Abstract
Many phytochemicals possess cancer-preventive properties, some putatively through phase II metabolism-mediated mutagen/oxidant quenching. We applied human lung cells in vitro to investigate the effects of several candidate phytopreventive agents, including green tea extracts (GTE), broccoli sprout extracts (BSE), epigallocatechin gallate (EGCG), sulforaphane (SFN), phenethyl isothiocyanate (PEITC), and benzyl isothiocyanate (BITC), on inducing phase II enzymes glutathione S-transferase P1 (GSTP1) and NAD(P)H:quinone oxidoreductase 1 (NQO1) at mRNA and protein levels. Primary normal human bronchial epithelial cells (NHBE), immortalized human bronchial epithelial cells (HBEC), and lung adenocarcinoma cells (A549) were exposed to diet-achievable levels of GTE and BSE (0.5, 1.0, 2.0 mg/L), or individual index components EGCG, SFN, PEITC, BITC (0.5, 1.0, 2.0 μmol/L) for 24 h, 48 h, and 6 d, respectively. mRNA assays employed RNA-specific quantitative RT-PCR and protein assays employed Western blotting. We found that in NHBE cells, while GSTP1 mRNA levels were slightly but significantly increased after exposure to GTE or BSE, NQO1 mRNA increased to 2- to 4-fold that of control when exposed to GTE, BSE, or SFN. Effects on NQO1 mRNA expression in HBEC cells were similar. NQO1 protein expression increased up to 11.8-fold in SFN-treated NHBE cells. Both GSTP1 and NQO1 protein expression in A549 cells were constitutively high but not induced under any condition. Our results suggest that NQO1 is more responsive to the studied chemopreventive agents than GSTP1 in human lung cells and there is discordance between single agent and complex mixture effects. We conclude that modulation of lung cell phase II metabolism by chemopreventive agents requires cell- and agent-specific discovery and testing.
Introduction
Higher dietary intake of fruits and vegetables has been associated with protection against lung cancer and other lung disorders in recent large epidemiologic observational studies (1–4). Among the molecular mechanisms involved in this protection, phase II carcinogen and oxidant detoxifying enzyme induction play a major role in quenching phase I-bioactivated carcinogens and inhaled or endogenously produced oxidants. One strategy for lung cancer and other lung disease chemoprevention focuses on the use of natural or synthetic agents to modulate the metabolism and disposition of endogenous and environmental carcinogens and oxidants through upregulation of phase II enzymes (5,6).
Both glutathione S-transferase (GST)8 and NAD(P)H:quinone oxidoreductase 1 (NQO1) are well-known phase II metabolism enzymes catalyzing diverse reactions that collectively result in broad protection against electrophiles and oxidants. GST is composed of multiple cytosolic and membrane-bound isoforms and primarily functions as a detoxification enzyme by catalyzing the conjugation of glutathione to a variety of electrophilic compounds, including carcinogens and other cytotoxic chemicals (7). GSTP1, a GST isoform, has been evaluated as a major metabolic phase II enzyme in nonmalignant human lung by mRNA and protein expression (8,9) and by activity (10). NQO1, a cytosolic flavoprotein, is best known for protecting cells against the toxicities of quinones and their metabolic precursors by catalyzing obligatory 2-electron reduction of these compounds, as well as acting as a coenzyme Q (ubiquinone) reductase and facilitating the conversion of α-tocopherolquinone to α-tocopherolhydroquinone, contributing to the maintenance of these important endogenous antioxidants (11,12). Many studies have shown that elevation of these enzymes correlates with protection against chemical-induced carcinogenesis in animal models (13,14). Knockout of either GSTP1 or NQO1 in mice led to significant increases in both carcinogen-induced and spontaneous tumorigenesis (15–18). Epidemiological studies in humans have shown an increased risk of lung cancers in individuals who carry a null genotype or a genotype that causes a significant decrease in enzyme activity of a GST isoform or NOQ1 (19–22).
The isothiocyanates (ITC) are a family of compounds with potential cancer chemoprevention activity. It is thought that an important mechanism by which ITC inhibit tumorigenesis is to suppress proliferation of oncogenic cells by inducing apoptosis and arresting cell cycle progression. Extensive literature suggests that a major component of chemopreventive activity of ITC occurs via induction of genes encoding phase II enzymes (23–25). Naturally occurring ITC found abundantly in cruciferous vegetables, including sulforaphane (SFN), phenethyl-isothiocyanate (PEITC), and benzyl-isothiocyanate (BITC) have demonstrated the induction of phase II genes, such as GSTP1 and NQO1 (26,27). Additionally, green tea and 1 of its components, epigallocatechin gallate (EGCG), are prepared with minimal oxidation of polyphenols and have been shown in animal studies and human epidemiological studies to prevent cancer, including lung cancer (28). Extensive laboratory studies in a variety of cell culture systems and in limited animal models have further demonstrated that green tea polyphenols afford protective effects from diverse types of carcinogens and induce phase II enzyme activity that could lead to enhanced detoxification process (29,30).
However, little testing has yet been reported in normal lung cells, which are target cells for such agents. Furthermore, in many cases, the effects of the chemopreventive agents in cultured cells or tissues are only achievable at supraphysiological concentrations; such concentrations might not be attained when the phytochemicals are administered as part of diet. Additionally, the differential effects of crude extracts and purified phytochemicals on stimulating gene expression are seldom addressed. In an effort to identify candidate chemopreventive agents for experimentally augmenting GSTP1 and NQO1 expression at diet-achievable levels [e.g. 1.0 μmol/L EGCG and SFN (31–34)], we tested a panel of 6 dietary compounds and mixtures in 3 human cell lines at 3 concentration levels and 3 time points (24 h, 48 h, and 6 d). The 6 testing mixtures/compounds were: green tea extracts (GTE), broccoli sprout extracts (BSE), green tea-derived EGCG, crucifer-derived SFN, PEITC, and BITC. The 3 human cell lines were: primary normal human bronchial epithelial (NHBE) cells, immortalized human bronchial epithelial cells (HBEC), and overtly malignant lung adenocarcinoma A549 cells.
Materials and Methods
Chemicals and materials.
GTE, named Sunphenon 90DCF, was obtained from Taiyo International. According to the manufacturer's information, the GTE (purity >99%) powder contained >80% ployphenols, of which 82.66% were catechins, including 55.67% being the catechin EGCG and <1% caffeine. BSE (35) were kindly provided by Dr. Zhang in the Department of Chemoprevention at Roswell Park Cancer Institute, Buffalo, NY. SFN (purity 98.3%) was purchased from LKT Laboratories. BITC (purity 98%), PEITC (purity 99%), and EGCG (purity ≥ 97%) were purchased from Sigma-Aldrich.
Cell lines and culture.
NHBE cells (BioWhittaker) were maintained in bronchial epithelial cell growth medium containing Clonetics bronchial epithelial cell basal medium with supplements provided by BioWhittaker. Immortalized HBEC, courtesy of Dr. Minna at the University of Texas Southwestern Medical Center (36), were cultured with keratinocyte serum-free medium (Life Technologies) containing 50 mg/L bovine pituitary extract with 5 μg/L epidermal growth factor. A549 (American Tissue Culture Collection) were cultured in F-12K nutrient mixture (Invitrogen). Cells were routinely fed 24 h before exposure, then were incubated with 0.5, 1.0, 2.0 mg/L mixtures (1.0 mg/L GTE containing 1.2 μmol/L EGCG; 1.0 mg/L BSE containing 1.4 μmol/L total ITC) or 0.5, 1.0, 2.0 μmol/L single index compound for 24 h, 48 h, and 6 d, respectively [1.0 μmol/L is a diet-achievable serum concentration for EGCG and SFN (31–34)].
Assay for cell viability.
Because NHBE cells in pilot studies were most sensitive to the agents, cell viability was assessed for NHBE cells by visual inspection of the plates under light microscopy and the use of the 3–4,5-dimethylthiazol-2-yl-2, 5-diphenyl-tetrazolium bromide (MTT) Cell Growth Assay (Millipore). The MTT assay allows quantitative analysis of cell viability via endogenous mitochondrial metabolic activity. NHBE cells were plated into 96-well tissue culture plates and grown for 24 h. Medium was discarded and replaced with medium containing serial dilutions of phytochemicals. After incubation at 37°C for 48 h, 10 μL of MTT (5 g/L PBS) was added to each well and the plates were incubated at 37°C for another 4 h. Then 100 μL isopropanol with 0.4 mol/L HCl and 50 μL dimethyl sulfoxide (DMSO) were added to solubilize the formazan crystals at room temperature. Within 1 h, the absorbance was measured on an ELISA plate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm. Cells were tested in 2 independent assays (biological duplicates) with each containing 3 technical replicates.
Quantitative real-time RT-PCR.
Total RNA was prepared by the Mini RNA isolation kit (ZYMO Research) according to the manufacturer's instructions. RT was performed with our universal tagged RT primer using SuperScript II Reverse Transcriptase (Life Technologies), as previously described (8,37,38) The cDNA products were then applied to quantitative real-time PCR immediately or stored at −20°C until use.
The quantitative real-time PCR for GSTP1 and NQO1 was performed in Applied Biosystems 7500 Real-Time PCR System (Applied Biosystem) using a previously published RNA-specific strategy that is unconfounded by any contaminating genomic DNA pseudogene sequence encoding target transcripts (GSTP1 and NQO1) and reference “housekeeper” internal transcript (GAPDH, glyceraldehyde-3-phosphate dehydrogenase) (37). A primer pair 5′-TGA AGA AGA AAG GAT GGG AGG-3′ (sense) and 5′-AGG GGG AAC TGG AAT ATC AC-3′ (antisense) was used for amplification of the NQO1 gene. The primers for GSTP1 and GAPDH amplification and the component of PCR reactions, as well as PCR conditions, have been described elsewhere (38). All real-time PCR were performed in technical triplicates for each sample. Each exposure condition for mRNA analysis was reproduced and confirmed by 1 additional independent experiment, representing biological duplicates.
Western blot analysis.
The treated and untreated cells were washed 2 times with PBS (pH 7.0) and harvested after Trypsin/EDTA treatment. The cells were then lysed by incubation at 37°C for 10 min with a solution containing 0.8% digitonin and 2 mmol/L EDTA (pH 7.8). Each whole-cell lysate was centrifuged at 10956 × g, 4°C for 3 min, and the supernatant fluid was stored at −80°C for GSTP1 or NQO1 protein assay. Protein assay was performed by Western blotting using WesternBreeze Chemiluminescent Western blot immunodetection kit (Invitrogen). Briefly, after quantification of total protein level using BCA protein assay reagent kit (Pierce), each cell lysate sample (15 μg) was subjected to electrophoresis in 10% acrylamide NuPAGE Novex Bis-Tris denaturing gels (Invitrogen). Proteins were then blotted onto nitrocellulose membranes (Invitrogen). Blots were blocked and treated with the antibodies according to the manufacturer's instructions. Rabbit polyclonal to GSTP1 (Oxford Biomedical Research), NQO1 (Abcam), and GAPDH (Imgenex) primary antibodies were diluted at 0.1 mg/L in block solution for 2 h at room temperature with the blotted membranes. The secondary anti-rabbit antibodies, supplied in the WesternBreeze kit, were incubated with the membranes for 30 min at room temperature. Detection was accomplished with a ready-to-use CDP-Star chemiluminescent substrate for alkaline phosphatase kit exposed on Kodak X-OMAT film (Sigma-Aldrich). Western blotting assays were performed in 3 independent experiments representing biological triplicates.
Genotyping of the 3 cell lines for functional GSTP1 promoter haplotypes by cloning and sequencing.
Our laboratory previously identified 10 promoter polymorphisms in the GSTP1 gene, which were statistically grouped into 3 main haplotypes (Hap1, Hap2, and Hap3) (39). Given previously observed GSTP1 promoter haplotypes and unpredicted inhibitory chemopreventive agent-haplotype interactions (39), the GSTP1 promoter haplotypes of the 3 cell lines were determined through cloning and sequencing, as described previously (40). Briefly, a 1616-bp fragment of the GSTP1 promoter region from −1804 to −199 (containing all the known SNP variants previously identified) was amplified by PCR. The PCR products were purified and ligated into pCR8/GW/TOPO TA cloning vector (Invitrogen) and transfected into One Shot Mach1-T1R Chemically Competent Escherichia coli (Invitrogen). DNA from individual clones was sequenced on a Perkin-Elmer Biosystems ABI PRISM 377XL automated sequencer.
Data and statistical analysis.
The data from the MTT assay were normalized to the untreated control (100% viability). We initially used 2-way ANOVA to test the effects of agent, dose, and their interaction. We then separately tested agent and dose response effect by 1-way ANOVA with post hoc Dunnett's test for comparing groups to the control. Results were expressed as means ± SEM, n = 2 means of triplicate measures.
The mRNA expression data for the target gene GSTP1 and NQO1 were first quantitated relative to the expression of the housekeeping gene GAPDH following the ΔΔCt method (41) and then normalized to the background expression in the vehicle, DMSO- or deionized water- treated cells at the respective time points by using the following formula: Relative gene expression = (1/[2Ct(target) – Ct(housekeeper)] for treated group)/ (1/[2Ct((target) – Ct(housekeeper)] for DMSO or deionized water).
For evaluation of the modulation of GSTP1 and NQO1 mRNA, we initially used 4-way ANOVA with interaction terms to test if there was any agent, cell line, dose, time and their interaction effects. To address the dose effects in the fixed cell line, agent and time point, we tested dose response effect using post hoc Dunnett's test with residual variation estimated from the 4-way ANOVA to compare groups to the control. Results were expressed as means ± SEM, n = 2 means of triplicate measures.
Western blot was performed for 3 cell lines after exposure to 2.0 μmol/L or 2.0 mg/L of the agents for 24 h, 48 h, or 6 d. Protein expression was quantified by densitometry with a scanning laser densitometer (Molecular Dynamics). The relative density ratio of GSTP1 or NQO1 protein band to GAPDH was normalized by arbitrarily setting the ratio for untreated control at that time point as 1.0. For evaluation of the modulation of GSTP1 and NQO1 protein, we initially used 3-way ANOVA with interaction terms to test if there is any cell line, agent, time, and their interaction effect. To address the effects of agents in the fixed cell line and time point, we tested agent effect by post hoc Dunnett's test with residual variation estimated from the 3-way ANOVA to compare groups to the control. Results were expressed as means ± SEM, n = 3. ANOVA was performed using SAS 9.1 for Windows and the Dunnett's test was done manually. All differences were considered significant at P < 0.05.
Results
Inhibition of cell proliferation.
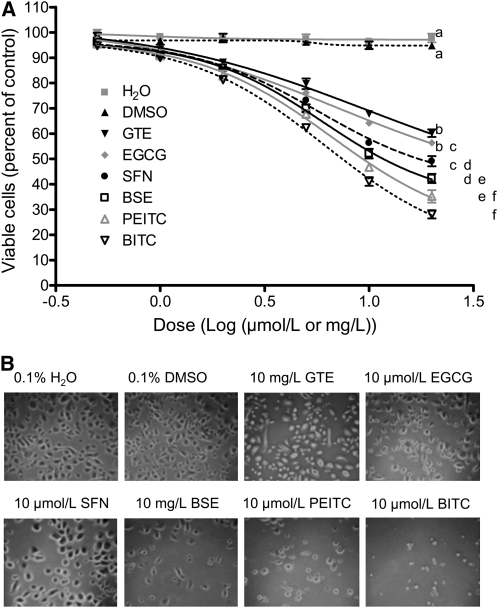
Exposure of NHBE cells to GTE, BSE, EGCG, SFN, PEITC, and BITC for 48 h at a concentration range of 0.5–20 μmol/L or mg/L caused a dose-dependent inhibition of cell proliferation, with the relative antiproliferative activity of BITC > PEITC > BSE > SFN > EGCG > GTE (Fig. 1A). Each agent had dose-dependent effects (P < 0.05). After 48-h exposure to 2.0 μmol/L BITC, PEITC, SFN, and EGCG, and 2.0 mg/L GTE and BSE, effects of the agents differed from one another (P < 0.05). NHBE cells were 41.1, 46.7, 56.3, 64.2, 67.8, and 52.1% nonviable after 48 h exposure to 10 μmol/L BITC, PEITC, SFN, and EGCG and 10 mg/L GTE and BSE, respectively. Morphologically, we observed that cell growth was inhibited after 48 h exposure to BITC or PEITC at 10 μmol/L or for BSE at 10 mg/L. However, SFN and EGCG at 10 μmol/L and GTE at 10 mg/L caused little change in cell morphology (Fig. 1B). We did not find apparent morphological changes in cells exposed to vehicle or 2.0 μmol/L EGCG, SFN, or PEITC or 2.0 mg/L GTE or BSE, respectively. Our results for the cytotoxicity of BITC, PEITC, and SFN are comparable with previous studies (42,43). In subsequent experiments, we chose concentrations of tested compounds whereby NHBE cells were ≥80% viable after 24 h exposure.
FIGURE 1 .
Cytotoxicity of GTE, BSE, EGCG, SFN, PEITC, and BITC in NHBE cells. (A) Cells were treated with each of the agents for 48 h. Data are means ± SEM, n = 2 means of triplicate measures. For each agent, the effect of the dose was significant. Agents differed significantly from one another at 2 units/L with differences shown at 20 units/L where means without a common letter differ, P < 0.05. (B) Phase contrast images of NHBE cells after 48 h treatments.
mRNA expression of GSTP1 and NQO1.
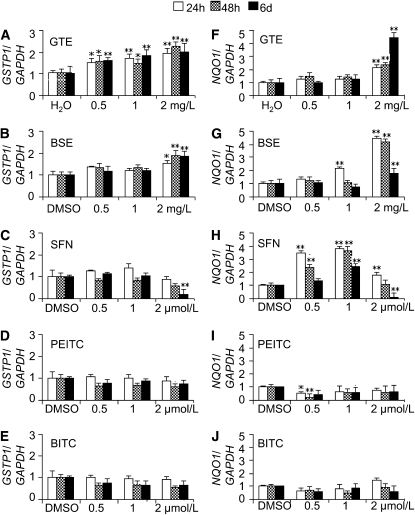
GSTP1 (Fig. 2A) and NQO1 (Fig. 2F) mRNA expression in NHBE cells showed increases after exposure to 2.0 mg/L GTE. There were 70 and 80% increases to 1.7 times (for 24 h) and 1.8 times (for 48 h) the control value for NQO1 mRNA expression after application of 2.0 mg/L GTE and a maximum increase of 3.3-fold after 6 d application of 2.0 mg/L GTE in NHBE cells. However, exposure to the index single compound EGCG component of GTE alone did not induce mRNA expression of GSTP1 and NQO1, even at the highest concentration of 2.0 μmol/L (data not shown).
FIGURE 2 .
Modulation of GSTP1 (A–E) and NQO1 (F–J) mRNA expression by the chemopreventive agents in NHBE cells. Values are expressed as fold of vehicle-treated control at that time and are means ± SEM, n = 2 means of triplicate measures. Different from the vehicle-treated control at that time, *P < 0.05.
Additionally, we found NQO1 mRNA expression increased after exposure to 2.0 mg/L BSE (containing SFN, PEITC, and BITC) (Fig. 2G) as well as to the BSE index component SFN alone (Fig. 2H) in NHBE cells. SFN showed a significant increase of NQO1 mRNA expression at the lower concentration of 0.5 and 1.0 μmol/L, whereas PEITC and BITC had consistent decreases of NQO1 expression at the concentrations of 0.5, 1.0, and 2.0 μmol/L (Fig. 2I, J). GSTP1 mRNA expression significantly increased upon exposure to 2.0 mg/L BSE (Fig. 2B) but not in response to the single components SFN, PEITC, and BITC (Fig. 2C–E).
In HBEC and A549 cells, we observed parallel effects of these agents on mRNA expression of GSTP1 and NQO1 (Supplemental Figs. 1 and 2). SFN exposure increased NQO1 mRNA expression in NHBE and HBEC cells but not in A549 cells, while GTE persistently induced GSTP1 mRNA expression in NHBE cells but not in HBEC3 and A549 cells.
Protein expression of GSTP1 and NQO1.
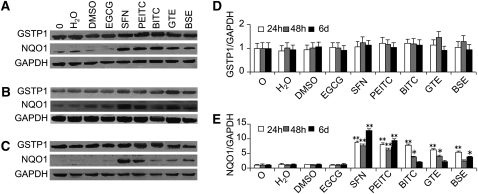
We determined the levels of GSTP1 and NQO1 protein in NHBE cells after exposure to the panel of agents for 24 h, 48 h, and 6 d (Fig. 3). NQO1 protein expression significantly increased after application of plant-derived mixtures of GTE and BSE as well as a single component SFN, PEITC, and BITC. It showed a maximum increase of 11.8-fold after 6 d of application of 2.0 μmol/L SFN. However, GSTP1 protein expression did not change in NHBE cells after exposure to the studied agents.
FIGURE 3 .
Modulation of GSTP1 and NQO1 protein expression by the chemopreventive agents in NHBE cells. (A–C) Cells were treated with 0.1% water or 0.1% DMSO vehicle and 2.0 μmol/L EGCG, SFN, PEITC, or BITC, and 2.0 ng/L GTE or BSE for 24 h (A), 48 h (B), and 6 d (C), respectively. D, E: Densitometry analysis of relative GSTP1 (D) and NQO1 (E) protein expression. Values are expressed as fold of the untreated control at that time and are means ± SEM, n = 3. Different from the untreated control at that time, *P < 0.05, ** P < 0.01. O, untreated control.
We also examined the modulation of GSTP1 and NQO1 protein levels in HBEC and A549 cells. We observed increases of NQO1 protein level in HBEC only at 1 time point, 48 h upon exposure to SFN, PEITC, BITC, GTE, or BSE, with a maximum increase of 7.5-fold by 2.0 μmol/L SFN (Supplemental Fig. 3). The level of GSTP1 and NQO1 protein did not show significant increases in A549 cells under any of the investigated conditions (Supplemental Fig. 4).
Genotypes of GSTP1 promoter haplotypes in the 3 cell lines.
NHBE, HBEC, and A549 cells were genotyped for previously reported GSTP1 promoter haplotypes (39) through cloning and sequencing. The 3 most frequent haplotypes (HAP1, HAP2, and HAP3) (40) were also found in these 3 cell lines. The NHBE primary cell line employed was HAP1/HAP3 heterozygous, HBEC was HAP1/HAP1 homozygous, and A549 was HAP2/HAP2 homozygous.
Discussion
In this investigation, we experimentally tested the hypothesis that commonly studied, naturally occurring mixtures and corresponding index component compounds persistently induce phase II metabolism enzymes in normal, immortalized, and malignant human bronchial cells. This initial exploration is unique in that it explores chemoprevention-induced expression modulation in primary lung cells at diet-achievable levels. It is apparent that phase II induction occurs on a lung cell line-specific basis and is not uniform across putative phase II active mixtures and index compounds.
We acknowledge that results in whole organisms may diverge from those in the cultured cells in part because of liver or other organ metabolism of ingested phytochemicals mixtures and agents, and therefore results in the cultured cells could be misleading if taken in isolation. Nonetheless, this study has potentially important implications for screening or evaluating chemopreventive agents for the lung, given that antioxidant phase II enzymes play a pivotal role in quenching phase I-bioactivated carcinogens, along with inhaled or endogenously generated oxidants. The machinery for this metabolism resides in both lung epithelial tissue and these lung epithelial cells in culture, as previously reported (8,39).
Several plant-derived mixtures/compounds had previously been identified as inducer of phase II enzymes in other cellular contexts. Among them, SFN has received much attention over the past decade, when it was found to be the most potent naturally occurring inducer of phase II enzymes in both animals and humans (6,44,45). In our current study, SFN had a significant inductive effect on NQO1 mRNA expression at the lower concentrations of 0.5 and 1.0 μmol/L. NQO1 protein expression in NHBE cells was induced up to 12-fold by SFN as well as less potent induction by PEITC, BITC, GTE, and ITC. There was a modest effect in HBEC cells and none in A549 cells. There are many possible reasons why responses could have differed from primary normal, to target gene immortalized, to overtly malignant lung cells. We speculate that the redox regulatory features of both immortalized (stable CDK and hTERT overexpressing) HBEC cells and malignant cells are somehow different from the native, nonimmortalized cells. The mechanisms driving these differences warrant further investigation but remain well beyond the scope of this study.
Time- and dose-dependent responses by SFN have been reported, but the induction levels, activities, and/or the type of phase II enzyme varied with the cell lines, in a study of 7 established malignant mammalian cell lines (46). Our current expression results in lung cells are in part consistent with previous reports. Yoxall et al. (47) showed that SFN at a typical dietary dose stimulated NQO1 in a dose-dependent fashion but failed to influence GST, epoxide hydrolase, and uridine diphosphate-glucuronosyl transferase activities in rat livers exposed to SFN in their drinking water for 10 d, equivalent daily doses of 3 and 12 mg/kg. McWalter et al. (48) also showed that treatment with SFN at 5 μmol/L induced NQO1 catalytic activity 4.5- and 5.2-fold in mouse hepatoma Hepa-1c1c7 and rat liver-derived RL-34 cells, respectively. By contrast, GST activity did not increase to the same extent in either cell line. Western blotting showed that a 10-fold increase of NQO1 protein can be readily achieved by the broccoli seed extracts at an estimated concentration of 0.6 μmol/L total ITC and 5 μmol/L SFN. Recently, Riedl et al. (49) reported that oral SFN increased phase II enzyme expression in human nasal lavage cells ranged from 101% for GSTP1 to 199% for NQO1 at the highest dose of 200 g broccoli sprout prepared as broccoli sprout homogenate. These results suggested that GST was notably less sensitive than NQO1 to induction by ITC, including SFN.
Why one ARE-containing regulatory apparatus (NQO1) in NHBE cells could drive responsiveness to an exogenous agent, but not another ARE-containing gene apparatus (GSTP1), is not clear. Competing transcription factors and motifs, or epigenetic mechanisms (methylation, histone modification, microRNA binding) could be at play. Parenthetically, we have previously shown subtle differences in upstream methylation in the GSTP1 promoter between NHBE and A549, concordant with the differing respective basal expression levels (50). Also, while not tested directly against other NHBE cell haplotypes, the GSTP1 response to the phytochemicals in NHBE from a donor heterozygous for Haplotype 3 did not suggest a major impact of GSTP1 promoter haplotype on in vitro responses to phytopreventive agents, which were modest overall. The observed inhibition of SFN and BITC for GSTP1 mRNA expression in NHBE cells was partially supported by our previous findings (39).
GSTP1 and NQO1 expression in A549 cells were constitutively high but not induced by the agents under all conditions. The reason for the nonresponsiveness of GSTP1 and NQO1 in A549 cells is unclear but could reflect constitutively high redox cycling, which is known to occur in malignant cells. Given that upregulation of phase II metabolism in malignant cells is known to confer resistance to several chemotherapeutic agents (e.g. platinum-based therapy) (51,52), this nonresponsiveness of GSTP1 would not appear to be, on the surface, detrimental for phase II-directed chemopreventive agent safety in this setting. That is, high-risk patients already harboring undetected malignant cells who might inadvertently be placed on chemoprevention agents prior to recognition and surgery/chemotherapy treatment for a clinical adenocarcinoma, we speculate, would presumably not suffer an iatrogenic chemopreventive agent-induced resistance to platinum-based or other chemotherapy by virtue of phase II pathway induction. Of course, this possibility would warrant direct human testing.
Although the GTE had some modest inductive effect uniquely on NQO1 expression, the component single compound tea component EGCG notably failed to induce mRNA expression of NQO1. For the Brassica mixture BSE components, where the overall mixture had some inductive effect, components PEITC and BITC may actually decrease the mRNA levels of GSTP1 and NQO1, although they may appear to elevate the protein level of NQO1 in NHBE uniquely. This discordance between single agent vs. complex mixture exposure effects is a common theme running through the phytopreventive literature (53,54). For example, Netsch et al. (53) showed that GTE, but not EGCG, significantly induced cytochrome P450 1A2 mRNA expression in LS-180 cells. Yan et al. (54), looking at tumor incidence and multiplicity, recently reported that aerosolized polyphenon E, containing 65% EGCG, 25% other catechins, and ∼0.5% caffeine, decreased lung tumor load by ∼59%, but aerosolized EGCG, both at the same and at a higher dose, failed to inhibit lung carcinogenesis in A/J mice treated with benzo(α)pyrene. It is possible that EGCG could still be an active compound, but for antitumor activity, it may require another component that is present in green tea or the polyphenon E formulation used, and, as such, none of the other components in the polyphenon E mixture may be biologically active without EGCG or vice versa. This speculation warrants further evaluation.
Overall, both GTE and BSE may be inducers of phase II metabolism, particularly NQO1, but they seem to be of relatively low potency in normal human lung cells. While the studies provide evidence for mild-to-moderate NQO1-based phase II induction by specific, commonly studied plant-derived agents uniquely in normal human lung cells (e.g. SFN), in the majority of conditions, these effects occur largely at doses above those easily achievable in conventional or modestly supplemented ad libidum diets. More potent inducers might offer more promise for augmentation of this phase II enzyme pathway in the prevention of lung cancer. Toward this discovery, high-throughput screening of a plant-derived library has commenced in our laboratory.
Supplementary Material
Acknowledgments
We thank Dr. Yuesheng Zhang in the Department of Chemoprevention at Roswell Park Cancer Institute for supplying BSE and Dr. John D. Minna at Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center at Dallas, for supplying the immortalized HBEC lines. X.L.T. and S.D.S. designed research; X.L.T., M.S., and W.H. conducted research; X.L.T. and H.T. analyzed data; X.L.T. and S.D.S. wrote the paper. X.L.T. and S.D.S. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by Prevent Cancer Foundation (research fellowship to X.L. Tan), by NIH-R21 CA 94714 (to S.D. Spivack), and by NIH-R01 CA 10618 (to S.D. Spivack).
Author disclosures: X. L. Tan, M. Shi, H. Tang, W. Han, and S. D. Spivack, no conflicts of interest.
Supplemental Figures 1–4 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BITC, benzyl isothiocyanate; BSE, broccoli sprout extract; DMSO, dimethyl sulfoxide; EGCG, epigallocatechin gallate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GST, glutathione S-transferase; GTE, green tea extract; HBEC, human bronchial epithelial cells; ITC, isothiocyanate; MTT, 3-4,5-dimethylthiazol-2-yl-2, 5-diphenyl-tetrazolium bromide; NHBE, normal human bronchial epithelial cell; NQO1, NAD(P)H:quinone oxidoreductase; PEITC, phenethyl isothiocyanate; SFN, sulforaphane.
References
- 1.Wark PA, Grubben MJ, Peters WH, Nagengast FM, Kampman E, Kok FJ, van 't Veer P. Habitual consumption of fruits and vegetables: associations with human rectal glutathione S-transferase. Carcinogenesis. 2004;25:2135–42. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, et al. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer. 2003;107:1001–11. [DOI] [PubMed] [Google Scholar]
- 3.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirayama F, Lee AH, Binns CW, Zhao Y, Hiramatsu T, Tanikawa Y, Nishimura K, Taniguchi H. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case-control study in Japan. Prev Med. 2009;49:184–9. [DOI] [PubMed] [Google Scholar]
- 5.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–7. [DOI] [PubMed] [Google Scholar]
- 6.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. [DOI] [PubMed] [Google Scholar]
- 7.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. [DOI] [PubMed] [Google Scholar]
- 8.Spivack SD, Hurteau GJ, Fasco MJ, Kaminsky LS. Phase I and II carcinogen metabolism gene expression in human lung tissue and tumors. Clin Cancer Res. 2003;9:6002–11. [PubMed] [Google Scholar]
- 9.Spivack SD, Hurteau GJ, Jain R, Kumar SV, Aldous KM, Gierthy JF, Kaminsky LS. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Res. 2004;64:6805–13. [DOI] [PubMed] [Google Scholar]
- 10.Coles B, Yang M, Lang NP, Kadlubar FF. Expression of hGSTP1 alleles in human lung and catalytic activity of the native protein variants towards 1-chloro-2,4-dinitrobenzene, 4-vinylpyridine and (+)-anti benzo[a]pyrene-7,8-diol-9,10-oxide. Cancer Lett. 2000;156:167–75. [DOI] [PubMed] [Google Scholar]
- 11.Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–40. [DOI] [PubMed] [Google Scholar]
- 12.Nioi P, Hayes JD. Contribution of NAD (P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004;555:149–71. [DOI] [PubMed] [Google Scholar]
- 13.Boone CW, Steele VE, Kelloff GJ. Screening for chemopreventive (anticarcinogenic) compounds in rodents. Mutat Res. 1992;267:251–5. [DOI] [PubMed] [Google Scholar]
- 14.Song LL, Kosmeder JW II, Lee SK, Gerhauser C, Lantvit D, Moon RC, Moriarty RM, Pezzuto JM. Cancer chemopreventive activity mediated by 4′-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer Res. 1999;59:578–85. [PubMed] [Google Scholar]
- 15.Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci USA. 1998;95:5275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long DJ II, Waikel RL, Wang XJ, Roop DR, Jaiswal AK. NAD (P)H:quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz[a]-anthracene-induced carcinogenesis in mouse skin. J Natl Cancer Inst. 2001;93:1166–70. [DOI] [PubMed] [Google Scholar]
- 17.Long DJ II, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, Jaiswal AK. Disruption of the NAD (P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–6. [PubMed] [Google Scholar]
- 18.Ritchie KJ, Henderson CJ, Wang XJ, Vassieva O, Carrie D, Farmer PB, Gaskell M, Park K, Wolf CR. Glutathione transferase pi plays a critical role in the development of lung carcinogenesis following exposure to tobacco-related carcinogens and urethane. Cancer Res. 2007;67:9248–57. [DOI] [PubMed] [Google Scholar]
- 19.Kolesar JM, Pritchard SC, Kerr KM, Kim K, Nicolson MC, McLeod H. Evaluation of NQO1 gene expression and variant allele in human NSCLC tumors and matched normal lung tissue. Int J Oncol. 2002;21:1119–24. [PubMed] [Google Scholar]
- 20.Saldivar SJ, Wang Y, Zhao H, Shao L, Lin J, Spitz MR, Wu X. An association between a NQO1 genetic polymorphism and risk of lung cancer. Mutat Res. 2005;582:71–8. [DOI] [PubMed] [Google Scholar]
- 21.Ye Z, Song H, Higgins JP, Pharoah P, Danesh J. Five glutathione s-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med. 2006;3:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen M, Raaschou-Nielsen O, Brasch-Andersen C, Tjonneland A, Overvad K, Autrup H. Interactions between GSTM1, GSTT1 and GSTP1 polymorphisms and smoking and intake of fruit and vegetables in relation to lung cancer. Lung Cancer. 2007;55:137–44. [DOI] [PubMed] [Google Scholar]
- 23.Wolf CR. Chemoprevention: increased potential to bear fruit. Proc Natl Acad Sci USA. 2001;98:2941–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates MS, Kensler TW. Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 2007;20:109–17. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90. [DOI] [PubMed] [Google Scholar]
- 26.Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133–48. [DOI] [PubMed] [Google Scholar]
- 27.Tan XL, Spivack SD. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer. 2009;65:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark J, You M. Chemoprevention of lung cancer by tea. Mol Nutr Food Res. 2006;50:144–51. [DOI] [PubMed] [Google Scholar]
- 29.Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–90. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 32.Baek WK, Jang BC, Lim JH, Kwon TK, Lee HY, Cho CH, Kim DK, Shin DH, Park JG, et al. Inhibitory modulation of ATP-sensitive potassium channels by gallate-ester moiety of (-)-epigallocatechin-3-gallate. Biochem Pharmacol. 2005;70:1560–7. [DOI] [PubMed] [Google Scholar]
- 33.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. [DOI] [PubMed] [Google Scholar]
- 34.Ho E, Clarke JD, Dashwood RH. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr. 2009;139:2393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, Fahey JW. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol Cancer Ther. 2006;5:935–44. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–34. [DOI] [PubMed] [Google Scholar]
- 37.Hurteau GJ, Spivack SD. mRNA-specific reverse transcription-polymerase chain reaction from human tissue extracts. Anal Biochem. 2002;307:304–15. [DOI] [PubMed] [Google Scholar]
- 38.Tan XL, Wang T, Xiong S, Kumar SV, Han W, Spivack SD. Smoking-related gene expression in laser capture-microdissected human lung. Clin Cancer Res. 2009;15:7562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cauchi S, Han W, Kumar SV, Spivack SD. Haplotype-environment interactions that regulate the human glutathione S-transferase P1 promoter. Cancer Res. 2006;66:6439–48. [DOI] [PubMed] [Google Scholar]
- 40.Tan XL, Moslehi R, Han W, Spivack SD. Haplotype-tagging single nucleotide polymorphisms in the GSTP1 gene promoter and susceptibility to lung cancer. Cancer Detect Prev. 2009;32:403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006;Chapter 15:Unit 15.8. [DOI] [PubMed]
- 42.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood). 2004;229:835–42. [DOI] [PubMed] [Google Scholar]
- 43.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA. 1992;89:2394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang ZQ, Chen C, Yang B, Hebbar V, Kong AN. Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers. Life Sci. 2003;72:2243–53. [DOI] [PubMed] [Google Scholar]
- 47.Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer. 2005;117:356–62. [DOI] [PubMed] [Google Scholar]
- 48.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD (P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:S3499–506. [DOI] [PubMed] [Google Scholar]
- 49.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han W, Cauchi S, Herman JG, Spivack SD. DNA methylation mapping by tag-modified bisulfite genomic sequencing. Anal Biochem. 2006;355:50–61. [DOI] [PubMed] [Google Scholar]
- 51.Goto S, Kamada K, Soh Y, Ihara Y, Kondo T. Significance of nuclear glutathione S-transferase pi in resistance to anti-cancer drugs. Jpn J Cancer Res. 2002;93:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii T, Teramoto S, Matsuse T. GSTP1 affects chemoresistance against camptothecin in human lung adenocarcinoma cells. Cancer Lett. 2004;216:89–102. [DOI] [PubMed] [Google Scholar]
- 53.Netsch MI, Gutmann H, Schmidlin CB, Aydogan C, Drewe J. Induction of CYP1A by green tea extract in human intestinal cell lines. Planta Med. 2006;72:514–20. [DOI] [PubMed] [Google Scholar]
- 54.Yan Y, Cook J, McQuillan J, Zhang G, Hitzman CJ, Wang Y, Wiedmann TS, You M. Chemopreventive effect of aerosolized polyphenon E on lung tumorigenesis in A/J mice. Neoplasia. 2007;9:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.