Abstract
Purpose
Up to 75% of women experience hot flashes, which can negatively impact quality of life. As hot flash physiology is not definitively understood, it cannot be assumed that effective agents represent class effects. Therefore, there is a continued need for rigorous evaluation to identify effective nonhormonal options for hot flash relief.
Methods
A randomized, double-blind trial evaluated citalopram at target doses of 10, 20, or 30 mg/d versus placebo for 6 weeks. Postmenopausal women with at least 14 bothersome hot flashes per week recorded hot flashes for 7 days before starting treatment and were then titrated to their target doses. The primary end point was the change from baseline to 6 weeks in hot flash score.
Results
Two hundred fifty-four women were randomly assigned onto this study. Data for hot flash scores and frequencies showed significant improvement in hot flashes with citalopram over placebo, with no significant differences among doses. Reductions in mean hot flash scores were 2.0 (23%), 7.0 (49%), 7.7 (50%), and 10.7 (55%) for placebo and 10, 20, and 30 mg of citalopram, respectively (P ≤ .002). Improvement in secondary outcomes, such as the Hot Flash Related Daily Interference Scale, was statistically superior in the 20-mg arm. Citalopram was well-tolerated, with no significant negative adverse effects.
Conclusion
Citalopram is an effective, well-tolerated agent in managing hot flashes. There does not appear to be a significant dose response above 10 mg/d, but broader helpful effects of the agent appear to be more evident at 20 mg/d.
INTRODUCTION
Hot flashes are a prevalent and distressing symptom associated with hormonal changes due to menopause or treatment for breast cancer, causing loss of sleep, productivity, and a desire for treatment.1–5 For some women, hot flashes are transient and subside within 2 years.3 For others, this symptom can persist for several years, often as a result of taking tamoxifen or aromatase inhibitor (AI) therapy for breast cancer.3,4,6 Effective, low-cost, low-toxicity, nonhormonal interventions are needed in order to have plenty of options available to provide relief to women throughout the time of need.
With regards to the physiology of this symptom, theories regarding the role of central neurotransmitters as triggers continue to thrive, based on the mechanism of action of the agents that have been found to positively impact hot flashes, namely serotonergic antidepressants.6–8 Randomized placebo controlled trials demonstrate at least a 50% reduction in hot flashes for paroxetine, fluoxetine, and venlafaxine.7–9 However, due to the unknown physiology of hot flashes and the lack of definitive research about how these agents reduce hot flashes, the efficacy cannot be attributed to a class effect.
There is at least one selective serotonin reuptake inhibitor (SSRI), sertraline, that has been evaluated for hot flashes by three independent researchers and has not been shown to yield effect sizes as large as the other agents.9–11 In addition, published articles call into question the veracity of the relief of serotonergic agents for hot flashes.12,13
One of the challenging clinical issues with utilizing SSRIs is that many of the effective ones evaluated for hot flashes, namely paroxetine and fluoxetine, are potent inhibitors of CYP2D6 and cannot be used to manage tamoxifen-associated hot flashes. Tamoxifen continues to be an important treatment in reducing the risk of primary or recurrent breast cancer and adverse effects, including vasomotor symptoms, remain one of the main reasons women decide against taking this treatment.14 Therefore, effective hot flash options that are safe to use in this population continue to be a critical need.
Citalopram, an antidepressant similar to fluoxetine and paroxetine,15 is considered to be an SSRI, readily absorbed following oral ingestion, reaching its peak serum concentration in 2 to 4 hours. Dosing commonly begins at 10-mg daily and can be increased to a maximum of 60-mg daily for treating depression. Citalopram is primarily hepatically metabolized by the CYP3A4 and CYP2C19 pathways17,18 and is a weak inhibitor of the CYP2D6 pathway. Since it is a weak inhibitor of CYP2D6, it is clinically feasible that this agent, if effective for hot flashes, would be able to be used to control hot flashes related to tamoxifen. In fact, preliminary data provide support that citalopram does not interfere with tamoxifen metabolism in a clinically significant way,16,17 with additional data being presented at the American Society of Clinical Oncology meeting in June 2009 consistent with this assertion.18,19
Pilot data have been published supporting citalopram's efficacy against hot flashes. Two small studies, involving 1820 and 2221 women, found a reduction of hot flash frequency of 58% and 45% (respectively) with a dose of 20 mg/d over 4 weeks. In contrast, a large, placebo controlled trial, evaluating up to 30 mg/d of citalopram and fluoxetine for hot flashes, concluded that neither citalopram or fluoxetine were effective.13 However, this study did not collect baseline hot flash data for a week before starting the study medications/placebo. Rather, day 1 data were used for baseline (E. Suvanto-Luukkonen, personal communication, April 2007).
Therefore, the purpose of this study was to evaluate three doses of citalopram (10, 20, and 30 mg) for its effectiveness in reducing hot flashes in a rigorous, placebo-controlled trial, in order to clarify whether another option for hot flash control exists for women who must avoid estrogen, particularly those on tamoxifen therapy.
METHODS
The primary purpose of this trial was to determine the optimal effective dose of citalopram for hot flash relief. In addition, secondary goals were to evaluate toxicity related to each dose, as well as to evaluate the impact of citalopram on mood and activity interference related to hot flashes.
Eligibility Criteria
Women eligible for this trial included those who were postmenopausal and reported to be bothered with at least 14 hot flashes per week for at least the past month. Women could not be on any antineoplastic therapy or receiving estrogen or testosterone. Women could have had a history of breast cancer, but must have had no evidence of active malignancy. The use of other treatments for hot flashes or the use of other antidepressants was not allowed. Stratification factors included age (< 50 or v 50 or older), number of hot flashes per day (2 to 3 to v 4 to 9 or v 10 or more), duration of hot flash symptoms (< 9 months v 9 months or longer), and current use of aromatase inhibitors, raloxifene, or tamoxifen (yes v no).
Intervention
Women were randomly assigned to receive one, two, or three pills daily, representing 10-mg citalopram/placebo, 20-mg citalopram/placebo or 30-mg citalopram/placebo. There was a two to one chance of receiving active agent compared with placebo. Study treatment duration was 6 weeks, after a baseline week in which no study medication was taken. Treatment for all participants was titrated to their assigned dose beginning with one tablet (10 mg/placebo) and increasing by one tablet per week (10 mg/placebo) up to their target dose, the largest of which was three tablets (30 mg/placebo) daily.
Analysis
The primary end point was the change from baseline to 6 weeks for the hot flash score, as measured by a prospective, self-report hot flash diary that women were asked to complete in real time.22 Hot flash scores were determined by assigning points to each hot flash based on reported severity with the following values: 1 = mild, 2 = moderate, 3 = severe and 4 = very severe. The points for each day were added together for each patient and then averaged across each week of the trial. Secondary end points included hot flash frequency, mood as measured by the Profile of Mood States (POMS)23 and activity interference, as measured by the Hot Flash Related Daily Interference Scale (HFRDIS).24
Toxicities were evaluated with a weekly self-report questionnaire as well as by National Cancer Institute Common Toxicity Criteria version 3.0. Adverse effects evaluated by self-report were appetite increase and loss, sleepiness, nausea, dizziness, fatigue, dry mouth, abnormal sweating, loose stools, blurred vision, trouble sleeping, nervousness, interest in sexual relations, vaginal dryness, trouble with orgasm, skin rash, and trouble concentrating. The POMS, HFRDIS, and adverse effects were rated on a 0- to 100-point scale where 0 is as bad as can be and 100 is as good as can be.
Although there were three different placebo dose levels, this was simply so that patients could not tell whether or not they were on active treatment due to the need to titrate antidepressant therapy. The three placebo doses were initially treated as a single group for comparison against the active treatment arms. Subsequently, a separate test for within placebo dose level effects was carried out via the same methods used to compare the three treatment arms to the joint placebo arm.
Multiple comparisons for the primary end point compared each of the three active arms with placebo, giving rise to three pairwise comparisons. This led to the adjustment of the P value to .05/3 = .0168. Therefore, each two-sided multiple comparison of the primary end point with 50 patients per treatment group at the end of 6 weeks of treatment had 80% power and 5% type I error rate to detect a difference of 0.82 standard deviations or 1.64 hot flashes per day, 4.10 units of hot flash score or a drop of 29% from the baseline score. This is considered a large effect size and is based on previous data with hot flash trials.22
RESULTS
A total of 254 patients were randomly assigned onto this study between November 3, 2006, and April 13, 2007. The flow of patients is depicted in the CONSORT diagram in Figure 1. Baseline characteristics, including all stratification variables, were well-balanced between arms with no statistically significant differences and are presented in Table 1.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Characteristics (N = 254)
| Variable | Placebo |
10 mg |
20 mg |
30 mg |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Mean age, years | 56.2 | 55.2 | 55.8 | 55.2 | ||||
| Standard deviation | 9 | 7 | 9 | 8 | ||||
| Race | ||||||||
| White | 75 | 90 | 51 | 90 | 50 | 88 | 49 | 86 |
| African American | 7 | 8 | 5 | 9 | 7 | 12 | 8 | 14 |
| Asian | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Concurrent treatment | ||||||||
| Current AI | 13 | 16 | 10 | 18 | 9 | 16 | 9 | 16 |
| Current raloxifene | 2 | 2 | 2 | 4 | 2 | 4 | 2 | 4 |
| Current tamoxifen | 5 | 6 | 6 | 11 | 5 | 9 | 4 | 7 |
| Breast cancer history | 26 | 31 | 20 | 35 | 21 | 37 | 20 | 35 |
| Estimated frequency at enrollment, HF/d | ||||||||
| < 4 | 11 | 13 | 8 | 14 | 7 | 12 | 8 | 14 |
| 4-9 | 40 | 48 | 29 | 49 | 28 | 49 | 27 | 47 |
| ≥ 10 | 32 | 39 | 21 | 37 | 22 | 39 | 22 | 37 |
| ≥ 9-month HF duration | 70 | 84 | 47 | 83 | 48 | 84 | 47 | 83 |
| No. of years since menopause | ||||||||
| 1 | 10 | 12 | 10 | 18 | 10 | 18 | 10 | 18 |
| 2 | 8 | 10 | 14 | 25 | 5 | 9 | 7 | 12 |
| 3 | 2 | 2 | 3 | 5 | 8 | 14 | 5 | 9 |
| 4 | 59 | 71 | 28 | 49 | 33 | 58 | 33 | 58 |
| 5 | 4 | 5 | 2 | 4 | 1 | 2 | 2 | 4 |
| Baseline week | ||||||||
| Actual HF score | 12 | 14 | 14 | 17 | ||||
| Actual HF frequency | 8 | 8 | 8 | 9 | ||||
Abbreviations: AI, aromatase inhibitor; HF, hot flash.
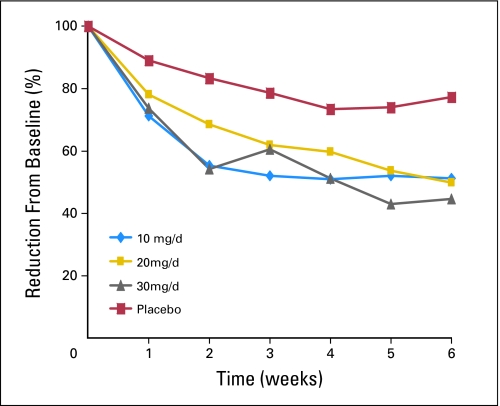
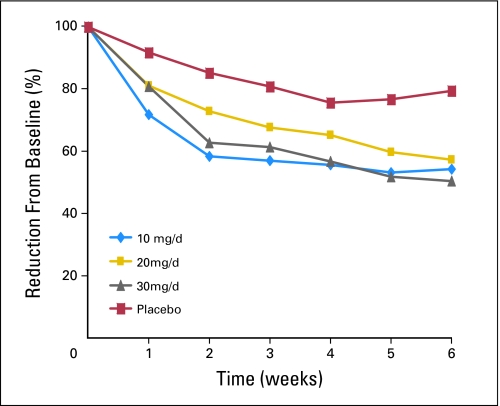
There was a statistically significant difference for the change in hot flash scores between each citalopram arm and the combined placebo arm (P < .001). Decreases from baseline to week 6 in daily hot flash scores were 7.0 (49%) for 10 mg/d of citalopram, 7.7 (50%) for 20 mg/d, 10.7 (55%) for 30 mg/d, and 2.0 (23%) for the placebo arms (P ≤ .002; Fig 2). Likewise, daily hot flash frequency was reduced 3.6 (46%), 3.9 (43%), 4.5 (50%), and 1.4 (20%) for 10 mg/d, 20 mg/d, 30 mg/d and placebo arms, respectively, (P < .001; Fig 3).
Fig 2.
Hot flash score percent reduction (mean daily reduction).
Fig 3.
Hot flash frequency percent reduction (mean daily reduction).
There was no statistically significant difference in the change in daily hot flash score or daily frequency among any of the three doses of citalopram. The analysis for differences across the placebo arms indicated that there were no differences in hot flash activity among the three levels of placebo (ie, one, two, or three placebo pills) with a mean score of −0.7 versus −4.2 versus −1.3, respectively, (P = .11).
Subset analyses were performed to evaluate whether citalopram's efficacy was impacted by whether a woman was on tamoxifen or an AI or had a history of breast cancer. There were equal reductions in hot flashes for all of these groups with a mean reduction in hot flash score for those on tamoxifen or an AI of 7.8 in versus 8.7 in women not using these agents (P = .80) and a mean reduction of 8.1 in women with a history of breast cancer versus 8.7 in those who did not have such a history (P = .84).
Toxicities graded per National Cancer Institute Common Toxicity Criteria version 3.0 did not reveal statistically significant differences in adverse events related to citalopram. Only one person at the 30-mg dose of citalopram reported grade 3 insomnia, and three reported grade 3 sedation. One person on placebo also experienced grade 2 sedation. Self-reported adverse effects (Table 2) did not reveal significant differences between placebo and any of the citalopram doses. Adverse events related to sexual health were somewhat worse (but not statistically significantly) in the 30-mg citalopram dose than the other doses or placebo. Using a single item numeric analog question, women on citalopram reported significantly less distress related to hot flashes (P = .003) and more satisfaction with hot flash control (P = .04).
Table 2.
Self-Reported Adverse Effects
| Variable | Placebo | 10 mg | 20 mg | 30 mg | P* |
|---|---|---|---|---|---|
| Potential benefit | |||||
| HF distress | 13.2 | 28.9 | 24.7 | 33.2 | .003 |
| Satisfaction with HF control | 22.1 | 40.9 | 35.9 | 39.8 | .04 |
| Adverse effect | |||||
| Dry mouth | −1.8 | 9.3 | −3.5 | −3.6 | .05 |
| Abnormal sweating | 10.3 | 23.3 | 20.2 | 25.5 | .05 |
| Loose stools | 2.5 | 1.4 | −8.1 | 0.9 | .06 |
| Sleepiness | 0.8 | 14.4 | 11.2 | 14.0 | .07 |
| Sexual relations | 11.2 | 9.5 | 0.8 | −5.0 | .07 |
| Appetite increase | 0.0 | 7.2 | −1.2 | −2.9 | .11 |
| Trouble sleeping | 8.9 | 17.2 | 23.3 | 18.9 | .15 |
| Nervousness | 3.8 | 7.7 | 10.9 | 6.4 | .30 |
| Fatigue | 3.1 | 10.0 | 2.6 | 9.3 | .45 |
| Appetite loss | 0.5 | 0.9 | −3.5 | −4.4 | .49 |
| Dizziness | 2.3 | 1.6 | −1.4 | −0.2 | .56 |
| Nausea | 2.5 | 0.2 | −1.9 | −0.7 | .57 |
| Blurred vision | 1.7 | 0.7 | 0.7 | 3.8 | .58 |
| Difficulty with orgasm | 0.9 | −5.9 | −3.5 | −8.3 | .59 |
| Skin rash | 0.6 | 0.0 | 1.8 | −0.7 | .68 |
| Trouble concentrating | 8.3 | 9.0 | 11.6 | 7.4 | .83 |
| Vaginal dryness/pain | −1.3 | 0.9 | −0.3 | −3.0 | .94 |
NOTE. Mean change from baseline (higher numbers indicate improvement, negative numbers indicate worse scores than at baseline).
Abbreviation: HF, hot flash.
Analysis of variance F-test for differences among the four treatment arms.
Secondary outcomes were similarly positive. Results from the POMS (Table 3) indicate that there were no statistically significant differences among any of the arms for mood disturbance based on the total scale score. However, two subscales were statistically significantly different among arms. The tension/anxiety subscale was significantly improved in the arm receiving citalopram 20 mg/d over the arm receiving either placebo or citalopram 30 mg/d and the anger/hostility subscale was significantly different between those receiving citalopram 10 mg/d versus placebo.
Table 3.
Mean Changes in the POMS and HFRDIS
| Variable | Placebo | 10 mg | 20 mg | 30 mg |
|---|---|---|---|---|
| POMS | ||||
| Total mood | 1.3 | 5.9 | 6.8 | 4.0 |
| Tension/anxiety | 3.3 | 5.8 | 12.9* | 4.1 |
| Depression/dejection | −0.1 | 6.0 | 5.2 | 6.5 |
| Confusion/bewilderment | 0.3 | 1.2 | 4.9 | 0.8 |
| Fatigue/inertia | 2.9 | 5.8 | 4.8 | 4.0 |
| Vigor/activity | 0.2 | 6.1 | 3.8 | 5.4 |
| Anger/hostility | 1.3 | 10.3* | 8.9 | 7.3 |
| HFRDIS | ||||
| Work | 3 | 15 | 21* | 9 |
| Social | 4 | 9 | 18* | 8 |
| Leisure | 4 | 9 | 16† | 13 |
| Sleep | 13 | 26 | 32* | 28* |
| Mood | 7 | 14 | 20* | 14 |
| Concentrate | 8 | 14 | 15 | 12 |
| Relationship | 2 | 8 | 14* | 9 |
| Sexuality | 2 | 4 | 16† | 4 |
| Enjoy life | 0 | 9 | 12† | 15* |
| Overall qualityof life | 4 | 11 | 17* | 16* |
NOTE. Higher numbers indicate improvement.
Abbreviations: POMS, Profile of Mood States; HFRDIS, Hot Flash Related Daily Interference Scale; ANOVA, analysis of variance.
ANOVA P ≤ .01, compared with the placebo arm.
ANOVA P = .02, compared with the placebo arm.
With respect to interference with activities due to hot flashes, statistically significant improvements were seen in six of 10 areas evaluated with the HFRDIS (Table 3). There were many areas of significant improvement. In areas which did not significantly improve, it should be noted that there were numerically better scores in every arm receiving citalopram as opposed to placebo. The total HFRDIS score was significantly different only between the 20-mg treatment arm and placebo (P < .01), however.
Data were explored in this study to look at what level of hot flash reduction was necessary to see a positive impact on activities of daily living. Hot flash scores in participants who realized at least 10 points of improvement on the HFDRIS were compared with those who did not. A 10-point difference is considered the minimally clinically significant difference.25 As is evident in Table 4, hot flash scores needed to be reduced by an average of 46% to 54% to see significant improvement in various areas of sleep, mood, work, social activity, and enjoyment of life.
Table 4.
Hot Flash Reduction Needed for > 10-Point Improvement in HFRDIS
| HFRDIS Change ≥ 10 | Reduction (%) | SD | P |
|---|---|---|---|
| Work | 54 | 36 | ≤ .01 |
| Social activity | 51 | 42 | ≤ .01 |
| Sleep | 49 | 42 | ≤ .01 |
| Mood | 50 | 46 | ≤ .001 |
| Concentration | 46 | 47 | |
| Relationship with others | 51 | 36 | |
| Sexual | 47 | 47 | |
| Enjoy life | 52 | 38 | ≤ .01 |
| Leisure activities | 51 | 38 | < .05 |
| Overall quality of life | 53 | 44 | ≤ .001 |
Abbreviations: HFRDIS, Hot Flash Related Daily Interference Scale; SD, standard deviation.
A responder analysis was completed for each arm in this trial, with those patients who did not complete the study or with missing data categorized as nonresponders. Patients who never began study treatment were not included. More participants (35% to 60%) on citalopram received a reduction in their hot flash score or frequency of ≥ 50% compared to those on placebo (Appendix Table A1, online only).
DISCUSSION
The results from this study support that citalopram, in a dose as low as 10-mg per day can significantly improve hot flash activity, with virtually no toxicity. This is contrary to the results of Suvanto-Luukkonen.13 In their 9-month study, the authors reported that there were no statistically significant differences in hot flashes between citalopram, fluoxetine, or placebo. However, this trial did not include a baseline week period to measure hot flash activity.26 The authors used hot flash information from day 1 as baseline, yet study medication also began on this day. This is problematic in that it has been demonstrated in a study evaluating venlafaxine, that on the very first day, hot flashes decreased by 30% compared to baseline. This issue is discussed in detail in two previous manuscripts.26,27
Clinically, it is reasonable to begin a patient on a 10-mg/d dose for a week or two and then determine whether titration up to 20 mg per day would be further beneficial. These data do show that although there was not a significant difference in hot flash reduction between doses of citalopram, a significantly beneficial impact on broader daily activities required 20 mg/d. The 30 mg/d dose was associated with a tendency for more toxicity with little increased hot flash improvement and, therefore, is not recommended.
It may seem surprising that overall mood disturbance as measured by the POMS was not significantly better in the citalopram arms since it is a mood modulator. However, participants in this study were recruited based on hot flashes, not mood disturbance. It could be that the lack of significance on mood reflects the lack of variability in this domain as well as floor effects. It is also important to note that the doses used to manage hot flashes are lower than those used to manage depression and therefore, may not produce an effect size large enough to be statistically significant. Both anger/hostility and tension/anxiety, however, were significantly impacted, being two things that are associated with effects of hot flashes.28 In addition, these findings provide further support that the decrease in hot flashes is likely not simply the result of overall mood improvements.
When evaluating treatments for hot flashes, it is important to know how much of a decrease in hot flashes is truly beneficial for women, namely, how much of a decrease would positively impact other areas of life. This study provides novel data related to this issue. Based on the results from the HFRDIS, a daily reduction of approximately 50% is needed in order for women to perceive general quality of life improvements. This reduction is more than what a placebo generally offers (25% to 30%) and therefore, may be one additional way to ascertain real versus placebo effects of an intervention.
The most feared adverse effects of SSRIs are negative sexual adverse effects. Due to the coexistence of hot flashes and sexual changes as a result of menopause, treatment for hot flashes with SSRIs may be particularly worrisome. There are currently no long-term studies with low-dose SSRIs for hot flashes that reveal the true incidence of sexual changes. Drug references for citalopram list a 1% to 6% incidence of sexual dysfunction, which, for females, includes 1% of patients noting anorgasm and 1.3% noting a reduced libido. Ejaculatory issues and impotence are a bit more common in men with 4% and 3% incidences, respectively.29 The incidence of sexual changes related to SSRIs is further complicated by the comorbidity of depression in the population from which the data are derived. This study does not provide any data to suggest that short-term changes in sexual function occurred due to citalopram. More research on long-term effects of antidepressants, in doses needed to treat hot flashes, is warranted.
As hot flashes can negatively impact some areas of life, such as sleep, it is important that health care providers assess both the positive and negative effects of each hot flash intervention for individual patients to make sure that the symptom improvement is not countered by other unwanted adverse effects. The improvement in the area of hot flashes interfering with sleep was profound on citalopram, with a 32-point improvement on a 100-point scale for the 20 mg/d dose.
Strengths of this study include the fact that it is a dose finding trial, only the third such trial done with the antidepressants. One limitation of this study is that it was 7 weeks long. While we have shown that effects of gabapentin and other effective antidepressants plateau between 4 and 12 weeks,30 long-term control of hot flashes or adverse effects with citalopram cannot be determined from this study.
Finally, consistent with a previous pooled analysis, efficacy of citalopram did not differ based on etiology or breast cancer history.31 Thus, in total, these data demonstrate that citalopram decreases hot flashes to a similar degree to what has been seen with venlafaxine, paroxetine, gabapentin,26 and pregabalin32 and is, therefore, another option for women in managing hot flashes.26,27
However, citalopram may have some advantages over the other agents. Citalopram, unlike paroxetine, can be given with tamoxifen.33 As little as 10 mg/d of citalopram is needed for a 46% reduction in daily frequency. This, coupled with the minimal adverse effect profile at this dose, makes this particularly useful for women who have difficulty tolerating pharmacologic agents and appears to be more tolerable than either gabapentin or pregabalin (although this would need confirmation ideally with a head to head trial). Citalopram is also only taken once per day and is available generically. Therefore, it has the dosing advantage of venlafaxine, extended release, without the cost.
Appendix
Additional participating institutions include: Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald Wender, MD); Carle Clinic CCOP, Urbana, IL (Kendrith Rowland Jr); Toledo Community Hospital Oncology Program (Paul L. Schaefer, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN 46601 (Robin Zon, MD); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, MD); Illinois Oncology Research Assn CCOP, Peoria, IL 61615-7828 (John W. Kugler, MD); Hematology/Oncology Centers of the Northern Rockies, Billings, MT 59101 (Benjamin T. Marchello, MD); Medical College of Georgia, Augusta, GA 30912 (Anand P. Jillella, MD); Medical Oncology and Hematology Associates – Pleasant, Des Moines, IA 50309 (Robert J. Behrens, MD); Rapid City Regional Hospital Inc, Rapid City, SD 57701 (Richard Charles Tenglin, MD); Duluth Clinic/Duluth CCOP, Duluth, MN 55805 (Daniel C. Nikcevich, MD); Columbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, MD, PhD); and Mayo Clinic Jacksonville, Jacksonville, FL.
Table A1.
Women Who Reported a 50% or More Reduction in Hot Flash Frequency and Score
| Variable | Placebo |
10 mg |
20 mg |
30 mg |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| No. of patients | 79 | 54 | 56 | 55 | |||||
| Score reduction ≥ 50% | 17 | 22 | 21 | 39 | 25 | 45 | 33 | 60 | < .001 |
| Frequency reduction ≥ 50% | 15 | 19 | 19 | 35 | 23 | 41 | 25 | 46 | .006 |
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health service Grants No. CA-25224, CA-37404, CA-63848, CA-35195, CA-37417, CA-35448, CA-35267, CA-63849, CA-35113, CA-35103, CA-35415, CA-35431, and CA 124477.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00363909.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Debra L. Barton, Jeff A. Sloan, Pamela J. Atherton, Brent Diekmann, Charles L. Loprinzi
Administrative support: Debra L. Barton, Charles L. Loprinzi
Provision of study materials or patients: Beth I. LaVasseur, Allen N. Stawis, Kathleen A. Flynn, Missy Dyar, David B. Johnson
Collection and assembly of data: Debra L. Barton, Jeff A. Sloan, Pamela J. Atherton, Brent Diekmann, Charles L. Loprinzi
Data analysis and interpretation: Debra L. Barton, Jeff A. Sloan, Pamela J. Atherton, Brent Diekmann, Charles L. Loprinzi
Manuscript writing: Debra L. Barton, Jeff A. Sloan, Pamela J. Atherton, Brent Diekmann, Charles L. Loprinzi
Final approval of manuscript: Debra L. Barton, Beth I. LaVasseur, Jeff A. Sloan, Allen N. Stawis, Kathleen A. Flynn, Missy Dyar, David B. Johnson, Pamela J. Atherton, Brent Diekmann, Charles L. Loprinzi
REFERENCES
- 1.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26:1311–1317. [PubMed] [Google Scholar]
- 2.Couzi RJ HK, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13:2737–2744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 3.Gracia CR, Freeman EW. Acute consequences of the menopausal transition: The rise of common menopausal symptoms. Endocrinol Metab Clin North Am. 2004;33:675–689. doi: 10.1016/j.ecl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Stearns V, Ullmer L, Lopez JF, et al. Hot flushes. Lancet. 2002;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JS, Johnson D, Wagner L, et al. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 6.Loprinzi C VS, Barton D. Centrally active nonhormonal hot flash therapies. Am J Med. 2005;118:118S–123S. doi: 10.1016/j.amjmed.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Berendsen H. Hot flushes and serotonin. Journal of the British Menopause Society. 2002;8:30–34. doi: 10.1258/136218002100321848. [DOI] [PubMed] [Google Scholar]
- 8.Shanafelt TD, Barton DL, Adjei AA, et al. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 9.Kimmick GG, Lovato J, McQuellon R, et al. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12:114–122. doi: 10.1111/j.1075-122X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 10.Gordon PR, Kerwin JP, Boesen KG, et al. Sertraline to treat hot flashes: A randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13:568–575. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 11.Wu MF, Hilsenbeck SG, Tham YL, et al. The efficacy of sertraline for controlling hot flashes in women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2009;118:369–375. doi: 10.1007/s10549-009-0425-y. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RC. SSRIs for menopausal hot flashes: A promise yet to be delivered. Menopause: The Journal of the North American Menopause Society. 2007;14:820–822. doi: 10.1097/gme.0b013e31812714c8. [DOI] [PubMed] [Google Scholar]
- 13.Suvanto-Luukkonen E, Koivunen R, Sundstrom H, et al. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: A prospective, randomized, 9-month, placebo-controlled, double-blind study. Menopause. 2005;12:18–26. doi: 10.1097/00042192-200512010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fagerlin A, Zikmund-Fisher B, Smith D, et al. Women's decisions regarding tamoxifen for breast cancer prevention: Responses to a tailored decision aid. Breast Cancer Res and Treat. 2010;119:613–620. doi: 10.1007/s10549-009-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spratto GR, Woods AL, editors. PDR Nurse's Drug Handbook. Clifton Park, NY: Thomson Delmar Learning; 2006. Citalopram hydrobromide; pp. 288–289. [Google Scholar]
- 16.Lash T, Pedersen L, Cronin-Fenton D, et al. Tamoxifen's protection against breast cancer recurrence is not reduced by concurrent use of the SSRI citalopram. Be J Cancer. 2008;99:616–662. doi: 10.1038/sj.bjc.6604533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borges M, Zeruesenay D, Lang L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Holzman D. Tamoxifen, antidepressants, and CYP2D6: The conundrum continues. J Natl Cancer Inst. 2009;101:1370–1371. doi: 10.1093/jnci/djp366. [DOI] [PubMed] [Google Scholar]
- 19.Aubert RE, Stanek EJ, Yao J, et al. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J Clin Oncol. 2009;27(suppl):15S. abstr CRA508. [Google Scholar]
- 20.Barton DL, Loprinzi CL, Novotny P, et al. Pilot evaluation of citalopram for the relief of hot flashes. J Support Oncol. 2003;1:47–51. [PubMed] [Google Scholar]
- 21.Loprinzi C, Flynn P, Carpenter LA, et al. Pilot evaluation of citalopram for the treatment of hot flashes in women with inadequate benefit from venlafaxine. J Pallat Med. 2005;8:924–930. doi: 10.1089/jpm.2005.8.924. [DOI] [PubMed] [Google Scholar]
- 22.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 23.Curran S, Andrykowski M, Stadts J. Short form of the profile of mood states (POMS-SF): Psychometric information. Psycho Assess. 1995;7:80–83. [Google Scholar]
- 24.Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 25.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 26.Loprinzi C, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loprinzi C, Barton D, Sloan J, et al. Newer antidepressants for hot flashes - should their efficacy still be up for debate? Menopause. 2009;16:184–187. doi: 10.1097/gme.0b013e31817dfd2b. [DOI] [PubMed] [Google Scholar]
- 28.Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21:261–274. doi: 10.1016/j.bpobgyn.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.citalopram, Thompson Micromedex; 2006. USPDI: USPDI Drug Information for the Health Care Professional. [Google Scholar]
- 30.Thomson Reuters. Micromedex: Citalopram hydrobromide. http://www.thomsonhc.com.
- 31.Bardia A, Novotny P, Sloan J, et al. Efficacy of nonestrogenic hot flash therapies among women stratified by breast cancer history and tamoxifen use: A pooled analysis. Menopause. 2009;16:477–483. doi: 10.1097/gme.0b013e31818c91ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loprinzi C, Qin R, Baclueva E, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. J Clin Oncol. 2010;28:641–647. doi: 10.1200/JCO.2009.24.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]