Abstract
Purpose
This study was undertaken to evaluate the effect of prior treatment with radiation therapy or chemotherapy for unilateral Wilms tumor (WT) diagnosed during childhood on pregnancy complications, birth weight, and the frequency of congenital malformations in live-born offspring.
Patients and Methods
We reviewed pregnancy outcomes among female survivors and partners of male survivors of WT treated on National Wilms Tumor Studies 1, 2, 3, and 4 by using a maternal questionnaire and a review of both maternal and offspring medical records.
Results
We received reports of 1,021 pregnancies with duration of 20 weeks or longer, including 955 live-born singletons, for whom 700 sets of maternal and offspring medical records were reviewed. Rates of hypertension complicating pregnancy (International Classification of Diseases [ICD] code 642), early or threatened labor (ICD-644) and malposition of the fetus (ICD-652) increased with increasing radiation dose in female patients. The percentages of offspring weighing less than 2,500 g at birth and of those having less than 37 weeks of gestation also increased with dose. There was no significant trend with radiation dose in the number of congenital anomalies recorded in offspring of female patients.
Conclusion
Women who receive flank radiation therapy as part of the treatment for unilateral WT are at increased risk of hypertension complicating pregnancy, fetal malposition, and premature labor. The offspring of these women are at risk for low birth weight and premature (ie, < 37 weeks gestation) birth. These risks must be considered in the obstetrical management of female survivors of WT.
INTRODUCTION
Approximately 90% of all children with Wilms tumor (WT) survive for 5 years.1 Treatment with radiation therapy to part or all of the abdomen may adversely affect reproductive function. Early studies demonstrated an increased risk of fetal death in women who received abdominal irradiation.2–5 These and other studies included too few offspring born to women treated with chemotherapy or lower radiation therapy doses to have the statistical power to exclude an effect of this treatment on pregnancy outcome.6–11
Women who participated in the Childhood Cancer Survivor Study (CCSS) and received radiation therapy to a volume in which the ovaries were included or were near had neither an increased risk of stillbirth nor miscarriage. The offspring of women who received pelvic irradiation were more likely to weigh less than 2,500 g,12 and the offspring of women who received doses greater than 0.5 Gy were more likely to have infants born before 37 weeks of gestation.13
The National Wilms Tumor Study Group (NWTSG) completed four studies that evaluated various doses of radiation therapy and several different combination chemotherapy regimens for the treatment of children with various stages of WT.14–18 The NWTSG undertook a rigorous evaluation of the late effects of therapy in successfully treated patients (ie, Long-Term Follow-Up Study ([LTFUS]).
A previous report from the LTFUS demonstrated that women had increased risks of early or threatened labor and malposition of the fetus. In offspring, higher maternal radiation dose was associated with increased rates of low birth weight (LBW) and premature birth and with higher numbers of congenital malformations.19
This study was undertaken to more thoroughly evaluate the effect of various doses of flank radiation therapy on the risk of pregnancy complications, premature birth, and congenital malformations in the offspring of women and the partners of men treated for WT during childhood.
PATIENTS AND METHODS
The original consent documents for National Wilms Tumor Study (NWTS) -1, -2, -3 and -4, which were approved by the institutional review boards of all participating institutions, included consent for follow-up of surviving patients for evaluation of long-term outcomes. Patients became eligible for the LTFUS on the second anniversary of their date of diagnosis of WT. Five thousand nine hundred ninety-two of 6,484 patients from US or Canadian institutions who were treated on NWTS-1 through NWTS-4 were enrolled. Four hundred thirty-eight patients are known to have died. Two thousand six hundred forty-three are in active follow-up. Four hundred sixteen are in tracking; 1,051 have been located but have not returned questionnaires within the last 2 years; 791 are lost to institutional follow-up; 254 are being observed at institutions that have declined to renew their institutional participation in the study; and 399 participants have voluntarily discontinued.
A total of 5,372 of the 5,554 patients not known to be dead by the end of 2006 also had their 15th birthday by December 31, 2006, the closing date for the study. The NWTS Data and Statistical Center received responses to questionnaires submitted after their 15th birthday from 2,060 (81%) of 2,532 of the men and 2,369 (83%) of 2,840 of the women in this subgroup of patients. The methods for maintaining follow-up, evaluation of maternal and offspring medical records, and coding of congenital malformations have been described previously.19
NWTS radiation treatment protocol specified that the lateral, superior, and inferior limits of the treatment portals were to encompass the site of the kidney and associated tumor as visualized on the preoperative excretory urogram or abdominal computed tomography scan. The field was extended across the midline to include the vertebral bodies. Radiation doses were age adjusted in NWTS-1 and -2.14,15 In NWTS-3 and -4, lower radiation treatment doses were employed.16,17 Radiation dosimetry for the flank, gonads, and contralateral kidney was available for 75 participants of NWTSG LTFUS who were also participants in the CCSS.20 The CCSS radiation doses were estimated by using previously published methods and are shown in Appendix Table A1 (online only).21,22
Statistical Methods
Linear associations between radiation dose category, using the radiation dose to the renal fossa reported on the radiation therapy checklist,14 and specific pregnancy outcomes were examined with exact tests for trends in binomial proportions for correlated data.23 These tests account for within-family correlation and are appropriate for small samples. The trend analyses in this study did not consider patients who received whole-abdomen irradiation but only those who received no or flank (tumor bed) irradiation with or without radiation to extra-abdominal sites as part of the initial treatment.
Associations between the number (0 to ≥ 4) of congenital malformations found in the offspring with increasing radiation therapy dose to the parent were evaluated by using an exact test for linear-by-linear association in a two-way table of frequencies. US national reference data for determining percentile of birth weight for gestational age were those of Oken et al.24 Exact Wilcoxon tests were used to compare distributions of birth weight for gestational age in LTFUS offspring with those for the US reference population.
All exact tests were performed with StatXact version 8 (Cytel, Cambridge, MA). Statistical adjustments for the effects of one risk factor after accounting for those of another were performed by using logistic regression with generalized estimating equations to account for potential within-family correlation.25,26
RESULTS
The patients reported 1,021 pregnancies of 20 weeks or longer gestation. There were seven reported miscarriages, two elective abortions, 20 stillbirths, 955 live-born singleton infants, and 34 live-born infants from twin gestations. Twin gestations were excluded from the analysis because of the known higher risk for prematurity and LBW. Maternal and child medical records of 700 pregnancies of 20 weeks or longer duration were reviewed. Twenty-three pregnancies were excluded because of ambiguity in the site of abdominal irradiation, which left 677 for tabular analysis. Thirty-one additional pregnancies were excluded from the trend analyses, because the NWTS parent received whole-abdomen irradiation and could not be assigned to a flank-irradiation dose category.
Among those included in the analysis, patients who sired a singleton pregnancy were 53.4 ± 38.1 months of age at diagnosis and 32.9 ± 5.5 years of age at follow-up. Mothers of a singleton pregnancy were 55.7 ± 40.3 months of age at diagnosis and 31.2 ± 5.2 years of age at follow-up.
Complications of pregnancy and labor, including hypertension complicating pregnancy (International Classification of Diseases [ICD] code 642), early or threatened labor (ICD-644), malposition of fetus (ICD-652), premature rupture of membranes (ICD-658.1), obstructed labor (ICD-660), abnormality of forces of labor (ICD-661), and umbilical cord complications (ICD-663), were examined.
In partners of male patients, there was no significant trend in the risk of any complication with the radiation dose received by the father. Female patients had significantly increased risk of hypertension complicating pregnancy (P < .001), early or threatened labor (P = .002), and malposition of the fetus (P = .04) with increasing radiation dose (Table 1). The dose-response trend for malposition of the fetus was not statistically significant, however, when analysis was adjusted for gestational age (< 37 weeks or ≥ 37 weeks; P = .20). The frequency of hypertension complicating pregnancy among the partners of the male patients was 8.4% (15 of 178 patients) compared with 12.3% (23 of 187 patients) among the female patients who did not receive radiation (P = .17; Table 2). No woman who received whole-abdomen radiation therapy developed hypertension complicating pregnancy, but the number of pregnancies was small (Table 1).
Table 1.
Relationship Between Flank Radiation Therapy Dose and Labor Complications by Sex of Wilms Tumor Parent
| Parent Sex and Radiation Dose, Gy | No. of Offspring | Labor Complication |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD-642: Hypertension Complicating Pregnancy |
ICD-644: Early or Threatened Labor |
ICD-652: Malposition of Fetus |
ICD-658.1: Premature Rupture of Membranes |
ICD-660: Obstructed Labor |
ICD-661: Abnormality of Forces of Labor |
ICD-663: Umbilical Cord Complications |
|||||||||
| % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | ||
| Female | |||||||||||||||
| None | 187 | 12.3 | 23 | 15.0 | 28 | 4.3 | 8 | 5.9 | 11 | 6.4 | 12 | 7.5 | 14 | 19.3 | 36 |
| 0.01-15.00 | 49 | 18.4 | 9 | 12.2 | 6 | 12.2 | 6 | 2.0 | 1 | 2.0 | 1 | 8.2 | 4 | 32.7 | 16 |
| 15.01-25.00 | 111 | 20.7 | 23 | 25.2 | 28 | 6.3 | 7 | 4.5 | 5 | 6.3 | 7 | 6.3 | 7 | 27.0 | 30 |
| 25.01-35.00 | 84 | 35.7 | 30 | 26.2 | 22 | 13.1 | 11 | 1.2 | 1 | 15.5 | 13 | 6.0 | 5 | 19.1 | 16 |
| > 35 | 50 | 24.0 | 12 | 30.0 | 15 | 10.0 | 5 | 4.0 | 2 | 4.0 | 2 | 4.0 | 2 | 20.0 | 10 |
| Whole abdomen | 18 | 0.0 | 0 | 44.4 | 8 | 11.1 | 2 | 11.1 | 2 | 0.0 | 0 | 0.0 | 0 | 27.8 | 5 |
| Exact trend test P | < .001 | .002 | .04 | .25 | .23 | .40 | .89 | ||||||||
| Male | |||||||||||||||
| None | 55 | 5.5 | 3 | 14.6 | 8 | 3.6 | 2 | 1.8 | 1 | 16.4 | 9 | 14.6 | 8 | 20.0 | 11 |
| 0.01-15.00 | 11 | 9.1 | 1 | 0.0 | 0 | 18.2 | 2 | 0.0 | 0 | 18.2 | 2 | 27.3 | 3 | 27.3 | 3 |
| 15.01-25.00 | 36 | 11.1 | 4 | 13.9 | 5 | 5.6 | 2 | 0.0 | 0 | 11.1 | 4 | 11.1 | 4 | 19.4 | 7 |
| 25.01-35.00 | 41 | 2.4 | 1 | 2.4 | 1 | 0.0 | 0 | 4.9 | 2 | 7.3 | 3 | 9.8 | 4 | 26.8 | 11 |
| > 35 | 22 | 13.6 | 3 | 13.6 | 3 | 9.1 | 2 | 0.0 | 0 | 9.1 | 2 | 13.6 | 3 | 18.2 | 4 |
| Whole abdomen | 13 | 23.1 | 3 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 7.7 | 1 | 0.0 | 0 | 23.1 | 3 |
| Exact trend test P | .56 | .52 | 1.0 | .95 | .23 | .59 | .90 | ||||||||
Abbreviation: ICD, International Classification of Diseases.
Table 2.
Tests for Differences in Adverse Pregnancy Outcomes Between the Partners of all Male Patients on NWTS and Female Patients on NWTS Who Received No Abdominal Radiation Therapy
| Variable | Unirradiated Female Patients (%) | Partners of Male Patients (%) | P* |
|---|---|---|---|
| Hypertension complicating pregnancy | 12.3 | 8.4 | .17 |
| Early or threatened labor | 15.0 | 9.6 | .20 |
| Malposition of fetus | 4.3 | 4.5 | 1.00 |
| Premature rupture of membranes | 5.9 | 1.7 | .09 |
| Obstructed labor | 6.4 | 11.8 | .15 |
| Abnormality of forces of labor | 7.5 | 12.4 | .14 |
| Umbilical cord complications | 19.3 | 21.9 | .63 |
| Low birth weight | 9.1 | 3.9 | .05 |
| Premature | 10.2 | 5.1 | .12 |
| One or more congenital malformations | 8.8 | 5.1 | .25 |
Abbreviation: NWTS, National Wilms Tumor Study.
Two-sided P value from exact test for a difference between correlated proportions.
The percentage of infants born before 37 weeks of gestation increased from 10.2% in female patients who received no radiation therapy to 22% in those who received greater than 35 Gy (P = .001). One third of women who received whole-abdomen irradiation had infants born before 37 weeks of gestation (Table 3). For partners of male patients, the corresponding increase was from 3.6% to 13.6% (P = .27; Table 3). Percentages of infants with a birth weight less than 2,500 g also increased with radiation dose, from 9.1% in female patients who received no radiation therapy to 16% in those who received greater than 35 Gy (P = .01), and from 0% for the partners of the male patients who received no radiation therapy to 9.1% for the partners of those who received greater than 35 Gy (P = .07; Table 4) One third of the infants born to women who received whole-abdomen irradiation had a birth weight less than 2,500 g (Table 4).
Table 3.
Relationship Between Radiation Therapy Dose and Frequency of Prematurity by Sex of Wilms Tumor Parent
| Parent Sex and Radiation Dose, Gy | No. of Offspring | Premature (20-36 weeks of gestation) |
|
|---|---|---|---|
| No. | % | ||
| Female* | |||
| None | 187 | 19 | 10.2 |
| 0.01-15.00 | 49 | 3 | 6.1 |
| 15.01-25.00 | 111 | 23 | 20.7 |
| 25.01-35.00 | 84 | 19 | 22.6 |
| > 35 | 50 | 11 | 22.0 |
| Whole abdomen | 18 | 6 | 33.3 |
| Male† | |||
| None | 55 | 2 | 3.6 |
| 0.01-15.00 | 11 | 0 | 0.0 |
| 15.01-25.00 | 36 | 3 | 8.3 |
| 25.01-35.00 | 41 | 1 | 2.4 |
| > 35 | 22 | 3 | 13.6 |
| Whole abdomen | 13 | 0 | 0.0 |
NOTE. P values were exact tests for trend of premature birth with radiation dose (correlated data).
P = .001.
P = .27.
Table 4.
Relationship Between Radiation Therapy Dose and Frequency of Low Birth Weight by Sex of Wilms Tumor Parent
| Parent Sex and Radiation Dose, Gy | No. of Offspring | Birth Weight < 2,500 g |
|
|---|---|---|---|
| No. | % | ||
| Female* | |||
| None | 187 | 17 | 9.1 |
| 0.01-15.00 | 49 | 4 | 8.2 |
| 15.01-25.00 | 111 | 14 | 12.6 |
| 25.01-35.00 | 84 | 18 | 21.4 |
| > 35 | 50 | 8 | 16.0 |
| Whole abdomen | 18 | 6 | 33.3 |
| Male† | |||
| None | 55 | 0 | 0.0 |
| 0.01-15.00 | 11 | 0 | 0.0 |
| 15.01-25.00 | 36 | 3 | 8.3 |
| 25.01-35.00 | 41 | 2 | 4.9 |
| > 35 | 22 | 2 | 9.1 |
| Whole abdomen | 13 | 0 | 0.0 |
NOTE. P values are exact tests for trend of low birth weight with radiation dose (correlated data).
P = .01.
P = .07.
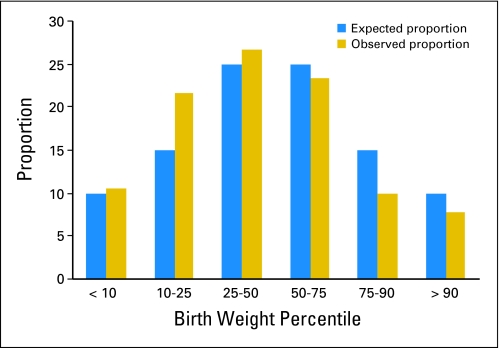
The frequency distribution of birth weight percentiles, determined from US reference population birth weights by gestational age for the offspring of female patients, was shifted left relative to the population norm (60% less than the US median; P = .003 by Wilcoxon test; Fig 1), which indicated that NWTS offspring were relatively small for gestational age. This shift to the left was observed for offspring of both women who receive irradiation (59% less than the median; P = .02) and women who did not (61% less than the median; P = .05). By contrast, the birth weight by gestational age distribution for offspring of the partners of male patients was shifted to the right (57% greater than the median; P = .29).
Fig 1.
Offspring of female patients who received irradiation.
Forty-four of 499 singleton offspring of female participants of LTFUS and nine of 178 singleton offspring of male participants of LTFUS for whom medical records were reviewed had one or more congenital malformations (Table 5; Appendix Table A2, online only). The percentages of offspring with one or more congenital malformations whose NWTS parent had received at least 35 Gy of irradiation were 14% (seven of 50 participants) for women and 18% (four of 22 participants) for men. The trend in the number of congenital malformations with increasing radiation dose was not statistically significant (P = .94) for women but was for men (P = .005; Table 5).
Table 5.
Relationship Between Flank Radiation Therapy and Number of Congenital Malformations in Offspring by Sex of Wilms Tumor Parent
| Parent Sex and Radiation Dose, Gy | No. of Offspring |
|||||
|---|---|---|---|---|---|---|
| Overall | No. of Congenital Malformations |
|||||
| 0 | 1 | 2 | 3 | ≥ 4 | ||
| Female* | ||||||
| None | 187 | 171 | 10 | 4 | 2 | 0 |
| 0.01-15.00 | 49 | 46 | 3 | 0 | 0 | 0 |
| 15.01-25.00 | 111 | 100 | 9 | 1 | 0 | 1 |
| 25.01-35.00 | 84 | 78 | 6 | 0 | 0 | 0 |
| > 35 | 50 | 43 | 5 | 2 | 0 | 0 |
| Whole abdomen | 18 | 17 | 1 | 0 | 0 | 0 |
| Male† | ||||||
| None | 55 | 54 | 1 | 0 | 0 | 0 |
| 0.01-15.00 | 11 | 11 | 0 | 0 | 0 | 0 |
| 15.01-25.00 | 36 | 36 | 0 | 0 | 0 | 0 |
| 25.01-35.00 | 41 | 38 | 2 | 1 | 0 | 0 |
| > 35 | 22 | 18 | 3 | 0 | 1 | 0 |
| Whole abdomen | 13 | 12 | 1 | 0 | 0 | 0 |
NOTE. P values are exact linear-by-linear association tests of number of congenital malformations by radiation dose.
P = .94.
P = .005.
DISCUSSION
We undertook this study to evaluate the effect of treatment for WT on complications of pregnancy and pregnancy outcome. Previously, we reported that the frequency of fetal malposition and premature labor was increased among female WT survivors.19 We have now demonstrated that the frequency of hypertension complicating pregnancy is also increased.
The pregnancies identified in this study ended with 955 live-born singleton infants of known gestational age, of whom 677 were included in the statistical analyses; this represents, to our knowledge, the largest study conducted to date of offspring of patients with WT.2–5,27,28 We did not evaluate the outcome of pregnancies of less than 20 weeks gestation because of the known inaccuracy of patient recall of such events.29,30
Pregnancy-associated hypertension (pre-eclampsia [eclampsia without chronic hypertension] and transient hypertension) occurred in 12.3% of pregnancies of women who did not receive irradiation, compared with 8.4% of pregnancies of the partners of the male survivors, which suggests that nephrectomy was unlikely to be a contributing factor to the increased frequency (23.7%) observed among the female WT survivors who had received irradiation. The rate among the women who did not receive irradiation was consistent with those reported for indigent women (13%)31 and nulliparous women (10% to 20%).32–35 However, the rate was higher than that reported by the National Center for Health Statistics, which was 3.9% for all ethnicities combined.36 The National Center for Health Statistics data are based on birth certificates. Several studies demonstrated that pregnancy-induced hypertension is under-reported on birth certificates.37,38
The mechanism responsible for the increased risk of pregnancy-associated hypertension is not known. Some survivors of WT have syndromes associated with WT1 mutations or deletions that predispose to renal disease. Breslow et al39 reported that the risk of end-stage renal disease in WT survivors 20 years after diagnosis was 1% for those with unilateral tumor and 12% for those with bilateral tumors. Patients with Denys-Drash syndrome, WT aniridia syndrome, or associated genitourinary anomalies had end-stage renal disease risks as high as 90%.39
Survivors of WT who have undergone nephrectomy may be at risk for hyperfiltration injury and/or hypertension. Compensatory hypertrophy of the remaining kidney is a well-documented finding after nephrectomy,40–43 although hypertrophy may be less robust if the kidney is irradiated.40,44 Although this adaptation may initially increase glomerular filtration capacity, the later development of glomerulosclerosis45,46 and interstitial injury47 may ultimately lead to deterioration of renal function.
Clinically significant reductions in glomerular filtration rate (GFR) after nephrectomy have been seen in a minority of survivors of WT who did not receive irradiation.40,42,48,49 A comparison between children with WT who did or did not receive whole-abdomen irradiation demonstrated lower GFR in the irradiated group (73% of normal) than in the nonirradiated group (95% of normal).50 However, others did not identify a difference in GFR between WT survivors who did or did not receive irradiation.42,51,52
GFR and renal compensatory growth were assessed a minimum of 5 years after nephrectomy in 22 children with WT who had received abdominal radiation and in 15 children who underwent nephrectomy for congenital hydronephrosis. The estimated size of the remnant kidney was increased by 25% in the WT group compared with 42% in the hydronephrosis group. Mean GFR was significantly lower in the WT group compared with that of the hydronephrosis group (82% and 92% of healthy controls, respectively).43 Long-term follow-up of children (mean and standard deviation, 12.9 ± 3 years after therapy) with WT found a low GFR (less than 80 mL/min/1.73 m2 as measured by chromium-51–EDTA clearance) in 19%. Children whose GFR measurements were decreased were more likely to have received higher doses of radiation to the kidney and demonstrated poorer renal growth, as measured by renal ultrasound.40
The prevalence of microalbuminuria, which is indicative of glomerular hyperfiltration, after nephrectomy for WT has been reported in 5%,40 9.4%,40 12.5%,53 and 84%41of those studied. Diastolic hypertension may also be a late effect of treatment that includes nephrectomy. In an analysis of 1,171 children treated for WT whose blood pressure was measured 5 years after diagnosis, 83 (7%) had a diastolic blood pressure greater than the 95th percentile for age.54
The offspring of women who received flank irradiation for WT were more likely to have a birth weight of less than 2,500 g than were those born to women who did not receive flank irradiation. This finding confirmed the results of several previous studies,2–5 including that of Chiarelli et al,55 who reported an increased relative risk of offspring with LBW among women treated with greater than 25 Gy of abdominal-pelvic radiation, and that of the CCSS, which reported an increased risk of birth weight less than 2,500 g among women who received pelvic irradiation.12 None of these studies reported the effect of the different doses received by the musculoskeletal structures, uterus, or ovaries from the various treatment volumes included in their analyses on outcome.
The mechanism responsible for LBW is unknown. The uterine volume is smaller in adult female survivors of childhood cancer who received radiation therapy below the diaphragm (median, 34 mL; range, 8 to 77 mL) than among those who received no radiation therapy (median, 47 mL; range, 22 to 88 mL).56 Others demonstrated that damage to both the uterine vasculature and myometrium contributed. Uterine length was significantly less in 10 women with ovarian failure who had been treated with whole-abdomen irradiation. Endometrial thickness did not increase in response to hormone replacement therapy, and no flow was detectable with Doppler ultrasound through one or both uterine arteries of these women.57,58 Six of eight women who received total-body irradiation during childhood or adolescence for treatment of leukemia had ovarian failure. Four had significantly reduced uterine volume and no significant endometrial tissue. Three had no uterine blood flow. Uterine volume, endometrial thickness, and uterine blood flow all increased significantly after 3 months of physiologic sex steroid replacement, although uterine volume remained reduced compared with that of women who did not receive irradiation.59 Others have confirmed the finding of reduced uterine volume despite sex steroid replacement therapy. In addition, uterine blood flow did not normalize in three of nine women, despite treatment with sex steroid replacement therapy.60
We previously reported an increase in the numbers of congenital anomalies in offspring of female patients on NWTS with increasing dose of radiation that attained borderline statistical significance, but we found no such increase for offspring of male patients.18 With the larger sample size of this study, we find no evidence for an increase in offspring of women, but instead we find a statistically significant increase in offspring of men. In view of the small numbers of patients involved, especially male patients, we are inclined to attribute both the earlier and present results to the play of chance.
Prior studies have generally not identified higher rates of congenital malformations in the offspring of cancer survivors. The number of congenital malformations identified in the offspring of the women who received irradiation was not confirmed to increase with increasing radiation dose. Byrne et al9 reported no statistically significant increase in the frequency of simple malformations among the offspring of female cancer survivors or of the partners of male cancer survivors compared with the offspring of their siblings. Chiarelli et al55 reported no increase in the risk of congenital anomalies among the offspring of women treated with abdominal or pelvic irradiation compared with those treated with surgery only, chemotherapy with an alkylating agent, or treatment with an alkylating agent and abdominal or pelvic irradiation.
Hawkins et al61 reported congenital anomalies in 3.6% of offspring born to women and in 2.6% of offspring born to the partners of men exposed to potentially mutagenic therapy (ie, radiotherapy involving direct exposure of the abdomen or gonads or treatment with an alkylating agent). These data were compared with 2.1% of offspring of unexposed women and 2.6% of the partners of unexposed men.
Our study was limited by the use of a mailed questionnaire for the ascertainment of pregnancy. Our results may be confounded by both under-ascertainment and ascertainment bias, with women whose pregnancies were complicated by one or more of the analyzed end points or whose infant had one or more congenital malformations possibly being more or less likely to participate.
We conclude that female survivors of WT who received irradiation are at increased risk for hypertension complicating pregnancy and early or threatened labor and fetal malposition and that their offspring are more likely to be premature (ie, < 37 weeks gestation) and of LBW (ie, < 2,500 g). The obstetrical management of the pregnancies of these women should take these elevated risks into consideration.
Acknowledgment
We thank the investigators of the Pediatric Oncology Group and the Children's Cancer Group, and we thank the health professionals who supervised the children entered onto the studies.
Appendix
Table A1.
Estimated Organ or Region Radiation Dose From Nominal Flank/Whole Abdomen Radiation Fields
| Parent Sex and NWTS Fossa Dose Category, Gy | No. of Patients | CCSS Radiation Dose by Location |
|||||
|---|---|---|---|---|---|---|---|
| Flank (Gy) |
Contralateral Kidney (Gy) |
Maximum Gonad (Gy) |
|||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Female | |||||||
| None | 2 | 5.82 | 3.13-8.51 | 5.82 | 3.13-8.51 | 0.14 | 0.10-0.17 |
| 0.01-15.00 | 8 | 10.90 | 10.90-11.30 | 1.30 | 1.25-2.48 | 0.39 | 0.27-0.49 |
| 15.01-25.00 | 20 | 21.35 | 20.28-22.50 | 2.35 | 2.18-3.30 | 0.82 | 0.55-1.53 |
| 25.01-35.00 | 19 | 30.20 | 27.20-32.00 | 3.70 | 3.10-4.40 | 1.35 | 0.76-3.48 |
| > 35 | 6 | 38.00 | 35.68-41.75 | 4.00 | 3.90-4.40 | 1.40 | 1.13-2.80 |
| Whole abdomen | 2 | 20.40 | 20.05-20.75 | 11.65 | 11.43-11.88 | 15.20 | 13.00-17.40 |
| Male | |||||||
| None | 0 | ||||||
| 0.01-15.00 | 2 | 10.55 | 10.53-10.58 | 6.00 | 3.70-8.30 | 0.13 | 0.09-0.16 |
| 15.01-25.00 | 5 | 19.50 | 18.20-21.00 | 2.80 | 2.20-3.00 | 0.26 | 0.15-0.27 |
| 25.01-35.00 | 8 | 30.10 | 28.92-30.78 | 3.50 | 3.28-4.20 | 0.52 | 0.19-0.69 |
| > 35 | 2 | 41.10 | 38.85-43.35 | 3.70 | 3.65-3.75 | 0.16 | 0.15-0.17 |
| Whole abdomen | 1 | 15.90 | 15.90-15.90 | 15.90 | 15.90-15.90 | 1.00 | 1.00-1.00 |
Abbreviations: CCSS, Childhood Cancer Survivor Study; NWTS, National Wilms Tumor Study; IQR, interquartile range.
Table A2.
Congenital Malformations in Offspring of Wilms Tumor Survivors Who Did and Did Not Receive Irradiation
| Sex and Radiation Group of NWTSG LTFUS Participant | Congenital Malformation |
|---|---|
| Female | |
| No abdominal irradiation | |
| Cardiac | Double outlet right ventricle, VSD, overriding aorta |
| Peripheral pulmonary stenosis | |
| VSD | |
| Genitourinary | Cryptorchidism, 3rd degree hypospadias |
| Deviation of chordee at hood of penis, nonpatent right narus | |
| Duplicated collecting system, left kidney, hydronephrosis, hemihypertrophy | |
| 1 degrees hypospadias, inv (11q) | |
| Duplicated collecting system, right kidney | |
| Bilateral vesicoureteral reflux | |
| Other | Achondroplasia, cavernous hemangioma (thigh) |
| Cleft lip, VSD, ASD | |
| Hypolastic mandible | |
| Developmental dysplasia, hip | |
| Abnormal karyotype [46, XX, t(5q;16q)] | |
| Imperforate anus | |
| Genu valgum | |
| Pyloric stenosis | |
| Bilateral Wilms tumor | |
| Deformity of skull, NOS | |
| Neurofibromatosis, optic glioma | |
| Abdominal irradiation | |
| Cardiac | Tetralogy of Fallot |
| PDA, talipes equinovarus | |
| VSD - four | |
| VSD, hypospadias | |
| Peripheral pulmonary stenosis | |
| Total anomalous pulmonary venous return | |
| Total anomalous pulmonary venous return, VSD | |
| ASD - two | |
| Genitourinary | Duplicated collecting system, left kidney |
| Hypospadias - two | |
| Ureteropelvic obstruction, right | |
| Other | Cleft lip |
| Neurobalstoma | |
| Adducted thumbs | |
| Left cleft lip and palate - two | |
| Scoliosis | |
| Beckwith-Wiedemann syndrome | |
| Vitreous conenital anomalies | |
| Acetabular dysplasia, bilateral | |
| Preauricular sinus, right | |
| Micrognathia, macrocephaly, choroid plexus cysts, ptosis | |
| Schizencephaly | |
| Coloboma of optic disc, hearing deficit | |
| Pectus excavatum | |
| Metatarsus valgus, left talipes calcaneovalgus | |
| Male | |
| No abdominal irradiation | |
| Cardiac | VSD |
| Abdominal irradiation | |
| Cardiac | Aortic stenosis |
| Genitourinary | Hypospadias, tibial hemimelia (bilateral), claw foot, left |
| Posterior urethral valves, failed hearing test | |
| Other | Calcaneovalgus, left |
| Pectus excavatum | |
| Wilms tumor | |
| Cleft lip and palate, left | |
| Retinoblastoma | |
| Open lip schizencephaly (bilateral) with partial agenesis of corpus callosum, VSD, cataract, microencephaly |
Abbreviations: NWTSG, National Wilms Tumor Study Group; LTFUS, Long-Term Follow-Up Study; VSD, ventricular septal defect; ASD, atrial septal defect; NOS, not otherwise specified; PDA, patent ductus arteriosus.
Footnotes
Supported in part by US Public Health Service Grants No. CA-54498 (N.E.B., Principal Investigator), CA-42326 (D.M.G., Principal Investigator), CA-55727 (L.L.R., Principal Investigator), and CA-21765 (M.B.K., Principal Investigator); by the Children's Cancer Research Fund (to the University of Minnesota Cancer Center); and by the American Lebanese Syrian Associated Charities (ALSAC).
Presented in part at the 40th Annual Meeting of the International Society of Paediatric Oncology, October 2-6, 2008, Berlin, Germany.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel M. Green, Jane M. Lange, Norman E. Breslow
Financial support: Norman E. Breslow
Administrative support: Norman E. Breslow
Collection and assembly of data: Eve M. Peabody, Natalia N. Grigorieva, Susan M. Peterson, Norman E. Breslow
Data analysis and interpretation: Daniel M. Green, Jane M. Lange, Norman E. Breslow
Manuscript writing: Daniel M. Green, Jane M. Lange, Eve M. Peabody, Natalia N. Grigorieva, Susan M. Peterson, John A. Kalapurakal, Norman E. Breslow
Final approval of manuscript: Daniel M. Green, Jane M. Lange, Eve M. Peabody, Natalia N. Grigorieva, Susan M. Peterson, John A. Kalapurakal, Norman E. Breslow
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975-2005. [Google Scholar]
- 2.Green DM, Fine WE, Li FP. Offspring of patients treated for unilateral Wilms' tumor in childhood. Cancer. 1982;49:2285–2288. doi: 10.1002/1097-0142(19820601)49:11<2285::aid-cncr2820491114>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Byrne J, Mulvihill JJ, Connelly RR, et al. Reproductive problems and birth defects in survivors of Wilms' tumor and their relatives. Med Pediatr Oncol. 1988;16:233–240. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 4.Li FP, Gimbrere K, Gelber RD, et al. Outcome of pregnancy in survivors of Wilms' tumor. JAMA. 1987;257:216–219. [PubMed] [Google Scholar]
- 5.Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: Probable effects of abdominal irradiation. Int J Cancer. 1989;43:399–402. doi: 10.1002/ijc.2910430309. [DOI] [PubMed] [Google Scholar]
- 6.Wallace WH, Shalet SM, Crowne EC, et al. Ovarian failure following abdominal irradiation in childhood: Natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–79. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 7.Li FP, Fine W, Jaffe N, et al. Offspring of patients treated for cancer in childhood. J Natl Cancer Inst. 1979;62:1193–1197. [PubMed] [Google Scholar]
- 8.Hawkins MM, Smith RA, Curtice LJ. Childhood cancer survivors and their offspring studied through a postal survey of general practitioners: Preliminary results. J R Coll Gen Pract. 1988;38:102–105. [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne J, Rasmussen SA, Steinhorn SC, et al. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet. 1998;62:45–52. doi: 10.1086/301677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DM, Zevon MA, Lowrie G, et al. Congenital anomalies in children of patients who received chemotherapy for cancer in childhood and adolescence. N Engl J Med. 1991;325:141–146. doi: 10.1056/NEJM199107183250301. [DOI] [PubMed] [Google Scholar]
- 11.Green DM, Fiorello A, Zevon MA, et al. Birth defects and childhood cancer in offspring of survivors of childhood cancer. Arch Pediatr Adolesc Med. 1997;151:379–383. doi: 10.1001/archpedi.1997.02170410053007. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–1080. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 13.Signorello LB, Cohen SS, Bosetti C, et al. Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98:1453–1461. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms' tumor: Results of the National Wilms' Tumor Study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.D'Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms' tumor: Results of the Second National Wilms' Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.D'Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms' tumor: Results of the Third National Wilms' Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 18.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:3744–3751. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 19.Green DM, Peabody EM, Nan B, et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: Gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 23.Corcoran C, Ryan L, Senchaudhuri P, et al. An exact trend test for correlated binary data. Biometrics. 2001;57:941–948. doi: 10.1111/j.0006-341x.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis with generalized linear models. Biometrics. 1985;41:582–583. abstr. [Google Scholar]
- 26.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Hawkins MM, Winter DL, Burton HS, et al. Heritability of Wilms' tumor. J Natl Cancer Inst. 1995;87:1323–1324. doi: 10.1093/jnci/87.17.1323. [DOI] [PubMed] [Google Scholar]
- 28.Sankila R, Olsen JH, Anderson H, et al. Risk of cancer among offspring of childhood-cancer survivors. N Engl J Med. 1998;338:1339–1344. doi: 10.1056/NEJM199805073381902. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox AJ, Horney LF. Accuracy of spontaneous abortion recall. Am J Epidemiol. 1984;120:727–733. doi: 10.1093/oxfordjournals.aje.a113940. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham FG, Leveno KJ. Management of pregnancy induced hypertension. In: Rubin PD, editor. Handbook of Hypertension: Hypertension in Pregnancy. Amsterdam, the Netherlands: Elsevier; 1988. [Google Scholar]
- 32.Pollak VE. Pre-Eclampsia and Kidney Disease. Bethesda, MD: National Institutes of Health; 1978. NIH publication 78-855. [Google Scholar]
- 33.Robinson N. Salt in pregnancy. Lancet. 1958;i:178–181. doi: 10.1016/s0140-6736(58)90665-2. [DOI] [PubMed] [Google Scholar]
- 34.MacGillivray I. Some observations on the incidence of preeclampsia. J Obstet Gynaecol Br Commonw. 1958;65:536–539. doi: 10.1111/j.1471-0528.1958.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomson AM, Chun D, Baird DD. Perinatal mortality in Hong Kong and in Aberdeen, Scotland. J Obstet Gynaecol Br Commonw. 1963;70:871–877. doi: 10.1111/j.1471-0528.1963.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin JA, Hamilton BE, Sutton PD, et al. Births: Final Data for 2005. vol 56. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 37.Bradford HM, Cardenas V, Camacho-Carr K, et al. Accuracy of birth certificate and hospital discharge data: A certified nurse-midwife and physician comparison. Matern Child Health J. 2007;11:540–548. doi: 10.1007/s10995-007-0178-3. [DOI] [PubMed] [Google Scholar]
- 38.Zollinger TW, Przybylski MJ, Gamache RE. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol. 2006;16:1–10. doi: 10.1016/j.annepidem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: Results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972–1975. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitt GA, Yeomans E, Dicks Mireaux C, et al. Renal size and function after cure of Wilms' tumour. Br J Cancer. 1992;66:877–882. doi: 10.1038/bjc.1992.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivas M, Agarwala S, Padhy AK, et al. Somatic growth and renal function after unilateral nephrectomy for Wilms' tumor. Pediatr Surg Int. 1998;14:185–188. doi: 10.1007/s003830050482. [DOI] [PubMed] [Google Scholar]
- 42.Bailey S, Roberts A, Brock C, et al. Nephrotoxicity in survivors of Wilms' tumours in the North of England. Br J Cancer. 2002;87:1092–1098. doi: 10.1038/sj.bjc.6600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wikstad I, Pettersson BA, Elinder G, et al. A comparative study of size and function of the remnant kidney in patients nephrectomized in childhood for Wilms' tumor and hydronephrosis. Acta Paediatr Scand. 1986;75:408–414. doi: 10.1111/j.1651-2227.1986.tb10222.x. [DOI] [PubMed] [Google Scholar]
- 44.Cassady JR, Lebowitz RL, Jaffe N, et al. Effect of low dose irradiation on renal enlargement in children following nephrectomy for Wilms' tumor. Acta Radiol Oncol. 1981;20:5–8. doi: 10.3109/02841868109130183. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez-Millet V, Nieto J, Praga M, et al. Focal glomerulosclerosis and proteinuria in patients with solitary kidneys. Arch Intern Med. 1986;146:705–709. [PubMed] [Google Scholar]
- 46.Welch TR, McAdams AJ. Focal glomerulosclerosis as a late sequela of Wilms' tumor. J Pediatr. 1986;108:105–109. doi: 10.1016/s0022-3476(86)80781-8. [DOI] [PubMed] [Google Scholar]
- 47.Mitus A, Tefft M, Fellers FX. Long term follow up of renal function of 108 children who underwent nephrectomy for malignant disease. Pediatrics. 1969;44:912–921. [PubMed] [Google Scholar]
- 48.Mpofu C, Mann JR. Urinary protein/creatinine index in follow up of patients with Wilms' tumour after nephrectomy. Arch Dis Child. 1992;67:1462–1466. doi: 10.1136/adc.67.12.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhisitkul DM, Morgan ER, Vozar MA, et al. Renal functional reserve in long-term survivors of unilateral Wilms tumor. J Pediat. 1991;118:698–702. doi: 10.1016/s0022-3476(05)80029-0. [DOI] [PubMed] [Google Scholar]
- 50.de Graaf SS, van Gent H, Reitsma-Bierens WC, et al. Renal function after unilateral nephrectomy for Wilms' tumour: The influence of radiation therapy. Eur J Cancer. 1996;32A:465–469. doi: 10.1016/0959-8049(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 51.Walker RD, Reid CF, Richard GA, et al. Compensatory renal growth and function in postnephrectomized patients with Wilms tumor. Urology. 1982;19:127–130. doi: 10.1016/0090-4295(82)90564-7. [DOI] [PubMed] [Google Scholar]
- 52.Di Tullio MT, Casale F, Indolfi P, et al. Compensatory hypertrophy and progressive renal damage in children nephrectomized for Wilms' tumor. Med Pediatr Oncol. 1996;26:325–328. doi: 10.1002/(SICI)1096-911X(199605)26:5<325::AID-MPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 53.Barrera M, Roy LP, Stevens M. Long-term follow-up after unilateral nephrectomy and radiotherapy for Wilms' tumour. Pediatr Nephrol. 1989;3:430–432. doi: 10.1007/BF00850221. [DOI] [PubMed] [Google Scholar]
- 54.Finklestein JZ, Norkool P, Green DM, et al. Diastolic hypertension in Wilms' tumor survivors: A late effect of treatment? A report from the National Wilms' Tumor Study Group. Am J Clin Oncol. 1993;16:201–205. [PubMed] [Google Scholar]
- 55.Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology. 2000;11:161–166. doi: 10.1097/00001648-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Larsen EC, Schmiegelow K, Rechnitzer C, et al. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83:96–102. doi: 10.1111/j.1600-0412.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 57.Critchley HO, Wallace WH, Shalet SM, et al. Abdominal irradiation in childhood: The potential for pregnancy. Br J Obstet Gynaecol. 1992;99:392–394. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 58.Critchley HOD. Factors of importance for implantation and problems after treatment for childhood cancer. Med Pediatr Oncol. 1999;33:9–14. doi: 10.1002/(sici)1096-911x(199907)33:1<9::aid-mpo3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 59.Bath LE, Critchley HOD, Chambers SE, et al. Ovarian and uterine characteristics after total body irradiation in childh od and adolescence: Response to sex steroid replacement. Br J Obstet Gynaecol. 1999;106:1265–1272. doi: 10.1111/j.1471-0528.1999.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 60.Holm K, Nysom K, Brocks V, et al. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant. 1999;23:259–263. doi: 10.1038/sj.bmt.1701569. [DOI] [PubMed] [Google Scholar]
- 61.Hawkins MM. Is there evidence of a therapy-related increase in germ cell mutation among childhood cancer survivors? J Natl Cancer Inst. 1991;83:1643–1650. doi: 10.1093/jnci/83.22.1643. [DOI] [PubMed] [Google Scholar]