Abstract
Microsatellite instability (MSI) is a clonal change in the number of repeated DNA nucleotide units in microsatellites. It arises in tumors with deficient mismatch repair due to the inactivation of one of the four mismatch repair genes: MSH2, MLH1, MSH6, and PMS2. In order to determine the MSI status of a tumor, microdissection and polymerase chain reaction–based detection strategies are required. For practical purposes, MSI is equivalent to the loss of staining by immunohistochemistry (IHC) of one of the mismatch repair genes since both signify an abnormality in mismatch repair. Of all colorectal cancers (CRCs), 15% to 20% display MSI or abnormal IHC (often referred to as microsatellite instability [MIN] pathway). The remaining 80% to 85% of CRCs are microsatellite stable but most are characterized by chromosomal instability (CIN pathway). Almost all Lynch syndrome tumors have MSI or abnormal IHC and they account for up to one third of all MIN CRCs (3% to 5% of all CRCs). The remaining MIN tumors are sporadic as a result of somatic inactivation of the MLH1 gene caused by methylation of its promoter. Thus, the presence of a MSI/IHC abnormality prompts further investigations to diagnose Lynch syndrome, whereas its absence excludes Lynch syndrome. We recommend screening all CRC tumors for IHC or MSI. MIN tumors have a more favorable outcome than CIN tumors, and fluorouracil-based adjuvant chemotherapy does not improve the outcome of stage II or stage III MIN tumors. More data are needed to determine how best to treat patients with stage II and stage III MIN CRCs.
DEFINITION OF MICROSATELLITES: OCCURRENCE
Microsatellites are stretches of DNA sequence where a single nucleotide (mononucleotides) or units of two or more nucleotides (eg, di-, tri-, tetra-, or pentanucleotides) are repeated in the genome. Currently, there is no general consensus on the minimum number of repeated nucleotide units necessary to define a microsatellite, the maximum number of repeated nucleotide units allowed to still be considered a microsatellite, or the minimum number of repeated nucleotide units necessary to define a minisatellite. Repeats with units as few as three and as many as several hundred have been classified as microsatellites. Examples of typical microsatellites are presented in Table 1.
Table 1.
Examples of Microsatellites
| Repeat Type | Sequence | Name and Location |
|---|---|---|
| Mononucleotide | AGGTAAAAAAAAAAAAAAAAAAAAAAAAAAGGGT | BAT26, Intron 5 of MSH2 gene |
| (A)26 shown | Chromosome 2 | |
| Dinucleotide | TGTACACACACACACATCGA | D5S346 |
| (CA)6 shown | Chromosome 5 | |
| Tetranucleotide | ATATTCTATCTATCTATCTATCTATCTG | D14S608 |
| (TCTA)5 shown | Intergenic region chromosome 14 |
There are at least 500,000 microsatellites in the human genome. They can occur in intergenic regions as well as in genes. They are commonly located in the introns of genes but there are numerous examples of microsatellites in promoters, untranslated terminal regions, and in the coding exons themselves. When exonic microsatellites are translated into proteins, this can occur with or without repeated amino acids, depending on the particular reading frame situation. Examples of coding microsatellites in the cancer literature include a GCG trinucleotide repeat in exon 1 of the TGFBR1 gene that encodes multiple alanines in the signal peptide, a mononucleotide (A)10 repeat in TGFBR2, and a (G)8 repeat in BAX.1–3 In many microsatellites the monotonous repetition of the unit is occasionally broken (eg, …CACACACAACACACACACACA).
MONOMORPHIC VERSUS POLYMORPHIC MICROSATELLITES
A monomorphic microsatellite is one in which all individuals share the same number of repeat units. The BAT26 marker (Table 1) is comprised of 26 adenines in more than 99% of ethnic Europeans, whereas alleles with different numbers of adenines at this location (eg, 15, 20, 22, 23) are seen in up to 25% of ethnic Africans, including African Americans.4 As a result, BAT26 has been called quasimonomorphic. It might be more accurately described as monomorphic in Europeans and polymorphic in Africans.
A polymorphic microsatellite is one in which more than 1% of the population display heterozygosity for the number of repeat units. Another often used definition is that the minor allele frequency exceeds 0.01 or 1%. In fact, many microsatellites include alleles with repeat sizes that occur with heterozygosity frequencies of 50% or higher. These are highly informative markers for mapping, allelic association, and loss of heterozygosity studies.
DESCRIPTION OF MICROSATELLITE INSTABILITY
The number of repeats contained in any one particular microsatellite is, in principle, the same in every cell of the body. Microsatellite instability (MSI) is said to occur when some cells display one or two alleles with different numbers of repeats. For this to be detectable (ie, present in enough cells to be identified by the common detection methods), the aberrant cells must be clonal. Hence, MSI is typically seen in tumors, which are almost always monoclonal or oligoclonal.
As an example, if a typical (CA)n dinucleotide microsatellite is polymorphic in the population, it might display a total of five different alleles, for example, (CA)22, (CA)20, (CA)17, (CA)16, and (CA)9. Any given individual will have two of these alleles. If homozygous, an individual might have two copies of the (CA)20 allele; if heterozygous, the individual might have one copy of (CA)20 and one of (CA)9.
If MSI is present, the (CA)20 homozygous individual's tumor might display (CA)20 and (CA)24. If both alleles were changed, the tumor might show (CA)24, (CA)20, and (CA)11. The remaining (CA)20 allele would most likely emanate from noncancer tissue contained in the sample, which occurs almost always. Likewise, the tumor of a heterozygous individual could display three or four different alleles with different sized repeats.
It is important to recognize that defective mismatch repair (MMR) does not by any means affect all microsatellites in the genome, but in a given tumor often affects only a proportion of them. Moreover, a low frequency of MSI can be seen in tumors without MMR deficiency.5,6 Such occasional MSI can be tentatively viewed as background noise and disregarded.
METHODS TO DETECT MSI
By far the most common method to detect MSI is to measure the length of a polymerase chain reaction amplicon containing the entire microsatellite. This requires DNA, a pair of primers of which one is fluorescently end labeled, a sequencer, and suitable software. Alternatively, if the amplicon is sequenced, one can simply count the number of repeat units. Examples of MSI analyses in colorectal cancer (CRC) are shown in Figures 1 and 2.
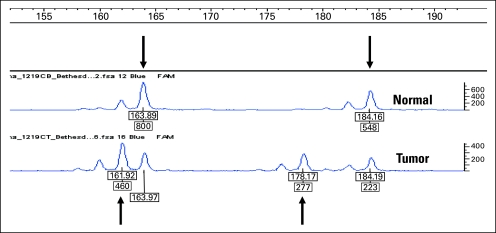
Fig 1.
Tracings of polymerase chain reaction (PCR) amplicons containing microsatellite D2S123, a highly polymorphic (CA)n dinucleotide. One PCR primer was end labeled with fluorescent dye, the amplicon was run on a sequencer, and it was analyzed by the Genotyper software. Lengths of the amplicons containing the microsatellite and the strength of the signal are indicated. The upper tracing is from blood DNA displaying heterozygosity, with one allele measuring 164 bp and the other allele measuring 184 bp in length (arrows). The lower tracing is from the same patient's colorectal tumor showing two new alleles, which measure 162 bp and 178 bp in length (arrows). This means that both of the germline alleles were mutated in the tumor. The persistence of the germline alleles most likely emanates from noncancerous tissue present in the tumor specimen (eg, tumor-infiltrating lymphocytes, stroma, blood vessels).
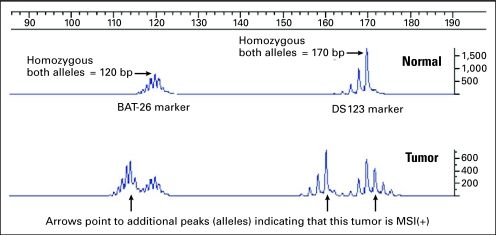
Fig 2.
Microsatellite instability (MSI) determination of two microsatellites in the same, multiplexed run. The upper tracing is from blood DNA showing (A)n mononucleotide marker BAT26 at 120 bp and (CA)n dinucleotide marker D2S123 at 170 bp. The patient is homozygous for both markers. The lower tracing is from the same patient's tumor DNA. Arrows point to the new alleles in BAT26 at 114 bp and in D2S123 at 172 and 160 bp. The fact that D2S123 displays two novel alleles suggests that the tumor consists of at least two different clonal expansions. Note the “stutter” bands that occur both in the dinucleotide marker and, more strongly, in the mononucleotide marker. These are believed to be produced during the polymerase chain reaction and do not occur in vivo.
DEFINITION OF MSI: NUMBER AND CHOICE OF MARKERS
Because defective MMR does not affect all microsatellites in a given tumor, it is important to study more than one microsatellite and to study microsatellites that are frequently affected by instability. In the early days of MSI study (from 1993 onward), researchers began to choose markers based on their own empirical experience. This led to considerable variation in the number and choice of markers. A conference was held in Bethesda, MD, to discuss the issues and make suggestions to promote consistency across studies. This resulted in a recommendation that has stood the test of time.7 A panel consisting of three dinucleotide repeats (D2S123, D5S346, D17S250) and two mononucleotide repeats (BAT26, BAT25) was proposed as a standard test for MSI. Moreover, it was proposed that MSI would be called if 40% or more of the markers tested were unstable. When using the five-marker panel, this means that MSI is called when at least two of them are positive; however, often four or all five are positive in tumors with MSI.
While most tumors show either a high degree of instability or no unstable markers, a minority of tumors display instability in fewer than 20% of the markers studied (or just one unstable marker in the case of the five-marker Bethesda panel). Although the biologic significance of this observation was not clear at the time, a classification of MSI low (MSI-L) was suggested, as presented in Table 2.
Table 2.
Proposed Classification of MSI7
| Description | Proportion of Unstable Markers | Positive Markers in the Bethesda Panel |
|---|---|---|
| MSI high | ≥ 40% | ≥ 2/5 |
| MSI low | < 40%; at least one | 1/5 |
| Microsatellite stable | 0% | 0/5 |
NOTE. After extensive debate the Bethesda Workshop did not precisely define the distinction between MSI low and microsatellite stable, except in cases where the recommended five markers are used.
Abbreviation: MSI, microsatellite instability.
The publication of these Bethesda guidelines brought about considerable standardization of procedures all over the world, many centers applying these guidelines as such. The guidelines also suggested that researchers might wish to use other panels of markers and other definitions. Numerous reports have been published about the possible superiority and ease of studying (eg, quasimonomorphic markers that in principle obviate the need to study unaffected tissue or panels containing tetra- or pentanucleotides that may be more sensitive to MMR than mono- and dinucleotide repeats).8–12 There is evidence that the sensitivity of MSI can be improved by including three or more mononucleotide markers; from 80% to 91% for tumors from patients with MLH1 mutations, from 84% to 87% for tumors from patients with MSH2 mutations, and from 55% to 77% for tumors from patients with MSH6 mutations.13 It is important to note that MSI is less strong in MSH6 mutation carriers possibly because mutations in this gene seem more likely to affect mononucleotide markers than dinucleotide markers.14 The inclusion of additional mononucleotide markers improves the detection rate for these tumors, but it is important to remember that these patients may occasionally present as microsatellite stable (MSS) or MSI-L. We are aware of the fact that some commercial laboratories routinely apply other markers, more markers, and fewer markers, but with the exception of data favoring the inclusion of three or more mononucleotide markers, we are not aware of systematic studies unequivocally favoring such panels.15 Remarkably, it appears that the five-marker Bethesda panel, perhaps with slight modifications, remains a verifiable and reproducible means of testing for MSI.7
CLINICAL SIGNIFICANCE OF MSI-L
Around 15% of unselected CRC tumors are MSI high (MSI-H). The proportion of MSI-L CRC tumors is lower, often 3% to 10%.16–19 While almost all MSI-H tumors are MMR deficient, most or all MSI-L tumors have no MMR defect.20,21 Only few studies have directly addressed the question of whether MSI-L ever signals MMR deficiency. This can best be done by testing random tumors for MSI followed by molecular analysis of the four MMR genes in both MSI-H and MSI-L tumors in search of mutations or promoter methylation. In one such study of 1,566 unselected patients with CRC, 199 tumors (12.7%) were found to be MSI-H and 107 tumors (6.8%) were found to be MSI-L.19,22 By mutational analysis of MSH2, MLH1, MSH6, and PMS2, 44 cases had deleterious germline mutations; all but one were in the MSI-H group. In several smaller studies, Lynch syndrome has been excluded or considered unlikely in MSI-L cases.21 By mathematical modeling it was suggested that most low-level MSI in CRC occurs without requiring an elevated slippage rate during neoplastic development.23 The very existence of a MSI-L group can be questioned based on the existence of a background mutation frequency.6 Hypothesizing that if many markers are studied, many if not most CRCs would show MSI in occasional markers, Laiho et al24 studied 90 CRC samples with 377 microsatellite markers. All cases were non–MSI-H based on stability at BAT26. As expected, as many as 71 (79%) of the cases displayed MSI at 1 to 11 loci. The authors concluded that there were no convincing differences between MSI-L and MSS.
A considerable number of researchers have used the Bethesda criteria to delineate MSI-L and asked whether MSI-L patients show distinguishing clinical or molecular features. This is complicated by the uncertain distinction between MSI-L and MSS.25 In a study of 183 patients with stage C CRC, the 51 MSI-L patients had significantly poorer outcome compared with both the 42 MSI-H and the 90 MSS cases.26 In a study of 657 CRCs from Korea, 30 MSI-L cases more often had poorly differentiated histology, mucinous appearance, and large tumor size in comparison with 574 MSS cases. In addition, MSI-L (n = 30) was accompanied by less frequent lymph node metastases and less advanced tumor stage than MSS.27 In contrast, Wright et al28 also studying stage C CRCs (n = 255) suggested that the MSI-L and MSS cases had comparable clinicopathologic features.
We conclude from these studies that the apparently contradictory results may be best explained by the lack of a conclusive cutoff between MSI-L and MSS described above. Convincing molecular or physiological differences do not appear to have been found between MSI-L and MSS tumors. Thus, after more than 15 years of research, the question about a clinically relevant distinguishable MSI-L phenotype is unanswered. It tentatively follows that if a difference exists, it is minor. For simplicity we shall hereafter refer to MSI-H simply as MSI.
CLINICAL SIGNIFICANCE OF MSI
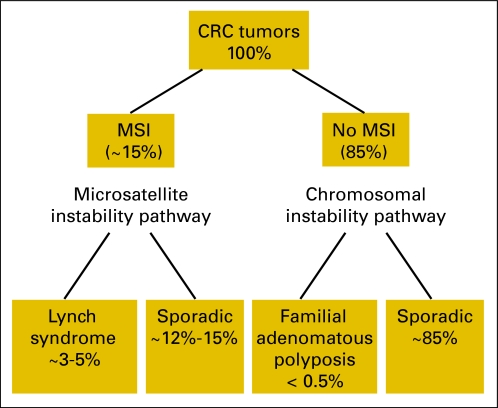
MSI occurs in around 15% of all CRC tumors in white populations.16–19 It arises as a result of defective MMR caused by the failure of one of the four main MMR genes, MSH2, MLH1, MSH6, or PMS2. On the cellular level, the mechanism is recessive.29 There are two different types of MMR gene failure: caused by an inherited germline mutation in one allele followed by somatic inactivation of the wild-type allele in a colonic mucosa cell (these individuals have Lynch syndrome and account for 3% to 5% of all CRCs), or failure caused by somatic inactivation of both alleles. These account for 10% to 15% of all CRCs. We shall discuss separately the diagnostic and prognostic implications of MSI (Fig 3).
Fig 3.
Schematic classification of colorectal cancers (CRCs). MSI, microsatellite instability.
Diagnostic: Lynch Syndrome
Lynch syndrome is the most common inherited cause of CRC. Making the diagnosis of Lynch syndrome in a patient with CRC is important to his future management since he will have a high risk for developing second primary cancers. It is also important to his family members since many of them will have also inherited Lynch syndrome and the attendant cancer risks. It has been shown that cancers and deaths can be prevented in individuals with Lynch syndrome through the appropriate management. More than 80% of tumors from patients with Lynch syndrome display MSI. This conclusion is based on the systematic study of tumors from patients belonging to families diagnosed with Lynch syndrome based on mutational analysis.13,30 There are two possible explanations for the absence of MSI in a small number of tumors from individuals with Lynch syndrome. The most common cause of this finding is that the MSI determination is false negative resulting from either an inadequate number of microsatellite markers or an inadequate proportion of tumor cells in the sample (Table 3). A less common cause of this finding is that the tumor is a phenocopy, that is a spontaneous (sporadic) tumor in an individual with Lynch syndrome. In practice, unless there is a strong family history of Lynch syndrome–associated cancer, patients whose tumors are MSS based on the five-marker Bethesda panel or another panel of high quality, do not need further evaluation for a possible diagnosis of Lynch syndrome. In patients with CRC, if their tumors are found to be MSS this excludes both Lynch syndrome and somatic inactivation of a MMR gene. In those 10% to 20% of Lynch syndrome tumors that are MSS, IHC of the MMR proteins sometimes shows the absence of one or two of the proteins, and the cause of this apparent discrepancy usually is not known.19,31 In a study of 500 cases where both MSI and IHC of all four proteins was done, there were 64 patients with MSI and 71 patients with abnormal IHC (including 56 with MSI). One case showing a discrepancy between MSI and IHC had Lynch syndrome (the tumor was MSI but IHC was normal).19
Table 3.
Sample Preparation for MSI Study and Sources of Uninformative Results
| For MSI to be readily detectable the proportion of tumor cells in the sample used to extract DNA must be sufficient; estimates for the minimum proportion vary greatly between laboratories (10% to 50%); as can be seen in Figures 1 and 2 even in clean tracings of the relevant amplicons, “stutter bands” occur that may make interpretation difficult |
| To accomplish as high a proportion of tumor cells as possible, most laboratories practice some form of “microdissection”; the pathologist examines a section of the paraffin block and marks the area with most tumor cells with a pen directly on the block; the tissue is collected from this area by “coring,” that is digging out a piece of tissue with the tip of a needle; alternatively, sections are cut, and the tissue outside the marked area is scraped off and discarded |
| One common reason for false negative MSI results is the low tumor cellularity found in mucinous tumors (which is a typical feature in CRCs with deficient mismatch repair) making the study vulnerable to false-negative results |
| If there are reasons to question a MSI result the best way of resolving the issue is to repeat the determination using cells from another tissue block or another microdissection of the original block or consider IHC for the four mismatch repair proteins; in cases where more than one family member might be affected, samples from as many patients as possible should be studied |
Abbreviations: MSI, microsatellite instability; CRC, colorectal cancer; IHC, immunohistochemistry.
Increasingly, IHC is replacing MSI as a screening method for deficient MMR. The sensitivity of MSI was discussed above. IHC has been found to have a sensitivity of 83% for patients with mutations in MSH2, MLH1, or MSH6.13 Specificity is 90.2% for MSI and 88.8% for IHC.13 While both tests have similar sensitivities and specificities, IHC is more convenient and cheaper. IHC only requires equipment and expertise that are available in most pathology departments. In addition, IHC has the major advantage of pinpointing directly the MMR gene that is likely to be mutated. As outlined in detail by Boland et al,32 the MMR proteins function in heterodimer pairs with MSH2 associating with MSH6 and some other MMR proteins while MLH1 associates with PMS2 and some other MMR proteins. MSH6 can only form a heterodimer with MSH2 and PMS2 can only form a heterodimer with MLH1. As a result, when MSH2 is inactivated IHC usually shows absence of both MSH2 and MSH6 protein. In contrast, when MSH6 is lost, IHC staining for MSH2 remains positive. Likewise, IHC loss of MLH1 and PMS2 signals mutational or epigenetic inactivation of MLH1, while IHC loss of PMS2 alone signals a mutation in PMS2 (Table 4).
Table 4.
Loss of Immunohistochemistry Staining in Relation to Affected Mismatch Repair Gene
| Immunohistochemical Loss of Staining | Affected Gene |
|||
|---|---|---|---|---|
| MSH2 | MSH6 | MLH1 | PMS2 | |
| MSH2 and MSH6 | + | Occasional | ||
| MSH6 | + | |||
| MLH1 and PMS2 | + | Rare | ||
| PMS2 | + | |||
Diagnostic: Sporadic CRC
As indicated, 60% to 80% of MMR-deficient tumors are caused by somatic events affecting both alleles, and therefore not inherited. The overwhelming majority of these are due to hypermethylation of the MLH1 promoter.33,34 The methylation-specific PCR method (MSP) designed by Herman et al35 is a relatively simple way of testing for methylation. When a patient's tumor does not stain for MLH1 by IHC or is found to have MSI, it is important to distinguish between the hereditary form (Lynch syndrome) and the sporadic form (methylation of the promoter). To exclude Lynch syndrome without resorting to mutational analysis, testing for mutations in the BRAF gene is advocated by some. The common somatic V600E mutation in BRAF is present in 40% to 60% of MSI positive tumors and in 69% of tumors with absence of MLH1 on IHC but virtually never in Lynch syndrome.13,36 Finding the mutation allows Lynch syndrome to be excluded; however, a negative result has no predictive value. Another distinguishing test should be to directly assess MLH1 promoter methylation (eg, by MSP). While a positive MSP test is highly suggestive of a sporadic tumor, it is only approximately 80% specific so it cannot completely rule out a diagnosis of Lynch syndrome since some MLH1 methylation has been reported in up to 46% of Lynch syndrome tumors.37 Ultimately, by mutation analysis of the MLH1 gene the presence or absence of germline mutations signaling Lynch syndrome can be determined.
MSI in Colorectal Adenomas
Large numbers of colonic adenomas are endoscopically removed during routine surveillance. Provided MMR repair deficiency is an early event in CRC tumorigenesis, the screening of adenomas for deficient MMR should be a useful strategy to detect Lynch syndrome. In an early study of 402 adenomas from 378 randomly ascertained patients, six patients had at least one MSI positive adenoma. Five of the six patients had germline Lynch syndrome mutations.38 This study suggested that MSI testing of adenomas might indeed be a useful way of diagnosing Lynch syndrome as had been suggested by others.39
Several smaller studies using various strategies and finding little or no MSI in adenomas have been published.40,41 Various arguments against using MSI to screen adenomas for Lynch syndrome have been advanced, mainly based on the finding that some adenomas in patients diagnosed with Lynch syndrome are MSI negative.42 Nevertheless, positive findings have recently been reported.43,44,45 MSI testing is more accurate in larger adenomas, with high-grade dysplasia, particularly tubulovillous adenomas.38 In summary, a reasonable proportion (50% or more) of adenomas from patients with Lynch syndrome show MSI and IHC evidence of MMR deficiency is highly suggestive of Lynch syndrome. There is little doubt that systematic analyses of all adenomas would lead to the detection of otherwise undetected Lynch syndrome. Whether comprehensive screening of this type would be deemed cost-effective should be evaluated in larger prospective trials. Obviously it will not be possible to study very small adenomas at all because of lack of material.
MSI AND CRC PROGNOSIS
The literature regarding the prognosis of patients with MSI CRC was reviewed in 2005 and the data was pooled.46 There were 32 eligible studies including a total of 7,642 CRC cases; 16.7% (1,277) of these tumors were MSI. Notably, in all but one of these studies, MSI-L tumors were grouped with the MSS tumors for the analysis. The overall survival hazard ratio (HR) associated with MSI was 0.65 (95% CI, 0.59 to 0.71) with no evidence for heterogeneity or publication bias. Patients with MSI-H CRCs had a better prognosis even when the data were restricted to clinical trial patients and those with locally advanced CRC. This study also evaluated progression-free survival (PFS) in nine data sets from eight of the published studies. The pooled HR for PFS was 0.67 (95% CI, 0.53 to 0.83), however, there was evidence of heterogeneity and the data set was too small for subset analysis.
Some recent studies have not found a better prognosis for patients with MSI CRCs.47,48 It has been noted that the differences in prognosis may be difficult to detect in more recent patient cohorts given the increased benefit from chemotherapy in the MSS group of patients.47 In addition, the MSI group of patients has a bimodal age distribution; those with MSI due to MLH1 promoter hypermethylation are older and may have other comorbidities versus those with MSI due to germline MMR gene mutations who are younger but less numerous and this may affect the survival data.49
Conversely, a meta-analysis of the association between CRC with chromosome instability (CIN) and prognosis was recently performed.50 This analysis of 63 studies included outcome data from 10,126 patients of whom 60% had CIN + CRC tumors. The overall HR for survival associated with CIN was 1.45 (95% CI, 1.35 to 1.55; P < .001). In patients with stage II and stage III CRCs, the HR was also 1.45 (95% CI, 1.27 to 1.65; P < .001). A similar effect was seen for progression-free survival (HR, 1.71; 95% CI, 1.51 to 1.94; P < .001). It is not clear whether the positive prognosis associated with MSI+ tumors is independent of the negative prognosis related to CIN+ tumors. In addition, the prognosis associated with CIN-/MSS CRCs or CpG island methylator phenotype CRCs is not yet known.
MSI AND CRC TREATMENT
While it has been relatively well-established that the prognosis is better for patients with MSI-H CRC, whether or not MSI status predicts response to adjuvant chemotherapy has been more controversial. Some of the controversy stems from the fact that early studies showing a better prognosis for patients with MSI CRCs did not include a control group of nontreated patients for comparison.51,52,53 Without untreated controls, it was not clear if the survival benefit was the result of the chemotherapy or the result of the inherent better survival for patients with MSI CRCs. On a molecular level, there is in vitro data supporting the fact that patients would need an intact MMR system to induce apoptosis of fluorouracil (FU) -modified DNA.54,55,56,57
The first major study indicating that patients with stage II or stage III MSI CRCs did not benefit from FU-based adjuvant therapy while patients with stage II or stage III MSS or MSI-L CRC did was Ribic et al58 in 2003. This study indicated that the overall survival for patients with MSI tumors who received FU was worse than for those who did not (HR, 2.17; 95% CI, 0.84 to 5.55; P = .10).58 In addition, when given FU, the prognosis for patients with MSI CRCs became indistinguishable from the patients with MSS or MSI-L CRCs (HR, 1.07; 95% CI, 0.62 to 1.86; P = .80).58 In the 2005 pooled data analysis by Popat et al,46 the authors also attempted to determine whether or not MSI status predicted benefit from adjuvant FU-based chemotherapy. Only two of the studies analyzed provided data that allowed for the benefit of FU-based chemotherapy to be assessed by MSI status. These studies confirmed a benefit in overall survival for patients with MSS CRCs who received FU adjuvant chemotherapy (HR, 0.72; 95% CI, 0.57 to 0.91; P = .007). However, the 184 patients with MSI CRCs did not appear to benefit from FU-based chemotherapy (HR, 1.24; 95% CI, 0.72 to 2.14) when compared with patients with MSI CRCs who did not receive chemotherapy. The numbers were small and not statistically significant at the time. Since then, several studies have been published including two prospective cohorts and a new meta-analysis of the data was performed in 2009.59
This most recent meta-analysis identified seven eligible studies which included 3,690 patients.59 Of these patients, 14% (454) were MSI-H, 810 were stage II, 2,444 were stage III, and 1,444 received FU-based chemotherapy. The MSI-H patients did not have a significant difference in recurrence-free survival whether or not they received chemotherapy (HR, 0.96; 95% CI, 0.62 to 1.49; P = .86). The MSI-H patients also did not have a signification difference in overall survival whether or not they were treated with adjuvant chemotherapy (HR, 0.70; 95% CI, 0.44 to 1.09; P = .12). No significant heterogeneity was found between the studies. There was not enough data to perform a subset analysis for the patients with stage II and stage III CRC separately.
There was a significant interaction on survival between MSI status and chemotherapy status. This means that MSI-H CRC patients do not benefit from FU-based adjuvant chemotherapy while MSS CRC patients do benefit from FU-based chemotherapy. The HR for recurrence-free survival among the patients with MSS CRC was 0.77 (95% CI, 0.68to 0.87; P < .001).
It appears that MSI status is a marker of nonresponse to FU-based chemotherapy in CRC patients. Currently, all patients with stage III CRC and some patients with high-risk stage II CRC receive FU-based chemotherapy. Based on these data, it seems clear that patients with stage II MSI CRC should not receive FU-based chemotherapy. Given that most patients with stage II CRC already do not receive chemotherapy, this is a reasonable approach clinically. It also appears that patients with stage III MSI CRC should not receive FU-based chemotherapy, however, many clinicians may feel uncomfortable withholding treatment for these patients in the absence of any alternative therapy options so this approach has not been widely adopted at present. Finally, given the expense of genetic testing, most studies have not assessed whether or not the prognostic or treatment response findings differ among patients with MSI CRC tumors due to Lynch syndrome versus those due to acquired hypermethylation of the MLH1 promoter.
SIGNIFICANCE
Given the implications of MSI for the prognosis, treatment, and diagnosis of Lynch syndrome, it has been recommended by us19,23 and others that all patients with newly diagnosed CRC should be screened for MSI either directly through MSI testing or indirectly through IHC of the four MMR proteins. This could have a significant public health impact in the reduction of CRC diagnoses and deaths due to CRC over time as at risk individuals are identified and receive appropriate surveillance.
Footnotes
Supported in part by Grants No. CA67941 and CA16058 from the National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Albert de la Chapelle, Heather Hampel
Administrative support: Albert de la Chapelle, Heather Hampel
Collection and assembly of data: Albert de la Chapelle, Heather Hampel
Data analysis and interpretation: Albert de la Chapelle, Heather Hampel
Manuscript writing: Albert de la Chapelle, Heather Hampel
Final approval of manuscript: Albert de la Chapelle, Heather Hampel
REFERENCES
- 1.Pasche B, Luo Y, Rao PH, et al. Type I transforming growth factor ß receptor maps to 9q22 and exhibits a polymorphism and a rare variant within a polyalanine tract. Cancer Res. 1998;58:2727–2732. [PubMed] [Google Scholar]
- 2.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-ß receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 3.Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 4.Pyatt R, Chadwick RB, Johnson CK, et al. Polymorphic variation at the BAT-25 and BAT-26 loci in individuals of African origin: Implications for microsatellite instability testing. Am J Pathol. 1999;155:349–353. doi: 10.1016/S0002-9440(10)65131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hienonen T, Sammalkorpi H, Enholm S, et al. Mutations in two short noncoding mononucleotide repeats in most microsatellite-unstable colorectal cancers. Cancer Res. 2005;65:4607–4612. doi: 10.1158/0008-5472.CAN-05-0165. [DOI] [PubMed] [Google Scholar]
- 6.Sammalkorpi H, Alhopuro P, Lehtonen R, et al. Background mutation frequency in microsatellite-unstable colorectal cancer. Cancer Res. 2007;67:5691–5698. doi: 10.1158/0008-5472.CAN-06-4314. [DOI] [PubMed] [Google Scholar]
- 7.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 8.Hoang J-M, Cottu PH, Thuille B, et al. BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res. 1997;57:300–303. [PubMed] [Google Scholar]
- 9.Zhou X-P, Hoang J-M, Cottu P, et al. Allelic profiles of mononucleotide repeat microsatellites in control individuals and in colorectal tumors with and without replication errors. Oncogene. 1997;15:1713–1718. doi: 10.1038/sj.onc.1201337. [DOI] [PubMed] [Google Scholar]
- 10.Suraweera N, Duval A, Reperant M, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123:1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 11.Mead LJ, Jenkins MA, Young J, et al. Microsatellite instability markers for identifying early-onset colorectal cancers caused by germ-line mutations in DNA mismatch repair genes. Clin Cancer Res. 2007;13:2865–2869. doi: 10.1158/1078-0432.CCR-06-2174. [DOI] [PubMed] [Google Scholar]
- 12.Ahrendt SA, Decker PA, Doffek K, et al. Microsatellite instability at selected tetranucleotide repeats is associated with p53 mutations in non-small cell lung cancer. Cancer Res. 2000;60:2488–2491. [PubMed] [Google Scholar]
- 13.Palomaki GE, McClain MR, Melillo S, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genetics in Medicine. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plaschke J, Engel C, Kruger S, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: The German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol. 2004;22:4486–4494. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KM, Zhang S, Geiger T, et al. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006;8:305–311. doi: 10.2353/jmoldx.2006.050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;38:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 17.Salovaara R, Loukola A, Kristo P, et al. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000;18:2193–2200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]
- 18.Samowitz WS, Curtin K, Lin HH, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–838. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 19.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among colorectal cancer patients. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaltonen LA, Peltomäki P, Mecklin J-P, et al. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994;54:1645–1648. [PubMed] [Google Scholar]
- 21.Mueller J, Gazzoli I, Bandipalliam P, et al. Comprehensive molecular analysis of mismatch repair gene defects in suspected Lynch syndrome (hereditary nonpolyposis colorectal cancer) cases. Cancer Res. 2009;69:7053–7061. doi: 10.1158/0008-5472.CAN-09-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 23.Graham T, Halford S, Page KM, et al. Most low-level microsatellite instability in colorectal cancers can be explained without an elevated slippage rate. J Pathol. 2008;215:204–210. doi: 10.1002/path.2351. [DOI] [PubMed] [Google Scholar]
- 24.Laiho P, Launonen V, Lahermo P, et al. Low-level microsatellite instability in mot colorectal carcinomas. Cancer Res. 2002;62:1166–1170. [PubMed] [Google Scholar]
- 25.Halford S, Sasieni P, Rowan A, et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002;62:53–57. [PubMed] [Google Scholar]
- 26.Kohonen-Corish MR, Daniel JJ, Chan C, et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. J Clin Oncol. 2005;23:2318–2324. doi: 10.1200/JCO.2005.00.109. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH, Min BH, Choi HK, et al. Sporadic colorectal carcinomas with low-level microsatellite instability in Korea: Do they form a distinct subgroup with distinguished clinicopathological features? J Surg Oncol. 2009;99:351–355. doi: 10.1002/jso.21239. [DOI] [PubMed] [Google Scholar]
- 28.Wright CM, Dent OF, Newland RC, et al. Low level microsatellite instability may be associated with reduced cancer specific survival in sporadic stage C colorectal carcinoma. Gut. 2005;54:103–108. doi: 10.1136/gut.2003.034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons R, Li G-M, Longley MJ, et al. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 30.Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: History, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–1994. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 32.Boland CR, Koi M, Chang DK, et al. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: From bench to bedside. Fam Cancer. 2008;7:41–52. doi: 10.1007/s10689-007-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. et al: 1997. [PubMed] [Google Scholar]
- 34.Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. 1998;58:3455–3460. [PubMed] [Google Scholar]
- 35.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bessa X, Balleste B, Andreu M, et al. A prospective, multicenter, population-based study of BRAF mutational analysis for Lynch syndrome screening. Clin Gastrenterol Hepatol. 2008;6:206–214. doi: 10.1016/j.cgh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Kuismanen SA, Holmberg MT, Salovaara R, et al. Genetic and epigenetic modification accounts for a major share of microsatellite-unstable colorectal cancers. Am J Pathol. 2000;156:1773–1779. doi: 10.1016/S0002-9440(10)65048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loukola A, Salovaara R, Kristo P, et al. Microsatllite instability in adenomas as a marker for hereditary nonpolyposis colorectal cancer. Am J Pathol. 1999;155:1849–1853. doi: 10.1016/S0002-9440(10)65503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe T, Kanazawa T, Kazama Y, et al. Microsatellite instability in adenoma as a possible marker to identify HNPCC patients. Am J Gastorenterol. 2006;101:204. doi: 10.1111/j.1572-0241.2006.00393_6.x. [DOI] [PubMed] [Google Scholar]
- 40.Velayos FS, Allen BA, Conrad PG, et al. Low rate of microsatellite instability in young patients with adenomas: Reassessing the Bethesda guidelines. Am J Gastroenterol. 2005;100:1143–1149. doi: 10.1111/j.1572-0241.2005.40862.x. [DOI] [PubMed] [Google Scholar]
- 41.Koh DC, Luchtefeld MA, Kim DG, et al. Microsatellite instability and MLH1 hypermethylation – incidence and significance n colorectal polyps in young patients. Colorectal Disease. 2007;9:521–526. doi: 10.1111/j.1463-1318.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 42.Müller A, Beckmann C, Westphal G, et al. Prevalence of the mismatch-repair-deficient phenotype in colonic adenomas arising in HNPCC patients: Results of a 5-year follow-up study. Int J Colorectal Dis. 2006;21:632–641. doi: 10.1007/s00384-005-0073-6. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira S, Claro I, Lage P, et al. Colorectal adenomas in young patients: Microsatellite instability is not a useful marker to detect new cases of Lynch syndrome. Dis Colon Rectum. 2008;51:909–915. doi: 10.1007/s10350-008-9224-5. [DOI] [PubMed] [Google Scholar]
- 44.Pino MS, Mino-Kenudson M, Wildemore BM, et al. Deficient DNA mismatch repair is common in Lynch syndrome-associated colorectal adenomas. J Mol Diagn. 2009;11:238–247. doi: 10.2353/jmoldx.2009.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira AM, Westers H, Sousa S, et al. Mononucleotide precedes dinucleotide repeat instability during colorectal tumor development in Lynch syndrome patients. J Pathol. 2009;219:96–102. doi: 10.1002/path.2573. [DOI] [PubMed] [Google Scholar]
- 46.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clinl Oncol. 2005;23:609–617. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 47.Jover R, Zapater P, Castells A, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer. 2009;45:365–373. doi: 10.1016/j.ejca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Kim GP, Colangelo LH, Wieand S, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2007;25:767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 49.Boland CR. Clinical uses of microsatellite instability testing in colorectal cancer: An ongoing challenge. J Clin Oncol. 2007;25:754–756. doi: 10.1200/JCO.2006.09.4607. [DOI] [PubMed] [Google Scholar]
- 50.Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: A meta-analysis. Gut. 2008;57:941–950. doi: 10.1136/gut.2007.135004. [DOI] [PubMed] [Google Scholar]
- 51.Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 52.Hemminki A, Mecklin JP, Järvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 53.Elsaleh H, Powell B, Soontrapornchai P, et al. p53 gene mutation, microsatellite instability and adjuvant chemotherapy: Impact on survival of 388 patients with Dukes' C colon carcinoma. Oncology. 2000;58:52–59. doi: 10.1159/000012079. [DOI] [PubMed] [Google Scholar]
- 54.Tajima A, Hess MT, Cabrera BL, et al. The mismatch repair complex hMutS alpha recognizes 5-fluroruracil-modified DNA: Implications for chemosensitivity and resistance. Gastroenterology. 2004;127:1678–1684. doi: 10.1053/j.gastro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Arnold CM, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 56.Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouricil. Gastroenterology. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyers M, Wagner MW, Hwang HS, et al. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–5201. [PubMed] [Google Scholar]
- 58.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from Fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Des Guetz G, Schischmanoff O, Nocalas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Euro J Cancer. 2009;45:1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]