nathaniel Landau, Eric Freed, and Hirofumi Akari for plasmids; Klaus Strebel and Bing Sun for Vpr antibody; Lixin Zheng, Andrew Snow, and Keiko Sakai for helpful discussions; and Daniel Douek and Andrew Snow for critical reading of the manuscript. DNA sequencing was performed at NIAID Rocky Mountain Laboratories.
D.L.B. was a participant in the FAES (NIH)-Johns Hopkins University Cooperative Graduate Program in Biomedical Sciences. R.A.B. was a student in the NIH-University of Pennsylvania Graduate Partnership Program in Immunology. F.W. is the recipient of NIH K99/R00 grant CA137171. This study was supported by the division of intramural research of the NIAID, NIH.
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Adams, S. R., A. T. Harootunian, Y. J. Buechler, S. S. Taylor, and R. Y. Tsien. 1991. Fluorescence ratio imaging of cyclic AMP in single cells. Nature 349:694-697. [DOI] [PubMed] [Google Scholar]
- 2.Agostini, I., S. Popov, T. Hao, J. H. Li, L. Dubrovsky, O. Chaika, N. Chaika, R. Lewis, and M. Bukrinsky. 2002. Phosphorylation of Vpr regulates HIV type 1 nuclear import and macrophage infection. AIDS Res. Hum. Retrovir. 18:283-288. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., M. M. Addo, R. L. Eldridge, X. G. Yu, S. Thomas, A. Khatri, D. Strick, M. N. Phillips, G. B. Cohen, S. A. Islam, S. A. Kalams, C. Brander, P. J. Goulder, E. S. Rosenberg, and B. D. Walker. 2001. Vpr is preferentially targeted by CTL during HIV-1 infection. J. Immunol. 167:2743-2752. [DOI] [PubMed] [Google Scholar]
- 4.Bachand, F., X. J. Yao, M. Hrimech, N. Rougeau, and E. A. Cohen. 1999. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J. Biol. Chem. 274:9083-9091. [DOI] [PubMed] [Google Scholar]
- 5.Belzile, J. P., G. Duisit, N. Rougeau, J. Mercier, A. Finzi, and E. A. Cohen. 2007. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton, D. L., R. A. Barnitz, K. Sakai, and M. J. Lenardo. 2008. 14-3-3 theta binding to cell cycle regulatory factors is enhanced by HIV-1 Vpr. Biol. Direct. 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton, D. L., B. I. Hahn, E. A. Park, L. L. Lehnhoff, F. Hornung, and M. J. Lenardo. 2002. Death of CD4+ T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J. Virol. 76:5094-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton, D. L., and M. J. Lenardo. 2007. Vpr cytopathicity independent of G2/M cell cycle arrest in human immunodeficiency virus type 1-infected CD4+ T cells. J. Virol. 81:8878-8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Builder, S. E., J. A. Beavo, and E. G. Krebs. 1980. The mechanism of activation of bovine skeletal muscle protein kinase by adenosine 3′-5′-monophosphate. J. Biol. Chem. 255:3514-3519. [PubMed] [Google Scholar]
- 11.Cao, J., I. W. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70:1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartier, C., B. Hemonnot, B. Gay, M. Bardy, C. Sanchiz, C. Devaux, and L. Briant. 2003. Active cAMP-dependent protein kinase incorporated within highly purified HIV-1 particles is required for viral infectivity and interacts with viral capsid protein. J. Biol. Chem. 278:35211-35219. [DOI] [PubMed] [Google Scholar]
- 13.Chen, R., E. Le Rouzic, J. A. Kearney, L. M. Mansky, and S. Benichou. 2004. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J. Biol. Chem. 279:28419-28425. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, H. C., B. E. Kemp, R. B. Pearson, A. J. Smith, L. Misconi, S. M. Van Patten, and D. A. Walsh. 1986. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J. Biol. Chem. 261:989-992. [PubMed] [Google Scholar]
- 15.Chijiwa, T., A. Mishima, M. Hagiwara, M. Sano, K. Hayashi, T. Inoue, K. Naito, T. Toshioka, and H. Hidaka. 1990. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 265:5267-5272. [PubMed] [Google Scholar]
- 16.Christensen, A. E., F. Selheim, J. de Rooij, S. Dremier, F. Schwede, K. K. Dao, A. Martinez, C. Maenhaut, J. L. Bos, H. G. Genieser, and S. O. Doskeland. 2003. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 278:35394-35402. [DOI] [PubMed] [Google Scholar]
- 17.Chui, C., P. K. Cheung, C. J. Brumme, T. Mo, Z. L. Brumme, J. S. Montaner, A. D. Badley, and P. R. Harrigan. 2006. HIV VprR77Q mutation does not influence clinical response of individuals initiating highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 22:615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 19.DeHart, J. L., E. S. Zimmerman, O. Ardon, C. M. Monteiro-Filho, E. R. Arganaraz, and V. Planelles. 2007. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol. J. 4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T-cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 21.Felzien, L. K., C. Woffendin, M. O. Hottiger, R. A. Subbramanian, E. A. Cohen, and G. J. Nabel. 1998. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 coactivator. Proc. Natl. Acad. Sci. U. S. A. 95:5281-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn, R. D., J. Tate, J. Mistry, P. C. Coggill, S. J. Sammut, H. R. Hotz, G. Ceric, K. Forslund, S. R. Eddy, E. L. Sonnhammer, and A. Bateman. 2008. The Pfam protein families database. Nucleic Acids Res. 36:D281-D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer, A., C. Lejczak, C. Lambert, F. Roman, J. Servais, E. Karita, S. Allen, J. C. Schmit, and V. Arendt. 2004. Is the Vpr R77Q mutation associated with long-term non-progression of HIV infection? AIDS 18:1346-1347. [DOI] [PubMed] [Google Scholar]
- 24.Flatten, K., N. T. Dai, B. T. Vroman, D. Loegering, C. Erlichman, L. M. Karnitz, and S. H. Kaufmann. 2005. The role of checkpoint kinase 1 in sensitivity to topoisomerase I poisons. J. Biol. Chem. 280:14349-14355. [DOI] [PubMed] [Google Scholar]
- 25.Fukumori, T., H. Akari, A. Yoshida, M. Fujita, A. H. Koyama, S. Kagawa, and A. Adachi. 2000. Regulation of cell cycle and apoptosis by human immunodeficiency virus type 1 Vpr. Microbes Infect. 2:1011-1017. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginty, D. D., J. M. Kornhauser, M. A. Thompson, H. Bading, K. E. Mayo, J. S. Takahashi, and M. E. Greenberg. 1993. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260:238-241. [DOI] [PubMed] [Google Scholar]
- 28.Glass, D. B., H. C. Cheng, L. Mende-Mueller, J. Reed, and D. A. Walsh. 1989. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J. Biol. Chem. 264:8802-8810. [PubMed] [Google Scholar]
- 29.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez, G. A., and M. R. Montminy. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675-680. [DOI] [PubMed] [Google Scholar]
- 31.Gummuluru, S., and M. Emerman. 1999. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J. Virol. 73:5422-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagiwara, M., P. Brindle, A. Harootunian, R. Armstrong, J. Rivier, W. Vale, R. Tsien, and M. R. Montminy. 1993. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. Cell. Biol. 13:4852-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. U. S. A. 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho, C. C., W. Y. Siu, J. P. Chow, A. Lau, T. Arooz, H. Y. Tong, I. O. Ng, and R. Y. Poon. 2005. The relative contribution of CHK1 and CHK2 to adriamycin-induced checkpoint. Exp. Cell Res. 304:1-15. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann, B., P. Nishanian, T. Nguyen, P. Insixiengmay, and J. L. Fahey. 1993. Human immunodeficiency virus proteins induce the inhibitory cAMP/protein kinase A pathway in normal lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 90:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hrecka, K., M. Gierszewska, S. Srivastava, L. Kozaczkiewicz, S. K. Swanson, L. Florens, M. P. Washburn, and J. Skowronski. 2007. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl. Acad. Sci. U. S. A. 104:11778-11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacquot, G., E. Le Rouzic, P. Maidou-Peindara, M. Maizy, J. J. Lefrere, V. Daneluzzi, C. M. Monteiro-Filho, D. Hong, V. Planelles, L. Morand-Joubert, and S. Benichou. 2009. Characterization of the molecular determinants of primary HIV-1 Vpr proteins: impact of the Q65R and R77Q substitutions on Vpr functions. PLoS One 4:e7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, S. A., and T. Hunter. 2005. Kinomics: methods for deciphering the kinome. Nat. Methods 2:17-25. [DOI] [PubMed] [Google Scholar]
- 40.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kase, H., K. Iwahashi, S. Nakanishi, Y. Matsuda, K. Yamada, M. Takahashi, C. Murakata, A. Sato, and M. Kaneko. 1987. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 142:436-440. [DOI] [PubMed] [Google Scholar]
- 42.Kondo, E., F. Mammano, E. A. Cohen, and H. G. Gottlinger. 1995. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J. Virol. 69:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs, E. G., and J. A. Beavo. 1979. Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 48:923-959. [DOI] [PubMed] [Google Scholar]
- 45.Lavallee, C., X. J. Yao, A. Ladha, H. Gottlinger, W. A. Haseltine, and E. A. Cohen. 1994. Requirement of the Pr55gag precursor for incorporation of the Vpr product into human immunodeficiency virus type 1 viral particles. J. Virol. 68:1926-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenardo, M. J., S. B. Angleman, V. Bounkeua, J. Dimas, M. G. Duvall, M. B. Graubard, F. Hornung, M. C. Selkirk, C. K. Speirs, C. Trageser, J. O. Orenstein, and D. L. Bolton. 2002. Cytopathic killing of peripheral blood CD4+ T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76:5082-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Rouzic, E., N. Belaidouni, E. Estrabaud, M. Morel, J. C. Rain, C. Transy, and F. Margottin-Goguet. 2007. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle 6:182-188. [DOI] [PubMed] [Google Scholar]
- 48.Le Rouzic, E., and S. Benichou. 2005. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, P. L., T. Wang, K. A. Buckley, A. L. Chenine, S. Popov, and R. M. Ruprecht. 2005. Phosphorylation of HIV Nef by cAMP-dependent protein kinase. Virology 331:367-374. [DOI] [PubMed] [Google Scholar]
- 50.Lu, Y. L., R. P. Bennett, J. W. Wills, R. Gorelick, and L. Ratner. 1995. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J. Virol. 69:6873-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansky, L. M., S. Preveral, L. Selig, R. Benarous, and S. Benichou. 2000. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J. Virol. 74:7039-7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 54.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 55.Mologni, D., P. Citterio, B. Menzaghi, B. Zanone Poma, C. Riva, V. Broggini, A. Sinicco, L. Milazzo, F. Adorni, S. Rusconi, M. Galli, and A. Riva. 2006. Vpr and HIV-1 disease progression: R77Q mutation is associated with long-term control of HIV-1 infection in different groups of patients. AIDS 20:567-574. [DOI] [PubMed] [Google Scholar]
- 56.Moore, M. J., J. A. Adams, and S. S. Taylor. 2003. Structural basis for peptide binding in protein kinase A: role of glutamic acid 203 and tyrosine 204 in the peptide-positioning loop. J. Biol. Chem. 278:10613-10618. [DOI] [PubMed] [Google Scholar]
- 57.Muller, B., U. Tessmer, U. Schubert, and H. G. Krausslich. 2000. Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells. J. Virol. 74:9727-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neuberger, G., G. Schneider, and F. Eisenhaber. 2007. pkaPS: prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol. Direct 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nokta, M., and R. Pollard. 1991. Human immunodeficiency virus infection: association with altered intracellular levels of cAMP and cGMP in MT-4 cells. Virology 181:211-217. [DOI] [PubMed] [Google Scholar]
- 60.Nokta, M. A., and R. B. Pollard. 1992. Human immunodeficiency virus replication: modulation by cellular levels of cAMP. AIDS Res. Hum. Retrovir. 8:1255-1261. [DOI] [PubMed] [Google Scholar]
- 61.Olsen, S. R., and M. D. Uhler. 1991. Inhibition of protein kinase-A by overexpression of the cloned human protein kinase inhibitor. Mol. Endocrinol. 5:1246-1256. [DOI] [PubMed] [Google Scholar]
- 62.Paxton, W., R. I. Connor, and N. R. Landau. 1993. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J. Virol. 67:7229-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 64.Poppe, H., S. D. Rybalkin, H. Rehmann, T. R. Hinds, X. B. Tang, A. E. Christensen, F. Schwede, H. G. Genieser, J. L. Bos, S. O. Doskeland, J. A. Beavo, and E. Butt. 2008. Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods 5:277-278. [DOI] [PubMed] [Google Scholar]
- 65.Puntervoll, P., R. Linding, C. Gemund, S. Chabanis-Davidson, M. Mattingsdal, S. Cameron, D. M. Martin, G. Ausiello, B. Brannetti, A. Costantini, F. Ferre, V. Maselli, A. Via, G. Cesareni, F. Diella, G. Superti-Furga, L. Wyrwicz, C. Ramu, C. McGuigan, R. Gudavalli, I. Letunic, P. Bork, L. Rychlewski, B. Kuster, M. Helmer-Citterich, W. N. Hunter, R. Aasland, and T. J. Gibson. 2003. ELM server: a new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res. 31:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajan, D., S. Wildum, E. Rucker, M. Schindler, and F. Kirchhoff. 2006. Effect of R77Q, R77A and R80A changes in Vpr on HIV-1 replication and CD4 T-cell depletion in human lymphoid tissue ex vivo. AIDS 20:831-836. [DOI] [PubMed] [Google Scholar]
- 67.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogel, M. E., L. I. Wu, and M. Emerman. 1995. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakai, K., J. Dimas, and M. J. Lenardo. 2006. The Vif and Vpr accessory proteins independently cause HIV-1-induced T-cell cytopathicity and cell cycle arrest. Proc. Natl. Acad. Sci. U. S. A. 103:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 71.Schrofelbauer, B., Y. Hakata, and N. R. Landau. 2007. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc. Natl. Acad. Sci. U. S. A. 104:4130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shabb, J. B. 2001. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 101:2381-2411. [DOI] [PubMed] [Google Scholar]
- 73.Siegel, R. M., F. K. Chan, D. A. Zacharias, R. Swofford, K. L. Holmes, R. Y. Tsien, and M. J. Lenardo. 2000. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci. STKE 2000:PL1. [DOI] [PubMed] [Google Scholar]
- 74.Songyang, Z., S. Blechner, N. Hoagland, M. F. Hoekstra, H. Piwnica-Worms, and L. C. Cantley. 1994. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4:973-982. [DOI] [PubMed] [Google Scholar]
- 75.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. U. S. A. 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan, L., E. Ehrlich, and X. F. Yu. 2007. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J. Virol. 81:10822-10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor, S. S., J. A. Buechler, and W. Yonemoto. 1990. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59:971-1005. [DOI] [PubMed] [Google Scholar]
- 79.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 80.Wang, L., S. Mukherjee, F. Jia, O. Narayan, and L. J. Zhao. 1995. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and transactivation of viral long terminal repeat. J. Biol. Chem. 270:25564-25569. [DOI] [PubMed] [Google Scholar]
- 81.Wen, X., K. M. Duus, T. D. Friedrich, and C. M. de Noronha. 2007. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J. Biol. Chem. 282:27046-27057. [DOI] [PubMed] [Google Scholar]
- 82.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou, Y., and L. Ratner. 2000. Phosphorylation of human immunodeficiency virus type 1 Vpr regulates cell cycle arrest. J. Virol. 74:6520-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]