Abstract
Background
Most insulin autoantibody assays for both human and animal models are in a radioassay format utilizing 125I-insulin, but despite the radioassay format international workshops have documented difficulty in standardization between laboratories. There is thus a need for simpler assay formats that do not utilize radioactivity, yet retain the high specificity and sensitivity of radioassays.
Methods
To establish an easier enzyme-linked immunosorbent assay (ELISA) for insulin autoantibodies of non-obese diabetic (NOD) mice, we used an ELISA format, competition with unlabeled insulin, europium-avidin, and time-resolved fluorescence detection (competitive europium insulin autoantibody assay).
Results
The competitive europium assay of insulin autoantibodies when applied to sera from NOD mice had high sensitivity and specificity (92% sensitivity, 100% specificity) compared to our standard insulin autoantibody radioassay (72% sensitivity, 100% specificity) in analyzing blind workshop sera. It is noteworthy that though the assay has extremely high sensitivity for murine insulin autoantibodies and utilizes human insulin as target autoantigen, human sera with high levels of insulin autoantibodies are not detected.
Conclusions
Our results clearly indicate that low levels of insulin autoantibodies can be detected in an ELISA-like format. Combining a europium-based ELISA with competition with fluid-phase autoantigen can be applicable to many autoantigens to achieve high specificity and sensitivity in an ELISA format.
Introduction
Of the three major anti-islet autoantibody assays (autoantibodies reacting with glutamic acid decarboxylase [GAD] 65, insulinoma antigen 2, and insulin), only insulin autoantibodies were confirmed as specifically detectable in blinded workshops studying sera of non-obese diabetic (NOD) mice and control strains.1,2 Nevertheless, the assay for insulin autoantibodies has proven the most difficult to standardize with relatively wide discrepancies between laboratories in sensitivity and specificity, especially for human samples and in workshops with many participating laboratories.3–6 A direct enzyme-linked immunosorbent assay (ELISA) format (binding of antigen to plate and detection of bound autoantibody with labeled anti-antibodies) has proven difficult to develop, and to date only one GAD ELISA that utilizes capture of solution-phase GAD by one chain of immunoglobulin (Ig) while being bound by its other chain to plate-bound GAD has demonstrated sensitivity and specificity similar to fluid-phase radioassays.
Fluid-phase radioassays for insulin autoantibodies as noted above have been the most difficult of the assays to standardize. Initial insulin autoantibody assays utilized a large volume of sera and poly(ethylene glycol) precipitation of autoantibody-bound 125I-insulin. Williams et al.7 developed a micro-insulin autoantibody (mIAA) assay that utilized protein A for precipitation, and Yu et al.8 modified this assay for performance in 96-well filtration plates with direct counting in a multichannel β-counter. Though the latter assay can be performed in a semiautomated manner, reagent costs are high with the cost of 125I-insulin and protein A-Sepharose and filtration plates, and one must handle radioactive insulin. There are reports of ELISAs for insulin autoantibodies. In studies of human sera an early workshop demonstrated that although ELISAs readily detected the insulin antibodies that follow subcutaneous insulin therapy, they failed to detect insulin autoantibodies of individuals with prediabetes and new-onset diabetes.9 Standard insulin autoantibody ELISAs applied to the NOD mouse have given variable results with questions related to specificity in workshop analysis using absorption studies.6
While evaluating an ELISPOT assay for insulin autoantibody-producing B lymphocytes, we unexpectedly noted that with a europium assay we could readily detect insulin autoantibodies of NOD mice, comparing signal with and without wells coated with insulin. The induced fluorescence of europium, a lanthanide, is long-lived and thus allows time-resolved fluorescence readers to differentiate short-lived “background” fluorescence from specific signals provided by europium. Therefore, europium has been utilized in ELISA-format assays to provide high sensitivity, comparable to radioassays, with time-resolved fluorescent detection of photons (results reported as counts per second [cps]).10,11 Some control sera we analyzed, however, gave false-positives (greater signal with insulin on the plate than without plate-bound insulin), and we further modified the europium assay to incorporate competition with excess fluid-phase insulin, with results, and thus positivity, determined by the difference with and without competition. The final competitive europium insulin autoantibody assay (CE-IAA) analyzing sera from mice is superior in sensitivity to our standard mIAA assay, and much simpler, requiring relatively inexpensive reagents and 1-s counting and not requiring radioactive compounds or chemically modified autoantigens.
Subjects and Methods
Samples
Serum was obtained from NOD mice, NOD mice with a knockout of the insulin 2 gene (2KO), BALB/c mice, C57BL/6 mice, and New Zealand Black (NZB) mice. We also obtained sera of BALB/c mice immunized with the B:9–23 insulin peptide. Mice were housed in a pathogen-free animal colony at the Barbara Davis Center for Childhood Diabetes (Aurora, CO) with an approved protocol from the University of Colorado Health Sciences Center Animal Care and Use Committee. All mice had free access to tap water in an air-conditioned room (22–25°C) with a 12-h light–dark cycle (6:00–18:00 h). We also used 49 coded sera kindly provided by Dr. Clive Wasserfall from an international animal models workshop (the Second Immunology of Diabetes Society (IDS) Animal Models Workshop, October 2002) and 34 human samples, which were obtained with informed consent and institutional review board oversight at the University of Colorado. Serum samples were stored at −20°C or prior to testing.
Standard mIAA assay
As previously described,8 the mIAA assay was performed using a 96-well filtration plate-based radioimmunoassay. The assay requires 26 μL of serum (6.5-μL duplicates with and without unlabeled insulin). Human 125I-insulin (20,000 cpm) was incubated with mouse serum overnight at 4°C, precipitated with protein A/G-Sepharose, and counted in a TopCount β-counter (PerkinElmer Inc., Waltham, MA). The upper limit of normal is an index of 0.01 relative to a standard positive serum.
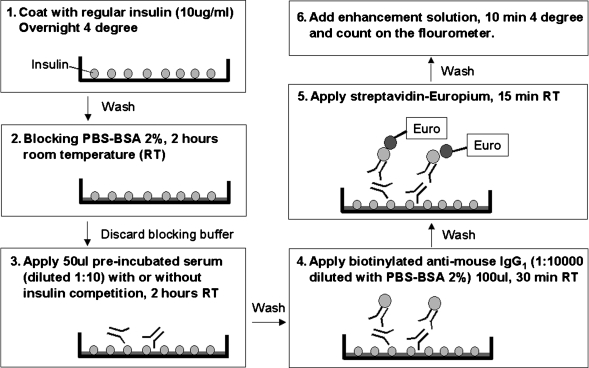
CE-IAA (Fig. 1) and noncompetitive europium insulin autoantibody assay (E-IAA)
FIG. 1.
The newly developed CE-IAA.
CE-IAA for mouse sera. Corning (Corning, NY) high-binding clear 96-well plates (Costar® 3590) were coated with 100 μL of human (recombinant) insulin (100 U/mL, Humulin R®, Eli Lilly and Co., Indianapolis, IN) in coating buffer (pH 8) overnight at 4°C at a concentration of 10 μg/mL (buffer details follow). The following day, the plate was washed three times with washing buffer and then incubated for 2 h with assay buffer containing phosphate-buffered saline (PBS) and 2% bovine serum albumin (BSA) at room temperature (RT) on a plate shaker as a plate-blocking procedure. For competition with insulin, mouse serum (5 μL) was diluted with 45 μL of assay buffer containing insulin (41 μL of assay buffer plus 4 μL of 100 U/mL Humulin R) for 1 h and then added to the wells for a 2-h incubation at RT on a shaker. Next, the wells were washed four times with washing buffer, and the plate was then briefly allowed to dry. Biotinylated anti-mouse IgG1 (ab11587) (Abcam Inc., Cambridge, MA) for secondary antibody was diluted 1:10,000 in assay buffer, and 100 μL was added to each well for 30 min at RT on a shaker. (The reason anti-mouse IgG1 was utilized as the secondary antibody was that IgG1 and IgG2b were reported to be the predominant insulin antibodies in the NOD mouse,12 and our preliminary experiments revealed relatively high background utilizing an anti-IgG antibody.) The wells were again washed four times with washing buffer, and then the wells were incubated for 15 min with 100 μL of europium-labeled streptavidin (PerkinElmer) (5 μL of streptavidin-europium added to 10 mL of assay buffer). The plate was washed five times, and then 200 μL of enhancement solution (PerkinElmer) was added to all wells and shaken for 10 min at 4°C in the dark. Finally, the plate was run on a Victor2V 1420 Multilabel Counter fluorimeter (Wallac, Turku, Finland) at one well per second. On a single plate, sera from an NOD mouse were used as standardized positive serum. Each sample was run in duplicate with and without competition using human insulin. For each sample, an index was calculated based on the average of the results: Index = (cps of test sample − cps of test sample with insulin competition)/(cps of positive standard serum − cps of positive standard serum with insulin competition). Intra- and inter-assay coefficients of variation were 6.8% (n = 15) and 16.4% (n = 19), respectively.
Buffers were made as follows: coating buffer, 0.5 M K2HPO4 (43.5 g of K2HPO4 [catalog number P288, Fisher Scientific, Fairlawn, NJ]) plus 500 mL of double distilled water) with 0.5 M KH2PO4 (34 g of KH2PO4 [catalog number P285, Fisher Scientific] plus 500 mL of double distilled water) added to pH 8; washing buffer, 50 mM Tris (pH 7.0–7.5) and 0.2% Tween-20 in distilled water; and assay buffer, 0.01% sodium azide and 2% BSA in PBS (pH 7.4).
CE-IAA for human sera
The procedure was same as that for mouse sera except for using biotinylated anti-human antibody and human standardized positive and negative sera for controls.
E-IAA for mouse sera
The differences between the CE-IAA and the E-IAA include: (1) for E-IAA plates were coated without or with human insulin; and (2) for E-IAA sera preincubation with insulin (competition) was not utilized. The E-IAA index was calculated as (cps of test sample with plate-bound insulin − cps of test sample without plate-bound insulin)/(cps of positive standard sera with plate-bound insulin − cps of positive standard sera without plate-bound insulin).
Results
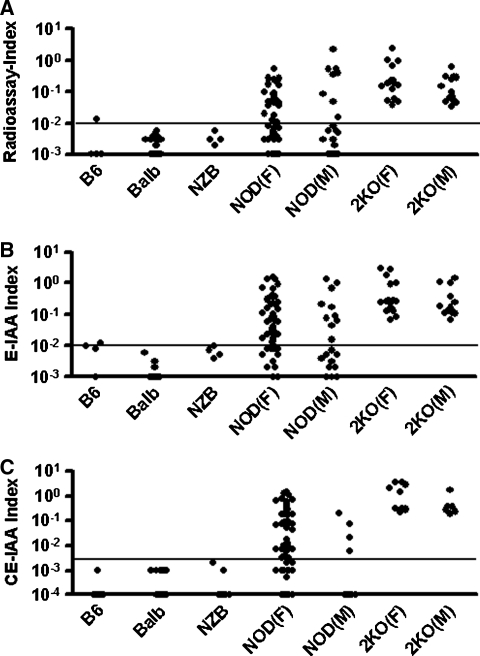
Figure 2 illustrates the results of testing mouse sera for insulin autoantibodies by our standard mIAA fluid-phase radioassay (Fig. 2A), a “standard” europium-based ELISA (Fig. 2B) with subtraction of counts in the absence of plate-bound insulin from counts with plate-bound insulin, and the “final” CE-IAA (Fig. 2C). Results for three control strains—C57BL/6, BALB/c, autoimmune NZB mice, and female and male NOD as well as NOD 2KO mice (insulin 2 gene knockouts have increased insulin autoantibodies)—are illustrated. Control mice were in general “negative” for insulin autoantibodies with an index for positivity set at 0.01 for the mIAA assay and the noncompetitive E-IAA (in prior studies with the mIAA assay the assay cutoff was set at 0.01 based on the 99th percentile of sera from normal human controls and confirmed with mouse sera), and the cutoff for the CE-IAA was set just above the highest value for control animals (index = 0.002). Specificity in the mIAA assay, E-IAA, and CE-IAA was 96.4% (27 of 28), 96.4% (27 of 28), and 100% (46 of 46), respectively. Sensitivity for NOD mice combining male and female mice was 45.2% (33 of 73) for the mIAA radioassay, 67.1% (49 of 73) for the noncompetitive E-IAA, and 73.0% (46 of 63) for the CE-IAA. Note that a subset of NOD mice were 4 weeks old and might not have yet developed insulin autoantibodies. As expected, NOD 2KO mice had higher levels of insulin autoantibodies than regular NOD mice by all three assays, and all sera from knockout mice were positive.
FIG. 2.
(A) Insulin autoantibodies measured by our standard mIAA assay. The horizontal line gives the cutoff (0.01). (B) Standard E-IAA. The horizontal line gives the cutoff (0.01). (C) Competitive CE-IAA. The horizontal line gives the cutoff (0.002). B6, C57BL/6 mice (n = 4–13); Balb, BALB/c mice (n = 20); NZB mice (n = 4–13); NOD (F) and NOD (M), female and male NOD mice (n = 53–57 and n = 10–20, respectively); 2KO(F) and 2KO(M), female and male mice with 2KO (n = 9–17 and n = 6–13, respectively). The samples were randomly selected and not identical in each group (mIAA assay, E-IAA, and CE-IAA).
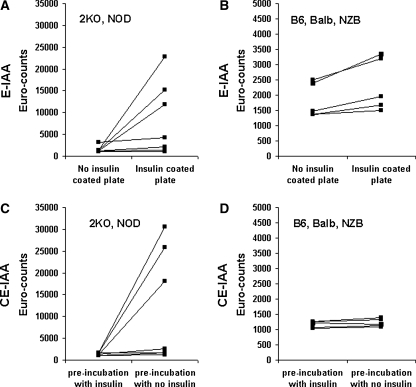
Figure 3 shows the actual cps values for NOD and 2KO sera with high and low levels of autoantibodies and for NOD sera negative for insulin autoantibodies as well as control mice with the E-IAA (Fig. 3A and B) and the CE-IAA (Fig. 3C and D). In both assay formats counts in the control situation (e.g., lacking insulin on the ELISA plate [top panels, left] or in the presence of an excess of insulin competition [bottom panels, left]) were low. One can also appreciate that for some control sera there are higher counts with insulin on the plate than without insulin on the plate (Fig. 3B), while with the competitive assay the background counts for control sera are unchanged in the presence of competition with insulin (Fig. 3D), thus giving no specific signal.
FIG. 3.
(A and B) Noncompetitive and (C and D) competitive europium assays using (A and C) NOD mice or mice with 2KO and (B and D) control (C57BL/6 [B6], BALB/c [Balb], or NZB) mice.
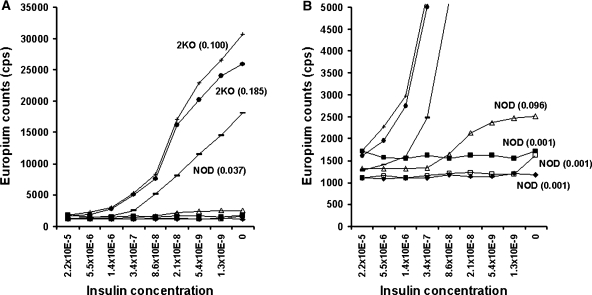
We evaluated the sensitivity of the CE-IAA to inhibition by different amounts of competing fluid-phase insulin. In general, approximately 2.1 × 10−8 M insulin inhibited binding by 50% (Fig. 4), and by 2.2 × 10−5 M insulin inhibition of specific binding was complete. Figure 4B shows the binding with an expanded y-axis scale. For a subset of NOD sera, there is no inhibition by competition with fluid-phase insulin, and these samples were all negative for insulin autoantibodies determined with the mIAA radioassay.
FIG. 4.
CE-IAA inhibition by different amounts of competing fluid-phase insulin. The initial competitive insulin concentration was 2.2 × 10−5 M, which is the same as that used in our standard CE-IAA, and was then diluted. The results are shown twice with different scales (A and B) to highlight the full range of values. Autoantibodies determined with the mIAA radioassay are shown in parentheses.
Figure 5A illustrates the correlation between results of the CE-IAA and the mIAA radioassay with data presented for NOD mice, 2KO mice, and control strains. There is a good correlation between the assays (note log scale), but there is a subset of NOD sera with positive CE-IAA levels that are negative with the radioassay.
FIG. 5.
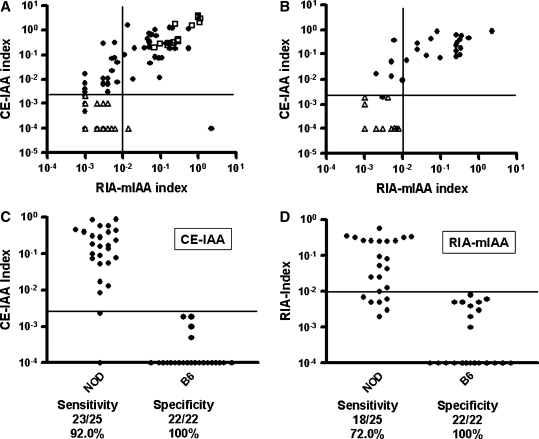
(A) Correlation between results of the CE-IAA assay and the mIAA radioimmunoassay (RIA) with data presented for NOD mice (solid circles, n = 68), 2KO mice (open squares, n = 16), and control strains (open triangles, n = 46). (B–D) Coded serum samples from an international animal models workshop (Second IDS Animal Models Workshop, October 2002, provided by Dr. Cliver Wasserfall). (B) Correlation between results of the CE-IAA and the mIAA radioassay with data presented for NOD (solid circles, n = 25) and C57BL/6 (open triangles, n = 22) mice. Sensitivity and specificity are given for (C) the CE-IAA and (D) the mIAA RIA.
To compare data with the prior international Animal Models workshop (Second IDS Animal Models Workshop, October 2002), Dr. Clive Wasserfall kindly provided coded sera samples from the workshop for measurement of insulin autoantibodies with the CE-IAA. In this workshop our mIAA radioassay from Denver had one of the highest sensitivities of the participating laboratories. The CE-IAA detected 23 of 25 NOD sera as positive (Fig. 5B and C) and none of the control sera utilizing a predetermined cutoff of an index of 0.002. Compared to the radioassay (Fig. 5C and D), the CE-IAA maintained 100% specificity with no loss of sensitivity (92% CE-IAA vs. 72% mIAA assay).
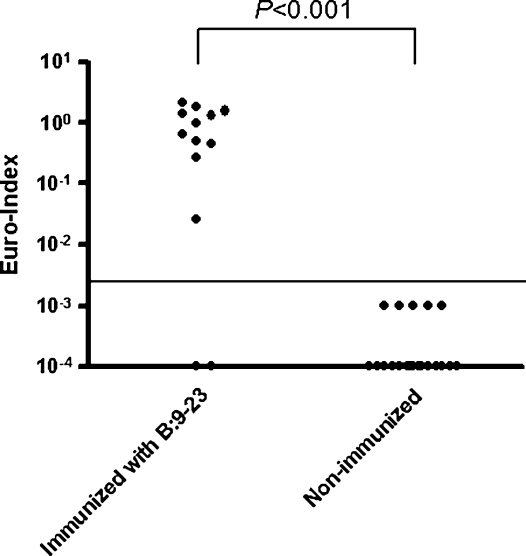
To assess whether insulin autoantibodies of non-NOD mice would also be detected with the CE-IAA, we evaluated sera of BALB/c mice immunized with the B:9–23 insulin peptide (Fig. 6). As previously reported such mice develop insulin autoantibodies detectable with the mIAA radioassay, but of note is that the antibodies are not inhibited by competition with the immunizing B:9–23 peptide but are blocked by competition with intact insulin. These insulin autoantibodies of BALB/c mice were readily detected with the CE-IAA.
FIG. 6.
Sera of BALB/c mice immunized with B:9–23 insulin peptide in complete Freund's adjuvant were measured with the CE-IAA. The horizontal line gives the cutoff for the CE-IAA (0.002). The difference between the two groups was tested by Mann-Whitney's U test.
We evaluated a series of human sera that with the fluid-phase radioassay that had high levels of insulin autoantibodies, and none reacted with insulin in the CE-IAA (data not shown).
Discussion
A large body of literature indicates that insulin is an important type 1 diabetes-relevant autoantigen, and recent studies from our and other laboratories suggest that insulin may be a primary autoantigen for the NOD mouse model.13–16 In humans, evidence includes the early appearance of insulin autoantibodies in children prospectively followed to the development of diabetes and the importance of insulin gene polymorphisms in determining diabetes risk.17,18 For the NOD mouse, diabetes is prevented with transgenic expression of proinsulin by antigen-presenting cells, and diabetes is accelerated with a knockout of the insulin 2 gene, 90% prevented with a knockout of the insulin 1 gene, and prevented in mice having no native insulin genes and only a mutated proinsulin transgene with a key epitope altered with the change of a single amino acid (B:16 tyrosine to B:16 alanine).13–16 Despite the importance of insulin autoimmunity, assays for insulin autoantibodies have been the most problematic in international workshops. We believe the bulk of the difficulty in standardization occurs because the separation of levels of insulin antibodies between controls and patients is very small compared to much stronger signals detected for GAD65 and insulinoma antigen 2 autoantibodies for the majority of individuals developing diabetes. Thus there is a major impetus to develop assays that both are easier to perform and have enhanced sensitivity (while preserving specificity).
For the NOD mouse we believe the current CE-IAA achieves both of the above goals. Its nonradioactive format is clearly an advantage as is the lack of expensive reagents and the ability to count wells for 1 s. The sensitivity using blinded workshop samples with essentially the same volume of sera utilized (CE-IAA, 5 μL per well; mIAA assay, 6.5 μL per well) was 92% compared to our previously reported sensitivity of 72% with our standard mIAA radioassay, while specificity was maintained at 100%. We believe both the utilization of europium for detection and the utilization of a fluid-phase competitive assay (taking as signal the difference between cps values with and without fluid-phase insulin competition) are both important for the high sensitivity and maintenance of specificity.
Given the excellent sensitivity of the ELISA-format CE-IAA compared to our mIAA radioassay, we believe that similar assays for other islet autoantigens and non-islet autoantigens can be developed for murine antibody assays. There are already assays using europium detection of human GAD65 and insulinoma antigen 2 autoantibodies.10,11 These assays are not standard ELISAs with autoantigen on the plate but utilize biotinylated and glutathionine S-transferase-modified autoantigens, and it is indicated that for the GAD assay that a standard assay with europium-labeled anti-human antibodies could not be developed.10 No standard ELISA has performed as well as their respective radioassays in international workshops of the IDS and Diabetes Autoimmunity Standardization Program (DASP), while a modified assay utilizing single chains of the antibody—one to bind to GAD on the well and the other to biotinylated GAD in solution—performed very well in the last DASP workshop. We have recently described a europium competitive assay for autoantibodies reacting with interferon-α that specifically detects autoantibodies present in patients with the autoimmune polyendocrine syndrome type I.19 With the competitive fluid-phase step this assay is extremely specific, and patients with related autoimmune disorders (e.g., isolated Addison's disease) are negative. Thus we believe this format of europium detection combined with ELISA format and fluid-phase competition is generalizable, including detection of human autoantibodies. Thus it is particularly interesting that the current insulin autoantibody assay (utilizing human insulin) does not detect anti-insulin autoantibodies of humans. We believe this relates to extreme specificity of the human insulin autoantibodies recognizing an epitope17,20 that is likely to be hidden when insulin is plate bound.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK32083), the Immune Tolerance Network (AI-15416), and the Children's Diabetes Foundation. We thank Clive Wasserfall, Gainesville, FL, for sending blinded samples from the prior Animal Islet Autoantibody Workshop.
Author Disclosure Statement
There are no any relevant conflicts for all authors.
References
- 1.Wilkin T. Palmer J. Kurtz A. Bonifacio E. Diaz JL. The second International Workshop on the Standardisation of Insulin Autoantibody (IAA) measurement. Held in New York, 27–30 October 1987. Diabetologia. 1988;31:449–450. doi: 10.1007/BF00271590. [DOI] [PubMed] [Google Scholar]
- 2.Kuglin B. Kolb H. Greenbaum C. Maclaren NK. Lernmark A. Palmer JP. The Fourth International Workshop on the Standardisation of Insulin Autoantibody Workshop. Diabetologia. 1990;33:638–639. doi: 10.1007/BF00400213. [DOI] [PubMed] [Google Scholar]
- 3.Bingley PJ. Bonifacio E. Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 4.Yu L. Eisenbarth G. Bonifacio E. Thomas J. Atkinson M. Wasserfall C. The second murine autoantibody workshop: remarkable interlaboratory concordance for radiobinding assays to identify insulin autoantibodies in nonobese diabetic mice. Ann N Y Acad Sci. 2003;1005:1–12. doi: 10.1196/annals.1288.002. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacio E. Atkinson M. Eisenbarth G. Serreze D. Kay TW. Lee-Chan E. Singh B. International Workshop on Lessons from Animal Models for Human Type 1 Diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice. Diabetes. 2001;50:2451–2458. doi: 10.2337/diabetes.50.11.2451. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacio E. Atkinson M. Eisenbarth G. Serreze D. Kay TW. Lee-Chan E. Singh B. International Workshop on Lessons from Animal Models for Human Type 1 Diabetes: analyzing target autoantigens of humoral immunity in nonobese diabetic mice. Ann N Y Acad Sci. 2002;958:1–2. doi: 10.1111/j.1749-6632.2002.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams AJ. Bingley PJ. Bonifacio E. Palmer JP. Gale EA. A novel micro-assay for insulin autoantibodies. J Autoimmun. 1997;10:473–478. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 8.Yu L. Robles DT. Abiru N. Kaur P. Rewers M. Kelemen K. Eisenbarth GS. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenbaum CJ. Palmer JP. Kuglin B. Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab. 1992;74:1040–1044. doi: 10.1210/jcem.74.5.1569152. [DOI] [PubMed] [Google Scholar]
- 10.Ankelo M. Westerlund-Karlsson A. Ilonen J. Knip M. Savola K. Kankaanpaa P. Merio L. Siitari H. Hinkkanen A. Time-resolved fluorometric assay for detection of autoantibodies to glutamic acid decarboxylase (GAD65) Clin Chem. 2003;49:908–915. doi: 10.1373/49.6.908. [DOI] [PubMed] [Google Scholar]
- 11.Westerlund-Karlsson A. Suonpaa K. Ankelo M. Ilonen J. Knip M. Hinkkanen AE. Detection of autoantibodies to protein tyrosine phosphatase-like protein IA-2 with a novel time-resolved fluorimetric assay. Clin Chem. 2003;49:916–923. doi: 10.1373/49.6.916. [DOI] [PubMed] [Google Scholar]
- 12.Koczwara K. Schenker M. Schmid S. Kredel K. Ziegler AG. Bonifacio E. Characterization of antibody responses to endogenous and exogenous antigen in the nonobese diabetic mouse. Clin Immunol. 2003;106:155–162. doi: 10.1016/s1521-6616(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M. Abiru N. Moriyama H. Babaya N. Liu E. Miao D. Yu L. Wegmann DR. Hutton JC. Elliott JF. Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeckel E. Lipes MA. von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5:1028–1035. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 15.Thebault-Baumont K. Dubois-Laforgue D. Krief P. Briand JP. Halbout P. Vallon-Geoffroy K. Morin J. Laloux V. Lehuen A. Carel JC. Jami J. Muller S. Boitard C. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French MB. Allison J. Cram DS. Thomas HE. Dempsey-Collier M. Silva A. Georgiou HM. Kay TW. Harrison LC. Lew AM. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach P. Koczwara K. Knopff A. Naserke H. Ziegler AG. Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugliese A. The insulin gene in type 1 diabetes. IUBMB Life. 2005;57:463–468. doi: 10.1080/15216540500163301. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L. Barker JM. Babu S. Su M. Stenerson M. Cheng M. Shum A. Zamir E. Badolato R. Law A. Eisenbarth GS. Anderson MS. A robust immunoassay for anti-interferon autoantibodies that is highly specific for patients with autoimmune polyglandular syndrome type 1. Clin Immunol. 2007;125:131–137. doi: 10.1016/j.clim.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castano L. Ziegler AG. Ziegler R. Shoelson S. Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes. 1993;42:1202–1209. doi: 10.2337/diab.42.8.1202. [DOI] [PubMed] [Google Scholar]