Abstract
A hallmark of energy restriction (ER) is a decrease in total body fat, which is thought to increase lifespan and maintain immune function. However, we have shown that during primary influenza infection, ER induces rapid weight loss, impairs natural killer (NK) cell function, and increases mortality in young and aged mice. To determine whether influenza-induced NK cell function could be restored in ER mice, young adult (6 mo) male C57BL/6 mice were fed an ER diet or re-fed (RF) control diet ad libitum for 2 wk before infection with PR8 influenza A. An initial hyperphagic response was observed in RF mice, characterized by increased food intake, rapid weight gain, and restoration of body fat and fat depots by 5–7 d of re-feeding to levels comparable to control ad libitum (AL) mice. Re-feeding improved survival and attenuated the decline in NK cell function during infection, evidenced by increased numbers, percentages, and CD69 expression by d 3 postinfection in RF mice. Interestingly, an altered metabolic phenotype was observed during infection of RF mice, with plasma leptin concentrations greater than in ER mice but less than in AL mice. In contrast, adiponectin concentrations of RF mice were lower than those of both ER and AL mice. These data suggest that re-feeding for a defined period before, and perhaps throughout, influenza season may provide the energy needed to counter the deleterious effects of ER on NK cell function, especially during exposure to newly emerging strains of influenza, for which vaccines are limited or unavailable.
Introduction
There is a critical need to evaluate the primary response to influenza infection, especially to newly emerging strains of influenza to which we have had no prior exposure. The recent emergence of the novel swine 2009-H1N1 influenza virus poses a real threat to a large population under the age of 65 y. This influenza strain has the potential to infect 30–50% of the population, leading to as many as 1.8 million hospitalizations and causing between 30,000 and 90,000 deaths. This is further complicated because the 2009-H1N1 vaccine supply has been either limited or unavailable and for most of the population, vaccination is likely to occur after the peak of influenza infection. It takes several weeks to develop protective immunity in vaccinated individuals. This delay in adaptive immunity is likely to diminish the usefulness of the vaccine in protecting from influenza infection (1) and puts a greater reliance on having a functional primary response to combat this virus. There is additional reason for concern, because both seasonal and H1N1 influenza strains are circulating simultaneously in the current 2009–2010 influenza season. Whereas ∼90% of influenza-related deaths occur from seasonal influenza in people 65 y and older, mortality from H1N1 during the early 2009 outbreak was highest among people 25–49 y of age (39%), followed by people 50–64 y of age (25%) and people 5–24 y of age (16%) (1). Therefore, a large percent of the population may be at risk for potentially contracting influenza from one or both strains.
Energy restriction (ER),8 also known as caloric restriction (2), is a nutritional paradigm that has been widely used to study life extension in rodents. Several studies have shown that diets ranging from 30–70% ER increase median and maximal lifespan of rodents by up to ∼65 and 50%, respectively, over those fed ad libitum (AL) diets (3,4). Similar results have been obtained in multiple rodent species and strains, dogs, and nonmammalian species such as fish and flies (4,5). Longitudinal studies of rhesus and squirrel monkeys fed a 30% ER diet suggest a comparable decrease in morbidity and mortality rates (4,5), but there are very limited data regarding the effects of ER on maximal lifespan in nonhuman primates.
ER has been repeatedly shown to have positive effects on both nonspecific and adaptive immunity in rodents (6). It is now accepted that in addition to life extension, one of the hallmarks of ER is to delay the onset of age-related changes in immune function. Many reports have indicated that ER reduces the incidence of spontaneous tumors and cancers in aged rodents (7) and maintains T cell proliferation, cytokine production, and cytotoxic T lymphocyte activity (8). ER has also has been shown to enhance splenic lymphocyte proliferation and improve the antibody response after vaccination of mice with influenza. However, our recent studies clearly indicate that young and aged mice fed an ER diet demonstrated decreased survival, delayed lung virus clearance, and markedly impaired natural killer (NK) cell function during an acute primary immune response to influenza infection (9,10). Therefore, this study was designed to determine whether short-term re-feeding of young adult ER mice with an AL diet was beneficial in response to an acute infection with influenza virus. Such studies are clinically relevant to identify possible nutritional paradigms to increase energy intake prior to and during influenza season, which occurs during the same defined time period each year. Further, increased energy intake may be essential to support the primary response of susceptible populations to newly emerging strains of influenza for which vaccines are limited or unavailable.
Materials and Methods
Mice and diets.
Specific pathogen-free young adult (6 mo) AL and young adult (6 mo) ER male C57BL/6 mice were purchased from the National Institute on Aging (NIA) colony maintained by Charles River Laboratories. The animal use protocol for this study was approved by Michigan State University Institutional Animal Care and Use Committee. Upon arrival, mice were housed individually in micro-isolator cages in the Association for the Assessment and Accreditation of Laboratory Animal Care-accredited containment facility at Michigan State University and were acclimated at least 10–14 d prior to the initiation of each experiment. Both ER (NIH-31/NIA-fortified) and AL (NIH-31) diets were purchased from the NIA; the compositions have been reported in detail previously (10). The composition of the ER diet is sufficient in micronutrients and minerals but results in restriction of total energy intake by ∼40%. The ER regimen was designed to gradually achieve 40% restriction in mice by 3 mo of age, such that they were weight stable upon arrival at 6 mo of age.
Feeding protocol.
Briefly, on d −14, ER mice were randomly assigned to 1 of the following dietary groups: 1) ER group, which continued to consume ER diet; or 2) RF group, in which ER mice were transferred onto NIH-31 diet, which they consumed freely. The 3rd diet group (AL) was comprised of mice consuming NIH-31 diet upon arrival and throughout the duration of the study. The feeding protocol continued until from d −14 until d 0 upon which mice were anesthetized and infected with influenza virus.
Food intake, body weight, and body composition.
Food intake and body weight were monitored daily during the 2-wk feeding protocol. All mice were weighed daily between 0800 and 0900, after which they were fed. Body composition was measured on d −14, −13, −11, −9, −7, −5, and 0. The protocol to assess body composition using the EchoMRI-500 (Echo Medical Systems) has been validated and described in detail previously (11). Briefly, after calibration using a rapeseed oil standard, individual mice are then placed in a holding tube to restrict movement. Body composition is determined within 30–45 s using standard programs validated for utilization in mice. The advantages of this system are that it is rapid, allows for repeated measurements, and does not require anesthesia, enabling mice to recover immediately after MRI.
Virus and infection.
The method to isolate mouse-adapted influenza A/Puerto Rico/8/34 (H1N1, PR8) from specific pathogen-free eggs (B & E Eggs) has been described in detail (12). For infection, mice were first anesthetized by intraperitoneal injection with Avertin (2,2,2-tribromoethanol, Sigma) and then infected intranasally with 104 × the 50% tissue culture infectious dose, calculated as 100 hemagglutinating units (13), of PR8 in saline. Previous studies in our laboratory (9,10) have routinely found that this dose of PR8 induces a measurable innate immune response with little to no mortality during d 3–4 postinfection (p.i.) Both nutritional and immune variables were assessed d 0–3 p.i.
Lymphocyte isolation.
The isolation of mononuclear cells from spleens and lungs has been described in detail (10). Cell suspensions from spleens and collagenase-digested lungs were layered on Histopaque-1083 (Sigma) for density gradient centrifugation and were resuspended at appropriate concentrations to quantify NK and CD8 cells by flow cytometry.
Flow cytometry.
Cells from lung or spleen were resuspended in FACS buffer (0.1% sodium azide, 1% fetal bovine serum, in dPBS) containing various combinations of the following fluorochrome-conjugated antibodies (eBioscience or BD Bioscience) at concentrations ranging from 1:100 to 1:300: CD3 (PerCP-Cy5.5), CD19 (PerCP-Cy5.5), CD8 (PerCP-Cy5.5), NK1.1 (PE-Cy7 or APC), B220 (APC), Nkp46 (AlexaFlour 647 or FITC), and CD69 (FITC). Cells were incubated in staining cocktails on ice in the dark for 30 min. Samples were then acquired on a LSR II flow cytometer (Becton Dickinson) or a C6 flow cytometer (Accuri) and analyzed using FlowJo software (Tree Star).
Leptin and adiponectin concentrations in plasma.
Plasma concentrations of leptin and adiponectin were quantified by ELISA according to the manufacturer's instructions (R&D Systems). Plates were read at 450 nm wavelength using a Synergy HT plate reader (Bio-Tek) and concentrations were determined using a standard curve for each protein (11).
Fat pad weight.
Fat pads were excised after 14 d re-feeding and before challenge with influenza. Animals were anesthetized followed by cervical dislocation. Inguinal and gonadal fat pads were immediately removed and were weighed immediately.
Quantifying adipocyte accumulation in bone marrow.
Femur and tibia from ER, re-fed (RF), and AL mice were harvested, fixed in 10% formalin, decalcified in 14% EDTA, soaked in a 1:1 solution of equal volumes of 2% aqueous osmium tetroxide (OsO4) and 5% potassium dichromate to stain marrow bone adipocytes. The intact bones then were imaged at 6 μm resolution using micro-focus conebeam X-ray computed tomography (μCT40, Scanco Medical AG) at 55 kV and 145 μA, collecting 2000 projections per rotation at 300 msec integration time. Three-dimensional images were reconstructed using standard convolution and back-projection algorithms with Shepp and Logan filtering and rendered within a 12.3-mm field of view at a discrete density of 4,629,630 voxels/mm3 (isometric 6 μm voxels). The resulting images revealed high contrast 3D image arrays of adipocytes distributed throughout the fixed bone marrow. Discrete adipocytes were segmented from background using a constrained Gaussian filter, summing total marrow fat volume for each sample. Figures illustrating images produced from bone marrow in ER, RF, and AL mice are presented in Supplemental Fig. 1.
Statistics.
Statistical analyses were performed using Sigma Stat (Systat). Values in the text are means ± SEM. Survival data were analyzed using the Kaplan-Meier estimates with censoring. Log rank tests were used to determine significance of survival curves among diet groups. Body composition, food intake, and weight were analyzed using repeated-measures ANOVA. Flow cytometric data and plasma adipokine concentrations were analyzed by 2-way ANOVA, with diet and time as main effects. Following 2-way ANOVA, differences in cell populations and adipokine concentration were determined using Student's t test for post hoc analysis of differences between diet groups and Tukey's test for post hoc analysis of changes over time. Differences in adipocytes in bone marrow were analyzed assessed using 1-way ANOVA followed by unpaired 2-tailed t tests, corrected for multiple comparisons. Nonparametric data were analyzed using the Kruskal Wallis test. Significance was accepted at P < 0.05.
Results
Short-term feeding increases food intake and restores body weight and percentage of body fat in RF mice.
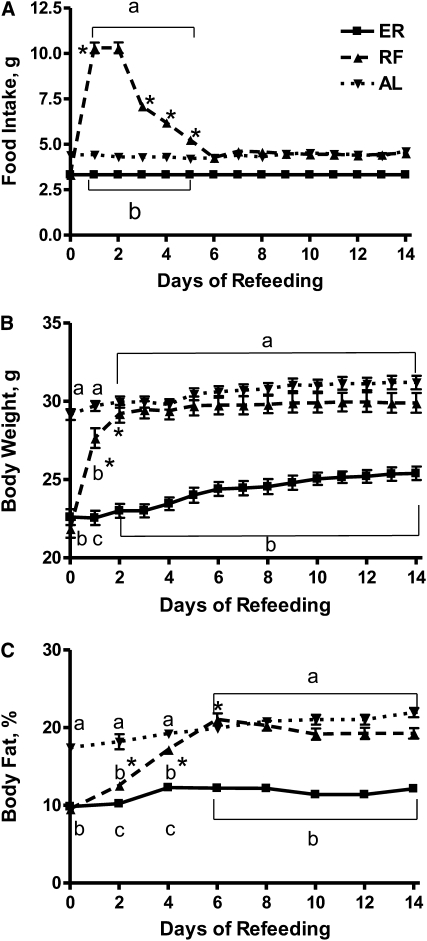
Food intake and body weight of ER, RF, and AL mice were monitored daily for 2 wk prior to influenza infection. During d 1 of re-feeding, RF mice initially demonstrated a hyperphagic response, during which food intake rapidly and markedly increased over that consumed at baseline (Fig. 1A). By d 5 of re-feeding, food intake steadily declined and was comparable to intakes of AL mice. Increased food intake was accompanied by a concomitant restoration of body weight (Fig. 1B) and percentage of body fat (Fig. 1C) in RF mice that were comparable to those seen in AL mice by d 6 of re-feeding. During the latter half of the re-feeding protocol, food intake, body weight, and body composition of RF mice were stable.
FIGURE 1 .
Food intake (A), body weight (B), and body fat percentages (C) of ER, RF, and AL mice during 14 d of feeding. Values are means ± SEM, n = 12–15. Means at a time without a common letter differ, P < 0.001. *Different from the preceding time point, P < 0.001.
Short-term re-feeding increases inguinal and gonadal fat pad depots but does not restore adipocyte accumulation in bone marrow of RF mice.
On d 0 of infection, we readily observed inguinal and gonadal fat pads in RF and AL but not in ER mice. Both inguinal and gonadal fat pad weights were quantified, indicating that fat weight was equivalent for RF and AL mice, but could not be detected in ER mice (Table 1).
TABLE 1.
Weights of inguinal and gonadal fat and adipocyte volume in bone marrow from femurs and tibias of ER, RF, and AL mice before and after influenza infection12
| ER | RF | AL | |
|---|---|---|---|
| Fat pat weight, g | |||
| Inguinal | NDb | 0.39 ± 0.07a | 0.26 ± 0.04a |
| Gonadal | NDb | 0.43 ± 0.03a | 0.42 ± 0.01a |
| Adipocyte volume, mm3 | |||
| Femur | 1.13 ± 0.71a | 1.10 ± 0.81a | 0.001 ± 0.007b |
| Tibia | 5.06 ± 1.37a | 4.51 ± 1.09a | 1.61 ± 0.006b |
Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.01.
ND, not detected.
The restoration of fat depots of RF mice prompted us to assess bone marrow-derived adipocyte volumes in femora and tibiae of ER, RF, and AL mice on d 0 of infection (Supplemental Fig. 1). Adipocyte volumes in both femurs and tibiae from AL mice were reduced compared with both ER and RF mice (Table 1). This indicates that RF had a phenotype more similar to ER rather than AL mice. These data suggest that re-feeding favors restoration of body fat depots to meet increased energy storage demands prior to infection but does not reduce the observed increase in bone marrow adiposity of ER mice (14).
Short-term feeding improves survival of RF mice during influenza infection.
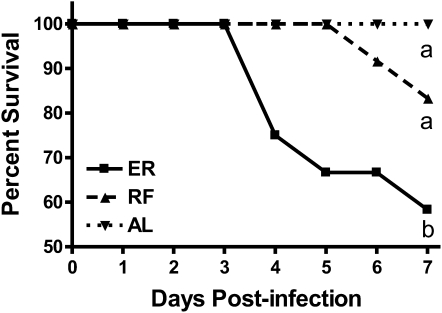
After the 2-wk feeding protocol, ER, RF, and AL mice were infected with PR8 and survival was monitored through d 7 p.i. (Fig. 2). Sixty percent of ER mice did not survive infection through d 7, having a median survival of 5 d, which was significantly less than in AL mice, all of which survived through d 7 p.i. In contrast, only 20% of RF mice succumbed to influenza infection by d 7 p.i. Importantly, the percent survival estimates of RF mice did not differ from AL mice through d 7 p.i.
FIGURE 2 .
Percent survival of ER, RF, and AL mice during the 7 d after influenza infection. Curves labeled with unlike letters differ, P < 0.05.
Short-term feeding attenuates the decline in percentages and numbers of NK cells during influenza infection of previously ER mice.
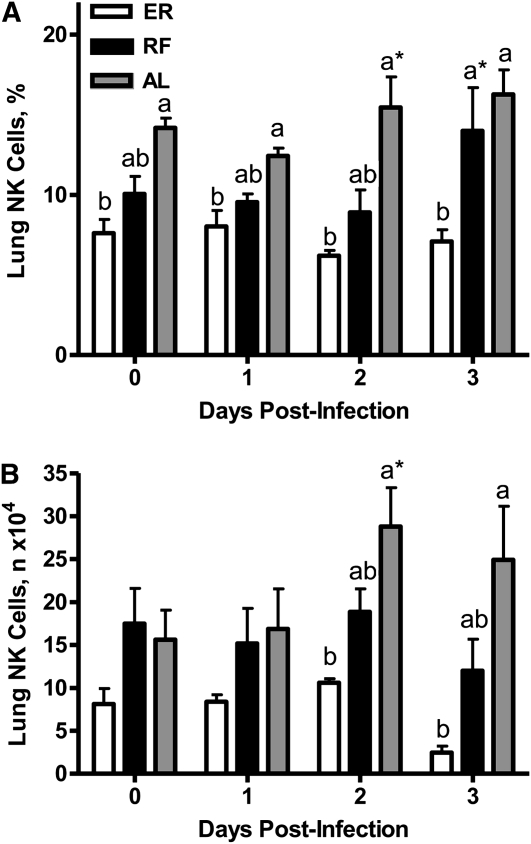
NK cell percentages (Fig. 3A) and numbers (Fig. 3B) were also quantitated in lungs of ER, RF, and AL mice through d 3 of infection. At baseline, neither the percentages nor numbers of NK cells significantly differed among the 3 diet groups. However, the percentages of NK cells in lungs of RF mice were significantly higher than in ER mice, but did not differ from AL mice on d 3 p.i. Whereas NK cell numbers were significantly lower in ER mice compared with AL and RF mice on d 2 and 3 of infection, these values did not differ between RF and ER mice.
FIGURE 3 .
NK cell percentages (A) and numbers (B) in lungs of ER, RF, and AL mice before and after influenza infection. Values are means ± SEM, n = 6. Labeled means at a time without a common letter differ, P < 0.01. *Different from the preceding time point, P < 0.05.
ER alters the kinetics, percentages, and numbers of NK cells expressing the activation marker CD69.
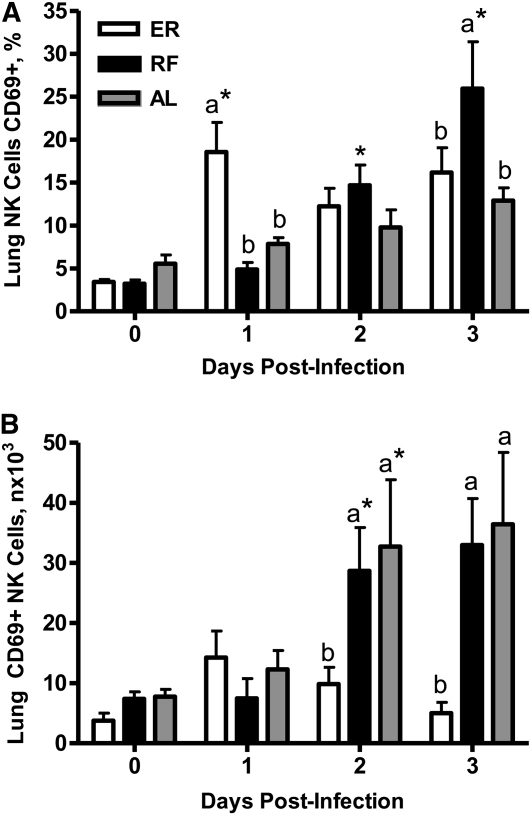
The surface marker, CD69, was used to assess early NK cell activation in lungs of ER, RF, and AL during influenza infection. Percentages (Fig. 4A), but not numbers (Fig. 4B), of CD69+ NK cells in ER mice on d 1 p.i. were greater than in RF and AL mice. However, ER mice could not maintain a large pool of activated NK cells during the first 3 d of infection, as indicated by significantly fewer CD69+ NK cells in lungs compared with RF and AL mice on d 2 and 3 p.i.
FIGURE 4 .
CD69 +NK cell percentages (A) and numbers (B) in lungs of ER, RF, and AL mice before and after influenza infection. Values are means ± SEM, n = 6. Labeled means at a time without a common letter differ P < 0.01. *Different from the preceding time point, P < 0.01.
Short-term feeding differentially affects leptin and adiponectin concentrations in plasma during influenza infection.
Adipokines function to both regulate energy metabolism and influence innate immunity. The adipokines, leptin and adiponectin, have both been shown to alter immunity, including NK cell function (15,16). The adipokine leptin is a hormone secreted mainly by adipocytes and regulates food intake and weight control; recently, leptin has been shown to support innate immunity, including NK cell function (16,17). However, adiponectin, an antiinflammatory adipokine, has also been shown to suppress inducible NK cell cytotoxicity and impair function (15). The concentrations of leptin and adiponectin were measured during the course of infection of ER, RF, and AL mice. The AL group exhibited a transient rise in plasma leptin concentrations on d 1 p.i. most likely due to the observed increased food intake upon recovery from anesthesia. ER mice had lower concentrations of leptin than RF and AL mice on d 0, 1, and 3 p.i. (Table 2). However, plasma leptin concentrations were comparable in RF and AL mice on 2 d p.i. when NK cell numbers were also greater than ER mice (2 d p.i.). In contrast, adiponectin concentrations in RF mice were lower on d 1 p.i. than in either ER or AL mice. By d 3 p.i., adiponectin concentrations were comparable between ER and RF mice but had steadily declined in AL mice such that they were lower than those of ER and RF mice.
TABLE 2.
Plasma concentrations of leptin and adiponectin before and after influenza infection1
| ER | RF | AL | |
|---|---|---|---|
| Plasma leptin, μg/L | |||
| d 0 | 1.46 ± 0.28b | 3.37 ± 0.34b | 7.84 ± 1.06a |
| d 1 | 1.24 ± 0.15b | 3.22 ± 0.15b | 11.26 ± 2.28a* |
| d 2 | 1.55 ± 0.27 | 3.56 ± 0.88 | 2.66 ± 0.61* |
| d 3 | 1.02 ± 0.16b | 2.71 ± 0.54a | 4.97 ± 1.15a |
| Plasma adiponectin, mg/L | |||
| d 0 | 12.14 ± 0.52 | 9.72 ± 3.10 | 12.94 ± 2.44 |
| d 1 | 16.06 ± 0.39a | 10.49 ± 0.68b | 16.34 ± 1.94a |
| d 2 | 18.38 ± 1.52 | 13.69 ± 2.19 | 13.76 ± 0.66 |
| d 3 | 17.72 ± 1.48a | 17.22 ± 0.26a* | 10.28 ± 2.11b |
Values are means ± SEM, n = 5. Means in a row with superscript letters without a common letter differ, P < 0.01. *Different from the preceding time point, P < 0.01.
Discussion
The present study was designed to elucidate the effects of short-term re-feeding of ER mice on metabolic parameters known to affect innate immune function during primary influenza infection. Our study indicates that re-feeding previously ER mice with AL diet induced a hyperphagic response as early as d 1 of re-feeding. This resulted in complete restoration of body weight and percent body fat comparable to AL mice by d 5 of the 14-d feeding protocol. In addition, quantifiable inguinal and gonadal fat depots were observed by d 0 of infection. The hyperphagic response after short- or long-term ER, characterized by a rapid increase in body weight, has been reported previously in humans and rodent models (18,19). However, to our knowledge, our study is the first to include comprehensive analysis of body composition to determine the kinetics of body fat restoration and relate these changes to outcome from acute virus infection.
In accord with previous observations (9,10), ER mice exhibited increased mortality, marked weight loss, and impaired NK cell function during the early innate response to primary influenza infection. Interestingly, RF mice exhibited anorexic effects of acute influenza infection, as evidenced by reduced food intake and lethargy (data not shown), that were more similar to that of ER, compared with AL mice. However, survival, and the percentages, numbers, and activation of NK cell in lungs were markedly improved in RF compared with ER mice. In addition, the percentages of activated NK cells in lungs of RF mice were greater than in ER mice at d 3 p.i.
The kinetics of the magnitude of influenza-induced NK cell responses was delayed by 1 d compared with our previous reports (9,10), in which the peak of NK cell function was seen by d 1 p.i. In addition, significant mortality was seen by d 4 p.i. previously (9,10) but not until d 5–7 p.i. in the present report. We attribute this delay in peak NK cell function to differences in the severity and kinetics of infection induced by the dose of virus used for each study. Therefore, our data suggest that at this virus dose, short-term re-feeding may not completely restore NK cell function but does maintain the activated NK cell pool throughout the early phase of infection.
Re-feeding also resulted in increased numbers and percentages of CD8+ T cells in the lungs by d 3 p.i. compared with ER mice (data not shown), which may contribute to improved survival of RF mice compared with ER mice through d 7 of infection.
Although it could be argued in the current study that re-feeding did not restore all aspects of NK cell function to levels comparable to that of AL mice, the more relevant comparison is between ER and RF mice. While the diet of AL mice was consistent through the entire study, ER and RF mice were fed the same diet up until the 2-wk feeding protocol. Therefore, it would not be expected for the responses of RF mice to be comparable to AL mice given the short duration of re-feeding. However, this re-feeding protocol clearly attenuated the decline in NK cell function observed in ER mice, resulting in overall improved outcome from acute influenza infection.
A critical observation in the current study indicated that the restoration of body weight, body fat percentage, and fat depots may be necessary to provide the energy necessary to support NK cell function during the early innate response to influenza infection. We hypothesized that this restoration of body fat may positively alter plasma concentrations of leptin and adiponectin, both of which have been shown to modulate energy metabolism and directly affect NK cell cytotoxicity and cytokine production (17,20).
Leptin is produced mainly by adipocytes and acts on the hypothalamus to regulate appetite and energy expenditure (21) and to suppress energy intake via a feedback mechanism (22). Thus, leptin-deficient ob/ob and leptin receptor-deficient db/db mice exhibit an obese phenotype (23,24). Leptin also plays an important role in immunity, because it is released during an infection, and the leptin receptor (Ob-Rb) is expressed by immune cells, including hematopoietic cells, T cells, B cells, NK cells, and macrophages (16,20). Further, leptin regulates NK cell cytotoxicity and apoptosis in db/db mice, which have impaired NK cell cytotoxicity and a greater percentage of NK cells undergoing apoptosis in bone marrow (17,24). In addition, inhibition of leptin signaling increases apoptosis of NK cells from wild-type animals (24).
Although body fat was restored in RF mice prior to infection, leptin concentrations were not statistically equivalent to AL until d 2 p.i, when the concentrations of plasma leptin in AL mice were comparable to levels in both ER and RF. Similarly, despite restoration of body weight, body fat percentage, and fat pad depots, high numbers of marrow adipocytes remained in RF mice. This phenomenon has been observed during ER and in ob/ob mice, suggesting that leptin has a critical role in regulating bone marrow adiposity (14,25). It is also reasonable to suggest that either leptin production and/or leptin receptor function on NK cells is not fully restored in RF mice, rendering them incapable of mounting an innate immune response equivalent to that of AL mice after influenza infection. This may account for the ability of RF mice to increase NK cell numbers and percentages relative to ER mice but not to comparable levels seen in AL mice through d 3 p.i. These data are supported by previous studies (26,27) indicating that exogenous leptin administration improves survival and reduces bacterial load in ob/ob mice.
It has been shown previously (28) that lower leptin output in response to decreased body fat is designed to improve survival under hostile conditions, such as starvation, by shifting energy utilization toward vital metabolic processes, such as cardiac and respiratory functions. In contrast, AL mice have large lipid-filled adipocytes that are programmed to rapidly release fatty acids for acute energy needs, such as during a primary infection. Thus, AL mice are able to respond to this acute stress via systemic catabolism of fat for energy by an increase in gluconeogenesis, lipolysis, and glycolysis to maintain an adequate immune response. Based upon these observations, it is likely that the combination of insufficient body fat and decreased circulating leptin concentrations impede the ability of ER mice to meet metabolic energy demands necessary to support NK cell function in the face of an acute infection.
Adiponectin is secreted from white adipose tissue into circulation and is inversely correlated with body fat percentage in adults (29). Therefore, we predicted that adiponectin levels in RF mice would be comparable to AL mice throughout infection. However, although our body composition data indicated that body fat was rapidly restored in RF mice, adiponectin levels were lower in RF compared with AL mice before infection. Interestingly, plasma concentrations of adiponectin steadily increased during infection of RF mice but sharply declined in AL mice, despite both groups losing a significant percentage of body weight during infection (data not shown). Thus, despite restoration of body fat, it is plausible that adiponectin may not be as responsive to short-term re-feeding.
It has been reported (30) that adipocyte type, function, gene expression, secretion levels, and deposition are altered following ER, which could explain the increased marrow adipocyte volume in ER mice in our study. Further, because NK cells develop and mature in bone marrow, we further speculate that ER also alters the microenvironment necessary for NK cell maturation. Indeed, adipocyte accumulation in bone marrow has negative impacts on hematopoesis, resulting in quiescent stem cells and negatively regulating hematopoetic activity. This may lead to limited expansion of short-term progenitors (13). Importantly, adiponectin has been reported to inhibit progenitor differentiation, resulting in a larger pool of undifferentiated hematopoetic stem cells (31). Thus, increased adiponectin concentrations during infection of ER and RF mice may further inhibit the development of NK cells during lymphopoesis in bone marrow. Previous reports support this hypothesis indicating adiponectin negatively regulates inducible NK cell cytotoxicity, decreasing IFN-γ production and reducing IL-2 induced Fas-L expression (15).
Future research will examine the role of longer term feeding and the effect on adipose tissue distribution after re-feeding. We will also address specific changes in NK cell development based upon the observation that the cellular components of the bone marrow appear altered. In addition, characterization of the adipose tissue changes in expression, function, and cell morphology are key to understanding how energy balance affects innate immune response to influenza infection. Taken together, these studies indicate that it may be beneficial to increase energy intake for a defined time period to prepare for yearly influenza season. This dietary intervention may improve the primary response of susceptible individuals to newly emerging strains of influenza, to which vaccination is limited or unavailable.
Supplementary Material
Acknowledgments
We thank Drs. Norman Hord and David Duriancik for their insightful discussions and critical reading of this manuscript. The authors are indebted to Dr. Mary K. Howett (1948–2008), whose unwavering support made this project possible. E.M.G. designed research, J.F.C., D.J.A., J.I.F, B.W.R. conducted research, J.F.C. and E.M.G. wrote the paper, and E.M.G. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by NIH Academic Research Enhancement Award (R15), AG029637-02
Author disclosures: J. F. Clinthorne, D. J. Adams, J. I. Fenton, B. W. Ritz, E. M. Gardner, no conflicts of interest.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AL, ad libitum group; ER, energy restriction; NIA, National Institute on Aging; NK, natural killer; p.i., postinfection; RF, re-fed group.
References
- 1.President's Council of Advisors on Science and Technology. Report to the President on U.S. Preparations for 2009 -H1N1 Influenza. White House Office of Science and Technology Policy. 2009 Aug 24.
- 2.McCay CM, Cromwell MF, Maynard LA. The effect of retarded growth upon the length of the lifespan and ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 3.Wanagat J, Allison DB, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol Sci. 1999;52:35–40. [DOI] [PubMed] [Google Scholar]
- 4.Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742–5. [DOI] [PubMed] [Google Scholar]
- 5.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40:884–93. [DOI] [PubMed] [Google Scholar]
- 6.Effros RB, Walford RL, Weindruch R, Mitcheltree C. Influences of dietary restriction on immunity to influenza in aged mice. J Gerontol. 1991;46:B142–7. [DOI] [PubMed] [Google Scholar]
- 7.Pahlavani MA. Caloric restriction and immunosenescence: a current perspective. Front Biosci. 2000;5:D580–7. [DOI] [PubMed] [Google Scholar]
- 8.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–94. [DOI] [PubMed] [Google Scholar]
- 10.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. 2008;138:2269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton JI, Nunez NP, Yakar S, Perkins SN, Hord NG, Hursting SD. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab. 2009;11:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129:223–30. [DOI] [PubMed] [Google Scholar]
- 13.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devlin M, Cloutier A, Thomas N, Panus D, Lotinun S, Pinz I, Baron R, Rosen C, Bouxsein M. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. Epub 2010 Mar 17. [DOI] [PMC free article] [PubMed]
- 15.Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, Lee MS, Lee JH, Yoon DY, et al. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. 2006;176:5958–64. [DOI] [PubMed] [Google Scholar]
- 16.Haas P, Straub RH, Bedoui S, Nave H. Peripheral but not central leptin treatment increases numbers of circulating NK cells, granulocytes and specific monocyte subpopulations in non-endotoxaemic lean and obese LEW-rats. Regul Pept. 2008;151:26–34. [DOI] [PubMed] [Google Scholar]
- 17.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. [DOI] [PubMed] [Google Scholar]
- 18.Hambly C, Mercer JG, Speakman JR. Hunger does not diminish over time in mice under protracted caloric restriction. Rejuvenation Res. 2007;10:533–42. [DOI] [PubMed] [Google Scholar]
- 19.Penas-Lledo EM, Loeb KL, Puerto R, Hildebrandt TB, Llerena A. Subtyping undergraduate women along dietary restraint and negative affect. Appetite. 2008;51:727–30. [DOI] [PubMed] [Google Scholar]
- 20.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–52. [DOI] [PubMed] [Google Scholar]
- 21.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–9. [DOI] [PubMed] [Google Scholar]
- 22.Rance KA, Johnstone AM, Murison S, Duncan JS, Wood SG, Speakman JR. Plasma leptin levels are related to body composition, sex, insulin levels and the A55V polymorphism of the UCP2 gene. Int J Obes (Lond). 2007;31:1311–8. [DOI] [PubMed] [Google Scholar]
- 23.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105:2017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo CK, Lam QL, Yang M, Ko KH, Sun L, Ma R, Wang S, Xu H, Tam S, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. 2009;6:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83. [DOI] [PubMed] [Google Scholar]
- 26.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects after acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2006;173:212–8. [DOI] [PubMed] [Google Scholar]
- 28.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39. [DOI] [PubMed] [Google Scholar]
- 29.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. [DOI] [PubMed] [Google Scholar]
- 30.Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–7. [DOI] [PubMed] [Google Scholar]
- 31.Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, et al. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.