Abstract
Purpose
We conducted a phase II trial to evaluate the efficacy and safety of single-agent sorafenib in chemotherapy-naïve patients with metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN). The primary end point was response probability (ie, confirmed complete and partial response [PR]).
Patients and Methods
Chemotherapy-naïve patients with metastatic, persistent, or recurrent SCCHN who received one induction or fewer or received an adjuvant chemotherapy regimen, who had adequate organ function, and who had a performance status ≤ 1 were eligible. Sorafenib was administered orally at 400 mg twice daily on a continuous basis in 28-day cycles. Responses were evaluated according to RECIST (Response Evaluation Criteria in Solid Tumors).
Results
Sorafenib was generally well tolerated. Of the 41 eligible patients assessed for adverse events, one experienced a grade 4 adverse event as a result of an asymptomatic pulmonary embolus. The most common grades 2 to 3 adverse events were fatigue, anorexia, stomatitis/oral pain, abdominal pain, hand-foot syndrome, weight loss, and hypertension. There was one confirmed PR and two unconfirmed PRs. The estimated confirmed response probability was 2% (95% CI, 0% to 13%). The estimated median progression-free survival was 4 months (95% CI, 2 to 4 months), and the estimated median overall survival was 9 months (95% CI, 7 to 14 months).
Conclusion
Sorafenib was well tolerated. Although response was poor, progression-free and overall survival times compare favorably with previous Southwest Oncology Group, phase II, single-agent trials.
INTRODUCTION
Recurrent squamous cell carcinoma of the head and neck (SCCHN) is a fatal disease that has a median survival time of 6 to 8 months.1,2 In a pooled analysis of patients with advanced or recurrent SCCHN enrolled on previous Southwest Oncology Group (SWOG), phase II, single-agent trials conducted in the 1990s and in the early 21st century, the median progression-free survival (PFS) was 3 months and the median overall survival (OS) was 7 months.3–5 Phase II trials of combinations of chemotherapy that contained a platinum during the same time period demonstrated a median PFS of 4 months and a median OS of 8 months.6,7 The disease responds poorly to chemotherapy. No accepted standard and effective therapy exists for these patients, and promising new regimens need to be evaluated.
Epidermal growth factor receptor (EGFR) is frequently overexpressed in head and neck cancers.8 A major downstream signaling route of the ErbB family is via the Ras-Raf-MAP-kinase pathway.9 Activation of Raf kinase, via activation of Ras, is thought to play an important role in carcinogenesis, and in vitro evidence suggests that the level of K-ras expression is a determinant of proliferation of SCCHN cell lines.10,11–13 SCCHN tumors also overexpress vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR-2), and VEGFR-3, which have been associated with a poor prognosis.14–17
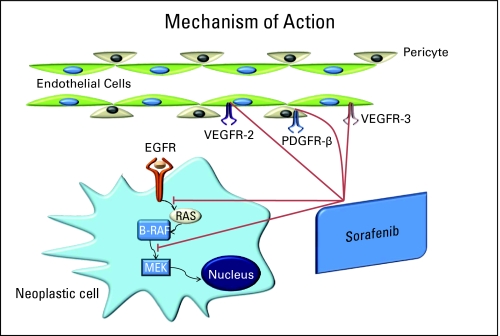
Sorafenib (NSC 724772, BAY 43-9006, Nexavar; Onyx Pharmaceuticals Inc, Everyville, CA; Bayer Healthcare Pharmaceuticals Inc, Wayne, NJ) is an inhibitor of wild-type and mutant B-Raf and c-Raf kinase isoforms in vitro.18 Sorafenib also inhibits in vitro several receptor tyrosine kinases that are involved in tumor progression, human VEGFR-2, murine VEGFR-2, murine VEGFR-3, murine platelet-derived growth factor receptor (PDGFR) -β, Flt-3, c-KIT, and p38α (MAPK family). In cellular assays, sorafenib was an inhibitor of human and murine VEGFR-2, murine VEGFR-3, and murine PDGFR-β receptor phosphorylation18,19 (Fig 1).
Fig 1.
Method of action of sorafenib. EGFR, epidermal growth factor receptor; VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor. Data adapted.19
Sorafenib has broad antitumor activity demonstrated through in vivo tumor models. It has demonstrated activity in human tumor xenograft models with cell lines that depend on K-Ras activation as well as in models that contain K-ras mutations. It has also demonstrated activity against the human SKOV-3 ovarian tumor cell line that contains a wild-type Ras but that overexpresses both EGFR and human epidermal growth factor receptor 2, which signal through the Ras/Raf/Mek pathway.18,19
Sorafenib may be of therapeutic value not only in human tumors containing Ras gene mutations but also in tumors overexpressing growth factor receptors in the Ras/Raf/Mek pathway, (as does EGFR) and by inhibiting tumor angiogenesis or neovascularization through inhibition of VEGFR-2, VEGFR-3, and/or PDGFR-β. Therefore, even though Ras mutations are not common in head and neck cancer, sorafenib may be an effective downstream inhibitor of one pathway of the EGFR signaling pathway.20 In addition, inhibition of tumor angiogenesis may also be a useful strategy for treating SCCHN; on the basis of these hypotheses, we felt sorafenib should be evaluated in this disease.
Sorafenib has manageable toxicity and has been demonstrated to improve PFS in patients with advanced clear cell renal carcinoma and hepatocellular carcinoma as well as OS in patients with hepatocellular carcinoma.21–24 Sorafenib is approved for the treatment of both of these diseases. We report here the results of the Southwest Oncology Group phase II trial of sorafenib in patients with advanced SCCHN.
PATIENTS AND METHODS
Patient Selection
Patients with histologically proven SCCHN that was metastatic at diagnosis or that persisted, metastasized, or recurred after definitive therapy and that was not amenable to surgical resection were eligible. Newly diagnosed patients with nonmetastatic disease were not eligible. Patients must not have received prior chemotherapy for recurrent or newly diagnosed metastatic disease but could have received one induction or adjuvant chemotherapy, provided at least 6 months had elapsed since the last course of chemotherapy. Prior radiation and surgery must have been completed at least 28 days before registration. Patients were required to have a Zubrod performance status (PS) of 0 or 1 and measurable disease as defined per Response Evaluation Criteria in Solid Tumors (RECIST)25; no other concurrent antitumor therapy was allowed while on study. All patients had to have adequate renal function (ie, serum creatinine < two times the institutional upper limit of normal; adequate hematologic values (ie, granulocyte count > 1,500/mm3, platelet count > 100,000/mm3); and adequate hepatic function, (ie, bilirubin, alkaline phosphatase, and AST or ALT ≤ two times the institutional upper limit of normal). Patients were excluded if they had active infection; active or prior CNS metastases; a prior malignancy (excluding adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, adequately treated stage I or II cancer in complete remission, or any other cancer from which they were disease free for 5 years); uncontrolled hypertension; significant history of cardiac disease within 6 months of registration or cardiac ventricular arrhythmias requiring medication; evidence of bleeding diathesis or on therapeutic anticoagulation; unable to take oral medications without crushing, dissolving, or chewing; pregnant or nursing; or on drugs known to be potent inhibitors of the CYP3A4 enzyme. Women and men of reproductive potential must have agreed to use an effective contraceptive method. The study was approved by the institutional review boards at each participating institution. All patients provided informed consent.
Study Design
The primary objective of this trial was to evaluate the response probability. Secondary objectives were to evaluate the median PFS, median OS, and qualitative and quantitative toxicity of this treatment in this patient population.
Patients received continuous oral treatment with sorafenib 400 mg (consisting of two 200-mg tablets) twice daily. Patients continued on study therapy until one of the following occurred: disease progression, unacceptable toxicity, significant symptomatic deterioration in the patient's condition, treatment delay of greater than 4 weeks, or patient request. Dose modifications were based on interval adverse events. These included two or more episodes of grades 1 to 4 thrombocytopenia (< 100,000/μL) or grades 3 or 4 neutropenia; persistent (ie, > 2 weeks) symptomatic grade 2 (> 110 mmHg diastolic) or any grades 3 or 4 hypertension uncontrolled with antihypertensive medication; grades 2 to 4 rash or hand-foot skin reaction; and any other grades 3 to 4 adverse event felt related to study drug. There were three dose levels: the full dose level was 400 mg administered orally twice daily; the −1 dose level was 200 mg administered orally twice daily; and the −2 dose was 200 mg administered orally once daily. No more than two dose reductions were allowed; if sorafenib was not tolerated at the −2 dose level, the patient was removed from study. No dose escalations were allowed after a dose reduction. If the drug was held for toxicity, appropriate supportive measures were provided, and toxicity assessed at least twice a week.
Assessments
Tumor measurements by physical examination, x-ray, computed tomography, or magnetic resonance imaging scans were obtained within 28 days before starting treatment and were repeated every 8 weeks while on study. Response was evaluated according to RECIST.25 Blood pressure, toxicity notation, and complete blood cell count, serum chemistry, and liver function studies were monitored weekly during the first 4 weeks of therapy and were repeated every 4 weeks with a history and physical examination while on study therapy.
The National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0, was used for the classification of adverse events. Patients were removed from study if treatment was held for any reason for greater than 4 weeks or for greater than 2 weeks if held for uncontrolled hypertension despite antihypertensive medications.
Fresh or archival tumor tissue was requested at the time of enrollment on the study, and biopsies of tumor tissue at the time of progression were requested for subsequent biomarker analysis. We obtained baseline specimens from 25 patients, but we obtained only two paired specimens. The data from these correlative studies is pending and will be reported separately.
Statistics
The primary objective of this trial was to evaluate the response probability (ie, confirmed complete and partial response [PR]) in patients with advanced metastatic head and neck cancer who were treated with sorafenib. Secondary objectives included PFS, OS, and the qualitative and quantitative toxicity of this treatment.
A two-stage design was used for patient accrual. It was assumed that this agent would not be of additional interest if the true response probability was less than 5% and that a true response probability of 20% or greater would be of interest. Twenty patients were required to be entered initially. If zero responses were observed, the study would be permanently closed, and the agent would be concluded to be inactive. If one or more responses were observed in the first 20 patients, an additional 20 patients would be accrued. Five or more responses of 40 patients would be considered evidence that this agent would warrant additional study, provided other factors, such as adverse events and survival, were also favorable. This design had a significance level of 5% and a power of 92%.
OS and PFS estimates were calculated by using the method of Kaplan-Meier,26 and 95% CIs for the medians were constructed by using the method of Brookmeyer-Crowley.27 Exact binomial CIs were calculated for response outcomes.
RESULTS
Patient Characteristics
From October 1, 2004 to March 1, 2006, 44 patients were registered from 18 participating institutions. Two patients were ineligible, both because of no documentation of measurable disease. One eligible patient did not receive any treatment because of noncompliance and was not analyzable for any study end point. Patient characteristics are listed in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Total No. of patients enrolled | 44 | |
| Eligible | 42 | |
| Ineligible | 2 | |
| Evaluable | ||
| Not evaluable and no treatment given | 1 | |
| Evaluable for response | 41 | |
| Evaluable for toxicity | 41 | |
| Age, years | ||
| Median | 63.5 | |
| Minimum | 31.1 | |
| Maximum | 84.2 | |
| Sex | ||
| Male | 34 | 83 |
| Female | 7 | 17 |
| Ethnicity | ||
| White | 39 | 95 |
| Black | 2 | 5 |
| Performance status | ||
| 0 | 16 | 39 |
| 1 | 24 | 59 |
| Primary site | ||
| Lip/oral cavity | 9 | 22 |
| Nasopharynx | 1 | 2 |
| Oropharynx | 8 | 20 |
| Hypopharynx | 4 | 10 |
| Larynx | 15 | 37 |
| Other/unknown | 4 | 10 |
| Disease status | ||
| Newly diagnosed | 2 | 5 |
| Persistent | 3 | 7 |
| Recurrent/metastatic | 36 | 88 |
Toxicity
Forty-one eligible patients were assessed for adverse events. The drug was generally well tolerated. There was one grade 4 adverse event caused by an asymptomatic pulmonary embolus. An additional 14 patients experienced grade 3 toxicities. The most common grade 2 or worse adverse events were fatigue, anorexia, stomatitis/oral pain, abdominal pain, hand-foot skin reaction, nausea, weight loss, and hypertension. The most commonly noted adverse events are listed in Table 2.
Table 2.
Common or Serious Adverse Events
| Toxicity | No. of Patients by Toxicity Grade |
||
|---|---|---|---|
| 3 | 4 | 5 | |
| ALT/AST | 0 | 0 | 0 |
| Anemia | 1 | 0 | 0 |
| Alopecia | 0 | 0 | 0 |
| Anorexia | 2 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Dry skin | 0 | 0 | 0 |
| Decubitus | 1 | 0 | 0 |
| Dry mouth | 1 | 0 | 0 |
| Esophagitis | 1 | 0 | 0 |
| Fatigue/malaise/lethargy | 1 | 0 | 0 |
| GI pain in abdomen | 0 | 0 | 0 |
| Hand-foot | 3 | 0 | 0 |
| Hypertension | 0 | 0 | 0 |
| Hyponatremia | 1 | 0 | 0 |
| Hypoxia | 1 | 0 | 0 |
| Leukopenia | 1 | 0 | 0 |
| Stomatitis/oral cavity pain | 2 | 0 | 0 |
| Nausea | 2 | 0 | 0 |
| Somnolence | 1 | 0 | 0 |
| Neurosensory | 1 | 0 | 0 |
| Rash | 0 | 0 | 0 |
| Thrombosis/embolism | 0 | 0 | 0 |
| Thrombosis/CVA | 0 | 1 | 0 |
| Vomiting | 0 | 0 | 0 |
| Weight loss | 1 | 0 | 0 |
| No. of events as maximum grade | 14 | 1 | 0 |
NOTE. Two patients did not experience any adverse events.
Abbreviation: CVA, cerebrovascular accident.
In addition, one of the ineligible patients experienced grade 4 cerebral ischemia (which was assessed as possibly caused by sorafenib, as this patient was taking sorafenib at the time of the adverse event). This patient was registered to the trial and started treatment before the determination of ineligibility. The primary objective of this trial was to evaluate the response rate. Two patients were excluded from the statistical analysis, because the documentation submitted did not support evidence of measurable disease as defined by RECIST. However, both were treated and observed per protocol, as eligibility was determined by a central review process that did not occur in real time. Although this patient was excluded from the analysis, we feel the event warrants mention in this report.
Response to Treatment
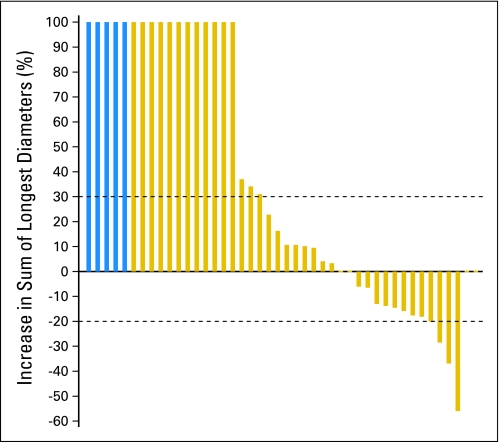
Forty-one eligible patients have been evaluated for response; five patients could not have an exact response determined because of inadequate assessment and were counted as nonresponders. There has been one confirmed PR (2%; 95% CI, 0% to 13%) and two unconfirmed PRs (7%; 95% CI, 2% to 20%). The estimated median PFS is 4 months (95% CI, 2 to 4 months), and the estimated median OS is 9 months (95% CI, 7 to 14 months). Figure 2 represents a waterfall plot of the maximum decrease in the sum of the longest diameters of target lesions observed while on treatment. Twenty-one patients had either stable disease or PR as their best response, for an estimated disease control rate of 51% (95% CI, 35% to 67%).
Fig 2.
Waterfall plot of the maximum decrease in the sum of the longest diameters of target lesions observed. Patients who experienced progression as a result of new lesions and/or clear worsening of nonmeasurable lesions are represented as a 100% increase. Patients whose exact responses could not be determined because of inadequate follow-up assessments are represented with blue bars on the far left of the figure.
To evaluate these results within the context of previous SWOG trials in this patient population with similar eligibility criteria, we pooled the data from three recent, single-agent, phase II trials and from two multi-agent, phase II trials to compare the PFS and OS rates. In the three previous phase II, single-agent trials, the median PFS was 3 months, and the median OS was 7 months. In addition, in SWOG phase II trials utilizing combinations of a platinum or taxane, the median PFS was 4 months, and the median OS was 8 months.
DISCUSSION
We have demonstrated that sorafenib is reasonably well tolerated in patients with advanced or metastatic SCCHN, as it has a toxicity profile similar to that observed in other trials with this agent. The adverse events primarily consist of hand-foot skin reaction, manageable hypertension, stomatitis, fatigue, rash, and anorexia. We did observe two serious thrombotic episodes. One was an asymptomatic pulmonary embolus identified on a dynamic computed tomography scan utilized for evaluation of the patient's response. The other was a cerebral thrombosis. This patient population is at increased risk for these types of events, and it is not clear that these were directly related to sorafenib, although agents that inhibit VEGF are known to increase the risk for venous and arterial thrombosis. Larger trials will be needed to more clearly discern the relationship of thromboembolism to this agent.
We demonstrated a confirmed PR rate of 2%, an unconfirmed PR rate of 7%, a median PFS of 4 months, and a median OS of 9 months. We also demonstrated a disease control rate of 51%. Unfortunately, we did not meet our predefined primary end point of a 20% confirmed response rate as indicative for additional study of the drug. However, it is now generally accepted that potential benefit from this agent and from other targeted agents that have a cytostatic rather than cytotoxic mechanism of action may not be ideally correlated by response rates. Sorafenib has been demonstrated to provide a clinically and statistically significant PFS and OS benefit in patients with renal and hepatocellular carcinomas without producing significant response rates (10% and 2%, respectively).23,24 As such, our choice of a defined response rate as the primary end point for this trial may not have been the best method for evaluating the potential effectiveness of this agent in this disease.
The disease control rate in this trial is encouraging, as are the PFS and OS rates (ie, secondary end points), when compared with the results obtained in previous SWOG trials. The PFS and OS rates demonstrated in this phase II trial appear comparable to those obtained in similarly designed SWOG phase II trials with agents with well-defined activity in SCCHN—platinums and taxanes. These results also appear superior to those obtained in similarly designed single-agent trials subsequently deemed to have minimal or no activity in SCCHN. The comparator SWOG trials had similar patient populations in terms of the following eligibility criteria: All received no prior systemic treatment for advanced metastatic head and neck cancer, had PS 0 to 1, and had most of the same labs. At the time, there may have been a selection bias, in which investigators put patients on the multidrug regimens if they felt those patients were better able to withstand the additional toxicity. However, this selection bias is difficult to measure. Given the limited sample size, this was an exploratory comparison and was not intended to make any definitive conclusions.
There are no trials of other targeted agents given as monotherapy in a population similar to ours. An open-labeled, multicentered, phase II trial of cetuximab was performed in 103 patients treated with one prior platinum-based regimen. In the single-agent phase of the trial, the response rate was 13%, disease control rate was 46%, and median time to progression was 70 days.28 These results were the basis for US Food and Drug Administration approval of cetuximab for second-line therapy in advanced SCCHN.
Elser et al29 evaluated sorafenib in 27 patients with SCCHN; however, this was a slightly different patient population from ours. They allowed patients to enroll if they had up to one prior systemic therapy for recurrent or metastatic disease and if they had a PS of 2 or less. One patient (3.7%) achieved a PR, and disease stabilization was maintained in 10 patients (37%). The median time to progression was 1.8 months, and the median OS was 4.2 months. They performed biomarker analysis on paired tumor samples from five patients before and after treatment with sorafenib. This analysis revealed decrease of pERK in all five patients and a decrease in Ki67 in four patients, consistent with a disruption of ERK signaling. The antiapoptotic protein Mcl-1 was downregulated in four patients, and there was also some—but not as convincing—evidence of antiangiogenic activity. Even though Elser et al29 were able to demonstrate biologic activity in these patients, no significant clinical benefit could be documented.
Taken together, our trial and the study by Elser et al29 demonstrate a modest level of activity of sorafenib as a single agent in SCCHN. The trial by Elser et al29 additionally demonstrates, in a preliminary fashion, a biologic effect of the drug with evidence of disruption of the EGFR-Ras-Raf-MEK-ERK signaling pathway, a pro-apoptotic effect, and, less convincingly, an effect on angiogenesis pathways. These are preliminary findings in only five patients and would need to be evaluated in a larger population of patients. Development of this agent in SCCHN will be dependent on additional correlative studies to define potential pretreatment biologic markers that may predict for response to sorafenib. Other strategies could include evaluation with irradiation or other targeted agents. Plastaras et al30 reported on the cell cycle and antitumor effects of a combination of sorafenib and radiation and establish in vivo that the optimal schedule would be sequential administration after irradiation rather than concurrent administration, as sorafenib slows cell cycle progression and prevents irradiated cells from reaching and accumulating at Gap2 (G2) to mitosis (M). Thus, an evaluation of sorafenib after radiation or chemoradiotherapy would be interesting. Sorafenib has shown preclinical synergy with other biologic antitumor agents, such as inhibitors of mammalian target of rapamycin,31 protein kinase Cδ,32 and the proteasome.33 Such combinations may be worthwhile for exploration if additional preclinical data were supportive. Interestingly, sorafenib has demonstrated antagonism with classic antineoplastic drugs utilized in the treatment of SCCHN, including paclitaxel, fluorouracil (FU), and platinum-containing drugs, which suggests that combination with any of these agents may not be a rational therapeutic strategy.34,35
In conclusion, additional evaluation of sorafenib as a single agent in SCCHN is not warranted. However, on the basis of the disease stabilization rate observed in this trial as well as its tolerability, additional study with other agents or modalities should be explored. We await the completion of the biomarker analysis on the tissue specimens obtained from patients enrolled on this trial, which may guide development of this agent in SCCHN.
Footnotes
Supported in part by the following Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA12644, CA45807, CA58658, CA35119, CA20319, CA42777, CA76448, CA35090, CA52654, CA45808, CA35431, CA67575, CA35128, CA46441, CA16385, and CA11083, awarded by the National Cancer Institute, Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00096512.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Stephen K. Williamson, Bayer Pharmaceuticals
AUTHOR CONTRIBUTIONS
Conception and design: Stephen K. Williamson, James Moon, Chao H. Huang, Michael LeBlanc, Susan G. Urba
Administrative support: Gregory T. Wolf
Provision of study materials or patients: Stephen K. Williamson, Chao H. Huang, Perry P. Guaglianone
Collection and assembly of data: James Moon
Data analysis and interpretation: Stephen K. Williamson, James Moon, Chao H. Huang, Michael LeBlanc
Manuscript writing: Stephen K. Williamson, James Moon, Chao H. Huang, Michael LeBlanc, Gregory T. Wolf
Final approval of manuscript: Stephen K. Williamson, James Moon, Chao H. Huang, Perry P. Guaglianone, Michael LeBlanc, Gregory T. Wolf, Susan G. Urba
REFERENCES
- 1.Forastiere AA, Leong T, Rowinsky E, et al. Phase III comparison of high-dose paclitaxel + cisplatin + granulocyte colony-stimulating factor versus low-dose paclitaxel + cisplatin in advanced head and neck cancer: Eastern Cooperative Oncology Group Study E1393. J Clin Oncol. 2001;19:1088–1095. doi: 10.1200/JCO.2001.19.4.1088. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: A Southwest Oncology Group study. J Clin Oncol. 1992;10:1245–1251. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 3.Samlowski WE, Gundacker H, Kuebler JP, et al. Evaluation of gemcitabine in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: A Southwest Oncology Group phase II study. Invest New Drugs. 2001;19:311–315. doi: 10.1023/a:1010657609609. [DOI] [PubMed] [Google Scholar]
- 4.Samlowski WE, Lew D, Kuebler PJ, et al. Evaluation of Tomudex in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: A Southwest Oncology Group study. Invest New Drugs. 1998;16:271–274. doi: 10.1023/a:1006178808095. [DOI] [PubMed] [Google Scholar]
- 5.Smith RE, Lew D, Rodriguez GI, et al. Evaluation of topotecan in patients with recurrent for metastatic squamous cell carcinoma of the head and neck: A phase II Southwest Oncology Group study. Invest New Drugs. 1996;14:403–407. doi: 10.1007/BF00180818. [DOI] [PubMed] [Google Scholar]
- 6.Samlowski WE, Moon J, Kuebler JP, et al. Evaluation of the combination of docetaxel/carboplatin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN): A Southwest Oncology Group Phase II study. Cancer Invest. 2007;25:182–188. doi: 10.1080/07357900701209061. [DOI] [PubMed] [Google Scholar]
- 7.Worden FP, Moon J, Samlowski W, et al. A phase II evaluation of a 3-hour infusion of paclitaxel, cisplatin, and 5-fluorouracil in patients with advanced or recurrent squamous cell carcinoma of the head and neck: Southwest Oncology Group study 0007. Cancer. 2006;107:319–327. doi: 10.1002/cncr.21994. [DOI] [PubMed] [Google Scholar]
- 8.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 9.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 10.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SL, Khosravi-Far R, Rossman KL, et al. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 12.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 13.Hoa M, Davis SL, Ames SJ, et al. Amplification of wild-type K-ras promotes growth of head and neck squamous cell carcinoma. Cancer Res. 2002;62:7154–7156. [PubMed] [Google Scholar]
- 14.Gold KA, Kim ES. Role of molecular markers and gene profiling in head and neck cancers. Curr Opin Oncol. 2009;21:206–211. doi: 10.1097/CCO.0b013e328329ac00. [DOI] [PubMed] [Google Scholar]
- 15.Neuchrist C, Erovic BM, Handisurya A, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003;25:464–474. doi: 10.1002/hed.10235. [DOI] [PubMed] [Google Scholar]
- 16.Neuchrist C, Erovic BM, Handisurya A, et al. Vascular endothelial growth factor receptor 2 (VEGFR2) expression in squamous cell carcinomas of the head and neck. Laryngoscope. 2001;111:1834–1841. doi: 10.1097/00005537-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005;86:347–363. doi: 10.1111/j.0959-9673.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayer Pharmaceuticals. Investigator's Brochure BAY 43-9006/Raf Kinase Inhibitor, Version No. 4.1. 2003 Aug 21; [Google Scholar]
- 19.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20(suppl 18):1S–13S. [PubMed] [Google Scholar]
- 21.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 22.Strumberg D, Voliotis D, Moeller JG, et al. Results of phase I pharmacokinetic and pharmacodynamic studies of the Raf kinase inhibitor BAY 43-9006 in patients with solid tumors. Int J Clin Pharmacol Ther. 2002;40:580–581. doi: 10.5414/cpp40580. [DOI] [PubMed] [Google Scholar]
- 23.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. NEJM. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. NEJM. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 28.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 29.Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 30.Plastaras JP, Kim SH, Liu YY, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–9454. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 31.Molhoek KR, Brautigan DL, Slingluff CL., Jr Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J Transl Med. 2005;3:39. doi: 10.1186/1479-5876-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jane EP, Premkumar DR, Pollack IF. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther. 2006;319:1070–1080. doi: 10.1124/jpet.106.108621. [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Friday BB, Lai JP, et al. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: Induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5:2378–2387. doi: 10.1158/1535-7163.MCT-06-0235. [DOI] [PubMed] [Google Scholar]
- 34.Heim M, Scharifi M, Zisowsky J, et al. The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anticancer Drugs. 2005;16:129–136. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Heim M, Sharifi M, Hilger RA, et al. Antitumor effect and potentiation or reduction in cytotoxic drug activity in human colon carcinoma cells by the Raf kinase inhibitor (RKI) BAY 43-9006. Int J Clin Pharmacol Ther. 2003;41:616–617. doi: 10.5414/cpp41616. [DOI] [PubMed] [Google Scholar]