Abstract
Purpose
In Total Therapy 2, after randomly assigning 323 patients with myeloma to thalidomide and 345 to a control arm, no difference was observed in overall survival, with a median follow-up of 42 months, although at 72 months, survival was superior on the thalidomide arm in the one third exhibiting cytogenetic abnormalities (CA). After further follow-up of 87 months, we examined, in reiterative analyses, the effect of increasing time intervals on clinical outcomes relevant to baseline prognostic variables and treatment randomization.
Patients and Methods
We investigated clinical trial end points as a function of increasing time intervals from protocol enrollment to determine consistencies of results by treatment and prognostic variables.
Results
The complete congruence of serial survival plots for both study arms combined attested to stable patient characteristics over the time of accrual and the quality of follow-up management. Presence of CA was associated with consistently inferior survival curves from year 3 onward. Although 80% of patients randomly assigned to thalidomide discontinued study drug after 2 years because of toxicity, its clinical benefit did not reach statistical significance until year 10. The relative ranking order in multivariate models of prognostic factors remained stable over time. Decline in initially high hazard ratio values of gene array–defined high risk is consistent with an initial crisis phase that is time limited.
Conclusion
Reporting potentially time-sensitive features as a part of clinical trial results will enable the critical reader to judge the robustness of prognostic factors and the time sensitivity of outcome predictors, with important implications for future trial designs.
INTRODUCTION
Myeloma survival has benefited from autotransplants1–3 and the discovery of several novel agents.4–6 Both approaches have raised the incidence of complete response (CR) to 40% and higher7–12 from lower than 5% in the melphalan and prednisone era. Since CR increments translated into significant survival prolongation with autotransplants, such high-quality response was deemed a valid surrogate trial end point for survival. Follow-up is too short for novel agent trials to judge whether, with the exclusive use of nongenotoxic drugs, high CR rates also impart durable disease control. These considerations raise the important issue of timing of trial reporting for meaningful survival implications to be derived. This topic is relevant in view of the recent retraction of reports on shorter survival with tandem versus single transplant plus thalidomide maintenance.13–16
Applying reiterative survival analyses spanning early to complete patient accrual and progressively longer follow-up, we determined the minimum follow-up times required to assure valid outcome projections. Total Therapy 2 (TT2) appeared well suited for this purpose because of the large sample size of 668 patients accrued over 64 months with a median follow-up currently of 87 months.7 This phase III trial investigated whether the addition of thalidomide upfront to a tandem autotransplantation trial would improve event-free survival (EFS) and overall survival (OS) by increasing the frequency of CR. Despite significantly higher CR rate and superior EFS, OS was similar at the time of the original publication with a median follow-up of 42 months,7 but with longer follow-up of 72 months was significantly prolonged in the one third of patients displaying cytogenetic abnormalities (CA).17 The availability of comprehensive baseline characteristics and detailed follow-up were well suited to investigate the stability of clinical outcomes relative to baseline prognostic factors and treatment randomization.
PATIENTS AND METHODS
TT2 accrued 668 patients between October 1998, and February 2004, of whom 345 were randomly assigned to the control group and 323 were randomly assigned to the thalidomide arm. The median follow-up of live patients was 87 months. As of April 17, 2009, 371 patients were alive and 253 had not suffered an event. Of 349 attaining CR (52%), 181 remain relapse free. Baseline and follow-up laboratory studies performed as part of these trials have been published. Gene-expression profiling (GEP) analysis of highly purified bone marrow plasma cells, available in 351 subjects, distinguished 13% with high-risk myeloma.18
Kaplan-Meier (KM) plots of OS and EFS from treatment initiation and, for those achieving CR, of CR duration (CRD) from its onset were portrayed at annual intervals from the start of initial enrollment through the present time,19 and log-rank tests were performed to determine their consistencies. In order to determine potentially time-dependent differences related to treatment randomization and prognostic variables, KM plots were also shown relevant to patients being randomly assigned to control or thalidomide arms and in relationship to the presence of CA. Serial multivariate analyses were performed for baseline variables and treatment arms in order to determine possible changes in independently significant parameters over the time course of enrollment and subsequent follow-up.20 Such data are portrayed, for better clarity, by forest plots.
Additional analyses examined the survival impact of thalidomide cumulative dosing, employing time-dependent variable methodology that also accounted for the four phases of protocol therapy (induction, transplantations, consolidation, and maintenance).
RESULTS
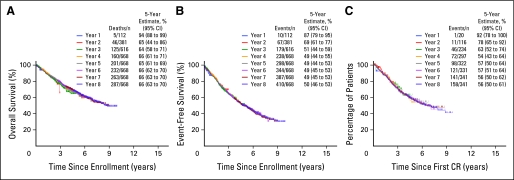
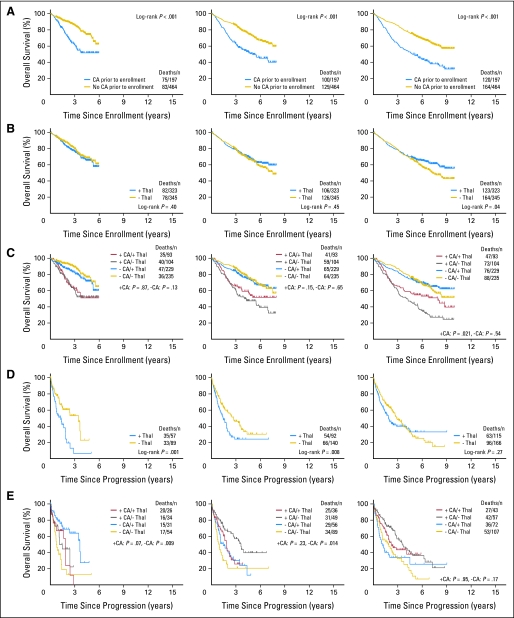
KM plots over time of OS, EFS, and CRD were completely superimposable, indicating absence of time-dependent differences in the characteristics of successively enrolled patients (Fig 1). Indeed, there was no difference during the time of trial accrual from year 1 to year 6 in the frequency of baseline laboratory parameters of recognized prognostic significance such as elevated serum levels of beta-2-microgobulin (B2M; ≥ 3.5 or > 5.5 mg/L), C-reactive protein (≥ 4.0 or ≥ 8 mg/L), lactate dehydrogenase (LDH; ≥ 190 U/L) and creatinine (≥ 2.0 mg/dL); low levels of albumin (< 3.5 g/dL) and hemoglobin (< 10 g/dL); and, importantly, the presence of CA and GEP-defined high-risk designation (data not shown). Reiterative KM plots according to potentially important variables are portrayed in Figure 2, covering years 6, 8, and 10 from enrollment or relapse. The presence of CA conferred inferior OS throughout (Fig 2A), which also pertained to EFS and CRD (not shown). Although discontinued due to toxicity in approximately 80% of patients within 2 years from starting protocol therapy,7 thalidomide's benefit became manifest at a significant level of P < .05 only after 7 years for EFS (not shown) and 10 years for OS (Fig 2B). The higher CR frequency on the experimental arm did not translate into longer CRD (P = .15; data not shown), thus not explaining the late survival benefit of patients randomly assigned to thalidomide. According to statistical analyses that tested for potential interaction, thalidomide's preferential benefit in CA-type myeloma17 was confirmed and became apparent for EFS in year 8 (not shown) and for OS in year 10 (Fig 2C). The initially observed shorter postrelapse survival (PRS) in case of random assignment to thalidomide7 disappeared with longer follow-up (Fig 2D), so that by year 10, PRS curves had crossed in favor of the thalidomide arm. Given thalidomide's preferential benefit in CA-type myeloma, PRS was also examined in the context of baseline CA status. While initially appearing superior in case of random assignment to the control arm, PRS curves for the thalidomide arm crossed those for the control arm by year 10 independent of CA status (Fig 2E). The similarity in PRS between the two study arms could not be attributed to differences in access to or utilization of lenalidomide and bortezomib as primary salvage therapies, which were employed in 43% and 9% of patients treated on the control arm and in 49% and 8% of those randomly assigned to thalidomide.
Fig 1.
Serial Kaplan-Meier plots of overall survival (A), event-free survival (B) and complete response (CR) duration (C) from various times of trial enrollment. The curves are superimposable, suggesting that patients' characteristics and quality of care provided over time were consistent. TT, Total Therapy; OS, overall survival; EFS, event-free survival.
Fig 2.
Survival plots depicted as a function of increasing time intervals to the present time (left column, year 6; middle column, year 8; right column, year 10). (A) The presence of cytogenetic abnormalities (CA) consistently affected survival adversely. (B) Random assignment to thalidomide (± Thal) emerged as a favorable feature only 10 years after trial inception. (C) Consideration of both CA and trial arm revealed that Thal's restricted benefit to patients with CA also did not emerge until year 10. (D) Postrelapse survival was significantly inferior in case of initial random assignment to Thal 6 and 8 years after starting protocol therapy, to disappear at year 10 as a result of crossing of curves, which also pertained when examined in the context of CA (E). TT, Total Therapy.
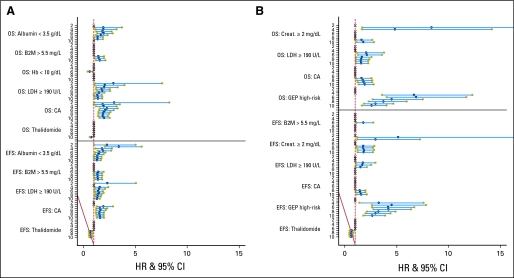
The results of serial multivariate analyses of baseline variables affecting OS and EFS are depicted in Figure 3. In the larger patient set without GEP data, low serum albumin levels, elevated LDH and B2M concentrations, and the presence of CA exerted fairly consistent adverse effects on both OS and EFS (Fig 3A). Thalidomide's favorable impact was detectable for EFS at year 7 and for OS not until year 10. Hazard ratio values for albumin and LDH, but not CA, decreased over time. In addition, when GEP data were considered, high-risk designation (13% of patients) dominated both OS and EFS models, whereas the deleterious roles of CA and LDH were less pronounced (Fig 3B). High B2M essentially failed to affect clinical outcomes, while serum creatinine levels of 2 mg/dL and greater imparted poor outcomes only early on. Random assignment to thalidomide was a favorable feature for EFS but not OS.
Fig 3.
Display of hazard ratio (HR) values of baseline prognostic variables on overall survival (OS) and event-free survival (EFS) as a function of years since enrollment. The HRs and 95% CIs are based on a multivariate Cox regression. An X indicates that the variable was not found significant in the multivariate analysis. The first row of the x-axis indicates the number of years since first enrollment. When examined among all patients (A, upper panel), regardless of vailability of gene expression profiling (GEP), HR values for albumin and lactate dehydrogenase (LDH), but not cytogenetic abnormalities, decreased over time. Thalidomide's favorable impact was detectable for EFS at year 7 but was not detectable for OS until year 10. When GEP data were also onsidered (B, lower panel), high-risk designation dominated both OS and EFS models. Random assignment to thalidomide was a favorable feature for EFS but not OS. B2M, beta-2-microglobulin; Hb, hemoglobin; CA, cytogenetic abnormalities; Creat, creatinine.
Cumulative thalidomide dosing, whether considered across all treatment phases or separately by induction, peritransplant, consolidation, and maintenance, did not affect OS, EFS, or CRD, regardless of CA status (data not shown).
DISCUSSION
To our knowledge, this is the first report to address the important issue of consistency of clinical outcomes as a function of increasing patient enrollment numbers and of subsequent follow-up times. Completely overlapping KM plots of OS, EFS, and CRD reflect the consistency of patient characteristics during successive enrollment intervals, patient follow-up, supportive care, and management of relapse and other complications. While affecting OS and EFS consistently over time, the magnitude of CA's adverse impact was diminished when GEP data were also considered. GEP risk implications were greatest in the early time segments and declined progressively through year 10. Despite its use upfront with the inception of induction therapy and discontinuation after 2 years in 80% of patients due to adverse effects, thalidomide's beneficial role especially in CA-type myeloma evolved gradually reaching significant levels for EFS at 7 years and for OS not until 10 years. The underlying mechanism remains obscure. Work is in progress to examine, by GEP analysis of whole bone marrow biopsies obtained during maintenance, whether thalidomide-unique microenvironment signature changes can be detected that may account for its observed delayed clinical benefit. The adverse role on PRS of initial random assignment to thalidomide applied regardless of CA status until year 10, when cross-over was observed in favor of the thalidomide arm.
The implications of our work for clinical trials practice can be summarized as follows: consistency of patient characteristics and management over time can be inferred from superimposable serial KM survival plots, the inclusion of which in clinical trial reporting appears desirable to guard against faulty early conclusions.14,16 Robustness of prognostic variables can be confirmed when their hazard ratio values compete effectively over time. Decreasing hazard ratio values over time in patients with high-risk GEP features suggest the need for close medical attention to this group during the initial crisis phase, beyond which survival chances seem to improve toward levels seen in low-risk myeloma.21
Footnotes
Supported by Grant No. PO1 CA55819 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Bart Barlogie, Celgene (C), International Myeloma Foundation (C), Multiple Myeloma Research Foundation (C), Southwest Oncology Group (C), Genzyme (C); Frits van Rhee, Celgene (C), Novartis (C); John D. Shaughnessy Jr, Myelogix (C), Genzyme (C), Novartis (C), Celgene (C) Stock Ownership: John D. Shaughnessy Jr, Myelogix Honoraria: Bart Barlogie, Millennium, Celgene, International Myeloma Foundation; Frits van Rhee, Celgene, Millennium, Novartis; John D. Shaughnessy Jr, Myelogix, Genzyme, Novartis, Celgene Research Funding: Bart Barlogie, Millennium, Celgene, Novartis; Frits van Rhee, Centocor Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Bart Barlogie, John D. Shaughnessy Jr, John Crowley
Provision of study materials or patients: Elias Anaissie, Frits van Rhee
Collection and assembly of data: Nathan Petty
Data analysis and interpretation: Jackie Szymonifka, Antje Hoering, John Crowley
Final approval of manuscript: Bart Barlogie, Elias Anaissie, Frits van Rhee, John D. Shaughnessy Jr, Jackie Szymonifka, Antje Hoering, Nathan Petty, John Crowley
REFERENCES
- 1.Barlogie B, Tricot G, van Rhee F, et al. Long-term results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:1365–2141. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau J-L, Stoppa A-M, et al. L: A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 7.Barlogie B, Tricot G, Anaissie E, et al. Effect of adding thalidomide to the treatment of multiple myeloma with tandem autotransplants. N Engl J Med. 2006;10:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 8.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 9.Cavo M, Tacchetti P, Patriarca F, et al. Superior complete response rate and progression-free survival after autologous transplantation with up-front velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly diagnosed multiple myeloma. Blood. 2008;112 abstr 158. [Google Scholar]
- 10.Richardson PG, Mitsiadis C, Schlossman R, et al. New drugs for myeloma. The Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo A, Bringhen S, Rossi D, et al. A prospective, randomized, phase III study of bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and predisone (VMP) in elderly newly diagnosed myeloma patients. Blood. 2008;112 abstr 652. [Google Scholar]
- 12.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 13.Abdelkefi A, Torjman L, Ben Romdhame N, et al. First-line thalidomide-dexamethasone therapy in preparation for auto-SCT in young patients (< 61 years) with symptomatic multiple myeloma. Bone Marrow Transplant. 2005;36:193–198. doi: 10.1038/sj.bmt.1705050. [DOI] [PubMed] [Google Scholar]
- 14.Abdelkefi A, Torjman L, Ben Romdhane N, et al. First-line thalidomide-dexamethasone therapy in preparation for auto-SCT in young patients (< 61 years) with symptomatic multiple myeloma: Retraction. Bone Marrow Transplant. 2009;43:893. doi: 10.1038/bmt.2009.113. [DOI] [PubMed] [Google Scholar]
- 15.Abdelkefi A, Ladeb S, Torjman L, et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: Results of a multicenter randomized clinical trial. Blood. 2008;111:1805–1810. doi: 10.1182/blood-2007-07-101212. [DOI] [PubMed] [Google Scholar]
- 16.Abdelkefi A, Ladeb S, Torjman L, et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantion in multiple myeloma: Results of a multicenter randomized clinical trial: Retraction. Blood. 2009;113:6265. doi: 10.1182/blood-2007-07-101212. [DOI] [PubMed] [Google Scholar]
- 17.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaughnessy J, Zhan F, Burington B, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome I. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from imcomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Cox DR. Regression tables and life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 21.Hoering A, Crowley J, Shaughnessy JD, Jr, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114:1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]