Abstract
Purpose
Understanding delays in cancer diagnosis requires detailed information about timely recognition and follow-up of signs and symptoms. This information has been difficult to ascertain from paper-based records. We used an integrated electronic health record (EHR) to identify characteristics and predictors of missed opportunities for earlier diagnosis of lung cancer.
Methods
Using a retrospective cohort design, we evaluated 587 patients of primary lung cancer at two tertiary care facilities. Two physicians independently reviewed each case, and disagreements were resolved by consensus. Type I missed opportunities were defined as failure to recognize predefined clinical clues (ie, no documented follow-up) within 7 days. Type II missed opportunities were defined as failure to complete a requested follow-up action within 30 days.
Results
Reviewers identified missed opportunities in 222 (37.8%) of 587 patients. Median time to diagnosis in cases with and without missed opportunities was 132 days and 19 days, respectively (P < .001). Abnormal chest x-ray was the clue most frequently associated with type I missed opportunities (62%). Follow-up on abnormal chest x-ray (odds ratio [OR], 2.07; 95% CI, 1.04 to 4.13) and completion of first needle biopsy (OR, 3.02; 95% CI, 1.76 to 5.18) were associated with type II missed opportunities. Patient adherence contributed to 44% of patients with missed opportunities.
Conclusion
Preventable delays in lung cancer diagnosis arose mostly from failure to recognize documented abnormal imaging results and failure to complete key diagnostic procedures in a timely manner. Potential solutions include EHR-based strategies to improve recognition of abnormal imaging and track patients with suspected cancers.
INTRODUCTION
Missed and delayed cancer diagnoses are associated with substantial disability and costs1–10 and are a frequent cause for ambulatory malpractice claims.11 Lung cancer is both common and lethal and has a particularly poor prognosis if not diagnosed early.12,13 Although efforts to promote earlier diagnosis and treatment of lung cancer have not yet demonstrated improved survival outcomes, research is underway to evaluate the benefits of screening in high-risk patients.14
Early diagnosis hinges on timely recognition and action on clinical clues.15–18 Although patient care-seeking delays are well documented,19–23 treatment delays may also be related to the diagnostic process following the patient's first presentation with signs and symptoms.9,12,16,18,21,22,24–27 Prolonged waiting times after the initial presentation are less well understood, but some contributing factors have been documented.16,18,28–30 For instance, busy frontline providers might miss early signs and symptoms of lung cancer. Scheduling delays for diagnostic tests, poor communication of abnormal results, or test misinterpretation may also impede the diagnostic work-up. Finally, patients may not adhere to scheduled appointments or procedures after the initial work-up, or they may seek care in a different health system where their test results are not available. Therefore, missed opportunities for early diagnosis of lung cancer can occur due to failure to recognize potential diagnostic clues or failure to complete the diagnostic work-up in a timely manner.
Previous literature offers limited information on the nature of missed opportunities for earlier lung cancer diagnosis. Many studies have relied on information extracted from paper-based medical records, which may be difficult to evaluate for evidence of breakdowns in communication and care coordination.10,12,18,22,24,26,31–33 Integrated electronic health records (EHRs), on the other hand, can provide ready access to progress notes, documentation of abnormal findings, and exchanges of information (eg, test results, referrals, and so on) among front-line primary care providers, consultants, and other diagnostic specialists.34 We hypothesized that using an EHR would provide new insights into the origin and prevention of diagnostic delays in lung cancer. Our objective was to evaluate characteristics and predictors of missed opportunities for earlier diagnosis of lung cancer in a health care system with an advanced integrated EHR.
METHODS
Setting
We used a retrospective cohort design to identify and evaluate all pathologically confirmed, newly diagnosed cases of primary lung cancer at two geographically dispersed Veterans Affairs (VA) medical centers. Our sample consisted of patients diagnosed between July 2005 and June 2007 at Site A and between July 2004 and June 2007 at Site B (a longer time period at the latter site allowed for more patients). Both sites are tertiary care referral centers with on-site multispecialty ambulatory care clinics and community-based satellite clinics that provide care to urban and rural populations. All patients are assigned a primary care provider, and most patients obtain their longitudinal care within these systems from academic and nonacademic providers and resident trainees. The study was approved by the local institutional review boards.
Data Collection Procedures
We performed a detailed review of progress notes, consultations, laboratory and radiology reports, discharge summaries, and additional relevant data in the EHR to evaluate the diagnostic processes for missed opportunities. Two trained physician raters independently reviewed each case using a standardized data collection instrument adapted from our previous work in colorectal cancer diagnosis (Table 1).35 Reviewers evaluated all relevant EHR data (in most patients as far back as 5 years) for the presence of predefined clinical clues that warrant a diagnostic work-up for lung cancer. Clues were derived from current literature19,36–39 followed by team consensus (Table 1). To ensure reliable and consistent data collection, the study team supervised and trained the reviewers during pilot testing, and all discordant judgments of missed opportunities were discussed to obtain consensus. Data on patient outcomes (harm, stage of diagnosis) were not collected to reduce hindsight bias.40
Table 1.
Summary of Data Collection Instrument
| Data Category | Description of Item(s) | Example |
|---|---|---|
| Patient characteristics | Age, race/ethnicity, sex, medical and psychological comorbidities, smoking | 77-year-old white male with coronary artery disease |
| Type of clue (symptom or sign that should prompt further work-up) | Presence of at least one of the following: | Abnormal chest x-ray showing nodule |
| Blood in sputum/hemoptysis | ||
| Hoarseness that lasts > 2 weeks | ||
| Recurrent bronchitis or pneumonia | ||
| Abnormal (ie, suggestive of possible neoplastic disorder) chest x-ray | ||
| Abnormal chest CT | ||
| Abnormal abdomen CT | ||
| Serial abnormal imaging | ||
| Abnormal sputum examination/sputum cytology | ||
| Unexplained effusion | ||
| Clubbing | ||
| New onset Cushing's symptoms/syndrome | ||
| New onset of hypercalcemia symptoms/syndrome | ||
| New onset of syndrome of inappropriate antidiuretic hormone | ||
| Superior vena cava obstruction | ||
| Worsening persistent cough/bronchitis or new description of chronic cough lasting > 8 weeks | ||
| Provider acknowledged unexplained weight loss or other unexplained weight loss > 10 lbs in addition to respiratory symptoms | ||
| Chest pain or rib pain | ||
| New onset/worsening pain in non-chest location | ||
| Date clue first appeared on medical record review | June 5, 2005 | |
| Date next step was requested (ordered) | September 9, 2005 | |
| Date next step was completed | September 15, 2005 | |
| Presence of type 1 or type 2 missed opportunity? | Type 1: No evaluation (or work-up) for lung cancer was initiated within 7 days of appearance of a predefined clinical clue | Yes: Type 1 |
| Type 2: Failure to complete within 30 days a diagnostic procedure or consultation or the follow-up action requested in response to a predefined clue | No follow-up CT scan ordered to evaluate nodule by June 13, 2005 | |
| Contributory factors | Provider, system, and/or patient | Provider |
| Type of personnel involved | Codes for personnel adapted from Gandhi et al11 | Staff physician |
| Setting of care | Codes for settings adapted from Gandhi et al11 | Primary care |
| Date of lung cancer diagnosis by pathology | September 20, 2005 |
Abbreviation: CT, computed tomography.
After review of the EHR, we excluded patients who had a recurrence of lung cancer within the previous 5 years. Also excluded were patients whose pathologic diagnoses were made outside the VA setting, provided that they had not presented to the VA earlier with any potentially diagnostic clues for lung cancer.
No timeliness standards for diagnosis currently exist in the United States. However, the British Thoracic Society recommends that patients with suspected lung cancer should undergo an initial evaluation within 1 week of primary care referral and should receive diagnostic tests within 2 weeks of the decision to perform a biopsy.41 Through team consensus and additional literature on test result follow-up,42 we defined two types of missed opportunities that could result in diagnostic delays: (1) type I missed opportunities, defined as episodes of care in which there was failure to recognize a predefined clinical clue (ie, no required action or work-up was initiated within 7 days of clue appearance); appropriate decisions to watch and wait were not considered missed opportunities; and (2) type II missed opportunities, defined as episodes of care in which there was failure to complete within 30 days a diagnostic procedure, consultation, or other requested follow-up action in response to a predefined clue.
We defined the first appearance of a diagnostic clue as the earliest date that the clue could have been recognized by the care providers, regardless of when the patient first started experiencing symptoms. For instance, if a patient had hemoptysis since June 1, 2006, but did not report it to the care provider until December 1, 2006, the first appearance of a diagnostic clue was dated December 1, 2006. We applied rigorous criteria to define missed opportunities that could occur along the diagnostic pathway of lung cancer (Appendix Fig A1, online only). When information in the EHR was vague or inconsistent with expected practices, we used conservative guidelines to avoid overestimating missed opportunities. For example, reviewers were instructed not to record missed opportunities if there was insufficient supporting documentation in the EHR, or when documentation supported an informed decision not to work up a particular clue. No missed opportunity was recorded when delays occurred solely in response to appropriate diagnostic attempts, such as repeated negative bronchoscopies. For each case, we collected information on provider types and specialties, types of diagnostic procedures used, and patient characteristics. We also classified contributing factors in each case into one of three categories (Table 1). “System factors” included scheduling delays, policies, and/or procedures that were judged to have contributed to a missed opportunity. “Provider factors” were attributed in situations when providers failed to recognize previously documented clues or did not follow standards of care or standard policies, resulting in a missed opportunity. Finally, we attributed missed opportunities to “patient factors” (eg, when patients did not adhere to medical advice or appointments).
Data Analysis
After evaluating reviewer agreement on the presence of missed opportunities and reaching consensus on discordant judgments, we identified two groups of patients: (1) those determined to have experienced at least one missed opportunity (of either type), and (2) those determined to have no missed opportunities. We compared these groups on demographic and clinical characteristics, location (Site A v Site B), and provider types and specialties. We then separately evaluated predictors for type I and type II missed opportunities. First, we compared the frequencies of diagnostic clues present in cases with type I missed opportunities and cases in the no missed opportunities group. We then similarly compared the frequencies of specific follow-up actions documented in cases with type II missed opportunities and cases in the no missed opportunities group. Finally, we calculated the median wait times associated with each type of diagnostic clue or follow-up action in type I and type II cases, respectively. We used Fisher's exact test for categoric variables when the assumptions for the χ2 test were not met (two-tailed) and the nonparametric Wilcoxon rank sum test to compare median times to pathological diagnosis.
Finally, we fit three multivariable logistic regression models. The first model predicted the presence of any missed opportunity from provider type and specialty. The other models tested whether particular diagnostic clues or actions were associated with increased risk for type I and type II missed opportunities, respectively. Each model was adjusted for baseline patient characteristics that were distributed unequally between cases with and without missed opportunities. Predictors entered into the initial models included variables that were statistically significant at the 0.1 level in univariate analysis. The final models included only significant predictors. We used SAS version 9.2 (SAS Institute, Cary, NC) for all analyses.
RESULTS
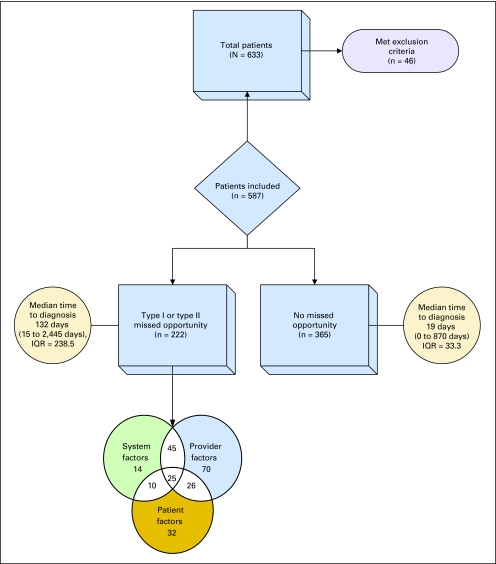
Of 633 new patient cases of lung cancer identified over the study period, 587 met inclusion criteria (Fig 1), and 222 (37.8%) were judged to have missed opportunities after consensus agreements. Before consensus, both reviewers independently agreed on the presence of at least one missed opportunity in 184 patients and on the absence of any missed opportunities in 284 patients (overall κ = 0.69).43 The median time elapsed from first appearance of a diagnostic clue to final pathologic diagnosis was 132.0 days (range, 15 to 2,445 days) in patients with at least one missed opportunity compared with 19.0 days (range, 0 to 870 days) in patients with no identified missed opportunities (P < .001). The outliers in the latter group included patients that required serial imaging and were appropriately followed up. The Venn diagram at the bottom of Fig 1 shows the distribution of provider-related, system-related, and patient-related factors in the 222 patients with at least one missed opportunity.
Fig 1.
Study flowchart. IQR, interquartile range.
Type I missed opportunities were judged to occur in 148 (25.2%) of 587 included patients; among these, the median time to pathologic diagnosis was 168 days (range, 15 to 2,445 days; interquartile range, 290 days). Type II missed opportunities occurred in 121 patients (20.6%); in these patients, the median time to pathologic diagnosis was 141.5 days (range, 38 to 2,445 days; interquartile range, 224 days).
We compared baseline characteristics of patients with and without at least one missed opportunity for subsequent inclusion in adjusted predictor models. At the 0.10 level of significance, three comorbidities were more frequent in patients with missed opportunities: hypertension, chronic obstructive pulmonary disease (COPD), and antisocial personality disorder (Table 2). However, only COPD remained statistically significant in subsequent logistic regression models.
Table 2.
Baseline Characteristics of Patients With and Without Missed Opportunities
| Characteristics | Patients With at Least One Missed Opportunity (n = 222) |
Patients With No Missed Opportunities (n = 365) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years* | |||||
| Median | 67.9 | 67.8 | |||
| < 65 | 91 | 40.9 | 152 | 41.6 | |
| 65-74 | 66 | 29.7 | 107 | 29.3 | |
| ≥ 75 | 64 | 28.8 | 106 | 29.0 | .99 |
| Race* | |||||
| White | 168 | 75.7 | 284 | 78.8 | |
| Black | 42 | 18.9 | 59 | 16.2 | |
| Other | 10 | 4.5 | 19 | 5.2 | .66 |
| Sex | |||||
| Male | 221 | 99.6 | 360 | 98.6 | |
| Female | 1 | 0.45 | 5 | 1.4 | .42 |
| Year of diagnosis* | |||||
| 2004 | 5 | 2.2 | 15 | 4.1 | |
| 2005 | 57 | 25.7 | 102 | 28.0 | |
| 2006 | 108 | 48.6 | 161 | 44.1 | |
| 2007 | 50 | 22.5 | 84 | 23.0 | .51 |
| Location | |||||
| Site A | 158 | 71.2 | 240 | 65.8 | |
| Site B | 64 | 28.8 | 125 | 34.2 | .17 |
| Comorbid medical diseases† | |||||
| Congestive heart failure | 19 | 8.6 | 32 | 8.8 | .93 |
| Coronary artery disease | 70 | 31.5 | 107 | 29.3 | .57 |
| Hypertension | 152 | 68.5 | 223 | 61.1 | .07 |
| Diabetes | 50 | 22.5 | 70 | 19.2 | .33 |
| Chronic obstructive pulmonary disease | 99 | 44.6 | 121 | 33.2 | .006 |
| Advanced cardiopulmonary disease with life expectancy < 1 year | 2 | 0.9 | 5 | 1.4 | .72 |
| Severely disabled due to medical problem | 13 | 5.9 | 19 | 5.2 | .74 |
| Cancer (prior to lung cancer) | 58 | 26.1 | 76 | 20.8 | .13 |
| HIV | 2 | 0.9 | 9 | 2.5 | .22 |
| Any of the above | 199 | 89.6 | 314 | 86.0 | .20 |
| Comorbid psychiatric disorders | |||||
| Depression | 42 | 18.9 | 53 | 14.5 | .16 |
| Anxiety | 12 | 5.4 | 21 | 5.8 | .86 |
| Dementia | 8 | 3.6 | 14 | 3.8 | .89 |
| Post-traumatic stress disorder | 6 | 2.7 | 15 | 4.1 | .37 |
| Schizophrenia | 3 | 1.4 | 3 | 0.82 | .68 |
| Bipolar disorder | 0 | 0.0 | 4 | 1.1 | .30 |
| Alcohol dependence | 30 | 13.5 | 52 | 14.2 | .80 |
| Antisocial personality disorder | 3 | 1.4 | 0 | 0.0 | .05 |
| Severely disabled due to psychiatric problem | 1 | 0.45 | 5 | 1.4 | .42 |
| Any of the above | 78 | 35.1 | 121 | 33.2 | .60 |
| Smoking status‡ | |||||
| Current smoker | 127 | 57.2 | 218 | 59.7 | |
| Prior smoker | 88 | 39.6 | 130 | 35.6 | |
| Nonsmoker | 7 | 3.2 | 17 | 4.7 | .47 |
Percentages may not add up to 100% due to missing data.
Medical record documentation was used to determine comorbid conditions. Advanced cardiopulmonary disease with life expectancy < 1 year determined by documentation of either advanced stage chronic obstructive pulmonary disease (eg, Stage IV), advanced heart failure (eg, Stage IV) or inoperable coronary artery disease combined with the mention of poor prognosis in the medical record.
Current smoker, patients who were actively smoking at the time of lung cancer diagnosis; prior smoker, patients who had smoked anytime in the past regardless of quantity and duration; nonsmoker, patients who had never smoked in the past.
Provider characteristics (Table 3) were associated with patients with one or more missed opportunities. In the final adjusted multivariable model, trainees were less likely to be associated with patients with missed opportunities (odds ratio [OR], 0.41; 95% CI, 0.27 to 0.62; referent, staff physician). Whereas emergency medicine providers were relatively unlikely to be associated with missed opportunities (OR, 0.52; 95% CI, 0.28 to 0.96), oncology and pulmonary specialists were overrepresented in patients with missed opportunities (OR, 18.72; 95% CI, 2.30 to 152.46 and OR, 2.35; 95% CI, 1.36 to 4.08, respectively; referent, primary care). For both oncologists and pulmonologists, type I missed opportunities were more frequent. Patient factors were associated with more than half of missed opportunities associated with pulmonary (20 [54%] of 37), but were associated with only two (2 [20%] of 10) missed opportunities related to oncology. Sample sizes were insufficient to test whether these relationships differed between sites.
Table 3.
Characteristics of Providers in Patients With and Without Any Missed Opportunities
| Characteristics | Patients With Any Missed Opportunity (n = 222) |
Patients With No Missed Opportunities (n = 365) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Type of provider* | |||||
| Staff physician | 119 | 53.6 | 136 | 37.3 | |
| Trainee | 50 | 22.5 | 145 | 39.7 | |
| Nurse practitioner | 14 | 6.3 | 18 | 4.9 | |
| Physician assistant | 32 | 14.4 | 31 | 8.5 | < .001 |
| Specialty* | |||||
| Generalist/primary care | 127 | 57.2 | 208 | 57.0 | |
| Oncology | 10 | 4.5 | 1 | 0.3 | |
| Pulmonary | 37 | 16.7 | 31 | 8.5 | |
| Other medical subspecialty† | 7 | 3.2 | 1.5 | 4.1 | |
| Emergency medicine | 17 | 7.7 | 53 | 14.5 | |
| Surgery‡ | 15 | 6.8 | 20 | 5.5 | |
| Other | 0 | 0.0 | 1 | 0.3 | < .001 |
NOTE. Adjustment variable, chronic obstructive pulmonary disease, was also significant (odds ratio, 1.56; 95% CI, 1.08 to 2.24).
Percentages may not add up to 100% due to missing data.
Other medical subspecialties include cardiology, nephrology, neurology, rheumatology/immunology, gastroenterology, dermatology, endocrinology, infectious disease, and intensive care.
Surgery includes general surgery, cardiothoracic surgery, orthopedic surgery, ophthalmology, otolaryngology, vascular surgery, neurosurgery, plastic surgery, and urology.
Table 4 shows χ2 comparisons of diagnostic clues in patients with type I missed opportunities and no missed opportunities. Median times to clue recognition for missed clues is also listed. An abnormal chest x-ray was the most frequently missed clue, followed by abnormal chest computed tomography scan, and new or worsening persistent cough > 8 weeks. When we relaxed the criterion for recognition from 7 days to 14 days, the total number of patients with type I missed opportunities decreased from 148 to 127. Only recurrent bronchitis was associated with type I missed opportunities in unadjusted and adjusted logistic regression models (adjusted OR, 3.31; 95% CI, 1.20 to 9.10; referent, no recurrent bronchitis). We further assessed whether nonsmokers experienced longer delays from type I missed opportunities (data not shown). We found that of 19 outlier patients,44 11 were smokers, seven were past smokers, and one had never smoked. Smoking history was not associated with outlier status.
Table 4.
Diagnostic Clues and Associated Median Time to Clue Recognition in Lung Cancer Patients With and Without Missed Opportunities
| Clues | Patients With Type I Missed Opportunities (n = 148) |
Patients Without Missed Opportunities* (n = 365) |
P | |||||
|---|---|---|---|---|---|---|---|---|
| Time to Clue Recognition in Type I Patients (days) |
No. | % | ||||||
| Median | Range | IQR | No. | % | ||||
| Blood in sputum/hemoptysis | 128.5 | 98.0-159.0 | 61.0 | 2 | 1.4 | 33 | 9.0 | .09 |
| Recurrent bronchitis or pneumonia | 109.0 | 22.0-293.0 | 136.0 | 5 | 3.4 | 7 | 1.9 | .04 |
| Abnormal chest x-ray | 89.0 | 8.0-2,011.0 | 162.5 | 92 | 62.2 | 280 | 76.7 | < .001 |
| Abnormal chest CT | 27.0 | 8.0-1,126.0 | 49.0 | 42 | 28.4 | 317 | 86.8 | < .001 |
| Abnormal abdomen CT | 10.0 | 8.0-67.0 | 59.0 | 3 | 2.0 | 18 | 4.9 | .0026 |
| Hoarseness lasting > 2 weeks | 109 | 109-109 | 0 | 1 | 0.7 | 10 | 2.7 | .23 |
| Unexplained effusion | 51.0 | 12.0-56.0 | 44.0 | 3 | 2.0 | 10 | 2.7 | .02 |
| Worsening persistent cough/bronchitis or new description of chronic cough lasting > 8 weeks | 51.0 | 8.0-177.0 | 138.0 | 11 | 7.4 | 64 | 17.5 | < .001 |
| Unexplained weight loss in addition to respiratory symptoms | 49.0 | 12.0-556.0 | 455.0 | 7 | 4.7 | 74 | 2.3 | < .001 |
| Chest pain or rib pain | 77.0 | 63.0-117.0 | 54.0 | 3 | 2.0 | 40 | 11.0 | .01 |
| New onset/worsening pain in non-chest location | 35.5 | 12.0-222.0 | 37.5 | 8 | 5.4 | 28 | 7.7 | < .001 |
NOTE. The following clues were not seen in any patients: clubbing, new onset Cushing's disease, or superior vena cava obstruction. The following clues were identified only in the no missed opportunities group: abnormal sputum examination/sputum cytology (3), new onset of hypercalcemia symptoms/syndrome (5), and syndrome of inappropriate [secretion of] antidiuretic hormone (2). Adjustment variable, chronic obstructive pulmonary disease, was significant (odds ratio, 1.83; 95% CI, 1.17 to 2.86).
Abbreviations: IQR, interquartile range; CT, computed tomography.
All clues were recognized in ≤ 7 days.
Table 5 compares the proportions of requested actions (procedures, consultations, or follow-up actions on clues) in patients with type II missed opportunities and no missed opportunities. For missed opportunities, median times to action completion are also listed. Patient factors were strongly associated with type II missed opportunities: completion of needle biopsies (15 [62.5%] of 24), completion of bronchoscopies (15 [100%] of 15), follow-up of abnormal chest x-rays (28 [38.9%] of 72), pulmonary consults (17 [65.4%] of 26), and follow-up of abnormal chest computed tomography scans (11 [61.1%] of 18). Follow-up of abnormal chest x-ray, completion of first needle biopsy, and follow-up of recurrent bronchitis were significant predictors of type II missed opportunities in the unadjusted logistic regression model. In the adjusted model, which controlled for the presence of COPD, only follow-up action on abnormal chest x-ray (OR, 2.07; 95% CI, 1.04 to 4.13; referent, no abnormal chest x-ray) and completion of first needle biopsy (OR, 3.02; 95% CI, 1.76 to 5.18; referent, no needle biopsy) were associated with type II missed opportunities. Appendix Table A1 summarizes logistic regression results for missed opportunities.
Table 5.
Requested Actions and Associated Median Time to Completion in Patients With and Without Missed Opportunities
| Requested Follow-Up Actions, Procedures, or Consultations | Patients With Type II Missed Opportunities (n = 121) |
Patients Without Missed Opportunities* (n = 365) |
P | |||||
|---|---|---|---|---|---|---|---|---|
| Time to Completion of Procedure or Consultation or Follow-Up Action Based on the Clue in Type II Patients (days) |
No. | % | ||||||
| Median | Range | IQR | No. | % | ||||
| Follow-up on blood in sputum | 63.0 | 46-279 | 233.0 | 3 | 2.5 | 33 | 9.0 | .01 |
| Follow-up on recurrent bronchitis or pneumonia | 1075 | 1,075.0-1,075.0 | 0 | 1 | 0.8 | 7 | 1.9 | .30 |
| Follow-up on hoarseness lasting > 2 weeks | 58.0 | 48-68 | 20.0 | 2 | 1.6 | 10 | 2.7 | .06 |
| Follow-up on abnormal chest x-ray | 48.0 | 32-548 | 23.0 | 72 | 59.5 | 279 | 76.4 | < .001 |
| Follow-up on abnormal chest CT | 42.5 | 31.0-366.0 | 59.0 | 18 | 14.9 | 314 | 86.0 | < .001 |
| Follow-up on abnormal abdomen CT | 39.0 | 39.0-39.0 | 0 | 1 | 0.8 | 18 | 4.9 | .10 |
| Follow-up on worsening bronchitis/cough | 117.5 | 31.0-204.0 | 173.0 | 2 | 1.6 | 64 | 17.5 | .03 |
| Follow-up on unexplained weight loss | 98.5 | 36.0-1,029.0 | 504.0 | 4 | 3.3 | 74 | 20.3 | < .001 |
| Follow-up on pain in non-chest location | 45.0 | 40.0-72.0 | 32.0 | 3 | 2.5 | 28 | 7.7 | .005 |
| Pulmonary consult | 50.0 | 33.0-588.0 | 26.0 | 26 | 21.5 | 283 | 77.5 | < .001 |
| First bronchoscopic biopsy | 85.0 | 31-387 | 171.0 | 13 | 10.7 | 240 | 65.8 | < .001 |
| Second bronchoscopic biopsy | 53 | 49.0-57.0 | 8.0 | 2 | 1.6 | 21 | 5.8 | .12 |
| First needle biopsy | 50.0 | 32-253.0 | 52.0 | 24 | 19.8 | 72 | 19.7 | < .001 |
| Thoracic surgery consult | 55.0 | 34-386.0 | 49.0 | 5 | 4.1 | 36 | 9.9 | .02 |
| Open lung biopsy | 37.5 | 33-63.0 | 16.5 | 4 | 3.3 | 9 | 2.5 | .08 |
NOTE. The following were not seen in any patients: clubbing, new onset Cushing's disease, or superior vena cava obstruction. The following clues/procedure/consultation were identified only in patients with no missed opportunities: chest pain (40), abnormal sputum examination (3), unexplained effusion (10), new onset of hypercalcemia symptoms/syndrome (5), new onset of syndrome of inappropriate [secretion of] antidiuretic hormone (2), third bronchoscopic biopsy (2), second needle biopsy (2), mediastinoscopy (8), thoracentesis (26), and positron emission tomography scan (15).
Abbreviations: IQR, interquartile range; CT, computed tomograhy.
All actions were completed in ≤ 30 days.
DISCUSSION
We used an advanced, integrated EHR to discover missed opportunities for an earlier lung cancer diagnosis and found evidence of missed opportunities in more than one-third (n = 222) of 587 patients diagnosed at two institutions. Missed opportunities led to significant delays in diagnosis. More than half of missed opportunities arose from failures to recognize diagnostic clues (in most patients, abnormal imaging results) already present in the EHR. Other missed opportunities resulted from failures to complete key diagnostic procedures or investigations in a timely manner; patient factors often contributed in these cases.
Previous studies of delayed lung cancer diagnosis have relied on interview and questionnaire data20–22,27,45 or reviews of paper-based medical records.12,21,27,46 However, recall bias and the large potential for missing information in paper-based records limit the utility of these methods for studying opportunities to improve diagnostic care. Furthermore, previous work has involved a significant amount of subjective judgment11,47,48 and yielded little insight about the frequency and origins of delayed cancer diagnoses.10 Our study overcomes many of these limitations and, to the best of our knowledge, is the largest of its kind.
In the VA system, abnormal imaging results are transmitted to ordering providers through an automated notification system in the EHR.49 Radiologists transmit the abnormal reports to an inbox where the clinician can access and act on the reports. Reports are always accessible to providers and are marked as abnormal to heighten awareness. However, for several reasons, clinicians may not always act on abnormal imaging results in a timely manner.50 It is unlikely that this problem is unique to the VA.13,45,51 Outcomes of imaging notification may actually be better within the VA system because of the integrated nature of its EHR, the presence of a state-of-the-art notification system, and clear policies and procedures for follow-up of diagnostic information.49,52
The origins of missed opportunities are multifactorial, and multidisciplinary strategies are needed to improve the timeliness of the diagnostic process. EHR-based strategies to reduce missed opportunities should target communication, recognition of abnormal imaging results, and monitoring of follow-up actions.49,50 For example, programs in the EHR could identify high-risk patients with abnormal imaging and no evidence of follow-up; the program could then generate a trigger (ie, a signal to alert providers to review the medical record53) to a responsible clinician.54 Second, strategies could be designed to improve recognition of clues that might otherwise stay buried in the wealth of the information available in the EHR. For instance, the documentation of “hemoptysis” or “blood in sputum” in a 60-year-old previous smoker could result in a trigger to initiate or continue the work-up of lung cancer through decision support and text-recognition rules.55
Third, patient transition among different settings of care10 (eg, scheduling and completing procedures) is a high-risk area for preventable breakdowns in communication and coordination. The association of missed opportunities with oncology and pulmonary subspecialties highlights this issue. While trainees were less likely to be associated with missed opportunities, this might be because critical information (such as a test result) transmitted to trainees is also transmitted to their supervising physicians. Nevertheless, current EHR systems may not be able to support the sophisticated degree of tracking providers need to ensure fail-safe follow-up of high-risk patients. While such systems are being designed, the use of lung nodule clinical pathways56 or other programs for patient navigation57 appears promising. Additionally, the VA has recently initiated a national lung cancer collaborative program to improve the timeliness of lung cancer care. These approaches could be particularly beneficial for patients who miss appointments or procedures and are at risk of being lost to follow-up.
Our study findings may not be generalizable outside the VA setting. Moreover, our results may not generalize to other, similar investigations, because our model building was not exclusively based on theory and experience but included predictors on the basis of their chance covariation with the outcome. Studies of diagnostic breakdown traditionally suffer from methodologic limitation of low reliability,58 which we addressed by using two independent reviewers followed by consensus agreements. We may have also missed clues or follow-up actions that were completed but either were not documented in the chart or were documented where the information was hard to find among hundreds of other notes. However, in our previous work, we found that fewer than 2% of providers failed to document follow-up actions related to abnormal imaging results, so it is unlikely that we significantly overestimated missed opportunities because of lack of documentation.50 Another limitation is the lack of comparison information from comparable health systems or from systems that use paper-based records. Hindsight bias40 is of particular concern in studies such as ours, and we tried to minimize it by omitting data collection on outcomes, such as stage at diagnosis and patient harm. Finally, it is not clear whether reducing these delays would improve outcomes.16 Nevertheless, timeliness is considered one of six aims for improving quality of health care.59 Specific strengths of our study included a reliable data collection methodology and a rigorous definition of missed opportunities. Most important, an integrated EHR of a closed health system facilitated collection of data relevant to the entire diagnostic process.
In summary, delays in lung cancer diagnosis are not infrequent. Reducing delays will require strategies to address multiple contributing factors. Potential solutions include using the EHR to improve clinician recognition of abnormal imaging results and instituting programs to track patients with suspicious findings.
Acknowledgment
We thank Annie Bradford, PhD, for assistance with technical writing.
Appendix
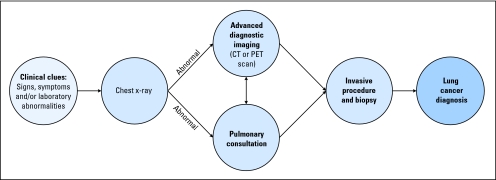
Fig A1.
Diagnostic flowchart for lung cancer. CT, computed tomography; PET, positron emission tomography.
Table A1.
Logistic Regression Results for Missed Opportunities for Lung Cancer Diagnosis
| Provider Characteristics | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Type of provider | |||
| Trainee | 0.41 | 0.27 to 0.62 | < .001 |
| Nurse practitioner | 0.99 | 0.46 to 2.14 | .99 |
| Physician assistant | 1.44 | 0.81 to 2.55 | .21 |
| Referent: staff physician | |||
| Specialty | |||
| Emergency medicine | 0.52 | 0.28 to 0.96 | .04 |
| Oncology | 18.72 | 2.30 to 152.46 | .006 |
| Pulmonary | 2.35 | 1.36 to 4.08 | .002 |
| Other subspecialties | 0.92 | 0.35 to 2.42 | .87 |
| Surgery | 1.86 | 0.89 to 3.87 | .10 |
| Other* | — | — | — |
| Referent: primary care | |||
| Diagnostic clues (type I) | |||
| Recurrent bronchitis | 3.31 | 1.20 to 9.10 | .02 |
| Requested follow-up actions, procedures, or consultations (type II) | |||
| Abnormal chest x-ray | 2.07 | 1.04 to 4.13 | .04 |
| First needle biopsy | 3.02 | 1.76 to 5.18 | < .001 |
No patients with missed opportunities.
Footnotes
Supported by K23 Career Development Award No. K23CA125585 from the National Institutes of Health (H.S.) and in part by the Houston Veterans Administration Health Services Research and Development Center of Excellence (HFP90-020).
Presented as an abstract at the 32nd Annual Meeting of the Society of General Internal Medicine, May 13-16, 2009, Miami, FL.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Hardeep Singh, Kamal Hirani, Himabindu Kadiyala, Olga Rudomiotov, Myrna M. Khan, Terry L. Wahls
Financial support: Hardeep Singh
Administrative support: Traber Davis
Provision of study materials or patients: Kamal Hirani, Himabindu Kadiyala, Olga Rudomiotov, Traber Davis, Terry L. Wahls
Collection and assembly of data: Hardeep Singh, Kamal Hirani, Himabindu Kadiyala, Olga Rudomiotov, Traber Davis, Terry L. Wahls
Data analysis and interpretation: Hardeep Singh, Olga Rudomiotov, Traber Davis, Myrna M. Khan, Terry L. Wahls
Manuscript writing: Hardeep Singh, Myrna M. Khan, Terry L. Wahls
Final approval of manuscript: Hardeep Singh, Kamal Hirani, Himabindu Kadiyala, Olga Rudomiotov, Traber Davis, Myrna M. Khan, Terry L. Wahls
REFERENCES
- 1.Phillips RL, Jr, Bartholomew LA, Dovey SM, et al. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13:121–126. doi: 10.1136/qshc.2003.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodson WH, III, Moore DH. Overall clinical breast examination as a factor in delayed diagnosis of breast cancer. Arch Surg. 2002;137:1152–1156. doi: 10.1001/archsurg.137.10.1152. [DOI] [PubMed] [Google Scholar]
- 3.Goodson WH, III, Moore DH. Causes of physician delay in the diagnosis of breast cancer. Arch Intern Med. 2002;162:1343–1348. doi: 10.1001/archinte.162.12.1343. [DOI] [PubMed] [Google Scholar]
- 4.Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129:397–403. doi: 10.1001/archsurg.1994.01420280073009. [DOI] [PubMed] [Google Scholar]
- 5.Kern KA. The delayed diagnosis of breast cancer: Medicolegal implications and risk prevention for surgeons. Breast Dis. 2001;12:145–158. doi: 10.3233/bd-2001-12115. [DOI] [PubMed] [Google Scholar]
- 6.Raab SS, Grzybicki DM, Janosky JE, et al. Clinical impact and frequency of anatomic pathology errors in cancer diagnoses. Cancer. 2005;104:2205–2213. doi: 10.1002/cncr.21431. [DOI] [PubMed] [Google Scholar]
- 7.Turkington PM, Kennan N, Greenstone MA. Misinterpretation of the chest x-ray as a factor in the delayed diagnosis of lung cancer. Postgrad Med J. 2002;78:158–160. doi: 10.1136/pmj.78.917.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust N Z J Surg. 2000;70:635–638. doi: 10.1046/j.1440-1622.2000.01916.x. [DOI] [PubMed] [Google Scholar]
- 9.Billing JS, Wells FC. Delays in the diagnosis and surgical treatment of lung cancer. Thorax. 1996;51:903–906. doi: 10.1136/thx.51.9.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh H, Sethi S, Raber M, et al. Errors in cancer diagnosis: Current understanding and future directions. J Clin Oncol. 2007;25:5009–5018. doi: 10.1200/JCO.2007.13.2142. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: A study of closed malpractice claims. Ann Intern Med. 2006;145:488–496. doi: 10.7326/0003-4819-145-7-200610030-00006. [DOI] [PubMed] [Google Scholar]
- 12.Salomaa ER, Sallinen S, Hiekkanen H, et al. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128:2282–2288. doi: 10.1378/chest.128.4.2282. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto A, Tsuji H, Takazakura E, et al. Reasons for the delays in the definitive diagnosis of lung cancer for more than one year from the recognition of abnormal chest shadows. Intern Med. 2002;41:95–102. doi: 10.2169/internalmedicine.41.95. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute, ClinicalTrials.gov: National Lung Screening Trial (NLST), Identifier NCT00047385, 2002. http://clinicaltrials.gov/ct/show/NCT00047385.%202010.
- 15.The NHS Cancer Plan. London, United Kingdom, Department of Health, 2000. http://www.uicc-community.org/templates/ccc/images/NCP%20UK.pdf.
- 16.Gould MK, Ghaus SJ, Olsson JK, et al. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133:1167–1173. doi: 10.1378/chest.07-2654. [DOI] [PubMed] [Google Scholar]
- 17.Kanashiki M, Satoh H, Ishikawa H, et al. Time from finding abnormality on mass-screening to final diagnosis of lung cancer. Oncol Rep. 2003;10:649–652. [PubMed] [Google Scholar]
- 18.O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141–144. doi: 10.1053/clon.2000.9139. [DOI] [PubMed] [Google Scholar]
- 19.Corner J, Hopkinson J, Fitzsimmons D, et al. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax. 2005;60:314–319. doi: 10.1136/thx.2004.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corner J, Hopkinson J, Roffe L. Experience of health changes and reasons for delay in seeking care: A UK study of the months prior to the diagnosis of lung cancer. Soc Sci Med. 2006;62:1381–1391. doi: 10.1016/j.socscimed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Ichinohe K, Takahashi M, Tooyama N. Delay by patients and doctors in treatment of Pancoast tumor. Wien Klin Wochenschr. 2006;118:405–410. doi: 10.1007/s00508-006-0615-0. [DOI] [PubMed] [Google Scholar]
- 22.Koyi H, Hillerdal G, Branden E. Patient's and doctors’ delays in the diagnosis of chest tumors. Lung Cancer. 2002;35:53–57. doi: 10.1016/s0169-5002(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 23.Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: A qualitative study. J Adv Nurs. 2008;61:336–343. doi: 10.1111/j.1365-2648.2007.04542.x. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of primary lung cancer. Acta Oncol. 2002;41:147–152. doi: 10.1080/028418602753669517. [DOI] [PubMed] [Google Scholar]
- 25.Devbhandari MP, Bittar MN, Quennell P, et al. Are we achieving the current waiting time targets in lung cancer treatment? Result of a prospective study from a large United Kingdom teaching hospital. J Thorac Oncol. 2007;2:590–592. doi: 10.1097/JTO.0b013e318070ccf0. [DOI] [PubMed] [Google Scholar]
- 26.Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3:951–957. doi: 10.1097/JTO.0b013e3181839b60. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz A, Damadoglu E, Salturk C, et al. Delays in the diagnosis and treatment of primary lung cancer: Are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113:287–296. doi: 10.3109/2000-1967-236. [DOI] [PubMed] [Google Scholar]
- 28.Weingart SN, Saadeh MG, Simchowitz B, et al. Process of care failures in breast cancer diagnosis. J Gen Intern Med. 2009;24:702–709. doi: 10.1007/s11606-009-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo DS, Zeldin RA, Skrastins R, et al. Time to treat: A system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol. 2007;2:1001–1006. doi: 10.1097/JTO.0b013e318158d4b6. [DOI] [PubMed] [Google Scholar]
- 30.Moody A, Muers M, Forman D. Delays in managing lung cancer. Thorax. 2004;59:1–3. [PMC free article] [PubMed] [Google Scholar]
- 31.Vinod SK, O'Connell DL, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol. 2008;3:871–879. doi: 10.1097/JTO.0b013e31818020c3. [DOI] [PubMed] [Google Scholar]
- 32.Månsson J, Bengtsson C. Pulmonary cancer from the general practitioner's point of view: Experience from the health centre area of Kungsbacka, Sweden. Scand J Prim Health Care. 1994;12:39–43. doi: 10.3109/02813439408997055. [DOI] [PubMed] [Google Scholar]
- 33.Porta M, Gallen M, Malats N, et al. Influence of “diagnostic delay” upon cancer survival: An analysis of five tumour sites. J Epidemiol Community Health. 1991;45:225–230. doi: 10.1136/jech.45.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh H, Naik A, Rao R, et al. Reducing diagnostic errors through effective communication: Harnessing the power of information technology. J Gen Intern Med. 2008;23:489–494. doi: 10.1007/s11606-007-0393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh H, Daci K, Petersen LA, et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104:2543–2554. doi: 10.1038/ajg.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buccheri G, Ferrigno D. Lung cancer: Clinical presentation and specialist referral time. Eur Respir J. 2004;24:898–904. doi: 10.1183/09031936.04.00113603. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: A structured review. Fam Pract. 2004;21:605–611. doi: 10.1093/fampra/cmh605. [DOI] [PubMed] [Google Scholar]
- 38.Lee DK. Suspected lung cancer: Its initial management and staging. Prim Care Respir J. 2007;16:106–111. doi: 10.3132/pcrj.2007.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiro SG, Gould MK, Colice GL. Initial evaluation of the patient with lung cancer: Symptoms, signs, laboratory tests, and paraneoplastic syndromes—ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:149S–160S. doi: 10.1378/chest.07-1358. [DOI] [PubMed] [Google Scholar]
- 40.Fischhoff B. Hindsight does not equal foresight: The effect of outcome knowledge on judgment under uncertainty—1975. Qual Saf Health Care. 2003;12:304–311. doi: 10.1136/qhc.12.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BTS recommendations to respiratory physicians for organising the care of patients with lung cancer—The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1–S8. doi: 10.1136/thx.53.suppl_1.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna D, Griswold P, Leape LL, et al. Communicating critical test results: Safe practice recommendations. Jt Comm J Qual Patient Saf. 2005;31:68–80. doi: 10.1016/s1553-7250(05)31011-7. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 44.Rosner B. Fundamentals of Biostatistics (ed 5) Pacific Grove, CA: Duxbury; 2000. [Google Scholar]
- 45.Bjerager M, Palshof T, Dahl R, et al. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56:863–868. [PMC free article] [PubMed] [Google Scholar]
- 46.Myrdal G, Lambe M, Hillerdal G, et al. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59:45–49. [PMC free article] [PubMed] [Google Scholar]
- 47.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493–1499. doi: 10.1001/archinte.165.13.1493. [DOI] [PubMed] [Google Scholar]
- 48.Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: Lessons from a multi-institutional collaborative project. Advances in Patient Safety. 2005;Vol. 2 [PubMed] [Google Scholar]
- 49.Singh H, Arora H, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc. 2007;14:459–466. doi: 10.1197/jamia.M2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh H, Thomas EJ, Mani S, et al. Timely follow-up of abnormal diagnostic imaging test results in an outpatient setting: Are electronic medical records achieving their potential? Arch Intern Med. 2009;169:1578–1586. doi: 10.1001/archinternmed.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169:1123–1129. doi: 10.1001/archinternmed.2009.130. [DOI] [PubMed] [Google Scholar]
- 52.Choksi VR, Marn CS, Bell Y, et al. Efficiency of a semiautomated coding and review process for notification of critical findings in diagnostic imaging. AJR Am J Roentgenol. 2006;186:933–936. doi: 10.2214/AJR.04.1913. [DOI] [PubMed] [Google Scholar]
- 53.Agency for Healthcare Research and Quality. Patient Safety Network. 2008 doi: 10.1080/15360280802537332. [DOI] [PubMed] [Google Scholar]
- 54.Singh H, Thomas EJ. Diagnostic event triggers: Current state of science and future directions. AHRQ Special Report. 2009 [Google Scholar]
- 55.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348:2526–2534. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 56.Holden WE, Lewinsohn DM, Osborne ML, et al. Use of a clinical pathway to manage unsuspected radiographic findings. Chest. 2004;125:1753–1760. doi: 10.1378/chest.125.5.1753. [DOI] [PubMed] [Google Scholar]
- 57.Battaglia TA, Roloff K, Posner MA, et al. Improving follow-up to abnormal breast cancer screening in an urban population: A patient navigation intervention. Cancer. 2007;109:359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 58.Thomas EJ, Lipsitz SR, Studdert DM, et al. The reliability of medical record review for estimating adverse event rates. Ann Intern Med. 2002;136:812–816. doi: 10.7326/0003-4819-136-11-200206040-00009. [DOI] [PubMed] [Google Scholar]
- 59.Committee on Quality of Health Care in America and Institute of Medicine. Washington, DC: National Academies Press; 2000. Crossing the Quality Chasm: A New Health System for the 21st Century. [PubMed] [Google Scholar]