Abstract
Aging is frequently accompanied by a proinflammatory state with adverse health consequences. This state is commonly assessed by markers in serum, either in isolation or ad hoc combination. We sought, alternatively, to develop scores summarizing multiple markers in accordance with biology on inflammatory regulation and evaluate their value added for discriminating functional outcomes in older adults. Data came from InCHIANTI (Invecchiare in Chianti; Aging in the Chianti Area) study participants age 65 years and older. Serum concentrations of seven inflammatory biomediators were subjected to latent variable analysis implementing a biological model of counterbalancing up- and down-regulation processes. Resulting process constructs were approximated by principal component scores; these, and individual markers, were evaluated as predictors of mobility impairment and frailty status in regression analyses, adjusting for key confounders. The biomediators' interrelationships were well predicted by the hypothesized biology. The up-regulation score was independently associated with worsened mobility functioning and frailty risk. For mobility, the association was stronger than, persisted independently of, and accounted for association with each biomediator. The down regulation score was associated with frailty outcomes. We conclude that systemic inflammation is relevant to the process that leads to functional loss in older persons and can be validly measured through biologically informed summary of inflammatory markers.
Introduction
Increasing evidence indicates that aging is associated with a mild proinflammatory state that directly influences pathophysiologic processes and contributes to chronic disease, frailty, disability and mortality.1–10 This state is thought to result from system dysregulation, because it can be detected in healthy older individuals. If this state could be accurately and precisely measured, treatments to ameliorate its adverse effects might be designed.
Nearly all studies linking chronic inflammatory activation to adverse health outcomes in older adults have relied on serum biomarkers; however, the vast majority has focused on single molecules under the assumption that these reflect overall changes in severity of activation. A few recent studies have found older adults with simultaneous elevations in two or more inflammatory biomarkers to be significantly more likely than those with fewer elevations to experience a host of adverse outcomes.11–15 Importantly, only one of these attempted to create aggregates of inflammatory markers to assess inflammatory regulation at a systemic level, and all employed data reduction techniques to effect aggregation.
In this study, we attempt to provide initial rationale for the biologically grounded development and validation of measures of inflammatory activation that combine several inflammatory proteins. There is strong rationale to this end but also drawbacks. As a drawback, the evidence for target and functional specificity of specific inflammatory molecules in animal models is overwhelming, suggesting that elevations of different cytokines reflect the up-regulation of different aspects of the inflammatory pathway. On the other hand, when a specific cytokine is released into the circulation, the resulting signal is convoluted through mixing with other inflammatory biomarkers in a large distribution volume, suggesting that circulating levels of inflammatory biomarkers reflect generalized inflammatory regulatory activity occurring in local tissues. If so, the question is whether by synthetically analyzing the biomarkers we can gather enough signal to determine the state of activation of the regulatory system. We address this question by developing biomediator indices measuring different aspects of inflammation.
To our knowledge, two aspects of our work are novel and unique. First, our work internally validates its proposed measurement vis-à-vis hypothesized mechanisms of cytokine release and inhibition that have been confirmed experimentally, but have not been shown to be detectable from biomarkers in serum. Second, it tests hypotheses that (1) resulting indices combining multiple biomarkers of inflammation provide additional information as to the risk of mobility impairments or frailty over any one of the biomarkers, and (2) no individual biomarker provides additional information as to these risks over the indices.
Methods
Study population
InCHIANTI (Invecchiare in Chianti; Aging in the Chianti Area) is a population-based cohort study on factors affecting mobility in older persons.3 The InCHIANTI protocol and the consenting procedures are in agreement with the principles stated in the Declaration of Helsinki and were approved by the Tuscany Region Institutional Review Board. Data analyzed herein were from the baseline assessment, completed in 1999. A total of 438 study participants who were younger than 65 years old or did not have complete data on inflammatory markers were excluded, as were 24 participants who reported taking steroidal drugs within the preceding 2 weeks, 8 participants with rheumatoid arthritis, and 16 persons with C-reactive protein (CRP) measurements greater than 30 g/L. The base analytic sample comprised N = 967 persons.
Inflammatory and antiinflammatory biomediators in serum
Blood samples were drawn in the morning after a 12-h fast and the participant had been sitting for at least 15 min. Aliquots of serum and plasma were processed immediately, stored in a deep freezer at −80°C, and had never been thawed prior to assaying now described.
For this report, data were analyzed on seven biomediators: tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-18, IL-1 receptor antagonist (IL-1RA), CRP, and transforming growth factor-β1 (TGF-β). Serum levels of IL-6, IL-1β, IL-1RA, and TNF-α were measured in duplicate by high-sensitivity enzyme-linked immunosorbent assays (ELISA) using commercial kits (BIOSOURCE International, Camarillo, CA). Serum IL-18 and TGF-β levels were detected in duplicate using highly sensitive quantitative sandwich assays (Quantikine HS, R&D Systems, Minneapolis, MN). CRP was measured in duplicate using an ELISA and colorimetric competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). Assay precisions and detectable limits have been reported elsewhere.5
Selection of biomarkers was based on availability and intention to assess inflammatory regulation in older adults. Along with IL-1β, TNF-α and IL-6 are part of the signaling pathway that triggers the acute-phase response, and together with IL-18 tend to be elevated in the low-grade inflammatory state.6,16 CRP is an acute-phase reactive protein that is mostly upregulated by IL-6. IL-1RA is produced by the liver contextually to, and antagonizes the effect of, IL-1β as a specific receptor blocker. However, because IL-1RA is produced in amounts about 100-fold higher than IL-1β, and remains in serum circulation longer, high IL-1RA is generally considered a marker of inflammation more reliable than IL-1β.9,17 Thus, we considered IL-1RA in our statistical analysis as a surrogate of IL-1β. TGF-β controls a diverse set of cellular processes.18
Physical function outcomes
Analyses were focused on two outcomes that have been hypothesized as targets of dysregulated inflammatory regulation: Mobility functioning and frailty. To define mobility, a z-score average was constructed of six variables: (1) Range of motion (ROM) of all principal movements of hips, knees, ankles, and shoulders were measured using standardized procedures.19 Mobility scoring used the average of standardized ROMs across different joints, movements and sides. (2) Lower extremity muscle power in watts (W) was measured using the Nottingham leg extensor power rig.20 (3) Mobility scoring used the standardized average of measures from right and left sides. (4) Isometric grip strength was assessed using a handheld dynamometer (Nicholas Manual Muscle Tester, model BK-5474; Fred Sammons, Burr Ridge, IL) following a standardized protocol.21 (5) Abnormal neurological signs were collected in a highly standardized examination.22 (6) To measure walking speed, participants were evaluated walking a 4-m course according to a rigorously standardized protocol with timing by activation of photocells. Walking speed was computed as the average over two walks, separately for usual and fast speed. Of the initial analytical sample, 243 participants were missing measurements on at least one of the functional variables and were excluded from the analysis.
Frailty was defined as having three or more of the following criteria: (1) exhaustion (self-reported feeling that ‘‘everything I did was an effort’’ at least three times a week in the last month, as per the Center for Epidemiologic Studies Depression (CES-D) scale23; (2) low physical activity (sedentary state or walking less than 1 h/week); (3) strength in the lowest gender and body mass index quartile-specific quintile of handgrip; (4) speed walking 15 feet in the lowest gender- and standing height-specific quintile; (5) self-report of having lost at least 3 kg over the previous 12 months. These criteria slightly modified those developed by Fried and colleagues.24 Of the initial analytical sample, 209 persons who were missing at least one frailty indicator were excluded.
Control variables
These comprised potential confounders known to relate to both inflammatory regulation and physical function. Age and sex were determined in participant interview. Smoking history was assessed by self-report and coded as pack years. Disease “absence,” “possibility,” and “presence” were assessed using slight modifications of ascertainment algorithms used in the Women's Health and Aging Study.25 Analyses reported here controlled for “presence” of angina, myocardial infarction, diabetes, and cancer.
Systemic inflammatory regulation: Conceptual and modeling framework
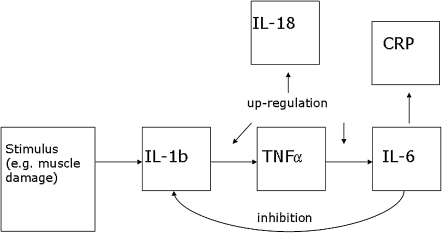
It is widely recognized that the inflammatory response is regulated by a delicate balance between stimulating and inhibitory signaling pathways involving many molecules. Our conceptual model is necessarily simplified and limited to biomarkers measured in InCHIANTI (Fig. 1). Proinflammatory events trigger release of IL-1β and TNF-α, with amplification by IL-1β via partial induction of TNF-α16 Release of IL-6 and IL-18 follow, directly and via activation of NF-κB.26–28 IL-6 induces the production of acute-phase proteins, including CRP.29 IL-18 amplifies the inflammatory cascade. It is increasingly recognized that the proinflammatory response is self-limited, as when IL-6 reaches a threshold level, a feedback inhibitory response is exerted on IL-1β and TNF-α.30–32 TGF-β is thought to be both pro- and antiinflammatory.18
FIG. 1.
Mechanism by which inflammatory system homeostasis is maintained. Not shown are interleukin-1 receptor antagonist (IL-1RA), which is treated as a surrogate for IL-1β, and transforming growth factor-β (TGF-β), which is thought to have both pro- and antiinflammatory effects. IL-1b, Interleukin-1β; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein.
Our analysis aimed to detect the two “constituents” of inflammatory regulation predicted by the theory just described: One of up-regulation, involving elevations of potentially all the biomediators, and another of down-regulation, contrasting elevations of IL-6 and CRP against depressed levels of biomediators preceding IL-6 in the release order. It employed a latent “factor” model33 envisioning each constituent as a “score,” gj, with values indicating “extent” of proinflammation, j = 1, 2. Then, biomediator values yk, k = 1, … ,7 are hypothesized to arise from inflammation scores as
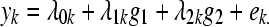 |
(Eq.1) |
“Loadings” λjk, when standardized, represent correlations between the underlying inflammation score gj and each kth measured biomediator. The theory predicts all g1 loadings to be positive, indicating “up-regulation.” For g2, the theory predicts loadings of different sign for IL-6 and its by-product than for biomediators earlier in the release order.
Data analysis
Variables were plotted and tabulated to inform choice of statistical methods. To correct for right skewness, natural logarithms of biomediator concentrations (henceforth, biomediator values) were used in all analyses. Scatter plots revealed 49 exceptionally outlying biomediator values. To preclude undue influence on multivariate analyses, outliers were truncated to values (m = number truncated): −3.0 < log(IL-6) <4.0 (m = 4); −3.0 < log(TNF-α) < 4.0 (m = 2); 2.4 < log(IL-1RA) <7.0 (m = 4); log(IL-1β) < −4.0 or > 2.0 (m = 39). Distributions of variables were contrasted between our eligible sample, analytic sample, and subsample with a valid mobility score. Wilcoxon rank sum tests were used for age and pack years of smoking; t-tests, with Bartlett correction as necessary, for biomediator values; and chi-squared tests, for all other variables.
To implement and evaluate our conceptual framework, latent factor models (Eq. 1) were fitted to biomediator values. Following the stated theory, the IL-1RA coefficient for the down-regulation component was constrained to equal zero. Scores, gj, and residual errors, ek, were assumed to be normally distributed and statistically independent—the latter, consistent with full accounting of biomediator interrelationships by up- and down-regulation components of inflammation at equilibrium mixing in serum. Models (Eq. 1) were evaluated for their ability to reproduce observed interrelationships using likelihood ratio goodness-of-fit tests and correlation matrix residual checking.34
To synthesize the biomediators into indices measuring up- and down-regulation components, principal components analysis (PCA)35 was used. PCA identifies weighted averages of (z-score standardized) biomediator values that have highest variance while being mutually uncorrelated, thus most efficiently distinguish individuals. For the model in (Eq. 1), biomediator “weights” (coefficients) in PCA approximate factor loadings, possibly after rotation.
To evaluate whether inflammatory biomediators inform the risk of adverse physical functioning in older adults, mobility and frailty outcomes were regressed on biomediator indices, all at once, and then separately on individual biomediators. For these latter regressions, models analyzing one biomediator at a time and all biomediators at once were compared. Outcome associations are reported per a one standard deviation inflammation increase; for the functioning analysis, they are additionally given in units of standard deviations of mobility score, as standardized betas.
Two analyses addressed whether individual biomarker values informed risk beyond the inflammation indices and vice versa. First, biomediators were added one at a time to models including all inflammation indices. Second, forward stepwise regression analyses treating indices, biomediator values, and control variables as candidates were run with model entry at p = 0.15, both forcing, and not forcing, the indices for model inclusion.
Finally, analyses were conducted to determine whether associations between inflammation measures and physical function outcomes generalized across the assessments comprising the outcomes, or were specific to selected assessments. Each functioning component assessment (six) and frailty criterion (five) was separately regressed on the inflammation indices.
All analyses adjusted for all control variables. Analyses excluding individuals who reported needing help in performing one or more Activities of Daily Living (ADL) are reported.36 Analyses without this exclusion differed by fewer than 10 individuals and minimally in findings. Our mobility score and all but one of its components were continuously scaled; these were analyzed using linear regression. The neurological intactness score was categorized as 0 impairments, 1–3 impairments, and more than 3 impairments, and then analyzed using ordinal logistic regression.37 All other outcomes were binary; they were analyzed using logistic regression. All analyses were diagnosed for fit and robustness to outlying values using residual plots.38
Results
Population characteristics
Our eligible sample (N = 967) included slightly more women than men and never- than ever-smokers (Table 1). No disease considered was present in more than 11% of individuals. Those with (n = 720), compared to those without (n = 247), a valid mobility score were significantly younger (p < 0.001), but there were no differences by sex, disease history, or smoking status. In analyses contrasting control variable distributions between our eligible sample and those age-eligible but lacking biomediator data (n = 188), those without biomediator data tended to be older (p < 0.001) and more frequently diabetic (p = 0.043), but otherwise were similar. Our mobility subsample was functionally robust, with mean usual walking speed of 1.03 meters per second (m/s), 95% confidence interval (CI) = (1.02,1.05). In our frailty subsample (n = 750), 11.6% were frail. Among individuals excluded from mobility analyses, all but 27 lacked data on either grip strength (n = 176 missing) or the fast-pace walk (n = 104 missing). A large majority of failures to obtain grip strength in the InCHIANTI baseline visit (70%) were due to a broken or unavailable dynamometer. Three quarters of those without data on the fast-pace walk were excluded from our primary analyses due to needing help in ADL tasks or lacking disease presence information.
Table 1.
Descriptive Characteristics of Eligible InCHIANTI Participants
| Full eligible sample (n = 967) | Mobility analysis sample (n = 720) | |
|---|---|---|
| Age cohort | ||
| Age, years‡ | 74.9 (7.34) | 73.9 (6.48) |
| 65–74 years‡ | 533 (55.1) | 431 (59.9) |
| 75–84 years‡ | 311 (32.2) | 229 (31.8) |
| 85+ years‡ | 123 (12.7) | 60 (8.33) |
| Sex | ||
| Female | 551 (57.0) | 411 (57.1) |
| Diseases | ||
| Angina | 66 (6.83) | 45 (6.25) |
| Myocardial infarction | 41 (4.49) | 30 (4.17) |
| Cancer | 64 (6.75) | 49 (6.81) |
| Diabetes | 103 (10.7) | 71 (9.86) |
| Cigarette use | ||
| Smoking, pack years | 11.8 (20.1) | 11.8 (19.9) |
| 0 | 578 (59.8) | 432 (60.0) |
| >0, <10 | 89 (9.20) | 60 (8.33) |
| 10–<30 | 128 (13.2) | 94 (13.1) |
| 30+ | 172 (17.8) | 134 (18.6) |
| Inflammatory markers | ||
| IL-1RA, pg/mL* | 133.6 (1.690) | 130.1 (1.650) |
| TNF-α, pg/mL* | 4.514 (2.334) | 4.371 (2.366) |
| IL-1β, pg/mL | 0.1360 (2.317) | 0.1334 (2.247) |
| IL-6, pg/mL‡ | 1.439 (2.197) | 1.342 (2.213) |
| CRP, g/L‡ | 2.669 (2.740) | 2.412 (2.603) |
| IL-18, pg/mL† | 383.7 (1.427) | 375.7 (1.416) |
| TGF-β, ng/mL | 9123 (2.228) | 9088 (2.254) |
Values are means (SD) for age in years and smoking in pack years, geometric means (geometric SD) for inflammatory markers, and n (%) otherwise. In tests of difference between the mobility analytic sample and its complement among eligible participants, p values exceeded 0.15 for sex, cigarette use, all disease variables, IL-1β and TGF-β, and otherwise were: *0.01 ≤ p < 0.05; †0.001 ≤ p < 0.01; ‡p < 0.001. Myocardial infarction diagnoses were available for n = 914, and cancer diagnoses were available for n = 948, in the eligible sample.
IL-1RA, Interleukin-1 receptor antagonist; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; TGF-β, transforming growth factor-β.
Biomediator value distributions
Geometric means are reported in Table 1. Intercorrelations among biomediator values were highest between IL-6 and CRP (r = 0.46 in the full eligible sample), IL-1RA (r = 0.32), and IL-18 (r = 0.21); and between IL-1RA and CRP (r = 0.35) and IL-18 (r = 0.26). Correlations between 0.10 and 0.20 were observed between TNF-α and IL-1RA, TNF-α and IL-18, and IL-18 and CRP. All of these, as well as smaller correlations between TNF-α and CRP and TGF-β and IL-1β, were statistically significant (p ≤ 0.05). Distributions differed significantly between our mobility subsample (n = 720) and the remainder of our analytic sample (n = 247) for IL-1RA, TNF-α, IL-6, IL-18, and CRP (Table 1); all biomediator concentrations tended to be lower in the mobility subsample.
Latent factor analyses of conceptual framework
As our theory predicted, a model with one inflammation factor did not adequately describe biomediator value interrelationships (goodness of fit rejected with p value <0.0001). There were large differences between observed correlations and those predicted by this model for TNF-α and IL-6, CRP and IL-18, and IL-1RA and IL-18. In contrast, the model implementing a theory of up- and down-regulation dimensions well fit the observed correlations, with likelihood ratio goodness of fit p values exceeding 0.5. Estimated correlations between the “up-regulation” factor and all biomediator values were positive (Table 2). For the “down-regulation” factor, they were negative with IL-6 and CRP, positive with TNF-α, and otherwise negligible. To ensure that correlations were not driven by aging effects on cytokines, we added age as a predictor of the latent inflammation constructs; results were very similar.
Table 2.
Measurement of Generalized Inflammatory Regulation
| |
Latent factors |
Principal components |
|||
|---|---|---|---|---|---|
| “Up”-regulation | “Down”-regulation | “Up”-regulation | IL-1/TGF | “Down”-regulation | |
| IL-1RA (pg/mL) | 0.68 | 0 | 0.51 | −0.079 | 0.11 |
| TNF-α (pg/mL) | 0.28 | 0.14 | 0.21 | −0.23 | 0.78 |
| IL-1β (pg/mL) | 0.08 | <0.010 | 0.09 | 0.71 | 0.09 |
| IL-6 (pg/mL) | 0.47 | −0.74 | 0.51 | −0.025 | −0.40 |
| CRP (g/L) | 0.50 | −0.30 | 0.52 | 0.010 | −0.30 |
| IL-18 (pg/mL) | 0.39 | −0.027 | 0.38 | −0.11 | 0.27 |
| TGF-β (ng/mL) | 0.10 | <0.010 | 0.11 | 0.65 | 0.23 |
Values for latent factors are estimated correlations with the biomediator values; for principal components (PCs), they are coefficients of biomediator values in the PCs. n = 967. For the PCs, respective percentages of variance explained are 28.1%, 15.0%, and 14.7%.
IL-1, Interleukin-1; TGF, transforming growth factor-β; IL-1RA, interleukin-1 receptor antagonist; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CRP, C-reactive protein; TGF-β, transforming growth factor-β.
Correlations between the first PCA index and the individual biomediators (PCA coefficients) were similar to those between “up-regulation” construct and individual biomediators (loadings; Table 2). However coefficients for the second PCA index did not well approximate the “down-regulation” loadings, but were highest for IL-1β and TGF-β. Instead, coefficients of the third PCA index were similar to the down-regulation loadings, albeit assigning relatively higher weight to TNF-α, and less to IL-6. The variances of the second two PCA indices were nearly identical to each other, with both eigenvalues greater than 1; therefore, we carried forward the first three PCA indices for use in analyses predicting adverse outcomes. Henceforth we refer to these respectively as “up-regulation,” “IL-1/TGF,” and “down-regulation” indices.
Analyses of inflammation with adverse outcomes
Increased up-regulation was statistically significantly associated with a mean mobility score reduction of 0.113 standard deviations, 95% CI = (−0.167, −0.060), and with a 32% increase in the odds of being frail, 95% CI for odds ratio (OR) = (1.03, 1.70). Neither adverse outcome was strongly associated with either the IL-1/TGF or down-regulation index.
In analyses predicting mobility by each individual inflammatory biomediator, IL-6, IL-1RA, and IL-18 were all significantly associated with worse mobility (Table 3; p < 0.05); the CRP association was borderline significant. These four biomediators most strongly correlate with the up-regulation construct. Standardized betas were 13%–42% smaller than for the up-regulation index. In analyses considering all biomediators simultaneously, only IL-6 remained significantly associated with worse functioning, with IL-1RA borderline so. No individual biomediator predicted mobility independently of the up-regulation index; the index remained significantly predictive independently of each biomediator.
Table 3.
Associations of Functioning with Individual Biomediators
| Univariable | Mutually adjusted | |
|---|---|---|
| IL-1RA | −0.082 (−0.14, −0.030) | −0.054 (−0.11, 0.003) |
| TNF-α | −0.022 (−0.074, 0.030) | −0.010 (−0.063, 0.043) |
| IL-1β | <0.001 (−0.053, 0.052) | 0.0053 (−0.047, 0.057) |
| IL-6 | −0.098 (−0.15, −0.044) | −0.083 (−0.14, −0.024) |
| CRP | −0.047 (−0.099, 0.0059) | 0.0059 (−0.052, 0.064) |
| IL-18 | −0.066 (−0.12, −0.010) | −0.047 (−0.10, 0.010) |
| TGF-β | −0.022 (−0.074, 0.031) | −0.014 (−0.067, 0.038) |
Values are standardized beta coefficients (95% confidence intervals). Both sets of models also included control variables specified in the Methods section. Univariable models entered one biomediator at a time; mutually adjusted models entered all biomediators simultaneously. n = 720.
IL-1RA, Interleukin-1 receptor antagonist; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CRP, C-reactive protein; TGF-β, transforming growth factor-β.
In analyses separately predicting frailty by the individual biomediators, the association with IL-6 was significant (OR = 1.32; 95% CI = [1.04, 1.69]), and the IL-1RA association nearly so (OR = 1.28; 95% CI = [0.999, 1.65]) (Table 4). In analyses simultaneously considering all biomediators, no biomediator retained significance, reflective of co-linearity given a relatively rare outcome. In stepwise analyses the up-regulation index was selected as a significant predictor of frail status, and no individual biomediator predicted frailty independently of the index. However the association did not remain significant independently of IL-6, IL-1RA, or CRP (added separately); the index and IL-6 had identical standardized strengths of association as separate predictors of frailty; and preferential selection of the index in stepwise regression was sensitive to the choice of covariates for which analyses controlled. In summary, up-regulation summarized that aspect of inflammation relating to frailty but did not afford prediction that was convincingly superior to IL-6 alone.
Table 4.
Associations of Frailty with Individual Biomediators
| Univariable | Mutually adjusted | |
|---|---|---|
| IL-1RA | 1.28 (0.999, 1.65) | 1.20 (0.908, 1.59) |
| TNF-α | 0.996 (0.773, 1.28) | 0.975 (0.752, 1.26) |
| IL-1β | 0.946 (0.740, 1.21) | 0.931 (0.725, 1.19) |
| IL-6 | 1.32 (1.04, 1.69) | 1.25 (0.954, 1.64) |
| CRP | 1.20 (0.940, 1.54) | 1.04 (0.784, 1.37) |
| IL-18 | 1.01 (0.774, 1.31) | 0.953 (0.723, 1.26) |
| TGF-β | 1.04 (0.811, 1.33) | 1.04 (0.810, 1.34) |
Values are multiplicative increases in odds of frailty per standard deviation increases in log serum biomarker concentrations, that is, odds ratios (95% confidence intervals). Both sets of models also included control variables specified in the Methods section. Univariable models entered one biomediator at a time; mutually adjusted models entered all biomediators simultaneously. n = 750.
IL-1RA, Interleukin-1 receptor antagonist; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CRP, C-reactive protein; TGF-β, transforming growth factor-β.
In analyses of the distinct functioning signs, exacerbated up-regulation was significantly associated with worse mobility for all signs except handgrip and lower extremity power. Only one of the 12 remaining associations between biomarker summary (PCAs 2 and 3) and mobility measures (six) was significant, consistent with no associations considering testing error. Individual analyses of the separate frailty indicators showed more interesting specificity (Table 5). Exacerbated up-regulation was implicated in lesser physical activity and slowness; inhibited down-regulation, in weight loss; and both, in weakness. This raised questions as to whether limited power might be obscuring a down-regulation association with frailty; in a post hoc ordinal logistic regression analysis, both the up- and down-regulation summaries were significant predictors of the number of frailty criteria, with ORs for a higher frailty count of 1.31 (95% CI [1.14,1.50]) and 1.18 (95% CI [1.03,1.35]) respectively.
Table 5.
Associations of Frailty Criteria With Inflammation Indices
| Up-regulation PC | IL-1/TGF PC | Down-regulation PC | |
|---|---|---|---|
| Weight loss | 0.839 (0.624, 1.13) | 0.941 (0.712, 1.24) | 0.705 (0.535, 0.928) |
| Exhaustion | 1.13 (0.964, 1.32) | 1.03 (0.890, 1.20) | 1.05 (0.905, 1.22) |
| Low physical activity | 1.38 (1.10, 1.72) | 0.993 (0.801, 1.23) | 0.962 (0.776, 1.19) |
| Slowness | 1.37 (1.03, 1.82) | 1.06 (0.807, 1.38) | 0.855 (0.647, 1.13) |
| Weakness | 1.34 (1.14, 1.59) | 1.08 (0.922, 1.26) | 0.779 (0.664, 0.914) |
Values are multiplicative increases in odds of manifesting frailty criteria per standard deviation increases in inflammation index values, that is, odds ratios (95% confidence intervals). Models also included control variables specified in the Methods section. n = 750.
PC, Principal component; IL-1, interleukin-1; TGF, transforming growth factor.
Discussion
We implemented a construct operationalizing distinct components of generalized inflammation using data collected in an epidemiological survey of a representative older adult population. Our construct was grounded in biology theorizing that inflammatory regulation is maintained via an up- and down-regulation feedback loop in a specific cytokine sequence. In latent factor analyses, observed interrelationships among seven serum-based inflammatory biomediators were highly consistent with the biological predictions. PCA of the biomediator values yielded an index approximating latent up-regulation, which was independently associated with worsened mobility functioning and frailty risk. Patterns of association between mobility and the individual cytokines were strongest for the biomediators involved in up-regulation. Association of mobility with the up-regulation index was stronger than, persisted independently of, and accounted for association with each individual biomediator. Two additional indices were not strongly associated with mobility; however, one approximating latent down-regulation was intriguingly associated with the number of positive frailty criteria and the individual criteria involving strength and weight loss. These findings support that systemic inflammation is a construct with internal biological validity and relevant to the process that leads to mobility disability and frailty in older persons. They also suggest that specific aspects of the inflammatory process can be measured through profiles of inflammatory biomarkers.
Previous publications have applied factor or principal components analysis to multiple inflammatory markers. Many have employed sample sizes in the tens,39–41 involved improved risk stratification together with noninflammatory factors in clinically diseased cohorts,42,43 or explored the value of expanding the metabolic syndrome.44,45 We are aware of three papers, besides ours, that have analyzed multiple cytokines toward improved measurement of inflammation per se. An exploratory factor analysis among 580 members of the Women's Ischemia Syndrome Evaluation cohort found “proinflammation,” “pro- and antiinflammation,” and “immunosuppressive” factors remarkably similar to our “up-regulation,” “down-regulation,” and “IL-1β/TGF” components.46 Similarly, a principal components analysis of 320 consecutive acute coronary syndrome patients found “systemic inflammation,” “antiinflammation,” and “local inflammation-endothelial function” components.47 That these papers and ours should have suggested three similar dimensions of inflammatory regulation is compelling. In contrast to these investigations, ours was focused on older adults, entailed a much larger sample, and involved a population-based cohort. A very recent paper14 shares these characteristics; it analyzed a somewhat different set of cytokines. Our research has uniquely validated a biologically motivated model of inflammatory regulation, beyond implementing empirical data reduction.
Association between frailty and the inflammation indices was not stronger than with IL-6 alone. One explanation is that IL-6 plays a critical role in the development of frailty. Alternatively, as suggested by Table 5, inflammation may participate in the genesis of frailty through multiple mechanisms that cannot be represented by a single index. It should be pointed out that our study is not powered to distinguish specific inflammatory regulation effects on frailty. Fewer than 100 participants were considered frail, and the prevalence of ADL disability was low.
Our analysis has appreciable data missing. The analysis of persons with complete biomediator information preferentially loses older individuals, and complete mobility and frailty analysis, individuals with higher biomarker values. Both lose individuals who may differ from those analyzed in ways the data cannot inform. We stand behind complete-case analysis for the analysis of the inflammatory construct because its findings were not sensitive to age adjustment and most individuals excluded for missing biomediator measures did not have valid measurements on any biomediator. Our analyses of mobility and frailty are valid, assuming that outcomes are missing in ways that may depend on inflammatory or control variable status but not on mobility or frailty given these other characteristics.48 This assumption may not hold. Because inflammation tended to be elevated among those lost from the functioning subsamples, we regard it more likely that our findings have been diluted than that spurious findings have been generated.
We derived inflammation indices via PCA and handled extreme values in biomarker variables via truncation, seeking to employ strategies easily replicable by other investigators. Structural equations analyses would more effectively account for errors in measuring inflammatory dimensions than PCA, thus the simplification to PCA stands to be conservative. Factor and PC analyses were not sensitive to truncation of outlying values versus utilizing raw values. In summary, we do not believe that methodological simplification has qualitatively distorted our findings.
This initial work aimed to create biomarker indices that can capture multiple aspects of inflammation. It indicates that this line of research should be fully pursued. Our finding should be confirmed in a larger population that includes substantial numbers of frail and disabled persons. The best biomediators to be included in global indices should be selected over a larger range of markers. A huge number of proteins could be considered using microarray or multiple beads methods, but this technology still shows low sensitivity and questionable reliability. Thus, short of relevant advancements, we believe the selection of markers should continue to be biologically motivated. Study of the biomediators' utility for longitudinal prediction of frailty outcomes should also be pursued; the InCHIANTI study will provide ideal data to this end when the longitudinal frailty outcomes have been adjudicated. The line of investigation we have pursued is crucial to a better and more specific understanding of the biology that underlies the human inflammatory response and ultimately promises to help identify older persons at high risk for adverse outcomes, identify potential treatments, and, perhaps, test treatment effects.
Acknowledgments
Yi Huang was a Ph.D. student in the Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health while the bulk of this research was conducted. The InCHIANTI study was supported as a targeted project (ICS 110.1/RS97.71) by the Italian Ministry of Health as well as by National Institute on Aging (NIA) contracts 263 MD 9164 13 and 263 MD 821336. Funding for this study was also provided by NIA grants R01 AG027012 and P50 AG021334-01.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cesari M. Penninx BW. Pahor M. Lauretani F. Corsi AM. Rhys WG. Guralnik JM. Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 2.Cohen HJ. Harris T. Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 3.Ferrucci L. Bandinelli S. Benvenuti E. Di Iorio A. Macchi C. Harris TB. Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. , [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L. Penninx BW. Volpato S. Harris TB. Bandeen-Roche K. Balfour J. Leveille SG. Fried LP. Guralnik JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L. Corsi A. Lauretani F. Bandinelli S. Bartali B. Taub DD. Guralnik JM. Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krabbe KS. Pedersen M. Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Leng S. Chaves P. Koenig K. Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DR. The relationship between functional status and inflammatory disease in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:995–998. doi: 10.1093/gerona/58.11.m995. [DOI] [PubMed] [Google Scholar]
- 9.Visser M. Pahor M. Taaffe DR. Goodpaster BH. Simonsick EM. Newman AB. Nevitt M. Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 10.Walston J. McBurnie MA. Newman A. Tracy R. Kop WJ. Hirsch CH. Gottdiener J. Fried LP Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: Results from the Cardiovascular Health Study. Arch Int Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 11.Harris TB. Ferrucci L. Tracy RP. Corti MC. Wacholder S. Ettinger WH Jr. Heimovitz H. Cohen HJ. Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M. Penninx BW. Newman AB. Kritchevsky SB. Nicklas BJ. Sutton-Tyrrell K. Rubin SM. Ding J. Simonsick EM. Harris TB. Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM. Ferrucci L. Liu K. Criqui MH. Greenland P. Green D. Guralnik JM. Ridker PM. Taylor LM. Rifai N. Tian L. Zheng J. Pearce WH. Schneider JR. Vonesh E. D-dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2005;53:1688–1696. doi: 10.1111/j.1532-5415.2005.53510.x. , [DOI] [PubMed] [Google Scholar]
- 14.Hsu FFC. Kritchevsky SB. Liu Y. Kanaya A. Newman AB. Perry SE. Visser M. Pahor M. Harris TB. Nicklas BJ Health ABC Study. Association between inflammatory components and physical function in the health, aging, and body composition study: A principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64:581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautmans I. Njemini R. Lambert M. Demanet C. Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci. 2005;60:361–367. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE. Baumgartner RN. Cytokine-related aging process. J Gerontol A Biol Sci Med Sci. 2004;59:M924–M929. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opal SM. DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 19.Kendall FP. McCreary EK. Muscles, Testing and Function. Williams & Wilkins; Baltimore: 1983. [Google Scholar]
- 20.Bassey EJ. Short AH. A new method for measuring power output in a single leg extension: Feasibility, reliability, and validity. Eur J Appl Physiol. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 21.Bandinelli S. Benvenuti E. Del Lungo I. Baccini M. Benvenuti F. Di Iorio A. Ferrucci L. Measuring muscular strength of the lower limbs by hand-held dynamometer: A standard protocol. Aging. 1999;11:287–293. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L. Bandinelli S. Cavazzini C. Lauretani F. Corsi A. Bartali B. Cherubini A. Launer L. Guralnik J.M. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116:807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Fried LP. Tangen CM. Walston J. Newman AB. Hirsch C. Gottdiener J. Seeman T. Tracy R. Kop WJ. Burke G. McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. , [DOI] [PubMed] [Google Scholar]
- 25.Fried LP. Kasper JD. Williamson JD. Skinner EA. Morris CD. Hochberg MC. Disease ascertainment algorithms. In: Guralnik JM, editor; Fried LP, editor; Simonsick EM, editor; Kasper JD, editor; Lafferty ME, editor. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institutes of Health, National Institute on Aging; Bethesda, MD: 1995. NIH Publication No. 95-4009. [Google Scholar]
- 26.Hanada T. Yoshimura A. Regulation of biomediator signaling and inflammation. Biomediator Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 27.Li Q. Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 28.Merola M. Blanchard B. Tovey MG. The kappa B enhancer of the human interleukin-6 promoter is necessary and sufficient to confer an IL-1 beta and TNF-alpha response in transfected human cell lines: Requirement for members of the C/EBP family for activity. J Interferon Cytokine Res. 1996;16:783–798. doi: 10.1089/jir.1996.16.783. [DOI] [PubMed] [Google Scholar]
- 29.Ershler WB. Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 30.Hurst SM. Wilkinson TS. McLoughlin RM. Jones S. Horiuchi S. Yamamoto N. Rose-John S. Fuller GM. Topley N. Jones SA. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. , [DOI] [PubMed] [Google Scholar]
- 31.Ulich TR. Guo KZ. Remick D. del Castillo J. Yin SM. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 32.Yanagawa Y. Kawakami M. Okada Y. Moderate hypothermia alters interleukin-6 and interleukin-1alpha reactions in ischemic brain in mice. Resuscitation. 2002;53:93–99. doi: 10.1016/s0300-9572(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 33.Bartholomew DJ. Latent Variable Models and Factor Analysis. Charles Griffin; London: 1987. p. 4. [Google Scholar]
- 34.Bollen KA. Structural Equations with Latent Variables. Wiley; New York: 1989. pp. 257–262. [Google Scholar]
- 35.Johnson RA. Wichern DW. Applied Multivariate Statistical Analysis. Prentice Hall; Englewood Cliffs, NJ: 1988. pp. 340–377. [Google Scholar]
- 36.Katz S. Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 37.McCullagh P. Regression models for ordinal data (with discussion) J Royal Stat Soc B. 1980;42:109–142. [Google Scholar]
- 38.Cook D. Weisberg S. An Introduction to Regression Graphics. Wiley; New York: 1994. [Google Scholar]
- 39.Grönke L. Kanniess F. Holz O. Jörres RA. Magnussen H. The relationship between airway hyper-responsiveness, markers of inflammation and lung function depends on the duration of the asthmatic disease. Clin Exp Allergy. 2002;32:57–63. doi: 10.1046/j.0022-0477.2001.01297.x. [DOI] [PubMed] [Google Scholar]
- 40.Aldonyte R. Eriksson S. Piitulainen E. Wallmark A. Janciauskiene S. Analysis of systemic biomarkers in COPD patients. COPD. 2004;1:155–164. doi: 10.1081/copd-120030828. [DOI] [PubMed] [Google Scholar]
- 41.Riechelmann H. Deutschle T. Rozsasi A. Keck T. Polzehl D. Bürner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186–1191. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 42.Folkerts U. Nagel D. Vogt W. The use of cluster analysis in clinical chemical diagnosis of liver disease. J Clin Chem Clin Biochem. 1990;28:399–406. doi: 10.1515/cclm.1990.28.6.399. [DOI] [PubMed] [Google Scholar]
- 43.Koukkunen H. Penttilä K. Kemppainen A. Halinen M. Penttila I. Rantanen T. Pyörälä K. C-reactive protein, fibrinogen, interleukin-6 and tumour necrosis factor-alpha in the prognostic classification of unstable angina pectoris. Ann Med. 2001;33:37–47. doi: 10.3109/07853890109002058. [DOI] [PubMed] [Google Scholar]
- 44.Sakkinen PA. Wahl P. Cushman M. Lewis MR. Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- 45.Hanley A.J. Festa A. D'Agostino R. B., Jr Wagenknecht L.E. Savage P.J. Tracy R.P. Saad M. F. Haffner S.M. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: Factor analysis using directly measured insulin sensitivity. Diabetes. 2004 Jul;53(7):1773–81. doi: 10.2337/diabetes.53.7.1773. [DOI] [PubMed] [Google Scholar]
- 46.Kip KE. Marroquin OC. Shaw LJ. Arant CB. Wessel TR. Olson MB. Johnson BD. Mulukutla S. Sopko G. Merz CN. Reis SE. Global inflammation predicts cardiovascular risk in women: A report from the Women's Ischemia Syndrome Evaluation (WISE) study. Am Heart J. 2005;150:900–906. doi: 10.1016/j.ahj.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Tziakas DN. Chalikias GK. Kaski JC. Kekes A. Hatzinikolaou EI. Stakos DA. Tentes IK. Kortsaris AX. Hatseras DI. Inflammatory and anti-inflammatory variable clusters and risk prediction in acute coronary syndrome patients: A factor analysis approach. Atherosclerosis. 2007;193:196–203. doi: 10.1016/j.atherosclerosis.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7:303–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]