Abstract
Purpose
We sought to determine whether the combination of ixabepilone plus capecitabine improved overall survival (OS) compared with capecitabine alone in patients with metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes.
Patients and Methods
A total of 1,221 patients with MBC previously treated with anthracycline and taxanes were randomly assigned to ixabepilone (40 mg/m2 intravenously on day 1) plus capecitabine (2,000 mg/m2 orally on days 1 through 14) or capecitabine alone (2,500 mg/m2 on the same schedule) given every 21 days. The trial was powered to detect a 20% reduction in the hazard ratio (HR) for death.
Results
There was no significant difference in OS between the combination and capecitabine monotherapy arm, the primary end point (median, 16.4 v 15.6 months; HR = 0.9; 95% CI, 078 to 1.03; P = .1162). The arms were well balanced with the exception of a higher prevalence of impaired performance status (Karnofsky performance status 70% to 80%) in the combination arm (32% v 25%). In a secondary Cox regression analysis adjusted for performance status and other prognostic factors, OS was improved for the combination (HR = 0.85; 95% CI, 0.75 to 0.98; P = .0231). In 79% of patients with measurable disease, the combination significantly improved progression-free survival (PFS; median, 6.2 v 4.2 months; HR = 0.79; P = .0005) and response rate (43% v 29%; P < .0001). Grade 3 to 4 neuropathy occurred in 24% treated with the combination, but was reversible.
Conclusion
This study confirmed a previous trial demonstrating improved PFS and response for the ixabepilone-capecitabine combination compared with capecitabine alone, although this did not result in improved survival.
INTRODUCTION
Metastatic breast cancer (MBC) remains an incurable disease causing more than 40,000 deaths in the United States annually, although disease-associated symptoms may be palliated and survival prolonged by cytotoxic chemotherapy, including the anthracyclines and taxanes.1 However, development of primary and acquired resistance to taxanes limits the use of such agents in subsequent therapies. Until recently, capecitabine was the only approved treatment option for such patients.2,3
The epothilones and their analogs are a new class of microtubule-stabilizing anticancer drugs that are active against multidrug-resistant cell lines and tumors.4 Although they stabilize microtubules in a manner similar to taxanes, they are structurally different and bind to microtubulin in a distinct manner that confers sensitivity in taxane-resistant human tumor models.5,6 Ixabepilone is a semisynthetic analog of epothilone B demonstrating particularly high antimicrotubule activity and low susceptibility to drug resistance mechanisms, such as overexpression of class III isoform of β-tubulin and efflux transporters (such as, multidrug-resistance protein-1, breast cancer resistance protein, and P-glycoprotein).7
Ixabepilone has shown notable efficacy as monotherapy in several phase II trials in patients with locally advanced breast cancer and MBC.8–10 On the basis of preclinical data demonstrating synergy between ixabepilone and capecitabine,11 a dose-finding phase I/II study showed safety and efficacy in anthracycline- and taxane-pretreated MBC.12 In a prior phase III trial (CA163-046, the A/T resistant study) comparing ixabepilone-capecitabine combination with capecitabine alone in patients with anthracycline- and taxane-resistant disease, progression-free survival (PFS; the primary end point for the study) was significantly improved and objective response rate doubled compared with capecitabine monotherapy.13,14
In this report, we describe the results of a second randomized, phase III study (the A/T pretreated study) comparing the ixabepilone-capecitabine combination with capecitabine alone in women with MBC. It was performed concurrently with the previous study and included patients with both nonmeasurable and measurable disease who were anthracycline and taxane pretreated but were not required to meet the specific chemotherapy resistance criteria used in the A/T resistant study. The primary objective of the study was to assess whether the combination improved survival compared with capecitabine monotherapy.
PATIENTS AND METHODS
Patients
Eligible women with measurable or nonmeasurable, metastatic or locally advanced disease, Karnofsky performance status (PS) score between 70% and 100%, and life expectancy ≥ 12 weeks were required to receive prior treatment with both an anthracycline-containing (doxorubicin or epirubicin) and a taxane-containing (paclitaxel or docetaxel) regimen. Patients were required to receive two or fewer prior chemotherapy regimens, including those administered in the neoadjuvant/adjuvant setting. For details of the eligibility criteria, study design, and dose reduction, please refer to the online-only Appendix.
Study Design
In this multinational, randomized, open-label, phase III study, patients were randomly assigned in a 1:1 ratio to receive ixabepilone plus capecitabine or capecitabine alone. The primary end point was an intent-to-treat analysis of overall survival (OS). Secondary end points included PFS (defined as the time from random assignment to disease progression or death) within patients with measurable disease, objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors (RECIST),15 time to response and response duration within response-evaluable patients, safety measures, and patient-reported outcomes. Response and progression were determined by the local investigator and were not confirmed by any independent review.
Patients received ixabepilone 40 mg/m2 as a 3-hour intravenous infusion on day 1 of a 21-day cycle, plus oral capecitabine 1,000 mg/m2, administered twice each day on days 1 through 14 of a 21-day cycle, or capecitabine alone, 1,250 mg/m2 twice each day on days 1 through 14 of a 21-day cycle.13 Treatment was continued until disease progression or unacceptable toxicity. All patients who received study drug were evaluated for safety. Adverse events were assessed according to Common Terminology Criteria of Adverse Events version 3.0.
Statistical Analysis
At least 846 events (deaths) were required to ensure the two-sided, α = 0.05 level, log-rank test to have 90% power to show statistically significant difference in OS between treatment groups with the hazard ratio (HR) of 0.8. Survival was estimated using Kaplan-Meier methods for the median and 95% CI; comparison of OS between the two arms was made using two-sided log-rank test stratified by the following factors used in randomization: taxane resistance, measurable disease, prior chemotherapy for metastatic disease, and anthracycline resistance. Survival HR (two-sided 95% CI) was computed using unadjusted and adjusted Cox proportional hazards model for group comparisons. Prespecified covariates (eg, age, PS, number of organ sites, visceral disease and estrogen receptor status, hepatic function, time from diagnosis) were used to investigate the association of the potential prognostic factors with OS and to adjust the treatment comparison for these factors.
Analyses of PFS and ORR were restricted to stratum of patients with measurable disease and response-evaluable patients, respectively, using two-sided .05 level tests. With an estimated 1,200 randomly assigned patients, the subset of patients with nonmeasurable disease was limited to a maximum of 450 to ensure inclusion of at least 750 patients with measurable disease. There was 90% power to detect differences in PFS when the true HR was 0.77 and 95% power to detect differences in response rate when the true ORR was 32% versus 20%. Similar analysis as in OS was conducted for PFS. The primary analysis of response rate was a stratified Cochran-Mantel-Haenszel test, with an associated odds ratio and 95% CI to compare the response rates between arms.
RESULTS
Patients
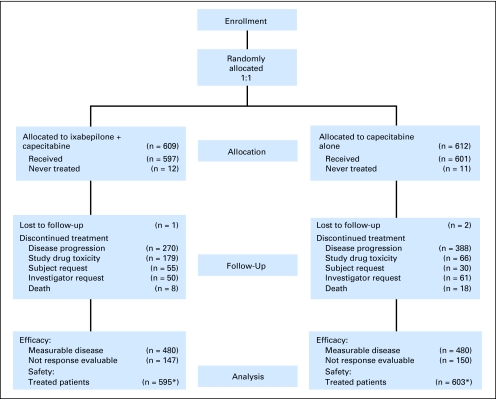
A total of 1,221 patients with metastatic or locally advanced breast cancer were randomly assigned to receive either ixabepilone plus capecitabine (n = 609) or capecitabine alone (n = 612) at 199 sites across 29 countries from November 2003 to August 2006. A total of 1,198 patients were treated: 595 with the combination and 603 with capecitabine alone (Fig 1 CONSORT diagram).
Fig 1.
CONSORT flowchart for CA163-048.
The majority of demographic characteristics were balanced between groups (Table 1), except for an imbalance in PS: the proportion of patients with an impaired PS (Karnofsky PS, 70% to 80%) was slightly higher in the combination group (32%) than in the capecitabine group (25%).
Table 1.
Baseline Patient Demographics and Disease Characteristics
| Characteristic | Ixabepilone Plus Capecitabine (n = 609) |
Capecitabine (n = 612) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 53 | 53 | ||
| Range | 23-78 | 24-81 | ||
| Measurable disease stratum | 480 | 480 | ||
| Karnofsky performance status, % | ||||
| 70-80 | 195 | 32 | 156 | 25 |
| 90-100 | 406 | 67 | 453 | 74 |
| < 70 | 2 | 0.3 | 2 | 0.3 |
| Not reported | 6 | 1 | 1 | 0.2 |
| Hormone receptor status | ||||
| ER positive | 341 | 56 | 330 | 54 |
| ER negative | 226 | 37 | 250 | 41 |
| HER2 positive | 85 | 14 | 100 | 16 |
| HER2 negative | 396 | 65 | 396 | 65 |
| ER-negative, PR-negative, HER2-negative | 122 | 20 | 134 | 22 |
| Site of visceral disease | ||||
| Liver | 273 | 45 | 276 | 45 |
| Lung | 221 | 36 | 217 | 35 |
| Extent of disease (No. of disease sites) | ||||
| ≥ 2 | 422 | 70 | 427 | 70 |
| < 2 | 184 | 30 | 185 | 30 |
| Prior regimens in the metastatic setting | ||||
| ≥ 3 | 2 | 0.3 | 3 | 0.5 |
| 2 | 112 | 18 | 107 | 17 |
| 1 | 371 | 61 | 388 | 63 |
| 0 | 124 | 20 | 114 | 19 |
| Prior chemotherapy and hormonal therapy | ||||
| Anthracycline | ||||
| Resistant* | 164 | 27 | 149 | 24 |
| Received minimum cumulative dose | 337 | 55 | 352 | 57 |
| Taxanes | ||||
| Resistance* | 299 | 49 | 286 | 48 |
| Progressive disease as best response to prior taxane (metastatic setting) | 101 | 17 | 105 | 17 |
| Trastuzumab | 48 | 8 | 48 | 8 |
| Hormonal therapy | 384 | 63 | 382 | 62 |
NOTE. Percentages may not add up to 100% due to rounding and/or unknown data for some patients.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Resistance = progression during treatment with or within 3 months of last dose (metastatic) or recurrence within 6 months of last dose (neoadjuvant/adjuvant).
Exposure
Patients in the combination group received a median of six cycles (range, one to 44 cycles); 89% received ≥ 70% of the intended dose of ixabepilone, and 62% received ≥ 70% of the intended dose of capecitabine. Patients in the capecitabine group received a median of five cycles (range, one to 50 cycles); 80% of patients received ≥ 70% of the intended dose of capecitabine.
In the combination group, 64% of patients had at least one dose reduction: both drugs in 34%, ixabepilone alone in 14%, and capecitabine alone in 15% of patients. In the capecitabine arm, 43% of patients received a reduced dose. Forty-five percent of patients in the combination group and 65% in the capecitabine group discontinued because of disease progression; 30% of the patients receiving the combination discontinued because of study drug toxicity as compared with 11% in the capecitabine arm; 26% discontinued one or both drugs because of peripheral neuropathy.
Efficacy
Overall survival: primary end point.
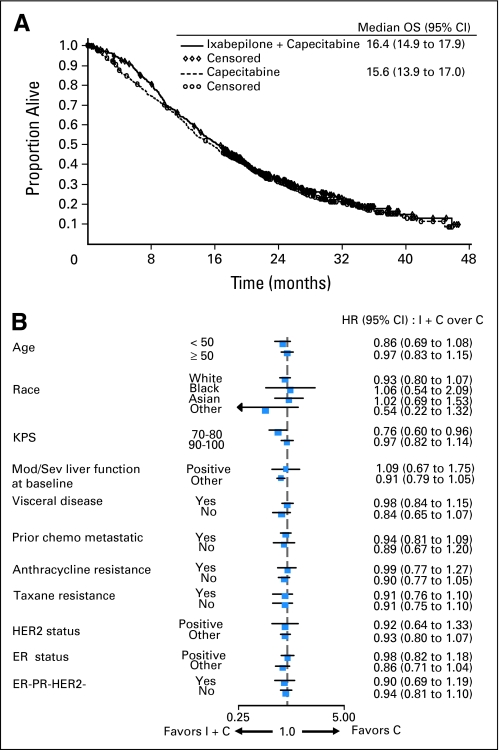
At the time of the analysis, there were 430 deaths (71%) in the combination group and 450 deaths (74%) in the capecitabine group. There was no significant difference in OS, the primary study end point, between the combination arm and capecitabine monotherapy arm (median 16.4 v 15.6 months; HR = 0.9; 95% CI, 078 to 1.03; P = .1162; Table 2, Fig 2A). As seen from the baseline patient characteristics (Table 1), a higher proportion of patients had impaired PS (Karnofsky 70% to 80%) in the combination group compared with the group receiving capecitabine alone (32% v 25%). Impaired PS was an indicator of poor prognosis regardless of treatment received; median survival was 13 months for PS 70% to 80% compared with 18 months for PS 90% to 100%. A secondary analysis adjusting OS for prespecified baseline covariates (including PS) showed improvement in OS for the combination arm (HR = 0.85; 95% CI, 0.75 to 0.98; P = .0231; Table 2). Exploratory analyses of OS unadjusted for multiple comparisons were also conducted across predefined subgroups. Among all the subgroups tested, it was worth noting that in symptomatic patients with a PS of 70% to 80%, median OS was 14.0 months for the combination and 11.3 months for capecitabine monotherapy (HR = 0.76; 95% CI, 0.60 to 0.96; Fig 2B).
Table 2.
Summary of Efficacy End Points of Ixabepilone Plus Capecitabine Combination
| Efficacy End Point | Ixabepilone Plus Capecitabine | Capecitabine |
|---|---|---|
| Overall survival (randomized patients) | ||
| No. of patients | 609 | 612 |
| OS, months | ||
| Median | 16.4 | 15.6 |
| 95% CI | 14.9 to 17.9 | 13.9 to 17.0 |
| No. of events | 430 | 450 |
| Hazard ratio | 0.90 | |
| 95% CI | 0.78 to 1.03 | |
| Stratified log-rank P | .1162 | |
| OS Adjusted Cox regression | 0.85* | |
| 95% CI | 0.75 to 0.98 | |
| P | .0231 | |
| PFS (measurable disease patients) | ||
| No. of patients | 480 | 480 |
| PFS | ||
| Median, months | 6.2 | 4.4 |
| 95% CI | 5.59 to 6.77 | 4.14 to 5.42 |
| Hazard ratio | 0.79 | |
| 95% CI | 0.69 to 0.90 | |
| Stratified log-rank P | .0005 | |
| ORR (response-evaluable patients) | ||
| No. of patients | 462 | 462 |
| Objective response rate, % | 43.3 | 28.8 |
| 95% CI | 38.7 to 47.9 | 24.7 to 33.2 |
| Odds ratio | 1.89 | |
| 95% CI | 1.44 to 2.50 | |
| Cochran-Mantel-Haenszel P | < .0001 | |
| Complete response | ||
| No. | 16 | 11 |
| % | 3 | 2 |
| Partial response | ||
| No. | 184 | 122 |
| % | 40 | 26 |
| Progressive disease | ||
| No. | 57 | 111 |
| % | 12 | 24 |
| Not determined | ||
| No. | 35 | 36 |
| % | 8 | 8 |
| Stable disease | ||
| No. | 170 | 182 |
| % | 37 | 39 |
| Month 6 stable disease rate | 15 | 15 |
| 95% CI | 11.8 to 18.5 | 11.4 to 18.0 |
Abbreviations: OS, overall survival; PFS, progression-free survival; ORR, objective response rate.
Adjusted for age, Karnofsky performance status, number of organ sites, estrogen receptor status, hepatic impairment, time from diagnosis, and visceral disease, which were all prespecified in the study protocol.
Fig 2.
(A) Overall survival (OS) Kaplan-Meier curves. OS distribution for the treatment arms receiving ixabepilone combination (I + C) versus capecitabine alone (C) are shown. (B) OS hazard ratio (HR) and 95% CI for subset analyses. HRs less than 1 favor combination therapy. KPS, Karnofsky performance status; Mod, moderate; Sev, severe; chemo, chemotherapy; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Approximately 80% of patients in each treatment group received subsequent therapy (chemotherapy, hormonal/immuno/biologic or radiotherapy) after termination of protocol therapy; 65% in the combination group received subsequent chemotherapy compared with 71% in the capecitabine-alone group. This imbalance was primarily reflected by a higher rate of subsequent taxane use (16% v 24%) in the capecitabine monotherapy arm, specifically in subsequent paclitaxel use (10% v 17%).
PFS.
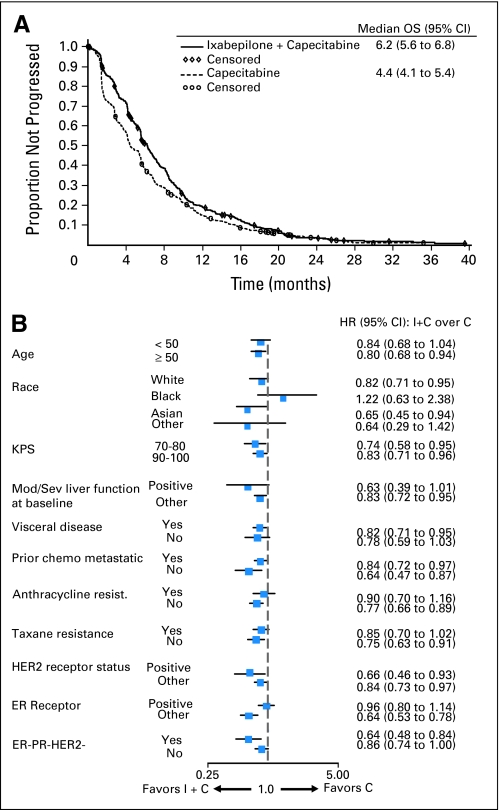
In the 79% of patients with measurable disease, ixabepilone plus capecitabine was superior to capecitabine for the secondary end point of PFS. Median PFS was prolonged to 6.24 months (95% CI, 5.59 to 6.97) for ixabepilone plus capecitabine compared with 4.4 months (95% CI, 4.14 to 5.42) for capecitabine (HR = 0.79; 95% CI, 0.69 to 0.90; P = .0005; Table 2; Fig 3A).
Fig 3.
(A) Investigator review of the progression-free survival (PFS) Kaplan-Meier curves. PFS distribution for patients with measurable disease receiving ixabepilone combination (I + C) versus capecitabine alone (C) are shown. (B) PFS hazard ratio (HR) and 95% CI for subset analyses. HRs less than 1 favor combination therapy. KPS, Karnofsky performance status; Mod, moderate; Sev, severe; chemo, chemotherapy; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Similar to OS, exploratory analyses on PFS were conducted across predefined subsets. These analyses indicated that benefit for the ixabepilone combination was maintained across various subgroups, with the exception of black patients; no conclusion can be drawn in this subset because of the small sample size (n = 40) and wide CI for the HR estimate (Fig 3B). In particular, PFS benefit was observed in patients with triple-negative tumors (HR = 0.64, 95% CI, 0.48 to 0.84; impaired PS: HR = 0.74, 95% CI, 0.58 to 0.95) and patients rapidly experiencing relapse within 12 months after adjuvant/neoadjuvant anthracycline and taxane treatment who received the combination as first-line therapy for metastatic disease (HR = 0.64; 95% CI, 0.47 to 0.87; Appendix Table A1, online only).
ORR.
In response-evaluable patients, ixabepilone plus capecitabine was also superior to capecitabine in the analysis of ORR (43% [95% CI, 39% to 48%] v 29% [95% CI, 25% to 33%]; P < .0001; Table 2). Duration of response was similar in both groups (6.1 v 6.3 months); time to response was also identical (6.6 weeks in both). Predefined subset analysis for response rate showed consistent benefit favoring the combination group (data not shown).
Safety
Incidences of treatment-related adverse events in this study were similar to the previously published report of this combination; the toxicity profile of the combination reflecting that of the individual agents.2,13,16 In the combination group, treatment-related adverse events were primarily mild to moderate (grade 1 to 2) in severity; the most frequently reported grade 3 to 4 nonhematologic adverse events were peripheral sensory neuropathy, hand-foot syndrome, fatigue, diarrhea, myalgia, arthralgia, and stomatitis (Table 3). The most frequent grade 3 to 4 adverse events in the capecitabine group were hand-foot syndrome, diarrhea, and stomatitis. There were fewer deaths on-study in the combination group than in the capecitabine group (3% v 7%); the incidence of death attributed to study drug toxicity was low and similar between the treatment groups (four deaths [0.7%] v two deaths [0.3%], respectively). In the combination group, these deaths were attributed to sepsis. Myelosuppression was common in patients treated with the combination and consisted primarily of leukopenia and neutropenia, with a low incidence of febrile neutropenia (7%; Table 3). Though not specified in the protocol, growth factor support (filgrastim) was administered to 18% of patients in the combination group and 3% of patients in the capecitabine group. To assess the impact of dose reductions on efficacy, a retrospective analysis for PFS and OS was conducted. Patients treated with combination therapy were analyzed for early dose reductions (dose reduction within first four courses) versus none or late dose reductions (dose reduction after four courses or no dose reduction). The results indicated that the efficacy (both PFS and OS) were similar in both these groups.
Table 3.
Most Common Treatment-Related Adverse Events and Hematologic Abnormalities
| Adverse Event* | Ixabepilone Plus Capecitabine (n = 595) |
Capecitabine (n = 603) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Any Grade |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Any Grade |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Nonhematologic abnormality | ||||||||||||||||||||
| Peripheral neuropathy | 98 | 17 | 151 | 25 | 140 | 24 | 4 | 0.7 | 393 | 66 | 103 | 17 | 14 | 2.3 | 7 | 1.2 | 0 | 0 | 124 | 21 |
| Peripheral sensory neuropathy† | 97 | 16 | 154 | 26 | 130 | 22 | 4 | 0.7 | 385 | 65 | 101 | 17 | 14 | 2.3 | 5 | 0.8 | 0 | 0 | 120 | 20 |
| Peripheral motor neuropathy | 16 | 3 | 15 | 3 | 22 | 4 | 0 | 0 | 53 | 9 | 6 | 1 | 2 | 0.3 | 2 | 0.3 | 0 | 0 | 10 | 2 |
| Hand-foot syndrome | 122 | 21 | 136 | 23 | 125 | 21 | NA | 383 | 64 | 123 | 20 | 168 | 28 | 121 | 20 | NA | 412 | 68 | ||
| Nausea | 156 | 26 | 117 | 20 | 29 | 5 | 0 | 0 | 302 | 51 | 148 | 25 | 61 | 10 | 10 | 2 | 1 | 0.2 | 220 | 37 |
| Diarrhea | 129 | 22 | 83 | 14 | 41 | 7 | 1 | 0.2 | 254 | 43 | 111 | 18 | 66 | 11 | 52 | 9 | 3 | 0.5 | 232 | 39 |
| Fatigue | 94 | 16 | 85 | 14 | 64 | 11 | 5 | 0.8 | 248 | 42 | 72 | 12 | 45 | 8 | 17 | 3 | 1 | 0.2 | 135 | 22 |
| Vomiting | 104 | 18 | 93 | 16 | 36 | 6 | 0 | 0 | 233 | 39 | 90 | 15 | 39 | 7 | 12 | 2 | 0 | 0 | 141 | 23 |
| Alopecia | 69 | 12 | 174 | 29 | NA | NA | 243 | 41 | 11 | 2 | 1 | 0.2 | NA | NA | 12 | 2 | ||||
| Nail disorder | 98 | 17 | 67 | 11 | 23 | 4 | 0 | 0 | 188 | 32 | 54 | 9 | 16 | 3 | 0 | 0 | 0 | 0 | 70 | 12 |
| Anorexia | 99 | 17 | 55 | 9 | 6 | 1 | 0 | 0 | 160 | 27 | 18 | 3 | 3 | 0.5 | 0 | 0 | 81 | 13 | ||
| Myalgia | 49 | 8 | 62 | 10 | 26 | 4 | 1 | 0.2 | 138 | 23 | 6 | 1 | 5 | 0.8 | 0 | 0 | 0 | 0 | 11 | 2 |
| Stomatitis | 62 | 10 | 48 | 8 | 12 | 2 | 0 | 0 | 122 | 21 | 32 | 5 | 31 | 5 | 3 | 0.5 | 3 | 0.5 | 69 | 11 |
| Constipation | 77 | 13 | 31 | 5 | 1 | 0.2 | 1 | 0.2 | 110 | 19 | 26 | 4 | 4 | 0.7 | 0 | 0 | 1 | 0.2 | 31 | 5 |
| Asthenia | 30 | 5 | 48 | 8 | 37 | 6 | 0 | 0 | 115 | 19 | 23 | 4 | 24 | 4 | 7 | 1 | 1 | 0.2 | 55 | 9 |
| Arthralgia | 38 | 6 | 37 | 6 | 17 | 3 | 0 | 0 | 92 | 16 | 4 | 0.7 | 1 | 0.2 | 1 | 0.2 | 0 | 0 | 6 | 1 |
| Mucositis | 47 | 8 | 23 | 4 | 10 | 2 | 0 | 0 | 80 | 13 | 36 | 6 | 11 | 2 | 5 | 0.8 | 1 | 0.2 | 53 | 9 |
| Hematologic abnormality‡ | ||||||||||||||||||||
| Leukopenia | 59 | 10 | 136 | 23 | 285 | 48 | 87 | 15 | 567 | 96 | 201 | 34 | 98 | 16 | 32 | 5 | 11 | 2 | 342 | 57 |
| Neutropenia | 36 | 6 | 76 | 13 | 202 | 34 | 229 | 39 | 543 | 92 | 118 | 20 | 113 | 19 | 33 | 6 | 16 | 3 | 280 | 47 |
| Anemia | 266 | 45 | 230 | 39 | 25 | 4 | 6 | 1 | 527 | 89 | 274 | 46 | 111 | 19 | 19 | 3 | 4 | 0.7 | 408 | 68 |
| Thrombocytopenia | 261 | 44 | 49 | 8 | 25 | 4 | 12 | 2 | 347 | 59 | 157 | 26 | 15 | 3 | 11 | 2 | 5 | 0.8 | 188 | 32 |
| Febrile neutropenia | 0 | 0 | 1 | 0.2 | 29 | 5 | 12 | 2 | 42 | 7 | 0 | 0 | 0 | 0 | 2 | 0.3 | 2 | 0.3 | 4 | 0.7 |
By patients' worse Common Terminology Criteria of Adverse Events version 3, except hand-foot syndrome, which was graded using Roche criteria.
Included the MedDRA version 9.1 terms of burning sensation, dysesthesia, hyperesthesia, hypoesthesia, neuralgia, neuritis, neuropathy, neuropathy peripheral, neurotoxicity, painful response to normal stimuli, paresthesia, pallanesthesia, peripheral sensory neuropathy, polyneuropathy, and polyneuropathy toxic.
For ixabepilone plus capecitabine, denominators for the individual tests were different: for leukopenia and anemia, n = 591, for neutropenia and thrombocytopenia, n = 590. For capecitabine alone, n = 597.
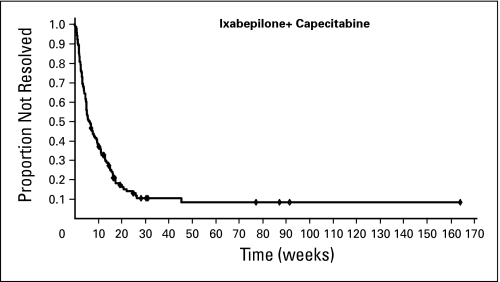
Peripheral neuropathy was common, primarily sensory, grade 1 to 2, cumulative, and generally reversible (Table 3). Sixty-six percent of patients in the combination group had treatment-related peripheral neuropathy (24% with grade 3 to 4); 65% had sensory neuropathy (22% grade 3 and 1% grade 4), and 9% had motor neuropathy (4% with grade 3). Patients who discontinued treatment received a significant course of treatment before discontinuation (median of six cycles). Neuropathy was managed by dose reduction and delay. Patients with persistent grade 2 to 3 neuropathy (162; 27%) were eligible for dose reduction; of these, 71% had dose reduction and received a median of three additional cycles (range, one to 32 cycles), and 75% had improvement or no worsening of their neuropathy. Of the 140 patients with treatment-related grade 3 to 4 peripheral neuropathy, 120 (86%) had resolution to grade 1 or baseline during the follow-up period in a median time of 6.2 weeks from the onset of the severe neuropathy (Fig 4). The median time to improvement of grade 3 or worse treatment-related neuropathy by at least 1 grade was 4.5 weeks.
Fig 4.
Time to resolution of grade 3 to 4 ixabepilone-related peripheral neuropathy to baseline or grade 1. Resolution was defined as return of symptoms to baseline or grade 1. Analysis included 120 ixabepilone-treated patients with peripheral neuropathy that occurred within 30 days of the last dose of ixabepilone and had a median resolution time of 6.2 weeks.
DISCUSSION
Anthracyclines and taxanes are the standard of care in the treatment of breast cancer, both in locally advanced and in the metastatic setting. Unfortunately, patients who develop progressive disease during or after anthracycline and taxane therapy have limited approved treatment options. Until recently, capecitabine was the only agent approved for patients with MBC resistant to anthracyclines and taxanes.2,3,17,18
In the previously reported phase III study, the A/T resistant study, the combination of ixabepilone plus capecitabine was compared with capecitabine alone in 752 women with MBC resistant to an anthracycline and a taxane.13,14 Anthracycline and taxane resistance was defined as tumor progression during treatment or within 3 months of last dose in the metastatic setting or recurrence within 6 months in the neoadjuvant or adjuvant setting.13 Ixabepilone plus capecitabine demonstrated significantly improved PFS (median, 5.8 v 4.2 months; HR = 0.75; P = .0003) and ORR (35% v 14%; P < .0001) compared with capecitabine alone.13,14 An improvement in OS was not observed.19
In contrast to the A/T resistant study, the A/T pretreated study, which is the subject of this report, was a substantially larger trial (N = 1,221), allowed patients with nonmeasurable disease (21% of the population), required prior anthracycline and taxane pretreatment but not resistance (74% not resistant to anthracycline and 52% not resistant to taxane), included patients receiving first-line chemotherapy for metastatic disease with early recurrence (20% of population), did not require central radiographic response confirmation, and was adequately powered to detect an improvement in OS. The A/T pretreated study confirmed the findings observed in the A/T resistant study; the combination was associated with significantly improved PFS (median, 6.2 v 4.2 months; HR = 0.79; P = .0005) and ORR (43% v 29%; P < .0001). PFS benefit was also observed for the combination in patients with triple-negative disease, impaired PS, and those receiving first-line therapy for metastatic disease. However, the trial failed to meet its primary end point of improved OS for the combination. An imbalance in patients with impaired PS in the combination arm (32% v 25%) may have contributed to the failure to observe a difference; a secondary prespecified analysis adjusting OS for baseline covariates including PS showed improvement in survival for the combination (HR = 0.85; P = .0231).
These results from both trials are similar to numerous previous trials demonstrating improved PFS and ORR with combination cytotoxic therapy compared with single-agent therapy. The availability of other treatment options that exhibit modest efficacy when administered sequentially probably contributes to the consistent failure to show improved survival.20–26 In this trial, although patients treated with capecitabine monotherapy did not cross over to receive ixabepilone, more patients in this group subsequently received taxanes (24% v 16%).
Although combination chemotherapy may be an appropriate choice for some patients with HER2-normal disease, many clinicians prefer single-agent cytotoxic therapy given sequentially (or single cytotoxic agents in combination with biologic agents such as bevacizumab), a preference that is consistent with current practice guidelines.20 Ixabepilone has demonstrated single-agent activity in patients with anthracycline-, taxane-, and capecitabine-resistant disease and is also approved in the United States for this indication,8 making this an appropriate choice for those patients. For patients who had less extensive chemotherapy but have advanced disease that is adversely impacting their performance status, combination cytotoxic therapy may also be an appropriate choice. For example, in both trials, in a subgroup of patients with impaired performance status (Karnofsky PS 70% to 80%), the combination of ixabepilone with capecitabine was associated with observed benefit in response, PFS, and OS in prespecified analyses, without a higher risk of toxicity.27 This analysis suggests a potential benefit in selecting combination therapy for patients who are more symptomatic because of tumor burden, which is common clinical practice, although additional studies are required for confirmation. Clinical trials are now ongoing to evaluate other ixabepilone schedules and combinations that may further improve its therapeutic index.
The safety profile of the combination of ixabepilone with capecitabine was manageable with appropriate dose modification; toxicity associated with the combination was greater than with capecitabine alone, but was consistent with those of the individual agents. The addition of ixabepilone to capecitabine therapy did not increase the frequency of severe capecitabine-related toxicities (hand-foot syndrome and diarrhea). In contrast to the A/T resistant study, in these less heavily pretreated patients, incidence of death due to study drug toxicity was low and similar between the treatment groups. As reported before, neuropathy was primarily sensory, cumulative, and reversible (effectively managed by dose reduction or delay, enabling a sufficient number of cycles to be administered to attain the observed levels of efficacy). For patients experiencing grade 3 to 4 peripheral neuropathy, median time to onset was four cycles. Grade 3 to 4 neuropathy improved by one or more Common Toxicity Criteria grade within a median of 4 weeks from onset, or resolved to baseline or grade 1 within a median of 6 weeks, after dose reduction.
In conclusion, the results of this trial provide additional evidence that the ixabepilone-capecitabine combination is more effective in prolonging PFS and improving response than capecitabine monotherapy in patients with anthracycline- and taxane-pretreated MBC, although it is associated with more toxicity and does not prolong survival. Clinicians and patients must consider both the potential benefits and risks when choosing a therapeutic option. The results of this trial confirm that the combination is an appropriate choice for those in whom a more effective treatment option than capecitabine monotherapy is indicated.
Acknowledgment
We thank the patients and all the investigators who participated in the study (see online-only Appendix for list of investigators) and Ananya Bhattacharya (employee of Bristol-Myers Squibb) for professional medical writing and editorial assistance.
Appendix
In addition to the authors, the following principal investigators participated in this study:
Argentina.
L. Arboit, S. Cagnolati, O. Duarte, M. Escudero, A. Ferro, C. Ivulich, M. Matwiejuk, N. Pilnik, C. Ramos, G. Salum, M. Tatangelo.
Australia.
E. Abdi, G. Beadle, D. Bell, J. Dewar, M. Green, P. Mainwaring, K. Pittman, S. Selva, A. Toner, N, Wilcken.
Austria.
F. Haslbauer, E. Kubista, H. Samonigg, C. Wiltschke.
Belgium.
J-F. Baurain, M. Borms, J-L. Canon, V. Cocquyt, L. Dirix, J. Mebis.
Brazil.
R. Hegg, A. Malzyner, A. Notari, N. Yamaguchi.
Canada.
J. Latreille, W. Miller, D. Vergidis.
Chile.
M. Alvarez, C. Vogel.
China.
J. Feng, Y. Gao, Z. Guan, Z. Jiang, S. Jiao, W. Liu, H. Pan, S. Qin, J. Ren, Z. Shen, Y. Wang, X. Wang, Y. Zhang.
Croatia.
G. Golcic, Z. Krajina, Z. Mrsic-Krmpotic.
Czech Republic.
J. Abrahamova, B. Melichar, K. Petrakova, L. Petruzelka, J. Prausova.
Denmark.
A. Knoop, D. Nielsen, L. Stenbygaard, U. Tange.
France.
S. Abadie-Lacourtoisie, B. Audhuy, J. Bloch, P. Bougnoux, M. Campone, P. Chollet, T. Delozier, N. Dohollou, T. Facchini, G. Ganem, A.-C. Hardy-Bessard, L. Miglianico, A. Monnier, M. Nabet, M. Spielmann, V. Trillet-Lenoir.
Germany.
K. Baumann, M. Beckmann, A. Du Bois, D. Hempel, W. Jonat, S. Kahlert, M. Kirschbaum, K. Krauss, R. Kreienberg, H. Kuhnle, W. Lichtenegger, H-G. Meerpohl, S. Seeber.
Greece.
V. Georgoulias, H. Kalofonos, N. Malamos, G. Rigatos, I. Stergiou.
Ireland.
D. Carney, J. Crown, L. Grogan, M. Keane, J. Kennedy, S. O'Reilly.
Israel.
R. Epelbaum, B. Kaufman, T. Peretz, M. Steiner, N. Yaal Hahoshen.
Italy.
L. Ciuffreda, F. Cognetti, F. Colucci, E. Cortesi, F. Di Costanzo, T. Gamucci, G. Gasparini, A. Latorre, E. Maiello, L. Manzione, A. Santoro A. Sobrero, M. Tonato, E. Villa.
Korea.
Y.-H. Im, J. Jeong, C-S. Kim.
Netherlands.
J. Coenen, J. Douma, H. Sleeboom, W. Smit.
Paraguay.
M. Alvarez, M. Tatangelo.
Portugal.
C. De Oliveira, M. Portela, L. Da Costa.
Russian Federation.
F. Akhmetzianov, M. Biakhov, R. Khasanov, S. Tjulandin.
Singapore.
P. Ang, A. Chang.
Spain.
A. Anton Torres, E. Aranda, J. Baena, L. Calvo, M. Constenla Figeiras, C. Crespo Massieu, M. Gil Gil, M. Godes, M. Gonzalez-Baron, R. Gonzalez Mancha, S. Gonzalez Jimenez, N. Lopez, A, Mena, B. Munarriz, A. Pelegri Sarle, C. Rodriguez, J. Salvador Bofill, P. Sanchez-Rovira, I. Trias De Bes.
South Africa.
A. Alberts, L. Dreosti, A. Dreyer, K. Maart, B. Rapoport, D. Vorobiof.
Sweden.
J. Bergh, Z. Einbeigi.
Switzerland.
S. Aebi, M. Fehr, D. Rauch.
Taiwan.
A.-L. Chen, R.-K. Hsieh, M-C. Liu, W.-C. Su.
Turkey.
A. Aydiner, E. Gokmen, Y. Ozisik, S. Serdengecti, U. Yilmaz.
United Kingdom.
R. Agrawal, P. Canney, P. Ellis, J. McAleer, D. Miles, C. Poole, R. Stein.
United States.
D. Abernathy, J. Atkins, K. Blackwell, R. Boccia, C. Brenin, C. Broome, P. Conkling, T. Dobbs, P. Eisenberg, J. Erban, P. Flynn, G. Grana, J. Hargis, K, Havlin, G. Houston, H. Jhangiani, A. Kallab, M. Karwal, M. Khan, S. Malamud, P. McAndrew, S. McCachren, M. Modiano, B. Mirtsching, R. Orlowski, I. Rabinowitz, Z. Rahman, R. Reilly, C. Ruud, L. Schwartzberg, C. Shapiro, H. Staszewski, D. Trent, C. Vogel, N. Vogelzang, T. Walters, P. Watson, K. Weeman, C. Weissman, A. Wood.
Methods
Key inclusion criteria included the following: Women ≥ 18 years of age with measurable or nonmeasurable, metastatic or locally advanced disease, and previously treated with both an anthracycline (doxorubicin or epirubicin) and a taxane (paclitaxel or docetaxel) were eligible. Anthracycline and taxane resistance was defined as (1) tumor progression during treatment or within 3 months of last dose in the metastatic setting, or (2) recurrence within 6 months in the neoadjuvant or adjuvant setting. Patients were not allowed to receive more than two prior chemotherapy regimens (including those administered in the neoadjuvant or adjuvant setting), with sequential neoadjuvant/adjuvant treatment counting as one regimen. The anthracycline and the taxane may have been given either as monotherapy or as part of a combination with another agent, in either the adjuvant or metastatic setting or both, and patients' not receiving treatment for metastatic disease had to have experienced relapse within 1 year. Karnofsky performance status of 70% to 100% and life expectancy ≥ 12 weeks were required. Patients were required to have adequate hematologic function (absolute neutrophil count > 1,500/μL, platelets > 125,000/μL), and hepatic function (serum bilirubin < 1.5× upper institutional limits and ALT or AST < 2.5× upper institutional limits).
Key exclusion criteria included the following: brain metastases; motor or sensory neuropathy ≥ grade 2; prior severe hypersensitivity to agents containing polyoxyethylated castor oil or hypersensitivity to fluoropyrimidine; known or suspected dihydropyrimidine dehydrogenase deficiency; prior radiation covering ≥ 30% of bone marrow–containing areas; concurrent active malignancy other than nonmelanoma skin cancer or carcinoma in situ of the cervix; history of previous malignancies (only included were patients disease-free for ≥ 5 years); concurrent chemotherapy or radiation therapy regimens; continued treatment with trastuzumab, hormonal anticancer agents, or other systemic treatment for cancer; prior epothilone or capecitabine therapy. The protocol was approved by the institutional review board of participating institutions, and all patients gave written informed consent.
Cross-over from capecitabine alone to combination therapy was not permitted.
Dose reduction or treatment discontinuations were based on tolerability; events necessitating ixabepilone dose reduction (from 40 to 32 to 25 mg/m2) were grade 3 neuropathy lasting less than 7 days, grade 2 neuropathy lasting ≥ 7 days and grade 3 hand-foot syndrome; ixabepilone was discontinued on the basis of grade 3 neuropathy lasting ≥ 7 days or grade 4 neuropathy. Capecitabine dose reductions were consistent with those specified by the guidelines for single-agent use.2 No dose re-escalation was allowed after a reduction. However, patients who required discontinuation of one study drug in the combination arm were allowed to remain on treatment for the other drug, similar to that used for CA163-046.13
Table A1.
Efficacy of Ixabepilone Plus Capecitabine Combination: Progression-Free Survival in Measurable Disease Stratum—Overall Population and Prespecified Subgroups
| Group | Ixabepilone Plus Capecitabine (n = 480) |
Capecitabine (n = 480) |
Hazard Ratio | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | No. of Subjects | PFS (months) |
No. of Events | No. of Subjects | PFS (months) |
|||||
| Median | 95% CI | Median | 95% CI | |||||||
| Overall population | 446 | 480 | 6.2 | 5.59 to 6.77 | 457 | 480 | 4.4 | 4.14 to 5.42 | 0.79 | 0.69 to 0.90 |
| Key subgroups | ||||||||||
| Triple negative | 93 | 100 | 4.24 | 3.45 to 5.49 | 108 | 112 | 1.81 | 1.51 to 2.92 | 0.64 | 0.48 to 0.84 |
| KPS 70%-80% | 138 | 149 | 5.59 | 4.30 to 6.77 | 120 | 121 | 3.9 | 2.79 to 4.80 | 0.74 | 0.58 to 0.95 |
| First line | 91 | 98 | 5.55 | 4.24 to 6.77 | 80 | 81 | 3.1 | 1.81 to 4.07 | 0.64 | 0.47 to 0.87 |
Abbreviation: PFS, progression-free survival; KPS, Karnofsky performance status.
Footnotes
Supported by Bristol-Myers Squibb.
Presented in part at the American Society of Clinical Oncology Breast Cancer Symposium, September 5-7, 2008, Washington, DC (abstr 186).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00082433.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Ronald Peck, Bristol-Myers Squibb (C); Valerie Poulart, Bristol-Myers Squibb (C) Consultant or Advisory Role: Joseph A. Sparano, Bristol-Myers Squibb (C); Oliver Rixe, Bristol-Myers Squibb (C); Pierfranco Conte, Bristol-Myers Squibb (C) Stock Ownership: Ronald Peck, Bristol-Myers Squibb Honoraria: Joseph A. Sparano, Bristol-Myers Squibb; Pierfranco Conte, Bristol-Myers Squibb Research Funding: Joseph A. Sparano, Bristol-Myers Squibb; Carlos Medina, Bristol-Myers Squib Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joseph A. Sparano, Oliver Rixe, Ronald Peck
Provision of study materials or patients: Joseph A. Sparano, Oliver Rixe, Binghe Xu, Alexey Manikhas, Carlos Medina, Susanne Crocamo Ventilari Da Costa, Jungsil Ro, Gonzalo Rubio, Monica Rondinon, Gumersindo Perez Manga, Pierfranco Conte
Collection and assembly of data: Eduard Vrdoljak, Binghe Xu, Carlos Medina, Susanne Crocamo Ventilari Da Costa, Ronald Peck
Data analysis and interpretation: Joseph A. Sparano, Ronald Peck, Valerie Poulart, Pierfranco Conte
Manuscript writing: Joseph A. Sparano, Eduard Vrdoljak, Oliver Rixe, Pierfranco Conte
Final approval of manuscript: Joseph A. Sparano, Eduard Vrdoljak, Oliver Rixe, Binghe Xu, Alexey Manikhas, Carlos Medina, Susanne Crocamo Ventilari Da Costa, Jungsil Ro, Gonzalo Rubio, Monica Rondinon, Gumersindo Perez Manga, Ronald Peck, Valerie Poulart, Pierfranco Conte
REFERENCES
- 1.Surveillance Epidemiology and End Results: Estimated new cancer cases and deaths for 2006. http://seer.cancer.gov/csr/1975_2003/results_resulsingle/resulsect_resul01_resultable.01.pdf.
- 2.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 3.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Lee JJ, Swain SM. Development of novel chemotherapeutic agents to evade the mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32:S22–S26. doi: 10.1053/j.seminoncol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Fumoleau P, Coudert B, Isambert N, et al. Novel tubulin-targeting agents: Anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol. 2007;18(suppl 5):v9–v15. doi: 10.1093/annonc/mdm173. [DOI] [PubMed] [Google Scholar]
- 6.Lee FY, Borzilleri R, Fairchild CR, et al. Preclinical discovery of ixabepilone, a highly active antineoplastic agent. Cancer Chemother Pharmacol. 2008;63:157–166. doi: 10.1007/s00280-008-0724-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee FY, Shen H, Lee H, et al. Ixabepilone overcomes multiple mechanisms of drug resistance including overexpression of class III ß tubulin and breast cancer resistance protein. Eur J Cancer. 2008;6(abstr):219. [Google Scholar]
- 8.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 9.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25:3415–3420. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 10.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 11.Lee FY, Camuso A, Castenada C, et al. Preclinical efficacy evaluation of ixabepilone (BMS 247550) in combination with cetuximab or capecitabine in human colon and lung carcinoma xenografts. J Clin Oncol. 2006;24(abstr 12017):597s. [Google Scholar]
- 12.Bunnell C, Vahdat L, Schwartzberg L, et al. Phase I/II study of ixabepilone plus capecitabine in anthracycline-pretreated/resistant and taxane-resistant metastatic breast cancer. Clin Breast Cancer. 2008;8:234–241. doi: 10.3816/CBC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 13.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 14.Thomas ES. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2008;26:2223. doi: 10.1200/JCO.2008.16.5019. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Pivot X, Dufresne A, Villanueva C. Efficacy and safety of ixabepilone, a novel epothilone analogue. Clin Breast Cancer. 2007;7:543–549. doi: 10.3816/CBC.2007.n.009. [DOI] [PubMed] [Google Scholar]
- 17.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–1768. doi: 10.1002/1097-0142(20011001)92:7<1759::aid-cncr1691>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi G, Perez E, Vrdoljak E, et al. Analysis of overall survival among patients with metastatic breast cancer receiving either ixabepilone plus capecitabine or capecitabine alone and a review of results from two randomized phase III trials. Presented at Am Soc Clin Oncol Breast Cancer Proceedings; September 5-7, 2008; Washington, DC. abstr 186. [Google Scholar]
- 20.National Comprehensive Cancer Network. Breast Cancer Guidelines V.2.2010. http://www.nccn.org/interactive/default.asp#breast. [Google Scholar]
- 21.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 22.Martín M, Ruiz A, Munoz M, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: Final results of the phase III Spanish Breast Cancer Res Group (GEICAM) trial. Lancet Oncol. 2007;8:219–225. doi: 10.1016/S1470-2045(07)70041-4. [DOI] [PubMed] [Google Scholar]
- 23.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 24.Petrelli F, Cabiddu M, Cazzaniga ME, et al. Targeted therapies for the treatment of breast cancer in the post-trastuzumab era. Oncologist. 2008;13:373–381. doi: 10.1634/theoncologist.2007-0173. [DOI] [PubMed] [Google Scholar]
- 25.Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–1986. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 26.Sargent DJ, Hayes DF. Assessing the measure of a new drug: Is survival the only thing that matters? J Clin Oncol. 2008;26:1922–1923. doi: 10.1200/JCO.2007.14.8064. [DOI] [PubMed] [Google Scholar]
- 27.Conte P, Roche H, Perez E, et al. Ixabepilone plus capecitabine improves overall survival in symptomatic patients with metastatic breast cancer previously treated with anthracycline and taxane in 2 large phase III studies. Cancer Res. 2009;69:6114. [Google Scholar]