Abstract
Purpose
To evaluate the prognostic significance of the integrin cell adhesion molecule very late antigen-4 (VLA-4) in acute myeloid leukemia (AML).
Patients and Methods
We prospectively quantified VLA-4 expression in 216 patients enrolled onto COG-AAML03P1 by flow cytometry and correlated expression levels with disease characteristics and clinical outcome.
Results
VLA-4 mean fluorescence intensity (MFI) varied 35-fold (range, 30 to 1,110; median, 219.5). High VLA-4 expression (> median MFI), compared with low expression, was associated with younger age (7.1 v 12.1 years, respectively; P < .001), lower FLT3 internal tandem duplication prevalence (4% v 21%, respectively; P < .001), and higher likelihood of extramedullary disease (16% v 5%, respectively; P = .013). In low- and high-expression groups, rates of remission (89% v 80%, respectively; P = .137) and minimal residual disease (29% v 25%, respectively; P = .700) were similar. Patients with low VLA-4 expression, compared with high expression, had a higher relapse rate (RR; 44% ± 10% v 24% ± 9%, respectively; P = .011) and lower disease-free survival (DFS; 48% ± 11% v 67% ± 10%, respectively; P = .023) after 3 years. Multivariate analyses showed that low VLA-4 expression was an independent adverse prognostic factor for DFS (hazard ratio [HR] = 1.98; P = .038) and RR (HR = 2.77; P = .009). Subgroup analyses indicated that the prognostic role of VLA-4 expression was most prominent in patients with standard-risk AML, in whom low VLA-4 expression was associated with inferior DFS (34% ± 16% v 69% ± 14% for high expression; P = .011) and higher RR (61% ± 16% v 26% ± 14% for high expression; P = .009). A similar trend was seen in low-risk but not high-risk patients.
Conclusion
High VLA-4 expression is associated with better clinical outcome in pediatric AML and is an independent predictor of relapse that may refine our abilities to stratify patients without identifiable cytogenetic or molecular risk factors.
INTRODUCTION
Integrins are receptors involved in cell adhesion to extracellular matrix proteins, cell surface ligands, and soluble ligands.1,2 Their pivotal role for many aspects of leukocyte biology has been well established.1,3,4 Recent studies have now highlighted the importance of integrins, in particular the prominent leukocyte α4β1 integrin very late antigen-4 (VLA-4; CD49d/CD29), for acute myeloid leukemia (AML), a disease in which VLA-4 is generally expressed to some degree.5 VLA-4 is central for binding of AML cells to bone marrow stromal elements as well as for cellular migration, and may mediate antiapoptotic signals and confer acquired drug resistance.6–8 Conversely, anti–VLA-4 antibodies can chemosensitize primary human AML cells and eliminate experimental minimal residual disease (MRD) in conjunction with chemotherapy.8
Thus, VLA-4 has emerged as a promising therapeutic target in AML. Still, its role as a clinical marker to define remission induction, disease recurrence, or prognosis remains controversial. Small studies on specimens from patients with AML other than acute promyelocytic leukemia (APL) indicated that VLA-4–positive patients had a significantly lower complete remission (CR) rate, higher relapse rate (RR), and worse overall survival (OS) and disease-free survival (DFS), implying an adverse prognostic role.8,9 In contrast, a recent retrospective study on frozen diagnostic samples from 175 adult patients with AML showed that VLA-4 function, as assessed by binding of the VLA-4 ligand, soluble vascular cell adhesion molecule-1 (sVCAM-1), was associated with improved OS.10 Unlike in adult AML, VLA-4 has not yet been evaluated in pediatric AML. Therefore, we performed a prospective evaluation of VLA-4 on diagnostic blast cells and correlated its expression with disease characteristics and clinical outcome in a large cohort of patients in a recent Children's Oncology Group (COG) AML protocol.
PATIENTS AND METHODS
Patient Samples
COG-AAML03P1 was a phase III pilot study to assess the safety and efficacy of gemtuzumab ozogamicin (GO) when added to standard chemotherapy. COG-AAML03P1 enrolled 340 children (age range, 1 month to 21 years) with newly diagnosed de novo AML, excluding patients with APL and Down syndrome. Treatment consisted of remission induction followed by intensification I, II, and III or intensification I and matched related donor hematopoietic cell transplantation with busulfan/cyclophosphamide as conditioning regimen. Cycle regimens were cytarabine/daunorubicin/etoposide plus GO (induction I), cytarabine/daunorubicin/etoposide (induction II), high-dose cytarabine and etoposide (intensification I), mitoxantrone/cytarabine plus GO (intensification II), and sequential high-dose cytarabine and asparaginase (intensification III).11 Diagnostic specimens from patients who consented to the biology studies and had marrow specimens available were shipped by overnight courier and immediately processed for prospective VLA-4 expression analysis on arrival at the centralized laboratory. Informed consent was obtained in accordance with the Declaration of Helsinki. The institutional review boards of all participating institutions approved the clinical protocol, and the COG Myeloid Disease Biology Committee approved this study.
Risk Stratification
A combination of cytogenetic and molecular abnormalities was used to stratify the patients into risk groups. A patient was considered low risk if a chromosomal abnormality/mutation was present in core-binding factors [CBF; t(8,21) or inv(16)/t(16,16)], nucleophosmin-1 (NPM1), or CEBPα (n = 73). Patients were classified as high risk if they had −5/5q–, monosomy 7, or FLT3 internal tandem duplication (ITD) with high allelic ratio (n = 25). All other patients with data sufficient for classification were considered standard risk (n = 101). Patients of unknown risk were those for whom cytogenetic/molecular data were insufficient for classification (n = 17).
Determination of VLA-4 Expression
Bone marrow cells were incubated with CD45-peridinin-chlorophyll-protein (clone 2D1), CD34-allophycocyanin (clone 8G12), and CD49d-phycoerythrin (clone 9F10; all from BD Biosciences, San Jose, CA) at saturating concentrations for 20 minutes in the dark followed by lysis of erythroid cells with NH4Cl (pH 7.2, 37°C) for 5 minutes. The cells were then washed in buffered saline and fixed with 1% paraformaldehyde. The flow cytometric data were collected immediately on one of two FACS Calibur cytometers (BD Biosciences) that had been cross-calibrated to provide identical fluorescence data. Quality control of these instruments used RFP and RCP rainbow beads (SpheroTech, Lake Forest, IL) to ensure identical sensitivity in all channels and between instruments on a daily basis. Additional laboratory quality control assays, taking advantage of the invariant CD4 expression intensities in normal individuals, showed identical CD4 fluorescence data throughout the study period, indicating consistent cytometer data acquisition properties over time without instrument drift. We also analyzed VLA-4 data over the study period and did not find any noticeable difference over time (median VLA-4 mean fluorescence intensity [MFI], 207.5 v 229.5 in the first half v second half of the study; P = .654). Data analysis was performed using WinList (Verity Software House, Topsham, ME). Identification of the viable tumor population was performed using CD45 versus side scatter (SSC) as an initial gate. We also assessed a secondary gate using CD34 but found no difference in the VLA-4 expression for CD34+ and CD34– subpopulations; thus, VLA-4 expression is presented for all leukemic cells based on CD45/SSC gating. The intensity of CD49d was generally homogeneous on the tumor cell populations, was unimodal, and approximated a log normal distribution (data not shown). The linear MFI12 was determined for each patient without knowledge of the clinical data.
Statistical Analysis
The Kaplan-Meier method13 was used to estimate OS (time from study entry to death), event-free survival (EFS; time from study entry until death, induction failure, or relapse), and DFS (time from end of induction I until death, induction failure, or relapse). Induction I treatment failure was defined as withdrawing from therapy as a result of relapse, persistent CNS disease, or refractory disease with more than 20% bone marrow blasts by the end of induction I. Estimates of the cumulative incidence of RR were calculated from the end of induction I to relapse, induction failure, or death as a result of progressive disease, where deaths from nonprogressive disease were considered competing events.14 The significance of predictor variables was tested using the log-rank statistic for OS, EFS, and DFS and Gray's statistic for RR. Children lost to follow-up were censored at their date of last known contact or at a cutoff 6 months before the date of analyses, which was June 24, 2009. Cox proportional hazards models15 were used to estimate the hazard ratio (HR) for specific univariate and multivariate analyses. Dichotomous variables defined from groupings of VLA-4 and the continuous variable of VLA-4 expression were analyzed by Cox models. Likelihood ratio tests of Cox models with VLA-4 included as a linear term versus a natural spline fit suggested that the linear term was adequate. For analyses yielding nonproportional hazards, accelerated failure time models with Weibull distributions were used. The χ2 test was used to test the significance of observed differences in proportions, and Fisher's exact test was used when data were sparse. Differences in medians were compared using the Mann-Whitney U test.
RESULTS
Study Cohort and VLA-4 Expression
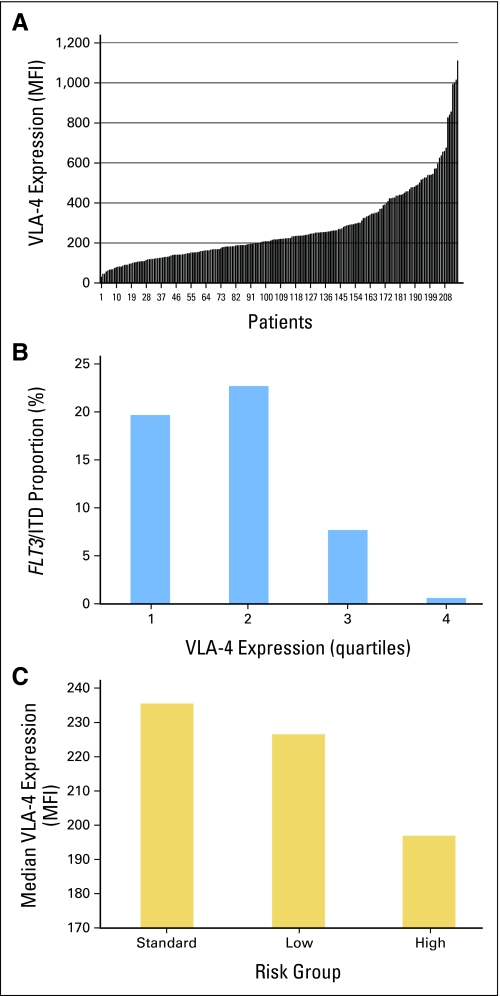
COG-AAML03P1 enrolled 340 children with de novo AML from December 2003 to November 2005. Of those, 216 patients (63.5%) consented to contribute to the biologic aims of the study and provided diagnostic marrow specimens for prospective evaluation of VLA-4 expression. As part of the immunophenotypic profiling of diagnostic specimens, the MFI of surface CD49d expression on leukemic blasts was measured. In the entire study cohort, VLA-4 expression, as shown by CD49d MFI, varied more than 35-fold (range, 30 to 1,110 MFI; median, 219.5 MFI; Fig 1A) with positive skewing. In individual patients, VLA-4 expression was generally homogeneous on tumor cells; unlike previous studies,8,10 we were unable to identify subpopulations of cells that were VLA-4 negative. Demographics, baseline laboratory findings, and pretreatment characteristics of all patients and of cohorts stratified by median VLA-4 MFI (low VLA-4 v high VLA-4) are listed in Table 1. Of note, patients with high VLA-4 expression (> median VLA-4 MFI), compared with low expression, were significantly younger (median age, 7.1 v 12.1 years, respectively; P < .001), with 34% v 13%, respectively, being younger than 3 years. Major cytogenetic subgroups were similarly distributed between patients with high and low expression with the exception of t(6;9), which was seen only in patients with low VLA-4 expression. Furthermore, there was a high association between FLT3/ITD and VLA-4 expression (21% in the low VLA-4 group v 4% in the high VLA-4 group; P < .001); in fact, none of the patients within the highest quartile of VLA-4 expression was positive for FLT3/ITD (Fig 1B). There was no apparent association between VLA-4 expression and the presence of CEBPα or NPM1 mutations; however, this analysis was limited by the small number of patients with such mutations. Overall, patients with high-risk disease had lower VLA-4 expression than patients with standard-risk or low-risk disease (P = .06; Fig 1C).
Fig 1.
(A) Distribution of very late antigen-4 (VLA-4) expression among the 216 patients in our study cohort. (B) Distribution of patients with FLT3 internal tandem duplication (ITD) across quartiles of VLA-4 expression. (C) Mean VLA-4 expression in patients with low-risk, standard-risk, and high-risk disease. MFI, mean fluorescence intensity.
Table 1.
Baseline Demographics and Clinical Characteristics of Study Cohort
| Demographic or Clinical Characteristic | All Patients (N = 216) |
Patients With Low VLA-4 (n = 108) |
Patients With High VLA-4 (n = 108) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | |||||||
| Median | 10.6 | 12.1 | 7.1 | < .001 | |||
| Range | 0.20-20.8 | 0.22-20.8 | 0.20-19.0 | ||||
| 0-2 | 51 | 24 | 14 | 13 | 37 | 34 | < .001 |
| 3-10 | 61 | 28 | 33 | 31 | 28 | 26 | .545 |
| ≥ 11 | 104 | 48 | 61 | 56 | 43 | 40 | .021 |
| Sex | |||||||
| Male | 119 | 55 | 61 | 56 | 58 | 54 | .784 |
| Female | 97 | 45 | 47 | 44 | 50 | 46 | |
| Diagnostic WBC, ×109/L | |||||||
| Median | 27.2 | 35.3 | 17.4 | .178 | |||
| Range | 0.8-495 | 0.9-323 | 0.8-495 | ||||
| Diagnostic hemoglobin, g/dL | |||||||
| Median | 8.2 | 8.2 | 8.1 | .565 | |||
| Range | 3.1-15.2 | 3.1-14.0 | 3.3-15.2 | ||||
| Diagnostic platelets, ×109/L | |||||||
| Median | 45.5 | 36.5 | 59.5 | .021 | |||
| Range | 4-578 | 4-578 | 4-520 | ||||
| Bone marrow blasts, % | |||||||
| Median | 70 | 72 | 69.8 | .421 | |||
| Range | 2-100 | 5-100 | 2-100 | ||||
| Cytogenetics | |||||||
| Normal | 37 | 19 | 21 | 22 | 16 | 16 | .385 |
| t(8;21)(q22;q22) | 27 | 14 | 11 | 11 | 16 | 16 | .475 |
| inv(16)/t(16;16)(p13.1;q22) | 31 | 16 | 15 | 16 | 16 | 16 | .901 |
| Abnormal 11 | 39 | 20 | 16 | 17 | 23 | 23 | .352 |
| t(6;9)(p23;q34) | 7 | 4 | 7 | 7 | 0 | 0 | .006 |
| –7/7q– | 6 | 3 | 2 | 2 | 4 | 4 | .683 |
| –5/5q– | 2 | 1 | 1 | 1 | 1 | 1 | 1.000 |
| Trisomy 8 | 20 | 10 | 13 | 14 | 7 | 7 | .202 |
| Other | 27 | 14 | 10 | 10 | 17 | 17 | .259 |
| Missing | 20 | 12 | 8 | ||||
| Risk group | |||||||
| Standard | 101 | 51 | 46 | 47 | 55 | 54 | .358 |
| Low | 73 | 37 | 33 | 34 | 40 | 40 | .471 |
| High | 25 | 13 | 19 | 19 | 6 | 6 | .008 |
| Unknown | 17 | 10 | 7 | ||||
| Molecular alterations | |||||||
| FLT3/ITD | 26 | 12 | 22 | 21 | 4 | 4 | < .001 |
| NPM1 mutation | 6 | 3 | 4 | 5 | 2 | 2 | .407 |
| CEBPα mutation | 12 | 6 | 5 | 5 | 7 | 7 | .835 |
| Extramedullary disease | 22 | 10 | 5 | 5 | 17 | 16 | .013 |
| CNS disease | 11 | 5 | 2 | 2 | 9 | 8 | .063 |
| Chloroma | 12 | 6 | 3 | 3 | 9 | 8 | .075 |
Abbreviations: VLA-4, very late antigen-4; ITD, internal tandem duplication.
Patients with high VLA-4 expression more commonly presented with extramedullary disease (EMD; chloroma or CNS involvement) at study entry (16% v 5% with low expression; P = .013), and 17 (77%) of 22 patients with EMD had high VLA-4 expression. Because EMD is more prevalent in patients with CBF AML, we investigated the association between EMD and VLA-4 expression in these patients and found that 3.8% of patients with CBF AML with low VLA-4 expression had EMD compared with 25% of patients with high VLA-4 expression (P = .033; n = 58). In contrast, in the patients who did not have CBF AML (n = 138), 4.3% with low VLA-4 expression versus 11.8% with high VLA-4 expression had EMD (P = .126).
VLA-4 Expression and Clinical Outcome
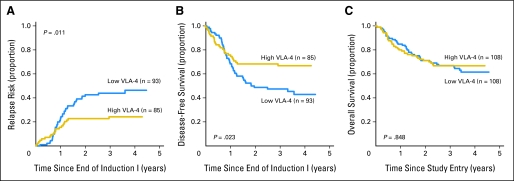
To investigate the relationship between VLA-4 expression and clinical outcome, we first studied responses to initial chemotherapy and found similar CR rates for patients with high (> median MFI) and low (< median MFI) VLA-4 expression (80% v 89%, respectively; P = .137) after one course of chemotherapy. Similarly, there was no difference in the incidence of MRD after induction I between patients with high and low VLA-4 expression (25% v 29%, respectively; P = .700). We subsequently evaluated how VLA-4 expression, as a continuous variable, correlated with parameters of long-term outcome. We found that higher VLA-4 levels were associated with longer DFS, longer EFS, and a lower RR. Specifically, over the range of VLA-4 expression, every 10-unit increase in MFI corresponded to a 2% decrease in RR (P = .023), a 1% increase in EFS (P = .049), and a 2% increase in DFS (P = .015). As with the initial analysis, we then stratified patients by median VLA-4 expression and determined outcomes for these two cohorts. Patients with high VLA-4 expression had a 3-year RR of 24% ± 9% compared with 44% ± 10% for patients with low VLA-4 expression (P = .011; Fig 2A). Interestingly, despite a lower likelihood of relapse, the median time to relapse tended to be shorter for patients with high VLA-4 expression relative to patients with low VLA-4 expression (285.5 v 362.5 days, respectively; P = .063), and survival after relapse tended to be shorter (2-year survival: 22% ± 16% v 44% ± 14%, respectively; P = .282). Three-year DFS for patients with high VLA-4 expression was 67% ± 10% compared with 48% ± 11% for patients with low VLA-4 expression (P = .023; Fig 2B). By comparison, 3-year OS was similar for patients with high and low VLA-4 expression (67% ± 9% and 68% ± 9%, respectively; P = .848; Fig 2C).
Fig 2.
Clinical outcome in patients with high and low very late antigen-4 (VLA-4) expression. Estimates of the probability of (A) relapse rate, (B) disease-free survival, and (C) overall survival in patients with high versus low VLA-4 expression.
Prognostic Factors
We next evaluated the potential role of VLA-4 expression, high-risk FLT3/ITD (ie, ITD with an allelic ratio > 0.4), WBC at diagnosis, and high-risk (–7, –5/del5q) and low-risk (CBF AML) cytogenetics as predictors of RR and DFS in univariate and multivariate Cox models (Table 2). In the univariate model, low VLA-4 expression was associated with a significantly higher RR and shorter DFS. Likewise, high-risk AML was associated with shorter DFS; conversely, low-risk AML was associated with a lower RR and longer DFS. MRD after induction I was associated with both lower RR and shorter DFS. In a multivariate model, low VLA-4 expression remained an independent prognostic factor for a higher RR (HR = 2.77; P = .009) and shorter DFS (HR = 1.98; P = .038), as did MRD after induction I (HR for RR = 2.57; P = .007; HR for DFS = 2.35; P = .006).
Table 2.
Univariate and Multivariate Cox Regression Models of DFS and RR for Entire Study Cohort
| Factor | DFS |
RR |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariate model | ||||||
| Low v high VLA-4* | 1.72 | 1.07 to 2.76 | .024 | 1.97 | 1.15 to 3.37 | .014 |
| Age, years | ||||||
| 0-2 | 1.00 | 1.00 | ||||
| 3-10 | 0.84 | 0.48 to 1.98 | .519 | 0.75 | 0.42 to 1.35 | .339 |
| ≥ 11 | 1.41 | 0.89 to 2.22 | .142 | 1.07 | 0.65 to 1.75 | .803 |
| Risk group | ||||||
| Low risk v standard risk | 0.58 | 0.37 to 0.93 | .025 | 0.47 | 0.28 to 0.82 | .007 |
| High risk v standard risk | 1.80 | 1.01 to 3.21 | .047 | 1.42 | 0.70 to 2.87 | .335 |
| WBC count, ≥ v < 100,000/μL | 0.78 | 0.45 to 1.34 | .364 | 0.93 | 0.53 to 1.65 | .807 |
| MRD positive at end of induction I | 2.44 | 1.54 to 3.86 | < .001 | 2.93 | 1.77 to 4.87 | < .001 |
| EMD positive at study entry | 0.97 | 0.52 to 1.80 | .915 | 0.88 | 0.43 to 1.83 | .734 |
| Multivariate model | ||||||
| Low VLA-4 | 1.98 | 1.04 to 3.78 | .038 | 2.77 | 1.28 to 5.97 | .009 |
| Age | ||||||
| 3-10 years | 1.24 | 0.48 to 3.21 | .659 | 0.91 | 0.34 to 2.47 | .853 |
| ≥ 11 years | 1.54 | 0.63 to 3.74 | .344 | 0.99 | 0.39 to 2.54 | .984 |
| Risk | ||||||
| Low | 0.66 | 0.36 to 1.21 | .177 | 0.56 | 0.29 to 1.11 | .095 |
| High | 1.45 | 0.64 to 3.28 | .369 | 1.08 | 0.39 to 3.05 | .879 |
| WBC count ≥ 100,000/μL | 0.68 | 0.31 to 1.48 | .333 | 0.77 | 0.35 to 1.72 | .525 |
| MRD positive at end of induction I | 2.35 | 1.29 to 4.29 | .006 | 2.57 | 1.30 to 5.06 | .007 |
| EMD at study entry | 1.54 | 0.63 to 3.77 | .350 | 1.29 | 0.43 to 3.87 | .645 |
Abbreviations: DFS, disease-free survival; RR, relapse rate; VLA-4, very late antigen-4; MRD, minimal residual disease; EMD, extramedullary disease.
Nonproportional hazards but accelerated failure time models yielded qualitatively similar results.
Correlation of VLA-4 Expression With Outcome in Risk Groups
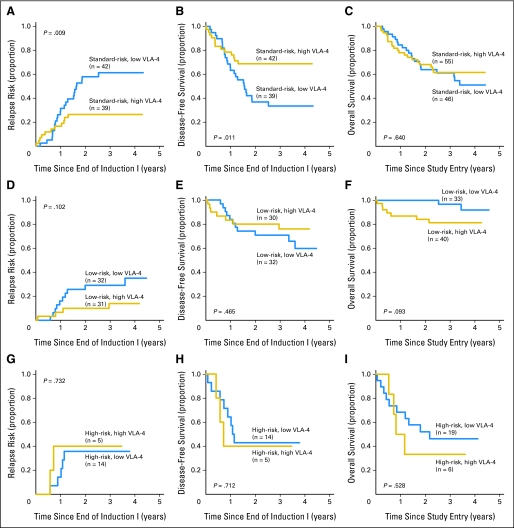
Finally, we performed post hoc subgroup analyses to investigate the potential role of VLA-4 expression as a predictor for outcome in specific disease risk groups. In standard-risk patients, CR rates after induction I were 76% ± 11% for patients with high VLA-4 expression and 87% ± 10% for patients with low expression (P = .294). RR after 3 years was significantly lower for patients with high VLA-4 expression versus low expression (26% ± 14% v 61% ± 16%, respectively; P = .009), whereas the DFS rate after 3 years was higher (69% ± 14% v 34% ± 16%, respectively; P = .011; Figs 3A and 3B), and OS rate after 3 years was similar (62% ± 14% v 61% ± 15%, respectively; P = .640; Fig 3C). In the multivariate model, low VLA-4 expression remained an independent prognostic factor for higher RR (HR = 6.86; P = .002) and shorter DFS (HR = 5.05; P = .032; Table 3). Similar to standard-risk patients, low-risk patients had a lower RR after 3 years if their AML expressed high levels of VLA-4 versus low levels (14% ± 13% v 29% ± 16%, respectively; Fig 3D). The small number of such patients resulted in a low power to detect a statistically significant difference in this outcome (P = .102). The 3-year DFS rates in patients with high versus low levels of VLA-4 were 76% ± 16% v 71% ± 16%, respectively (P = .465; Fig 3E), whereas OS rates after 3 years were 81% ± 13% v 97% ± 6%, respectively (P = .093; Fig 3F). In contrast, patients with high-risk disease had an RR that seemed to be independent of VLA-4 expression (36% ± 26% for low VLA-4 v 40% ± 44% for high VLA-4; P = .732; Fig 3G). Likewise, DFS and OS seemed to be independent of VLA-4 expression in this subgroup (DFS: 43% ± 26% for low VLA-4 v 40% ± 44% for high VLA-4; P = .712; Fig 3H; OS: 46% ± 23% for low VLA-4 v 33% ± 38% for high VLA-4; P = .528; Fig 3I), although the small sample size limited the precision of these estimates.
Fig 3.
Relapse risk (RR), disease-free survival (DFS), and overall survival (OS) in patients stratified by prognostic risk. Estimates of the probability of (A, D, G) RR, (B, E, H) DFS, and (C, F, I) OS are shown for patients expressing high or low very late antigen-4 (VLA-4) with (A, B, C) standard-risk acute myeloid leukemia (AML), (D, E, F) low-risk AML, and (G, H, I) high-risk AML.
Table 3.
Univariate and Multivariate Cox Regression Models of DFS and RR for Standard-Risk Patients
| Factor | DFS |
RR |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariate model | ||||||
| Low v high VLA-4* | 2.33 | 1.19 to 4.56 | .014 | 2.59 | 1.26 to 5.33 | .010 |
| Age, years | ||||||
| 0-2 | 1.00 | 1.00 | ||||
| 3-10 | 0.67 | 0.34 to 1.31 | .236 | 0.61 | 0.29 to 1.25 | .177 |
| ≥ 11 | 1.02 | 0.59 to 1.77 | .949 | 0.91 | 0.50 to 1.64 | .741 |
| WBC ≥ v < 100,000/μL | 0.73 | 0.33 to 1.60 | .429 | 0.85 | 0.38 to 1.87 | .679 |
| MRD positive at end of induction I | 2.50 | 1.32 to 4.76 | .005 | 2.63 | 1.35 to 5.13 | .005 |
| EMD positive at study entry | 0.85 | 0.34 to 2.12 | .729 | 0.79 | 0.28 to 2.18 | .644 |
| Multivariate model | ||||||
| Low VLA-4 | 5.05 | 1.72 to 14.8 | .032 | 6.86 | 2.05 to 22.9 | .002 |
| Age | ||||||
| 3-10 years | 0.49 | 0.13 to 1.88 | .297 | 0.35 | 0.08 to 1.49 | .154 |
| ≥ 11 years | 0.58 | 0.17 to 1.92 | .368 | 0.42 | 0.12 to 1.51 | .181 |
| WBC ≥ 100,000/μL | 0.57 | 0.19 to 1.75 | .329 | 0.60 | 0.19 to 1.90 | .386 |
| MRD positive at end of induction I | 2.78 | 1.19 to 6.48 | .018 | 2.87 | 1.15 to 7.17 | .024 |
| EMD positive at study entry | 1.35 | 0.25 to 7.32 | .731 | 1.54 | 0.27 to 8.87 | .629 |
Abbreviations: DFS, disease-free survival; RR, relapse rate; VLA-4, very late antigen-4; MRD, minimal residual disease; EMD, extramedullary disease.
Nonproportional hazards but accelerated failure time models yielded qualitatively similar results.
DISCUSSION
In this study, we found that VLA-4 expression is highly variable in pediatric AML and is associated with certain disease characteristics, most notably EMD, and clinical outcome, where patients with high VLA-4 expression have lower RR and longer DFS.
Previous studies on the prognostic significance of VLA-4 in AML reported conflicting findings. Initially, anti–VLA-4 antibodies were shown to chemosensitize human AML cells and to eradicate MRD in experimental mice when combined with chemotherapy, implicating VLA-4 in acquired chemotherapy resistance and MRD.8 These findings suggested that high VLA-4 expression might reduce chemosensitivity, resulting in poor remission induction, MRD, disease recurrence, and short survival.8 Indeed, results from a small series of non-APL AML samples supported this prediction.8 Similarly, univariate analyses from a study on 36 AML specimens indicated that high VLA-4 expression was associated with shorter survival.9 Subsequent studies failed to confirm these findings. In fact, a study of 175 AML samples indicated that high functional VLA-4 expression is associated with improved survival.10 Despite some methodologic differences, our main finding is consistent with that of Becker et al10 that patients with high VLA-4 expression had better long-term outcomes. However, our data differ slightly from those of Becker et al10 in that, in our cohort, superior outcome with high VLA-4 expression was primarily associated with increased DFS and reduced RR. Most notably, however, our data suggest that the effect of VLA-4 on outcome depends on other variables, most significantly cytogenetic risk. Specifically, the association of high VLA-4 expression with better outcomes was primarily seen in patients with standard-risk disease, with a similar trend toward lower RR in patients with low-risk disease, whereas DFS and RR estimates seemed to be independent of VLA-4 levels in high-risk patients. Therapeutics targeting VLA-4 are currently in clinical development for AML. Future studies need to determine whether the extent of response to these agents depends on VLA-4 expression levels; however, it is tempting to speculate that they might be particularly beneficial for patients with high VLA-4 expression and may allow use of less toxic chemotherapeutic regimens in this lower risk population.
The design of our study yielded several theoretical advances over previous studies. Most important, VLA-4 expression was determined on initial analysis of the diagnostic specimen on fresh cells without prior cryopreservation, avoiding potential experimental artifacts. Moreover, the study included a large number of patients with AML, thereby increasing the precision of outcome estimates for our cohort. Furthermore, all patients were treated in a uniform manner in a single one-arm clinical trial, thus facilitating patient comparisons. As a potential limitation of our analytic approach, the use of median VLA-4 expression levels may render standardization between different clinical laboratories challenging.
At first glance, our findings may seem counterintuitive, and it is interesting to speculate about their biologic basis. For example, it has previously been shown that soluble adhesion molecules, including sVCAM-1, are found at high levels in most AML patients.16 Because sVCAM-1 can overcome VLA-4–mediated chemotherapy resistance, it has been argued that soluble ligands could compete with adhesion in the marrow niche, dislodge AML cells from their protective environment, and enhance the effect of chemotherapy.10 However, high VLA-4 expression may merely be a surrogate for another prognostic factor that affects chemosensitivity and prognosis, either alone or in concert with VLA-4. Limited data suggest that VLA-4 expression may correlate with other molecules involved in adhesion and stroma cell interaction.7 In this respect, cooperation between VLA-4 and other mediators of AML-stroma interactions (eg, CXCR-4) has been well documented.7 Additional mechanistic and clinical studies will be necessary to fully understand this association between high VLA-4 expression and improved outcome in AML. Elucidation of the underlying mechanism will be important to address the possibility that patients with high VLA-4 expression are differentially sensitive to therapeutics targeting VLA-4, a finding that would go beyond the prognostic role of VLA-4.
Interestingly, we found an association between high VLA-4 expression and an increased prevalence of EMD. The significance of integrins and matrix metalloproteinases for tissue invasion of AML has recently been highlighted,17 and it is plausible that VLA-4 might be involved in the biology of the disease. However, similar to the association with improved outcome, future studies will be necessary to delineate whether this effect of expression is direct (ie, VLA-4 is involved in tissue invasion) or indirect (ie, high VLA-4 expression predicts for presence of other factors essential for EMD).
In summary, our analyses revealed significant heterogeneity of VLA-4 expression in pediatric AML. For outcome prediction, high VLA-4 expression defines a subgroup of standard-risk, and possibly low-risk, patients characterized by reduced RR and better DFS. The currently ongoing COG-AAML0531 randomized phase III trial will allow prospective validation of our findings. If the usefulness of VLA-4 expression for prognostication in patients without identifiable cytogenetic or molecular risk factors is confirmed, it may be integrated into the design of risk-adapted treatment strategies, for example, in upcoming COG trials.
Acknowledgment
We thank Sommer Castro and the Children's Oncology Group Acute Myeloid Leukemia (AML) Reference Laboratory for providing diagnostic AML specimens and David Galloway for editing the manuscript.
Supported by Grants No. U10-CA98543 (Chair's Grant), U10-CA98413 (Statistics and Data Center Grant), U24-CA114766 (Children's Oncology Group), R01-CA114563 (S.M.), and K23-CA137161 (R.B.W.) from the National Cancer Institute/National Institutes of Health, Bethesda, MD. A complete listing of grant support for research conducted by the Children's Cancer Group and Pediatric Oncology Group before initiation of the Children's Oncology Group grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Janet L. Franklin, Amgen (C); Michael R. Loken, Hematologics (C) Consultant or Advisory Role: None Stock Ownership: Janet L. Franklin, Amgen; Michael R. Loken, Hematologics Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Roland B. Walter, Franklin O. Smith, Michael R. Loken, Soheil Meshinchi
Financial support: Michael R. Loken
Provision of study materials or patients: Alan S. Gamis, Janet L. Franklin, Craig A. Hurwitz
Collection and assembly of data: Roland B. Walter, Robert B. Gerbing, Susana C. Raimondi, Betsy A. Hirsch, Michael R. Loken, Soheil Meshinchi
Data analysis and interpretation: Roland B. Walter, Todd A. Alonzo, Robert B. Gerbing, Phoenix A. Ho, Janet L. Franklin, Michael R. Loken, Soheil Meshinchi
Manuscript writing: Roland B. Walter, Todd A. Alonzo, Robert B. Gerbing, Franklin O. Smith, Susana C. Raimondi, Alan S. Gamis, Craig A. Hurwitz, Michael R. Loken, Soheil Meshinchi
Final approval of manuscript: Roland B. Walter, Todd A. Alonzo, Robert B. Gerbing, Franklin O. Smith, Betsy A. Hirsch, Alan S. Gamis, Janet L. Franklin, Craig A. Hurwitz, Michael R. Loken, Soheil Meshinchi
REFERENCES
- 1.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stucki A, Rivier AS, Gikic M, et al. Endothelial cell activation by myeloblasts: Molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97:2121–2129. doi: 10.1182/blood.v97.7.2121. [DOI] [PubMed] [Google Scholar]
- 6.Bendall LJ, Kortlepel K, Gottlieb DJ. Human acute myeloid leukemia cells bind to bone marrow stroma via a combination of beta-1 and beta-2 integrin mechanisms. Blood. 1993;82:3125–3132. [PubMed] [Google Scholar]
- 7.Burger JA, Spoo A, Dwenger A, et al. CXCR4 chemokine receptors (CD184) and alpha4beta1 integrins mediate spontaneous migration of human CD34+ progenitors and acute myeloid leukaemia cells beneath marrow stromal cells (pseudoemperipolesis) Br J Haematol. 2003;122:579–589. doi: 10.1046/j.1365-2141.2003.04466.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 9.Tavernier-Tardy E, Cornillon J, Campos L, et al. Prognostic value of CXCR4 and FAK expression in acute myelogenous leukemia. Leuk Res. 2009;33:764–768. doi: 10.1016/j.leukres.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Becker PS, Kopecky KJ, Wilks AN, et al. Very late antigen-4 function of myeloblasts correlates with improved overall survival for patients with acute myeloid leukemia. Blood. 2009;113:866–874. doi: 10.1182/blood-2007-12-124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin J, Alonzo T, Hurwitz CA, et al. COG AAML03P1: Efficacy and safety in a pilot study of intensive chemotherapy including gemtuzumab in children newly diagnosed with acute myeloid leukemia (AML) Blood. 2008;112:56. abstr 136. [Google Scholar]
- 12.Wells DA, Loken MR. Flow cytometric mean fluorescence intensity: The biophysics behind the number. Leuk Res. 2008;32:845–846. doi: 10.1016/j.leukres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. Hoboken, NJ: John Wiley & Sons; 2002. The Statistical Analysis of Failure Time Data. [Google Scholar]
- 15.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 16.Südhoff T, Wehmeier A, Kliche KO, et al. Levels of circulating endothelial adhesion molecules (sE-selectin and sVCAM-1) in adult patients with acute leukemia. Leukemia. 1996;10:682–686. [PubMed] [Google Scholar]
- 17.Stefanidakis M, Karjalainen K, Jaalouk DE, et al. Role of leukemia cell invadosome in extramedullary infiltration. Blood. 2009;114:3008–3017. doi: 10.1182/blood-2008-04-148643. [DOI] [PMC free article] [PubMed] [Google Scholar]