Abstract
Background
Choline is a dietary supplement that activates α7 nicotinic receptors. α7 nicotinic activation reduces cytokine production by macrophages and has antinociceptive activity in inflammatory pain models. We hypothesized that systemic administration of choline would reduce the inflammatory response from macrophages and have antinociceptive efficacy in a murine model of postoperative pain.
Methods
We studied the response of wild-type and α7 nicotinic knockout mice to heat and punctate pressure after a model surgical procedure. We investigated the effect of genotype and choline treatment on α-bungarotoxin binding to, and their production of tumour necrosis factor (TNF) from, macrophages.
Results
Choline provided moderate antinociception. The ED50 for choline inhibition of heat-induced allodynia was 1.7 mg kg−1 h−1. The ED50 for punctate pressure threshold was 4.7 mg kg−1 h−1 choline. α7 nicotinic knockout mice had no change in hypersensitivity to heat or pressure and were significantly different from littermate controls when treated with choline 5 mg kg−1 h−1 (P<0.05, 0.01). Choline 100 mM reduced binding of α-bungarotoxin to macrophages by 72% and decreased their release of TNF by up to 51 (sd 11)%. There was no difference by genotype in the inhibition of TNF release by choline.
Conclusions
Systemic choline is a moderately effective analgesic via activation of α7 nicotinic acetylcholine receptors. The antinocicepive effect may not be mediated by a reduction of TNF pathway cytokine release from macrophages. Although choline at millimolar concentrations clearly inhibits the release of TNF, this effect is not α7 subunit-dependent and occurs at concentrations likely higher than reached systemically in vivo.
Keywords: acetylcholine; acute pain, novel techniques; pharmacodynamics; pharmacology, dose–response; pharmacology, general
Key points.
Systemic choline is antinociceptive in a mouse model of acute postoperative pain.
Choline appears to have its antinociceptive action due to activation of α7 nicotinic acetylcholine receptors.
Choline inhibits TNF release from peripheral macrophages at high concentrations.
The concentrations of choline that inhibit TNF are not likely to be achieved in peripheral blood.
Despite the fact that cholinergic analgesia has been known for more than 70 yr,1 no treatment based on this mechanism has reached mainstream clinical use. Acetylcholinesterase inhibitors that increase concentrations of acetylcholine to activate both muscarinic and nicotinic receptors have analgesic efficacy, but are restricted to neuraxial use and even this mode may be limited by nausea, vomiting, and sedation in some settings.2,3
Systemic treatment with nicotine is effective in clinical trials,4–7 but is potentially limited by side-effects and concern for addiction. Nicotine activates a large family of nicotinic receptors, several of which have been implicated in its analgesic actions.8,9 Nicotine's addictive potential is at least partially as a result of activation of α4β2 type nicotinic receptors on presynaptic terminals of the nucleus accumbens.6 Activation of α4β2 type nicotinic receptors is also at least partially responsible for nicotine-associated nausea as it is recapitulated by the α4β2 selective agonist varenicline.10 Nicotine also has potential dose limitations related to autonomic side-effects related to α3β4-containing nicotinic receptors in the sympathetic nervous system, but surprisingly, haemodynamic side-effects were not found in the clinical trials cited above.
Studies in animal models have suggested that pharmacological activation of nicotinic receptors that contain α7 subunits decreases nociceptive responses in some settings but not others. Systemically administered α7 agonists seem to be preferentially effective in the setting of inflammation. Choline activates α7 nicotinic receptors,11 reduces hyperalgesia in the late phase of the formalin test, but not in hot plate or tail flick latency tests.12 Intraplantar choline is antinociceptive after carrageenan injection and its activity is associated with locally decreased tumour necrosis factor (TNF), swelling, and oedema.13 Our previous studies have suggested a role for α7-containing nicotinic receptors in a mouse model of postoperative pain.8 Intracerebroventricular and intrathecal administration of choline, a selective full agonist at α7 nicotinic receptors, is antinociceptive in a variety of pain models.14–16
Nicotinic α7-containing receptors are expressed in both central and peripheral sites. α7 nicotinic receptors are expressed by macrophages and monocytes where their activation attenuates the release of TNF and other downstream inflammatory cytokines.17 The preferential efficacy of α7 nicotinic agonists in the setting of inflammation could be a result of modulation of the release of cytokines by local macrophages. Choline reduces TNF release from macrophages by activating α7 nicotinic receptors.17 In the setting of inflammation, TNF sensitizes the NK1 receptor to substance P and other nociceptive stimuli.18,19 We hypothesized that choline would reduce hypersensitivity after surgery through its action as α7 nicotinic agonist and that the antinociceptive effects of choline might be due to inhibition of inflammatory cytokines released by macrophages after surgery because choline appears to be particularly effective in models in inflammatory pain.
Methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee at Columbia University. Wild-type female C57/Bl6 mice were used at 6–10 weeks of age for behavioural experiments and macrophage extraction (Jackson Laboratories, Bar Harbor, ME, USA). Additional experiments were performed on both male and female global nicotinic α7 knockout mice and their littermates on a C57/Bl6 background. Both male and female animals were used due to difficult breeding and a limited number of animals available (Jackson Laboratories). Genotype was identified using a polymerase chain reaction (PCR)-based analysis from DNA extracted from tail snips, described in detail below. Each animal underwent behavioural testing with continuous infusion of choline at only one concentration. The mice were housed in groups of five and had free access to food and water in an American Association of Laboratory Animal Care-approved facility.
Postoperative pain model
All mice were tested before surgery for sensitivity to heat and punctuate mechanical stimulus. Those mice with abnormal sensitivity to heat (<6 or >12 s) or punctuate pressure (<15 g) were not considered for further study. The incisional model for postoperative pain was implemented following the procedure originally described in mice by Pogatzki and Raja.20 Briefly, the mice were anaesthetized with isoflurane 1.5–2% from a nose cone. Adequacy of anaesthesia was determined as lack of pedal withdrawal response to paw pinch. The mice were allowed to recover from anaesthesia for 2 h before behavioural testing. There was a plan in place for animals that appeared to be in distress on emergence to be killed immediately. Animals that did not show adequate reduction in heat sensitivity (<4 s) or sensitivity to punctuate mechanical stimulus (<2 g) at 2 h after surgery were also killed. These values were determined in pilot studies as within 2 standard deviations (sds) of the mean response. Control animals underwent a sham surgical procedure with administration of isoflurane and recovery but had no surgical incision. At the end of all experiments, mice were killed using carbon dioxide.
Pump implantation surgery
After paw incision surgery and behavioural testing 2 h after surgery, alzet mini-Osmotic Pumps (Durect Corporation, Cupertino, CA, USA) were implanted to provide continuous steady-state s.c. infusion of choline. The pumps were loaded with saline or choline (at sufficient concentration to deliver between 0.2 and 100 mg kg−1 h−1 according to device instructions).
Behavioural testing
Mice underwent behavioural testing as described below at the following time points: before surgery, 2 h after paw surgery (or sham)—before drug treatment and after 24 and 48 h of continuous choline infusion. We measured hind-paw withdrawal latency in the unrestrained mice, housed individually in clear plastic chambers as described previously.21 The testing stimulus was 15% of maximal for the device and caused an average increase to 42°C before movement. Response to a punctate stimulus and righting reflex were also tested as previously described.21,22
Genotyping
Nicotinic knockout mice were a gift from Lorna Role at Columbia University. The breeding colony was derived from heterozygous matings of α7 nicotinic knockout mice on a C57/Bl6 background from Jackson laboratories that were originally created by Orr-Urtreger and colleagues.23,24 The mice have no major behavioural or pain-related phenotype. Littermates were studied without knowledge of genotype. DNA from tail clips was amplified by PCR using primers supplied by Jackson Laboratories to identify either the neo-cassette of the null mutation or the wild-type allele (forward: 5′ cc tgg tcc tgc tgt gtt aaa ctg ctt c—20 pmol, reverse: WT 5′ ctg ctg gga aat cct agg cac act tga g—10 pmol, mutant: 5′ gac aag acc ggc ttc cat ccg agt ac—25 pmol). PCR products identified as bands at 440 bp (wild-type) or 750 bp (mutant).
Peritoneal macrophage enrichment
Peritoneal macrophages were accumulated according to a protocol described by Edelson and Unanue25 designed to recruit blood macrophages to the peritoneal area for harvest. Macrophages were derived from mice that did not receive choline in vivo unless otherwise indicated. The cells were diluted to 50 000 macrophages/well and used acutely for TNF quantification or plated on cover slips and used the next day for immunocytochemistry.
Macrophage lipopolysacaride stimulation and TNF quantification
TNF release was stimulated with lipopolysacaride (LPS) from Escherichia coli 0111:b4 at concentrations between 0.1–100 ng ml−1.
The TNF concentration in the macrophage-containing media was measured after stimulation using Duoset ELISA Development System mouse TNF-alpha/TNFSFIA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. The minimum level of detection for TNF was 10 ng ml−1.
Macrophage immunohistochemistry
We studied the effect of choline on binding to mouse tissue macrophages to create a link between choline and the α7 nicotinic binding site on macrophages that inhibits TNF signalling.17 α-Bungarotoxin binds selectively to α7-, 8-, and 9-containing nicotinic receptors and muscle type nicotinic receptors;11,26 however, only α7 nicotinic subunits are known to be expressed by macrophages.17 Macrophages used for immunohistochemistry were derived from animals that did not undergo surgery. The macrophages were plated on plain glass cover slips and incubated overnight in DMEM (supplemented with 10% FBS and penicillin 100 U ml−1 and streptomycin 100 mg ml−1) at 37°C 1.7% CO2, 21% oxygen. They were treated with either florescent α-bungarotoxin-488 (αBgTx) (Molecular Probes; Invitrogen, Carlsbad, CA, USA), ER-MP58 (IgM primary antibody-derived against mouse macrophage cell lines; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), or both overnight at 4°C in 5% normal donkey serum (NDS) in PBS, diluted 1:500 and 1:50 from a 1 mg ml−1 stock, respectively. The samples were incubated with donkey-anti-rabbit-488 αBgTx and Texas Red donkey-anti-rat secondary antibodies for 1 h at 37°C in 5% NDS (diluted 1:500 and 1:50, respectively). The cells were visualized and photographed with a fluorescent microscope (Olympus IX50, Olympus Optical Co., Japan) with a ×40 power objective lens. The total number of cells was counted with light microscopy and then the per cent positive for macrophages (Texas Red), αBT-green, or both was determined. The total number of cells was counted with light microscopy and then the per cent positive for macrophage marker ER-MP58 (Texas Red), αBT-488 green, or both was determined. All chemicals and buffers were purchased from Sigma Corporation (St Louis, MO, USA) unless otherwise specified.
Data analysis
The time course of natural recovery from surgery was evaluated by comparing postoperative nociceptive reflexes with baseline responses using the Kruskal-Wallis test. The behavioural response to choline after 48 h infusion was fit with the sigmoidal equation: response=E0+(EMAX−E0)×[Choline]γ/([Choline]γ+C50γ) using non-linear mixed-effects modelling (NONMEM; Globomax, Ellicott City, MD, USA) and an additive error model. E0 is the response without choline, EMAX the maximal drug effect, C50 the choline dose at half-maximal effect, and γ the slope function. The result was compared with no drug effect using the change in −2 log likelihood, with a decrease of 3.84 considered statistically significant (χ2=0.05, 1 degree of freedom). Standard errors of the parameter estimates were calculated by NONMEM using the covariance step. Behavioural data are shown as means (sd). The effect of nicotinic α7 genotype on response to choline (5 mg kg−1 h−1) was compared with a two-tailed test.
The number of cells that stained positive for ER-MP58 or α-bungarotoxin-488 was compared with Fisher's exact test (GraphPad Software, San Diego, CA, USA). The amount of TNF released by the macrophages after surgery or sham surgery was compared with repeated-measures anova (GraphPad Software). A concentration–response curve for inhibition of TNF production by choline was derived according to the equation, y=100/[1+10(LOGx0−x)×p] where x0 is the EC50 and p the slope function. The values were computed and compared with Microcal Origin 8.0 (Northampton, MA, USA). TNF production was compared by genotype with a two-tailed t-test. Observations are summarized as mean (sds). When comparing groups, mean observations are reported and graphed using standard error of the mean. For derived parameters (ED50), standard error is reported. P<0.05 was considered significant in all statistical tests.
Results
Behavioural experiments
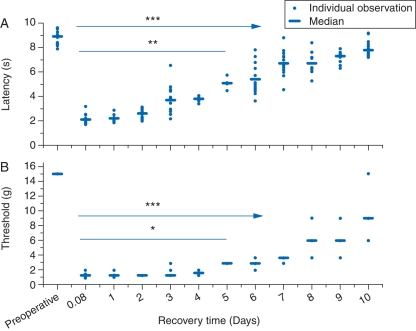
The surgical procedure enhanced nociceptive reflexes in response to heat and punctate mechanical stimulus (Fig. 1a and b). The reflex response to heat was significantly greater than immediately after surgery by postoperative day 5 (on day 6, P<0.01 and <0.001 thereafter; Kruskal–Wallis with Dunn's multiple comparisons test). The threshold for response to a punctate stimulus was also stable from immediately after surgery until postoperative day 5 (on day 6, P<0.05 and thereafter <0.001).
Fig 1.
In control experiments, (a) heat latency and (b) response to a punctate mechanical stimulus were significantly reduced after paw incision. The responses recovered over a 10 day period. Estimates for individual animals are shown with medians as a bar. Significant recovery of response compared with 2 h after surgery (*P<0.05; **P<0.01; ***P<0.001; Kruskal–Wallis; n=4–14 animals at each time point—118 observations/assay).
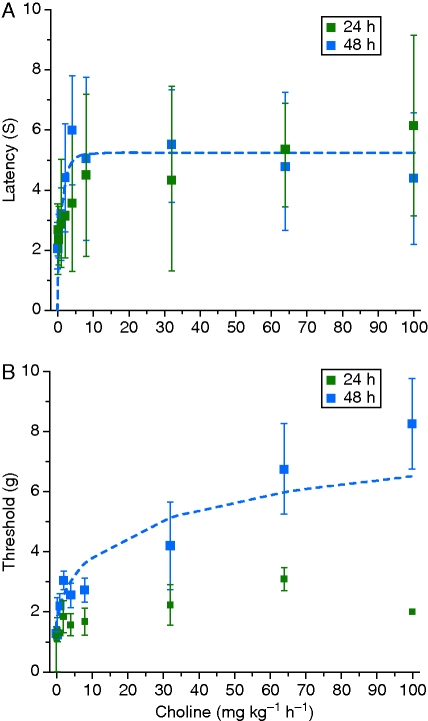
Chronic s.c. choline infusion reduced heat hypersensitivity after surgery with maximal efficacy after 48 h of infusion (Fig. 2a). The ED50 choline dose was 1.7 (0.3) mg kg−1 h−1. Choline was not fully efficacious but increased heat latency to a maximum of 5.3 (0.3) s (51 individual observations). Treatment with choline also increased withdrawal threshold to a punctate stimulus 48 h after surgery (Fig. 2b). The ED50 was 4.7 (3.4) mg kg−1 h−1 choline for the pressure stimulus, and the maximum threshold was 9.9 (0.0) g (53 individual observations). There was minimal change in response to the punctate stimulus 24 h after surgery with choline infusion. There were no gross behavioural changes at any concentration of choline. All mice retained righting reflex at all choline concentrations (data not shown).
Fig 2.
(a) Choline infusion dose-dependently increases heat latency at 24 and 48 h. The 48 h measurements are fit with the equation, Latency=E0+(EMAX−E0)×[Choline]γ/([Choline]γ+C50γ) in NONMEM (dashed blue line). (b) Choline infusion dose-dependently reduces hypersensitivity to a punctate mechanical stimulus after 48 but not 24 h of infusion. Measurements after 24 h of infusion and 48 h of continuous infusion are fit with the equation, Latency=E0+(EMAX−E0)×[Choline]γ/([Choline]γ+C50γ) (dashed blue line). Data are shown as mean (sd).
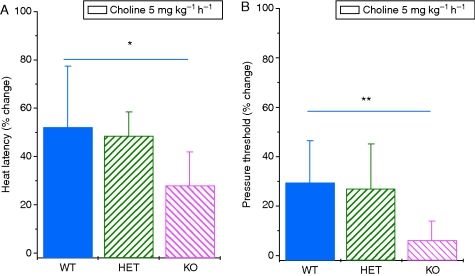
There was no difference in baseline heat or punctate stimulus responses among α7 knockout mice and their heterozygous and wild-type littermates [latency: WT, 8.0 (0.6); HET, 9.1 (0.9); KO, 8.5 (1.2) s]. No animals responded to the 15 g stimulus. Neither was there a difference in the hyperalgesia induced by the surgical procedure among genotypes [WT, 2.7 (0.4); HET, 2.8 (0.3); KO, 2.3 (0.2) s latency; WT, 1.7 (0.1); HET, 1.7 (0.1); KO, 2.1 (0.3) g pressure threshold]. However, the α7 nicotinic knockout mice had significantly less antinociceptive response to choline in both heat latency (P<0.05) and pressure threshold (P<0.01) when compared with the wild-type mice (Fig. 3a and b). Heterozygote animals had intermediate responses. There was no difference in response by sex (data not shown).
Fig 3.
Choline infusion at 5 mg kg−1 h−1 increases (a) heat latency and (b) pressure withdrawal in wild-type and heterozygous mice for the α7 deletion (P<0.001 heat, P<0.001 pressure). Choline did not affect heat latency and pressure threshold in mice-lacking α7 nicotinic acetylcholine receptors and their response was significantly different from that of wild-type mice (*P<0.05 heat, **P<0.01 pressure; mean (sd) n=9 WT, 14 HET, 4 KO).
Macrophage experiments
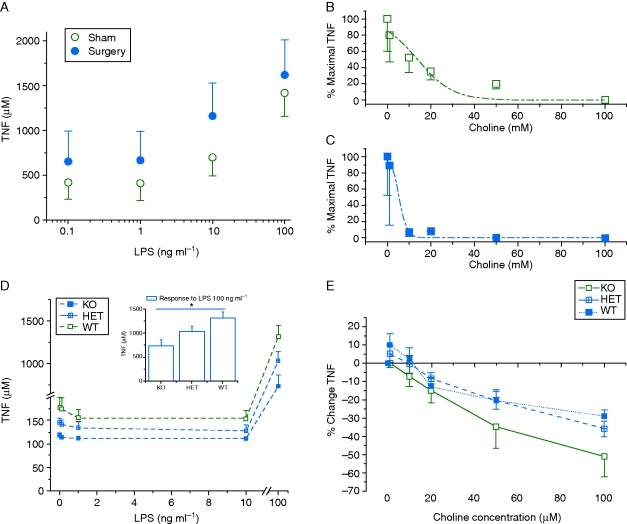
About 83–91% of cells derived from peritoneal lavage stained positive for the macrophage marker ER-MP58. There was no difference in the number of macrophages derived from wild-type and knockout mice. From wild-type mice, 85% of the cells that stained with the macrophage marker also were positive with α-BgTx-488. In the presence of choline (100 mM), staining for α-BgTx-488 was reduced (24%, P<0.001), but staining for the macrophage marker was not affected. Cells derived from α7 knockout mice had a similar number of macrophages with substantially less binding to α-BgTx-488 (31%, P<0.001).
Stimulation of macrophages with LPS resulted in enhanced TNF release. Macrophages derived from animals that had undergone surgery released more TNF than those that had undergone a sham procedure (Fig. 4a, anova P<0.001). Treatment with choline in vitro reduced the amount of TNF released with an EC50 of 13.9 (3.1) mM choline in sham surgical mice and 5.3 (0.9) mM choline in post-surgical animals (Fig. 4b and c).
Fig 4.
(a) More TNF was released from macrophages derived from animals that had undergone surgery than from those that had undergone a sham procedure (anova repeated measures, P<0.001). Exposure of macrophages to choline in vitro reduced TNF release from macrophages derived from sham (b) and surgical mice (c) in a dose-dependent manner. The data were fit with the following equation: y=A1+(A2–A1)/[1+10(LOGx0−x)×p]. (d) Macrophages from α7 nicotinic knockout mice produce less TNF when stimulated with LPS (Inset: at 100 mg kg−1, *P<0.05). (e) Choline caused a dose-responsive decrease in TNF release that did not differ among macrophages from the different genotypes.
Macrophages from α7 knockout animals produced less TNF than those from wild-type animals when stimulated with LPS 100 ng ml−1 (Fig. 4d, P<0.05). Millimolar concentrations of in vitro choline were effective in TNF reduction in macrophages from wild-type animals but also significantly reduced TNF concentrations in α7 knockout animals (Fig. 4e).
Discussion
Treatment with systemic choline has moderate antinociceptive efficacy after surgery in mice. Choline-treated animals responded as if they were tested on postoperative day 5. The decreased response to both heat and punctate stimuli is at least in part due to activity at α7-containing nicotinic acetylcholine receptors as it was significantly attenuated in α7 knockout mice.
In electrophysiological experiments, activation of α7 nicotinic receptors with choline results in a rapid inward current conducted by calcium and sodium that quickly desensitizes.11 Pilot experiments suggested that acute treatment with choline may reduce postoperative nociceptive reflexes (data not shown). However, if the effect quickly dissipated, this would be of little clinical utility. Continual exposure to the choline did not appear to dissipate over the 48 h period tested; rather the antinociceptive effect evolved over the 48 h studied. The fact that the antinociceptive effect of choline evolves more than 48 h of treatment may suggest an anti-inflammatory action or otherwise accelerated healing. One limitation of the paw incision model is that the wound that we induced was so small that it was difficult to classify erythema, oedema, and other clinical signs of wound integrity. Formal studies of wound strength and integrity would be interesting to pursue in the future.
α7 nicotinic acetylcholine receptors have been described on macrophages and microglia where they have anti-inflammatory effects through a pathway mediated by the inhibition of TNF release.17,27 The slowly developing antinociception could potentially be due to decreased wound inflammation after surgery. We have shown that TNF release is enhanced even by our relatively minor surgical procedure. Our immunocytochemistry studies show that high-dose choline prevents binding of α-bungarotoxin-488. However, macrophages from α7 knockout mice also had a small amount of residual α-bungarotoxin binding that could be due to the presence of other sensitive receptors, perhaps specific to macrophages. The original investigators that produced this line of α7 nicotinic knockout mice showed that there was no α-bungarotoxin binding in the hippocampus, but macrophages have not been studied in vitro.23 Other nicotinic subunits are expressed alveolar macrophages on the mRNA level including α3–α6, α9, and α10.28 Indeed, LPS stimulation causes up-regulation of both α7 and α10 nicotinic subunit expression,29 but it is not known what role activation of different receptor combinations by high-dose choline and bind α-bungarotoxin-488 might play in pain or inflammation.
Macrophage TNF release is inhibited by choline in vitro, but only at millimolar concentrations that are not likely to be achieved in vivo. Baseline choline concentrations in rodents (and humans) are ∼10 mM and have only been reported to be elevated by 10-fold with supplementation.30 The high concentrations of choline that we and others have used in vitro may have a non-specific action or action at another type of nicotinic receptor expressed by macrophages from α7 knockout mice because choline reduces TNF release irrespective of genotype. The work in sepsis models has shown the importance of α7 receptors in behavioural assays, but the macrophages studied in vitro were from the RAW macrophage-like cell line; macrophages from α7-receptor null animals have not been studied.31 Moreover, it is possible that the anti-inflammatory action of choline is not systemic, but rather local, in the wound, to prevent peripheral sensitization and perhaps improve wound integrity as suggested by the recent results of Gurun and colleagues.13 Choline loading before surgery may provide a natural analgesic adjuvant with low toxicity. Choline is currently available as a nutritional supplement in the USA and is thought to be important for pregnancy and lactation.32,33 However, as choline is not fully efficacious, evaluation of its interaction with other analgesic agents, including opioids and non-opioid analgesics, will be of interest. Wang and colleagues12 have previously suggested a synergistic anti-hyperalgesic interaction between choline and aspirin in response to a formalin challenge. Further studies will be required to determine whether choline supplementation may have anti-inflammatory activity, reduce pain, and the requirement for other analgesics after surgery in humans.
Conflict of interest
P.F. is married to Professor Steven Shafer, who is a member of the Editorial Board of the British Journal of Anaesthesia.
Funding
This study was funded in part with department funds and in part by National Institutes of Health grant R21AT004708 to P.F.
References
- 1.Davis L, Pollock I. Visceral pain. Surg Gynecol Obstet. 1932;55:418–27. [Google Scholar]
- 2.Klamt JG, Slullitel A, Garcia IV, Prado WA. Postoperative analgesic effect of intrathecal neostigmine and its influence on spinal anaesthesia. Anaesthesia. 1997;52:547–51. doi: 10.1111/j.1365-2222.1997.115-az0111.x. doi:10.1111/j.1365-2222.1997.115-az0111.x. [DOI] [PubMed] [Google Scholar]
- 3.Buvanendran A, Kroin JS. Useful adjuvants for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2007;21:31–49. doi: 10.1016/j.bpa.2006.12.003. doi:10.1016/j.bpa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hong D, Conell-Price J, Cheng S, Flood P. Transdermal nicotine patch for postoperative pain management: a pilot dose-ranging study. Anesth Analg. 2008;107:1005–10. doi: 10.1213/ane.0b013e318163204f. doi:10.1213/ane.0b013e318163204f. [DOI] [PubMed] [Google Scholar]
- 5.Habib AS, White WD, El Gasim MA, et al. Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg. 2008;107:999–1004. doi: 10.1213/ane.0b013e31816f2616. doi:10.1213/ane.0b013e31816f2616. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL. Nicotine and postoperative management of pain. Anesth Analg. 2008;107:739–41. doi: 10.1213/ane.0b013e3181813508. doi:10.1213/ane.0b013e3181813508. [DOI] [PubMed] [Google Scholar]
- 7.Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;101:1417–21. doi: 10.1097/00000542-200412000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Rowley TJ, Payappilly J, Lu J, Flood P. The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth Analg. 2008;107:1052–7. doi: 10.1213/ane.0b013e318165e0c0. doi:10.1213/ane.0b013e318165e0c0. [DOI] [PubMed] [Google Scholar]
- 9.Vincler MA, Eisenach JC. Knock down of the alpha 5 nicotinic acetylcholine receptor in spinal nerve-ligated rats alleviates mechanical allodynia. Pharmacol Biochem Behav. 2005;80:135–43. doi: 10.1016/j.pbb.2004.10.011. doi:10.1016/j.pbb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor S. Varenicline and its role in smoking cessation. Prev Cardiol. 2008;11:168–71. doi: 10.1111/j.1751-7141.2008.08258.x. [DOI] [PubMed] [Google Scholar]
- 11.Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–4. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. doi:10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108:1680–7. doi: 10.1213/ane.0b013e31819dcd08. doi:10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- 14.Abdin MJ, Morioka N, Morita K, et al. Analgesic action of nicotine on tibial nerve transection (TNT)-induced mechanical allodynia through enhancement of the glycinergic inhibitory system in spinal cord. Life Sci. 2006;80:9–16. doi: 10.1016/j.lfs.2006.08.011. doi:10.1016/j.lfs.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–91. doi: 10.1016/s0028-3908(00)00139-8. doi:10.1016/S0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 16.Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006;1117:92–100. doi: 10.1016/j.brainres.2006.07.118. doi:10.1016/j.brainres.2006.07.118. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. doi:10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 18.Miao FJ, Green PG, Benowitz N, Levine JD. Central terminals of nociceptors are targets for nicotine suppression of inflammation. Neuroscience. 2004;123:777–84. doi: 10.1016/j.neuroscience.2003.10.027. doi:10.1016/j.neuroscience.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Ware LB. Can nicotine treat sepsis? Nat Med. 2004;10:1161–2. doi: 10.1038/nm1104-1161. doi:10.1038/nm1104-1161. [DOI] [PubMed] [Google Scholar]
- 20.Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–7. doi: 10.1097/00000542-200310000-00041. doi:10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- 21.Flood P, Sonner JM, Gong D, Coates KM. Isoflurane hyperalgesia is modulated by nicotinic inhibition. Anesthesiology. 2002;97:192–8. doi: 10.1097/00000542-200207000-00027. doi:10.1097/00000542-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Flood P, Sonner JM, Gong D, Coates KM. Heteromeric nicotinic inhibition by isoflurane does not mediate MAC or loss of righting reflex. Anesthesiology. 2002;97:902–5. doi: 10.1097/00000542-200210000-00023. doi:10.1097/00000542-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Orr-Urtreger A, Goldner FM, Saeki M, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–71. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–12. doi: 10.1016/s1074-7613(01)00139-x. doi:10.1016/S1074-7613(01)00139-X. [DOI] [PubMed] [Google Scholar]
- 26.Tsetlin V, Utkin Y, Kasheverov I. Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:720–31. doi: 10.1016/j.bcp.2009.05.032. doi:10.1016/j.bcp.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Shytle RD, Mori T, Townsend K, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. doi:10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 28.Galvis G, Lips KS, Kummer W. Expression of nicotinic acetylcholine receptors on murine alveolar macrophages. J Mol Neurosci. 2006;30:107–8. doi: 10.1385/JMN:30:1:107. doi:10.1385/JMN:30:1:107. [DOI] [PubMed] [Google Scholar]
- 29.Chernyavsky AI, Arredondo J, Skok M, Grando SA. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int Immunopharmacol. 2009;10:308–15. doi: 10.1016/j.intimp.2009.12.001. doi:10.1016/j.intimp.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein J, Koppen A, Loffelholz K, Schmitthenner J. Uptake and metabolism of choline by rat brain after acute choline administration. J Neurochem. 1992;58:870–6. doi: 10.1111/j.1471-4159.1992.tb09337.x. doi:10.1111/j.1471-4159.1992.tb09337.x. [DOI] [PubMed] [Google Scholar]
- 31.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeisel SH. Choline, homocysteine, and pregnancy. Am J Clin Nutr. 2005;82:719–20. doi: 10.1093/ajcn/82.4.719. [DOI] [PubMed] [Google Scholar]
- 33.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009;89:673S–7S. doi: 10.3945/ajcn.2008.26811D. doi:10.3945/ajcn.2008.26811D. [DOI] [PMC free article] [PubMed] [Google Scholar]