Abstract
Purpose
We tested the hypothesis that Akt-Ser473 phosphorylation (pAkt) predicts benefit from the sequential addition of paclitaxel to adjuvant doxorubicin plus cyclophosphamide (AC) chemotherapy in patients with node-positive breast cancer participating in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trial.
Patients and Methods
Primary tumors from the NSABP B-28 trial tissue microarray were available from 1,581 of 3,060 patients who were randomly assigned to receive either four cycles of AC alone or followed by four cycles of paclitaxel. Immunohistochemistry and quantitative analysis of pAkt were performed at the National Cancer Institute blinded to clinical outcome. Association between pAkt and clinical outcome was assessed using multivariate Cox modeling adjusting for age, tumor size, number of positive nodes, tumor grade, estrogen receptor status, and human epidermal growth factor receptor 2 status.
Results
With a median follow-up of 9.1 years, there were no differences in disease-free survival (adjusted hazard ratio [HR], 1.02; P = .81) or overall survival (HR, 0.97; P = .80) with and without receiving paclitaxel among 975 patients with pAkt-negative tumors. In 606 patients with pAkt-positive tumors, the sequential addition of paclitaxel resulted in a 26% improvement in disease-free survival (HR, 0.74; P = .02) or a 20% improvement in overall survival (HR, 0.80; P = .17).
Conclusion
pAkt significantly predicts disease-free benefit from the sequential addition of paclitaxel to AC chemotherapy in patients with node-positive breast cancer. Patients with pAkt-negative breast tumors do not appear to benefit from the addition of paclitaxel.
INTRODUCTION
Adjuvant chemotherapy significantly improves disease-free survival (DFS) and overall survival (OS) in early-stage breast cancer.1 Anthracycline-containing compared with nonanthracycline-containing regimens further reduce recurrence and mortality rates.2 Over the past decades, taxanes (paclitaxel and docetaxel) have emerged as effective chemotherapy agents for breast cancer and other malignancies.3,4 Incorporation of taxanes into the adjuvant breast cancer setting has resulted in significant improvement in DFS and OS.2 The B-28 randomized clinical trial from the National Surgical Adjuvant Breast and Bowel Project (NSABP) evaluated whether the sequential addition of paclitaxel after doxorubicin plus cyclophosphamide (AC) compared with AC alone improved outcomes for patients with axillary node-positive breast cancer. The trial results demonstrated that the addition of paclitaxel significantly improved DFS but not OS.5
Akt is a serine/threonine protein kinase that has been implicated in the pathogenesis of cancer as well as essential cellular processes including metabolism, cell growth, proliferation, cell cycle progression, and survival.6 Recent preclinical studies report that Akt-Ser473 is phosphorylated by SIN1-rictor-mTOR (TORC2) complex, which is required for cellular functions such as survival7 and actin cytoskeletal reorganization.8,9 Akt via GSKbeta is implicated in the regulation of microtubule dynamics and organization.10 By directly phosphorylating and inactivating WEE1, Akt causes the activation of cdc2 and promotes the cell cycle progression at the G2-M transition, which may render cells more susceptible to mitotic inhibitors such as paclitaxel.11,12 Furthermore, inhibition of Akt phosphorylation by PI3K/Akt inhibitor enhances apoptosis induced by chemotherapy agents including paclitaxel.13 This combination approach produced greater apoptotic effect in cancer cells with higher levels than those with lower levels of active Akt. Importantly, paclitaxel and some other chemotherapy agents inactivate Akt, thus causing or enhancing apoptosis which leads to the reduced survival of cancer cells.14–17
Currently, there are no reliable biomarkers predictive of therapeutic benefit in patients who receive taxane-based adjuvant chemotherapy. A recent meta-analysis of adjuvant therapy trials found a significant DFS improvement from taxanes irrespective of hormone receptor status or human epidermal growth factor receptor 2 (HER2) status.2,18 Since not all patients benefit from taxanes and they are associated with significant toxicities such as neuropathy, it is critically important to identify biomarkers that reliably predict benefit specific to this class of drugs.
The role of Akt phosphorylation at Ser-473 (pAkt) on the outcome of patients with breast cancer who receive taxane-based chemotherapy has not been examined in clinical settings including adjuvant chemotherapy. Therefore, we designed and conducted this study that correlates pAkt status with clinical outcome in patients from the NSABP B-28 trial. We tested the hypothesis that pAkt predicts benefit from the sequential addition of paclitaxel to adjuvant AC chemotherapy in women with node-positive breast cancer.
PATIENTS AND METHODS
Patients
NSABP B-28 was an adjuvant chemotherapy trial in patients with early-stage breast cancer conducted from August 1995 to May 1998.5 In brief, 3,060 women with resected, node-positive breast cancer were randomly assigned to either four cycles of adjuvant AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) or to the same chemotherapy regimen followed by four additional cycles of paclitaxel (225 mg/m2). Eligible patients signed an approved informed consent which included tissue collection and research use of collected tissue conforming to federal and institutional guidelines. The NSABP B-28 clinical trial is registered at PDQ, number NSABP-B-28. The biomarker protocol for the evaluation of Akt phosphorylation in association with clinical outcome was approved by the institutional review boards of the National Cancer Institute (Bethesda, MD) and the NSABP.
Tissue Microarray Construction, and Determination of HER2 Status and Estrogen Receptor Status
Patient selection, assay performance, and data analysis are reported according to the Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria.19 Tissue microarray (TMA) were constructed from 1,982 cases that had the tumor blocks, which were representative of two treatment arms and tissue characteristics, and collected prospectively from patients who participated in NSABP B28 trial (Appendix Fig A1, online only). The B-28 microarray set contained 30 slides with 20 to 100 tissue cores per slide, which built a core per patient, and duplicate or triplicate cores per patient for 106 cases, which served as internal controls. HER2 status on the microarray sections was determined by the NSABP central pathology laboratory using PathVision fluorescent in situ hybridization (FISH) assay (Vysis, Downers Grove, IL) according to the US Food and Drug Administration–approved protocol per manufacturer. Data were expressed as the number of HER2 genes per chromosome 17 centromere; tumors with a ratio of ≥ 2 were classified as HER2 positive, and those with a ratio of less than 2 were defined as HER2 negative.20 Estrogen receptor (ER) status in TMA was also determined by the NSABP central pathology laboratory by immunohistochemistry according to the US Food and Drug Administration–approved protocol provided by Dako (PharmDx kit; Carpinteria, CA). A positive tumor cell nuclear staining for ER was defined as a total score of ≥ 3 (ranging from 0, 2 to 8). The total score is the sum of a proportion score from 0 to 5 and an intensity score of 0 to 3.21
pAkt Expression
pAkt status on formalin-fixed paraffin-embedded primary tumors from two complete sets of B-28 TMA was examined by immunohistochemistry using a standard avidin-biotin-peroxidase complex indirect immunoperoxidase procedure as previously described.22,23 In brief, epitope retrieval was performed in antigen retrieval buffer (pH10) and heated in a microwave oven for 15 minutes (Dako). This optimized antigen retrieval method for immunohistochemical detection of pAkt is shown in Appendix Fig A2 (online only).22,23 Sections were incubated with a rabbit polyclonal antibody specific to pAkt-Ser473 (Cell Signaling Technology, Beverly, MA) in a 1:100 dilution. Binding of the antibody to antigenic sites was amplified using Vectastain Elite avidin-biotin-peroxidase complex kits (Vector Laboratories, Burlingame, CA). Breast cancer cell line MDA-MB-468 and a breast cancer specimen that express pAkt were utilized as positive controls.24,25 Negative control was performed using isotype immunoglobulins appropriate to the primary antibody used (Zymed Laboratories, South San Francisco, CA). Similar staining results were obtained from the two sets of B-28 trial microarray and representative core by core comparison is shown in Appendix Fig A3 (online only). One representative set was analyzed in this study.
pAkt staining with cytoplasmic, membranous or nuclear signal were analyzed with the assistance of an Automated Cellular Imaging System (ACIS III, Dako) at the National Cancer Institute blinded to all clinical information.26 Missing cores and tissue cores with fewer than 5% of invasive tumor cells present were excluded for analysis (Fig A1). The intensity and percentage of stained tumor cells on each core was generated using a free-scoring tool by the digital imaging system. Staining index was determined by percentage (range, 0 to 94.1) multiplied by intensity of staining (range, 0 to 66.7) divided by 100 as previously described.23,27,28 The concordance for levels of pAkt on quality control cores in duplicates or triplicates was met for 75 (71%) of 106 cases; the cases with minor discordance were reviewed and reconciled between two investigators (S.Y., S.P.). Four cases, each in duplicate, could not be reconciled due to tumor heterogeneity and were excluded from the data analysis. The staining index of more than 2 (range, 0 to 62.8) was arbitrarily chosen as the cutoff for pAkt positivity.22,23,29,30 This cutoff included pAkt staining with a visual intensity of 1+, 2+, and 3+ as well as more than 6% of stained tumor cells. The correlation with clinical outcome was performed at the NSABP Biostatistical Center.
Statistical Analysis
Differences in the distribution of patient and tumor characteristics between the subset of patients with pAkt measurements and the total population were assessed using the χ2 test. DFS and OS curves by pAkt-positive group and pAkt-negative group treated with or without paclitaxel were estimated with the Kaplan-Meier method.31 The log-rank test was used to assess differences between treatment groups for outcomes.32 The events included in DFS were breast cancer recurrence, second primary cancer (excluding squamous or basal cell carcinoma of the skin, carcinoma in situ of the cervix, or lobular carcinoma in situ of the breast), and death from any cause without prior recurrence or second primary cancer. The end point included in OS was death from any cause. Multivariate Cox proportional hazards modeling was used to compute adjusted DFS and OS hazard ratios (HRs) with 95% CIs for pAkt and prognostic variables.33 The Wald statistic was used to determine P values for the adjusted HRs and the interaction terms. Kaplan-Meier curves that were adjusted for prognostic variables were determined using the method described by Xie and Liu.34 HRs and P values presented on the adjusted Kaplan-Meier curves were those obtained from multivariate Cox modeling.
RESULTS
Outcome, pAkt Status, and Patient Population With pAkt Measurement
The B-28 treatment trial results with approximate 5 years of follow-up have been reported previously.5 With a median follow-up of 9.1 years, the results were similar to those reported previously. The HRs comparing patients treated with paclitaxel to those not treated with paclitaxel were 0.89 (95% CI, 0.79 to 0.99; P = .034) for DFS and 0.92 (95% CI, 0.81 to 1.06; P = .25) for OS.
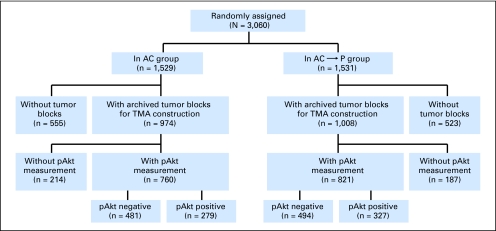
Primary breast tumors on B-28 tissue microarray were available for analyses from 1,581 patients, which represent 52% of B-28 trial population (Appendix Fig A1). Through a median follow-up of 9.1 years, the HRs for DFS and OS in 1,581 patients with pAkt data were 0.94 (95% CI, 0.81 to 1.10; P = .46) and 0.96 (95% CI, 0.79 to 1.15; P = .63), respectively. Expression of pAkt was observed in 606 (606 [38%] of 1,581) breast tumors, of which 279 (279/760; 37%) were in the AC group, and 327 (327/821; 40%) in the AC followed by paclitaxel group (Appendix Fig A1 and Table A1, online only). Appendix Figures A4A and A4B show the distribution of 1,581 patients by pAkt levels, and representative staining of pAkt-negative and pAkt-positive breast tumors as well as their corresponding staining indices.
To confirm that 1,581 patients with pAkt measurement in this study were representative of the entire population by treatment group of the B-28 trial, we compared age, tumor size, the number of involved lymph nodes, tumor grade, and ER status in patients with pAkt measurement and all treated patients (Table 1). There were no significant detectable differences in demographic or prognostic features except for tumor size and tumor grade in the group treated with AC alone. Approximately 84% of patients with pAkt measurement had received tamoxifen treatment, a proportion similar to that of 85% for the entire B-28 population.
Table 1.
Comparison of Outcome, Age, and Tumor Characteristics Between All NSABP B-28 Patients and Those With pAkt Measurement
| Variable | AC |
AC-P |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (n = 1,529) |
Patients With pAkt Data (n = 760) |
P* | All Patients (n = 1,531) |
Patients With pAkt Data (n = 821) |
P* | |||||||||
| No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | |||
| Age, years | .80 | |||||||||||||
| < 50 | 761 | 49.8 | 398 | 52.4 | .15 | 788 | 51.5 | 419 | 51.0 | |||||
| ≥ 50 | 768 | 50.2 | 362 | 47.6 | 743 | 48.5 | 402 | 49.0 | ||||||
| Tumor size† | .07 | |||||||||||||
| ≤ 2 | 917 | 60.0 | 417 | 55.0 | .017 | 894 | 58.4 | 447 | 54.5 | |||||
| 2.1-4.0 | 494 | 32.4 | 274 | 36.1 | 498 | 32.6 | 292 | 35.6 | ||||||
| ≥ 4.1 | 116 | 7.6 | 67 | 8.8 | 138 | 9.0 | 81 | 9.9 | ||||||
| Positive lymph nodes | .87 | |||||||||||||
| 1-3 | 1,068 | 69.8 | 525 | 69.1 | .88 | 1,068 | 69.8 | 568 | 69.2 | |||||
| 4-9 | 400 | 26.2 | 203 | 26.7 | 395 | 25.8 | 218 | 26.5 | ||||||
| ≥ 10 | 61 | 4.0 | 32 | 4.2 | 68 | 4.4 | 35 | 4.3 | ||||||
| Tumor grade | .13 | |||||||||||||
| Good | 147 | 9.6 | 51 | 6.7 | .002 | 149 | 9.7 | 70 | 8.5 | |||||
| Intermediate | 603 | 39.4 | 290 | 38.2 | 573 | 37.4 | 308 | 37.5 | ||||||
| Poor | 695 | 45.5 | 388 | 51.0 | 729 | 47.6 | 412 | 50.2 | ||||||
| Unknown | 84 | 5.5 | 31 | 4.1 | 80 | 5.2 | 31 | 3.8 | ||||||
| ER status | .20 | |||||||||||||
| Negative | 516 | 33.7 | 275 | 36.2 | .16 | 525 | 34.3 | 299 | 36.4 | |||||
| Positive | 1,013 | 66.3 | 485 | 63.8 | 1,006 | 65.7 | 522 | 63.6 | ||||||
| 5-year survival | ||||||||||||||
| Disease free | 72 | 70-74 | 70 | 66 to 73 | 76 | 73 to 78 | 73 | 69 to 76 | ||||||
| Overall | 85 | 83-87 | 83 | 81 to 86 | 85 | 84 to 87 | 84 | 81 to 86 | ||||||
| 10-year survival | ||||||||||||||
| Disease free | 58 | 55-60 | 56 | 52 to 60 | 62 | 59 to 64 | 58 | 54 to 61 | ||||||
| Overall | 71 | 69-74 | 71 | 67 to 74 | 74 | 72 to 76 | 72 | 69 to 75 | ||||||
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; pAkt, Akt-Ser473 phosphorylation; AC, doxorubicin plus cyclophosphamide; AC-P, AC followed by paclitaxel; ER, estrogen receptor.
χ2 goodness of fit test using all B-28 patients as the expected distribution.
Tumor size was unknown for two patients in the AC group and one in the AC-P group.
Paclitaxel and pAkt Status
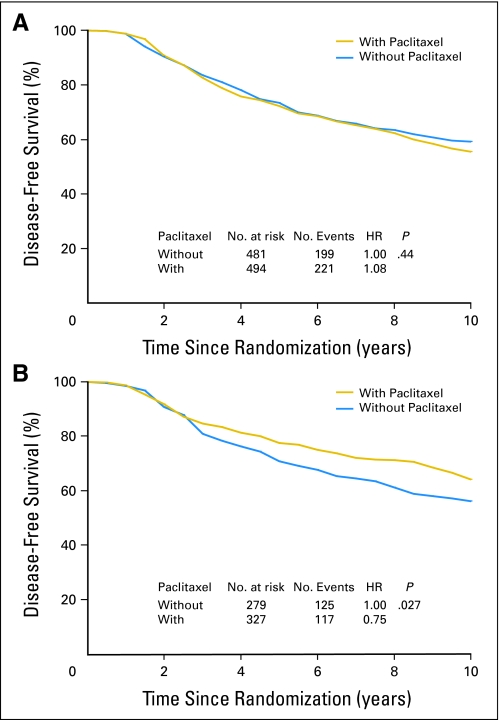
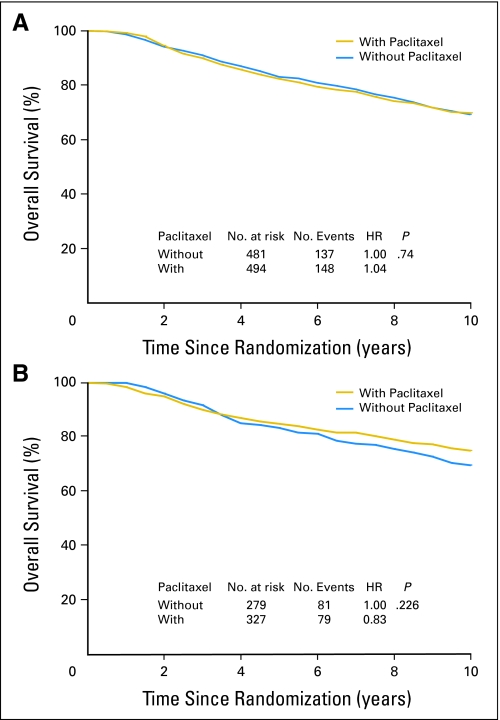
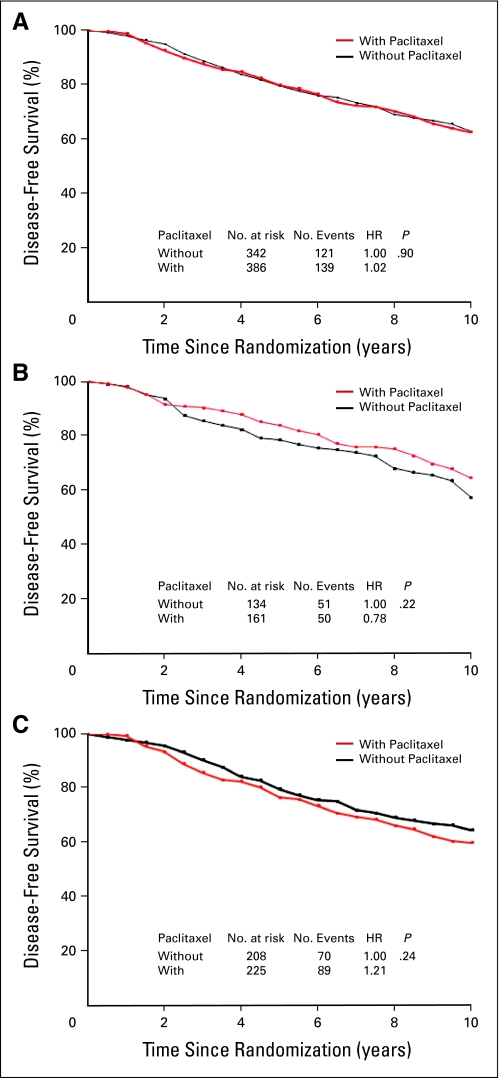
DFS and OS among patients who did or did not receive paclitaxel were analyzed according to pAkt status as established by immunohistochemistry (Appendix Fig A4). Among 975 patients with pAkt-negative tumors, DFS rate was similar in those treated with and without the addition of paclitaxel (HR, 1.08; P = .44; Fig 1A). However, in 606 patients with pAkt-positive tumors, the sequential addition of paclitaxel to AC significantly increased DFS as compared with AC alone (HR, 0.75; P = .027; Fig 1B). Moreover, there was no OS difference between treatment groups in patients with pAkt-negative tumors (HR, 1.04; P = .74; Appendix Fig A5A, online only), or statistically significant OS difference in patients with pAkt-positive cancer (HR, 0.83; P = .226; Fig A5B).
Fig 1.
Disease-free benefits in patients treated with or without paclitaxel according to Akt-Ser473 phosphorylation (pAkt) status. Disease-free survival according to (A) negative pAkt or (B) positive pAkt was analyzed with the use of the Kaplan-Meier method. HR, hazard ratio.
To evaluate the potential interaction between pAkt status and treatment for DFS, we tested the statistical significance of the interaction term in a proportional hazards model adjusted for age, tumor grade, tumor size, number of positive nodes, ER status, and HER2 status (Table 2). The formal test of the interaction between treatment and pAkt status reached borderline statistical significance (P = .056). However, there was no evidence of interaction between treatment effect and age, tumor size, positive lymph nodes, grade, ER status, or HER2 status. Furthermore, to investigate the influence of some imbalances in prognostic factors such as age, tumor grade, tumor size, number of positive nodes, ER status, and HER2 status on our results, we conducted a multivariate analysis using the Cox proportional hazards model with data stratified according to DFS. When comparing the adjusted DFS for patients treated with or without paclitaxel, the adjusted HRs were 1.02 (P = .81) for those with pAkt negative tumors and 0.74 (P = .02) for those with pAkt-positive tumors (Table 3). There was a 20% increase in OS by paclitaxel compared with no paclitaxel in patients with pAkt-positive tumors, but it did not reach statistical significance (Table 3). In addition, there were no significant differences for DFS and OS with or without the addition of paclitaxel in all patients with pAkt measurement (Table 3).
Table 2.
Results of Disease-Free Survival Analysis by Multivariate Cox Modeling in Patients With pAkt Measurement
| Variable | Disease-Free Survival |
|||
|---|---|---|---|---|
| HR | 95% CI | P | P for Treatment Interaction* | |
| pAkt group, treatment assignment | — | .056 | ||
| Negative, AC | 1.00 | |||
| Negative, AC followed by paclitaxel | 1.03 | 0.85 to 1.25 | ||
| Positive, AC | 1.16 | 0.92 to 1.45 | ||
| Positive, AC followed by paclitaxel | 0.88 | 0.70 to 1.10 | ||
| Age, years | .57 | .82 | ||
| < 50 | 1.00 | |||
| ≥ 50 | 0.96 | 0.82 to 1.12 | ||
| Tumor size† | .001 | .22 | ||
| ≤ 2 | 1.00 | |||
| 2.1-4.0 | 1.22 | 1.03 to 1.44 | ||
| ≥ 4.1 | 1.55 | 1.20 to 1.99 | ||
| Positive lymph nodes | < .0001 | .54 | ||
| 1-3 | 1.00 | |||
| 4-9 | 1.81 | 1.54 to 2.14 | ||
| ≥ 10 | 2.46 | 1.80 to 3.36 | ||
| Tumor grade | .04 | .35 | ||
| Good | 1.00 | |||
| Intermediate | 1.52 | 1.05 to 2.20 | ||
| Poor | 1.57 | 1.08 to 2.27 | ||
| Unknown | 1.04 | 0.60 to 1.82 | ||
| ER status | < .0001 | .42 | ||
| Negative | 1.00 | |||
| Positive | 0.69 | 0.58 to 0.81 | ||
| HER2 status | .07 | .75 | ||
| Negative | 1.00 | |||
| Positive | 1.27 | 1.03 to 1.55 | ||
| Unknown | 1.01 | 0.80 to 1.26 | ||
Abbreviations: AC, doxorubicin plus cyclophosphamide; HR, hazard ratio; pAkt, Akt-Ser473 phosphorylation; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
P for treatment-by-factor interaction.
Three patients with unknown tumor size excluded (two in the AC arm and one in the AC followed by paclitaxel group).
Table 3.
Adjusted HRs for Disease-Free Survival and Overall Survival by All Patients and by pAkt Status in This Study
| End Point | pAkt Positive Plus pAkt Negative (n = 1,581) |
pAkt Negative (n = 975) |
pAkt Positive (n = 606) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Disease-free survival | |||||||||
| AC | 1.00 | 1.00 | 1.00 | ||||||
| AC-P | 0.92 | 0.79 to 1.08 | .30 | 1.02 | 0.85 to 1.24 | .81 | 0.74 | 0.57 to 0.95 | .02 |
| Overall survival | |||||||||
| AC | 1.00 | 1.00 | 1.00 | ||||||
| AC-P | 0.94 | 0.78 to 1.13 | .51 | 0.97 | 0.77 to 1.23 | .80 | 0.80 | 0.59 to 1.10 | .17 |
NOTE. Adjusted for age, tumor size, number of positive nodes, tumor grade, estrogen receptor status, and human epidermal growth factor receptor 2 status.
Abbreviations: HR, hazard ratio; pAkt, Akt-Ser473 phosphorylation; AC, doxorubicin plus cyclophosphamide; AC-P, AC followed by paclitaxel.
Paclitaxel, and Expression of pAkt, ER, and HER2 Status
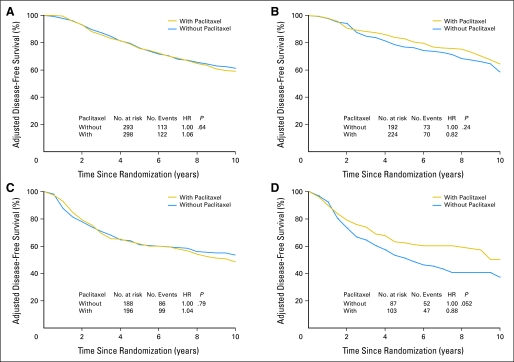
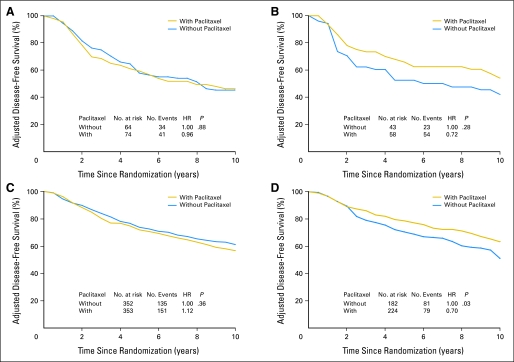
To explore the possible influence of ER status and HER2 status, as well as the effect of pAkt on paclitaxel benefit, we examined DFS in patients treated with or without paclitaxel according to pAkt status for patient subgroups stratified by ER status or HER2 status adjusting for age, tumor grade, tumor size, number of positive nodes, and either ER or HER2 status. Figure 2 provides the adjusted Kaplan-Meier plots for DFS in patients with pAkt-negative and pAkt-positive tumors stratified by ER status; Figure 3 shows the adjusted DFS curves in patients with pAkt-negative and pAkt-positive tumors stratified by HER2 status. In patients with pAkt-negative tumors, the effect of paclitaxel was not observed in any of the subgroups irrespective of ER status or HER2 status. The HRs were 1.06 for patients with ER-positive tumors (P = .64; Fig 2A), 1.04 for ER-negative tumors (P = .79; Fig 2C), 0.96 for HER2-positive tumors (P = .88; Fig 3A), and 1.12 for HER2-negative tumors (P = .36; Fig 3C). In contrast, for patients with pAkt-positive tumors, the HRs for the addition of paclitaxel versus no paclitaxel were 0.66 for those with ER-negative tumors (P = .052; Fig 2D) and 0.70 for those with HER2-negative tumors (P = .03; Fig 3D). The plots for patients with ER-positive tumors (HR, 0.82; P = .24; Fig 2B) and those with HER2-positive tumors (HR, 0.72; P = .28; Fig 3B) also showed a separation between treatment curves but the differences were not statistically significant. These results indicate that the interaction between paclitaxel treatment and pAkt status is still evident after stratification with ER status or HER2 status although the statistical power is reduced.
Fig 2.
Adjusted disease-free survival (DFS) among patients treated with or without paclitaxel according to estrogen receptor (ER) and Akt-Ser473 phosphorylation (pAkt) status. DFS was analyzed with the use of the Kaplan-Meier method by (A, C) negative pAkt or (B, D) positive pAkt, or by (A, B) positive ER or (C, D) negative ER expression.
Fig 3.
Adjusted disease-free survival (DFS) among patients treated with or without paclitaxel according to human epidermal growth factor receptor 2 (HER2) and Akt-Ser473 phosphorylation (pAkt) status. DFS was analyzed with the use of the Kaplan-Meier method by (A, C) negative pAkt or (B, D) positive pAkt, or by (A, B) positive HER2 or (C, D) negative HER2 expression.
DISCUSSION
Our findings indicate that pAkt independently predicts disease-free benefit from the sequential addition of paclitaxel to AC chemotherapy. The results from the entire B-28 population demonstrated that the sequential addition of paclitaxel significantly reduced DFS events by 11% while the population with pAkt measurement showed 6% reduction in DFS events. In contrast, the sequential addition of paclitaxel to AC significantly reduced DFS events by 25% in patients with pAkt-positive breast tumors.
We found an apparent interaction between pAkt and treatment with the addition of paclitaxel. The results are substantiated by preclinical findings that while Akt phosphorylation and its transducing downstream events play a central role in cell survival and cell cycle progression at the G2-M transition, paclitaxel inhibits Akt-Ser473 phosphorylation and induces mitotic arrest.15,16,35,36 Therefore, paclitaxel may have caused more damage to tumor cells that were dependent on pAkt for survival and cell cycle progression, significantly impacting outcome. Similar to the results of B28 reported previously, no interaction between treatment effect and any of the other prognostic factors was observed (Table 2).5
The use of archived tissue for pAkt detection has been described in small studies previously22,23,37; however, its clinical application has been hampered due to concerns of variation in specimen fixation and duration of fixation for phosphoproteins. Before this study, we optimized the immunohistochemistry procedure for pAkt detection. In addition, the use of digital imaging technology has strengthened the study results by increasing objectivity, reliability, and reproducibility.23,28 By these approaches, our results demonstrate the potential clinical utility of pAkt immunohistochemistry.
By primary and exploratory subset analyses, we observed that only patients with breast cancer with pAkt-positive tumors benefit from the addition of paclitaxel (Fig 1 and Appendix Fig A5); this appears true regardless of ER status or HER2 status (Figs 2 and 3). Of note, a significant improvement in DFS by paclitaxel versus no paclitaxel was achieved in the subgroup of patients with pAkt-positive and HER2-negative tumors, which represent one fourth of the study population (Fig 3D). It seemed that there was a paclitaxel benefit in the subset of patients with pAkt-positive and HER2-positive tumors as well but the patient number in this subgroup was small and only reached borderline statistical significance. Patients with both HER2-negative and HER2-positive tumors benefit from paclitaxel are in agreement with the results of Hayes et al and meta-analysis.38,39 When stratifying by ER status, there appeared to have a greater effect from paclitaxel in patients with pAkt-positive and ER-negative tumors than those with pAkt-positive and ER-positive tumors (Fig 2D v 2B). This is consistent with the findings of Berry et al40 on the analysis of ER status and chemotherapy outcome of three randomized trials conducted by Cancer and Leukemia Group B, and the US Breast Cancer Intergroup (9344, 8541, and 9741). In addition, the subgroup with HER2-negative and ER-positive tumors did not seem to benefit from paclitaxel (Appendix Fig A6A, online only). However, patients with pAkt-positive, HER2-negative, and ER-positive tumors, in contrast to those with pAkt-negative, HER2-negative, and ER-positive tumors, showed a trend for benefiting from paclitaxel (Appendix Figs A6B, A6C).
In the patient population with pAkt-positive breast tumors, the increase in OS by paclitaxel was 20% but it did not reach statistical significance (Table 3). This may be attributed to a reduced level of statistical power as the number of events in OS is fewer than those in DFS. A prolonged follow-up may hold answers for the impact of pAkt on OS from the addition of paclitaxel to AC chemotherapy.
Women with pAkt-negative tumors (62% in this study) did not appear to benefit from the addition of paclitaxel. Thus, the addition of paclitaxel to adjuvant chemotherapy regimen(s) could be spared for about two thirds of patients with node-positive breast cancer if our data are validated in Cancer and Leukemia Group B 9344 or E1199 trial or other prospective clinical trials. pAkt as a predictive biomarker will then likely contribute to an approach of individualized medicine.
Acknowledgment
We thank Wendy L. Rea from the NSABP for editorial assistance and Tito Fojo, MD, PhD, for his helpful discussion of this study.
Appendix
Fig A1.
Recommendations for Tumor Marker Prognostic Studies (REMARK) diagram on the distribution of patients with Akt-Ser473 phosphorylation (pAkt) measurement in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trial. AC, doxorubicin plus cyclophosphamide; AC-P, AC followed by paclitaxel; TMA, tissue microarray.
Fig A2.
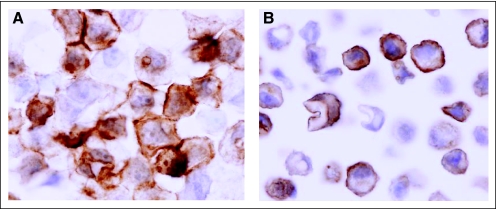
Akt-Ser473 phosphorylation (pAkt) immunohistochemistry in MDA-MB-468 breast cancer cells. Antigen retrieval was performed in high pH (pH 10) (A) antigen retrieval buffer versus (B) low pH (pH6) buffer. The optimized method showed good reproducibility with a 5.7% coefficient of variation (three independent staining in MDA-MB-468 cells); ×600.
Fig A3.
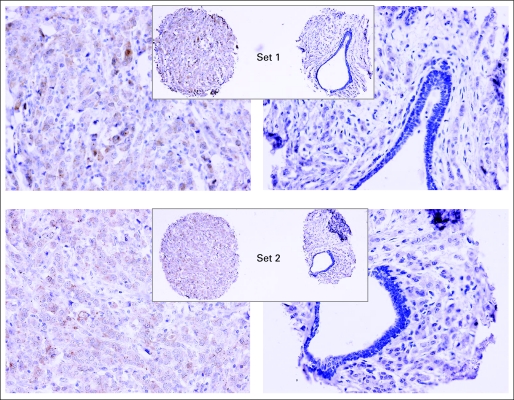
Representative Akt-Ser473 phosphorylation (pAkt) staining core by core comparison from the two sets of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 tissue microarray. The NSABP B-28 tissue microarray set 1 was used for data analysis and data presentation in this study. ×200; inset magnifications ×40.
Fig A4.
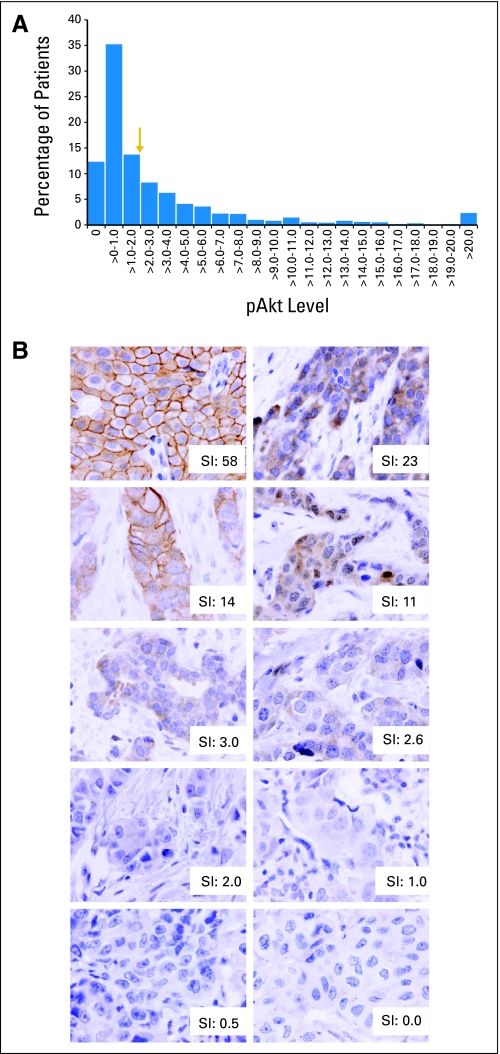
Distribution of 1,581 patients by (A) Akt-Ser473 phosphorylation (pAkt) levels and (B) pAkt staining in representative primary breast tumors from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 trial. The staining index (SI) for pAkt ranged from 0 to 62.8. The arrow (SI of > 2) indicates the positive cutoff, by which 38% of breast tumors are pAkt positive and 62% are pAkt negative.
Fig A5.
Overall survival (OS) among patients treated with or without paclitaxel according to Akt-Ser473 phosphorylation (pAkt) status. OS according to (A) negative pAkt or (B) positive pAkt was analyzed with the use of the Kaplan-Meier method. HR, hazard ratio.
Fig A6.
Disease-free survival (DFS) among patients treated with or without paclitaxel according to Akt-Ser473 phosphorylation (pAkt), human epidermal growth factor receptor 2 (HER2), or estrogen receptor (ER) status. DFS according to (A) negative HER2 and positive ER, (B) positive pAkt, negative HER2, and positive ER, or (C) negative pAkt, negative HER2 and positive ER was analyzed with the use of the Kaplan-Meier method. HR, hazard ratio.
Table A1.
Distribution of Patients With pAkt Measurement by Age and Tumor Characteristics in Each Treatment Group
| Variable | AC |
AC-P |
||||||
|---|---|---|---|---|---|---|---|---|
| pAkt Negative (n = 481) |
pAkt Positive (n = 279) |
pAkt Negative (n = 494) |
pAkt Positive (n = 327) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| < 50 | 253 | 52.6 | 145 | 52.0 | 255 | 51.6 | 164 | 50.2 |
| ≥ 50 | 228 | 47.4 | 134 | 48.0 | 239 | 48.4 | 163 | 49.8 |
| Tumor size* | ||||||||
| ≤ 2 | 262 | 54.5 | 155 | 55.8 | 256 | 51.8 | 191 | 58.6 |
| 2.1-4.0 | 173 | 36.0 | 101 | 36.3 | 184 | 37.3 | 108 | 33.1 |
| ≥ 4.1 | 45 | 9.3 | 22 | 7.9 | 54 | 10.9 | 27 | 8.3 |
| Unknown | 1 | < 0.1 | 1 | < 0.1 | 1 | < 0.1 | ||
| Positive lymph nodes | ||||||||
| 1-3 | 332 | 69.0 | 193 | 69.2 | 329 | 66.6 | 239 | 73.1 |
| 4-9 | 130 | 27.0 | 73 | 26.2 | 145 | 29.4 | 73 | 22.3 |
| ≥ 10 | 19 | 4.0 | 13 | 4.7 | 20 | 4.0 | 15 | 4.6 |
| Tumor grade | ||||||||
| Good | 29 | 6.0 | 22 | 7.9 | 35 | 7.1 | 35 | 10.7 |
| Intermediate | 178 | 37.0 | 112 | 40.2 | 166 | 33.6 | 142 | 43.4 |
| Poor | 259 | 53.9 | 129 | 46.2 | 275 | 55.7 | 137 | 41.9 |
| Unknown | 15 | 3.1 | 16 | 5.7 | 18 | 3.6 | 13 | 4.0 |
| ER status | ||||||||
| Negative | 188 | 39.1 | 87 | 31.2 | 196 | 39.7 | 103 | 31.5 |
| Positive | 293 | 60.9 | 192 | 68.8 | 298 | 60.3 | 224 | 68.5 |
| HER2 status | ||||||||
| Negative | 352 | 73.2 | 182 | 65.2 | 353 | 71.4 | 224 | 68.5 |
| Positive | 64 | 13.3 | 43 | 15.4 | 74 | 15.0 | 58 | 17.7 |
| Unknown | 65 | 13.5 | 54 | 19.4 | 67 | 13.6 | 45 | 13.8 |
Abbreviations: pAkt, Akt-Ser473 phosphorylation; AC, doxorubicin plus cyclophosphamide; AC-P, AC followed by paclitaxel; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Three patients with unknown tumor size excluded (two in AC arm and one in AC-P arm).
Footnotes
Supported in part by the Division of Cancer Treatment and Diagnosis and the Center for Cancer Research of the National Cancer Institute, National Institutes of Health (NIH); and by Public Health Service Grants No. U10-CA-12027, U10-CA-69651, U10-CA-37377, and U10-CA-69974 from the National Cancer Institute, NIH.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00896870.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Eleftherios P. Mamounas, Bristol-Myers Squibb (C), sanofi-aventis (C); Sandra M. Swain, Bristol-Myers Squibb (U), Abraxis BioScience, (U), Genentech (U), ImClone Systems (U), sanofi-aventis (U) Stock Ownership: None Honoraria: Eleftherios P. Mamounas, sanofi-aventis Research Funding: Sandra M. Swain, Genentech, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: Sandra M. Swain, Sanofi-Aventis
AUTHOR CONTRIBUTIONS
Conception and design: Sherry X. Yang, Joseph P. Costantino, Eleftherios P. Mamounas, Norman Wolmark, Soonmyung Paik, Sandra M. Swain
Administrative support: Sherry X. Yang, Joseph P. Costantino, Norman Wolmark, Soonmyung Paik
Provision of study materials or patients: Sherry X. Yang, Joseph P. Costantino, Chungyeul Kim, Eleftherios P. Mamounas, Dat Nguyen, Soonmyung Paik
Collection and assembly of data: Sherry X. Yang, Joseph P. Costantino, Chungyeul Kim, Eleftherios P. Mamounas, Soonmyung Paik
Data analysis and interpretation: Sherry X. Yang, Joseph P. Costantino, Eleftherios P. Mamounas, Jong-Hyeon Jeong, Kelley Kidwell, Soonmyung Paik, Sandra M. Swain
Manuscript writing: Sherry X. Yang, Joseph P. Costantino, Eleftherios P. Mamounas, Soonmyung Paik, Sandra M. Swain
Final approval of manuscript: Sherry X. Yang, Joseph P. Costantino, Chungyeul Kim, Eleftherios P. Mamounas, Dat Nguyen, Jong-Hyeon Jeong, Norman Wolmark, Kelley Kidwell, Soonmyung Paik, Sandra M. Swain
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 3.Montero A, Fossella F, Hortobagyi G, et al. Docetaxel for treatment of solid tumours: A systematic review of clinical data. Lancet Oncol. 2005;6:229–239. doi: 10.1016/S1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- 4.Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Buttrick GJ, Wakefield JG. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle. 2008;7:2621–2625. doi: 10.4161/cc.7.17.6514. [DOI] [PubMed] [Google Scholar]
- 11.Okumura E, Fukuhara T, Yoshida H, et al. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat Cell Biol. 2002;4:111–116. doi: 10.1038/ncb741. [DOI] [PubMed] [Google Scholar]
- 12.Katayama K, Fujita N, Tsuruo T. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol Cell Biol. 2005;25:5725–5737. doi: 10.1128/MCB.25.13.5725-5737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 14.MacKeigan JP, Taxman DJ, Hunter D, et al. Inactivation of the antiapoptotic phosphatidylinositol 3-kinase-Akt pathway by the combined treatment of taxol and mitogen-activated protein kinase kinase inhibition. Clin Cancer Res. 2002;8:2091–2099. [PubMed] [Google Scholar]
- 15.Asakuma J, Sumitomo M, Asano T, et al. Selective Akt inactivation and tumor necrosis actor-related apoptosis-inducing ligand sensitization of renal cancer cells by low concentrations of paclitaxel. Cancer Res. 2003;63:1365–1370. [PubMed] [Google Scholar]
- 16.Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Nakashio A, Fujita N, Rokudai S, et al. Prevention of phosphatidylinositol 3′-kinase-Akt survival signaling pathway during topotecan-induced apoptosis. Cancer Res. 2000;60:5303–5309. [PubMed] [Google Scholar]
- 18.Martin M, Mackey J, Vogel C. Benefit from adjuvant taxanes and endocrine responsiveness in breast cancer. Breast. 2007;16(suppl 2):S127–S131. doi: 10.1016/j.breast.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, et al. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 20.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing–National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 21.Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 22.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan AR, Yang X, Hewitt SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–3090. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Lin YZ, LaPushin R, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034–7045. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 25.Pervin S, Singh R, Chaudhuri G. Nitric-oxide-induced Bax integration into the mitochondrial membrane commits MDA-MB-468 cells to apoptosis: Essential role of Akt. Cancer Res. 2003;63:5470–5479. [PubMed] [Google Scholar]
- 26.Bauer KD, de la Torre-Bueno J, Diel IJ, et al. Reliable and sensitive analysis of occult bone marrow metastases using automated cellular imaging. Clin Cancer Res. 2000;6:3552–3559. [PubMed] [Google Scholar]
- 27.Yang SX, Simon RM, Tan AR, et al. Gene expression patterns and profile changes pre- and post-erlotinib treatment in patients with metastatic breast cancer. Clin Cancer Res. 2005;11:6226–6232. doi: 10.1158/1078-0432.CCR-05-0270. [DOI] [PubMed] [Google Scholar]
- 28.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 29.Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 30.Cappuzzo F, Magrini E, Ceresoli GL, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Statist Soc A. 1972;135:185–206. [Google Scholar]
- 33.Cox DR. Regression models and life tables. J R Statist Soc B. 1972;34:187–220. [Google Scholar]
- 34.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 35.Kawase T, Ohki R, Shibata T, et al. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136:535–550. doi: 10.1016/j.cell.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Kaplan DR, Cantley LC. PI3K: Downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 37.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard KI, Messersmith H, Elavathil L, et al. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–744. doi: 10.1200/JCO.2007.15.4716. [DOI] [PubMed] [Google Scholar]
- 39.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 40.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]