Abstract
Ethanol metabolism by liver generates short lived reactive oxygen species that damage liver but also affects distal organs through unknown mechanisms. We hypothesized that dissemination of liver oxidative stress proceeds through release of biologically active oxidized lipids to the circulation. We searched for these by tandem mass spectrometry in plasma of rats fed a Lieber-DeCarli ethanol diet or in patients with established alcoholic liver inflammation, steatohepatitis. We found a severalfold increase in plasma peroxidized phosphatidylcholines, inflammatory and pro-apoptotic oxidatively truncated phospholipids, and platelet-activating factor, a remarkably potent and pleiotropic inflammatory mediator, in rats chronically ingesting ethanol. Circulating peroxidized phospholipids also increased in humans with established steatohepatitis. However, reactive oxygen species generated by liver ethanol catabolism were not directly responsible for circulating oxidized phospholipids because the delayed appearance of these lipids did not correlate with ethanol exposure, hepatic oxidative insult, nor plasma alanine transaminase marking hepatocyte damage. Rather, circulating oxidized lipids correlated with steatohepatitis and tumor necrosis factor-α deposition in liver. The organic osmolyte 2-aminoethylsulfonic acid (taurine), which reduces liver endoplasmic reticulum stress and inflammation, even though it is not an antioxidant, abolished liver damage and the increase in circulating oxidized phospholipids. Thus, circulating oxidized phospholipids are markers of developing steatohepatitis temporally distinct from oxidant stress associated with hepatic ethanol catabolism. Previously, circulating markers of the critical transition to pathologic steatohepatitis were unknown. Circulating oxidatively truncated phospholipids are pro-inflammatory and pro-apoptotic mediators with the potential to systemically distribute the effect of chronic ethanol exposure. Suppressing hepatic inflammation, not ethanol catabolism, reduces circulating inflammatory and apoptotic agonists.
Keywords: Alcohol, Lipid Oxidation, Liver Injury, Mass Spectrometry (MS), Oxidative Stress
Introduction
Ethanol metabolism by liver cytochrome P450 2E1 leaks electrons to molecular oxygen to produce superoxide, O2˙̄ (1). This is an important side reaction because ablation of copper/zinc superoxide dismutase that metabolizes this reactive oxygen specie promotes lipid peroxidation and liver damage by even mild ethanol exposure (1). Consumption of alcohol increases whole body oxidant stress as shown by the increased urinary excretion of the predominantly free radical-generated arachidonate metabolite F2α-iii isoprostane (2), by increased circulating levels of phosphatidylcholine hydroperoxides (3), and by an increase in circulating oxidized low density lipoproteins (4). The source of these extracellular oxidized lipid species is not known but is assumed to reflect hepatocyte production of O2˙̄ by cytochrome P450 2E1.
Liver damage from chronic alcohol ingestion is associated with, and appears to derive from, oxidative stress, and in particular from mitochondrial oxidative stress (1). This chronic ethanol-related disturbance in mitochondrial health contributes to increased production of reactive oxygen species, a central abnormality responsible for liver injury and disease progression (5, 6). A critical watershed for alcoholics is the progression from benign fat accumulation in liver, steatosis, to the inflamed state, steatohepatitis, that is a precursor for end stage liver disease. The transition to steatohepatitis currently can only be established through invasive liver biopsy because there are no circulating markers of this critical event. Although liver is the primary organ metabolizing ethanol, chronic alcohol ingestion damages organs other than liver (7). An unanswered question is how oxidant stress is spread beyond the liver in individuals and animal models chronically exposed to dietary ethanol because reactive oxygen species are transient.
Circulating oxidation products, rather than oxidants themselves, could systemically disperse liver oxidative stress. A primary target of oxidizing radicals are polyunsaturated fatty acids (8), which oxidize to esterified lipid (hydro)peroxides that are esterified in cellular and lipoprotein complex lipids. Because polyunsaturated fatty acyl residues predominate at the sn-2 position of the glycerol backbone of phospholipids, common products of oxidative attack are phospholipids containing sn-2 hydroperoxyoctadecadienoyl ester (HpODE)2 from linoleoyl oxidation and hydroperoxyeicosatetraenoyl ester (HpETE) derived from arachidonoyl peroxidation. Phospholipid hydroperoxides themselves have no established signaling role, although this may be changing (9), but are relevant because they reflect endogenous oxidative stress and so act as circulating biomarkers of oxidative stress (3). Phospholipid hydroperoxides may also be relevant because they are precursors of a host of oxidatively truncated phospholipids, which are formed by fragmentation of the fatty acyl chain at the site of the (hydro)peroxy function (10), that can be potent inflammatory and apoptotic agonists.
Truncated phospholipid oxidation products display varied biologic activities relevant to thrombosis and the inflammatory response because they are agonists (11) of the G protein-coupled receptor for platelet-activating factor (PAF) expressed by all elements of the innate immune system. This class of truncated phospholipid favors a robust inflammatory response that would augment reactive oxygen species production. Oxidatively truncated phospholipids also affect cellular homeostasis by reducing mitochondrial integrity and function (12, 13). A common truncation product of HpODE residues esterified at the sn-2 position is an esterified sn-2 azelaoyl fragment, and this oxidatively truncated phospholipid is particularly mitotoxic and effectively initiates the intrinsic caspase cascade leading to apoptotic cell death (14, 15). Should these types of oxidation products accumulate in the circulation, production of reactive oxygen species in one organ would lead to mitochondrial damage, loss of mitochondrial function, and eventually cell death in distal tissues.
We therefore investigated the hypotheses that oxidized lipids and their biologically active truncation products accumulate in the circulation of animals chronically ingesting clinically relevant amounts of ethanol and that these result from liver oxidant stress imposed by ethanol catabolism. We verified the first hypothesis but found that rather than simply reflecting liver catabolism of ethanol, these circulating lipids instead mark the transition from fatty liver to active liver inflammation. We then verified the resulting hypothesis that suppressing liver inflammation suppresses circulating oxidized phospholipids.
EXPERIMENTAL PROCEDURES
Materials
Adult male Wistar rats weighing 170–180 g were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). Lieber-DeCarli high fat ethanol diet was purchased from Dyets (Bethlehem, PA). [acetyl-3H-]PAF was the product of PerkinElmer Life Sciences, PAF, [2H4]PAF, azelaoyl phosphatidylcholine, other truncated oxidized phospholipids, and all hydroxyoctadecadienoic acids (HODEs) and HETEs (2H-labeled or unlabeled) were from Cayman Chemical (Ann Arbor, MI). Hydroperoxyoctadecadienoyl phosphatidylcholine (HpODE-PC) and hydroxyoctadecadienoyl phosphatidylcholine (HODE-PC) were synthesized by Dr. Robert Solomon (Case Western Reserve University).
Animal Model and Ethanol Feeding
The chronic ethanol feeding model used in this study has been previously described (16) using animals that received humane care according to the criteria outlined in Ref. 52 were used in a protocol approved by the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC). Briefly, rats were randomly assigned to be ethanol-fed or assigned to a paired, isocaloric control group where maltose dextrin isocalorically substituted for ethanol in their liquid diet so the rats gained weight at the same rate. For the first 2 days of the protocol, rats in the ethanol group were fed with a liquid diet with 17% of the calories supplied as ethanol and then were provided an ad libitum liquid diet containing ethanol as 35% of total caloric value for 4 weeks (see Fig. 1). The amount of ethanol in the circulation is not excessive, being in the clinically relevant 0.1–0.15% range. 2-Aminoethylsulfonic acid (taurine) supplementation was at 30 g/liter as described (17). After the stated feeding period, animals were anesthetized, exsanguinated, and plasma-isolated and stored at −80° before batch analysis.
FIGURE 1.
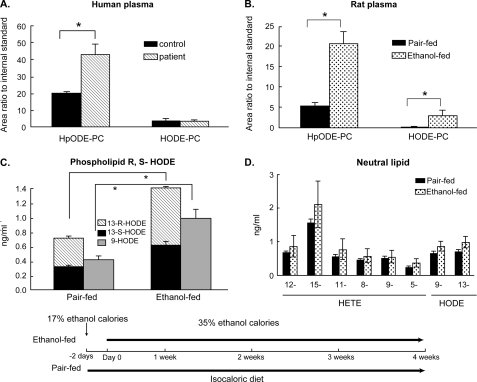
Hydroxy- and hydroperoxy-phospholipids increase in the circulation after chronic alcohol exposure. A, plasma phospholipid hydroperoxides from alcoholic patients. Human plasma was collected from patients with documented early stage alcoholic liver disease (n = 3) or healthy individuals (n = 5). Circulating levels of the oxidized phospholipids HpODE-PC and HODE-PC were measured as described under “Experimental Procedures.” B, plasma hydroperoxides in rats ingesting ethanol for 4 weeks. Plasma HpODE-PC and HODE-PC in rats (n = 5) chronically ingesting ethanol to provide 35% of their caloric intake or in paired control animals fed the same number of calories consumed by its paired ethanol-fed rat on the previous day (inset timeline). *, p < 0.05 comparing ethanol-fed to paired control. C, circulating oxidized phospholipids are a mixture of regio- and stereoisomers. Stereo- and regioisomers of H(p)ODE esterified in circulating phospholipids from rat plasma (n = 4) were determined by HPLC and mass spectrometry as described under “Experimental Procedures.” *, p < 0.05 comparing ethanol-fed to paired controls. D, oxidized fatty acyl residues in circulating neutral lipids. Regioisomers of arachidonoyl-derived HETEs and linoleoyl-derived HODEs in neutral lipids of rats maintained for 4 weeks on the Lieber-DeCarli ethanol diet or their isocaloric controls are shown. The inset depicts the experimental design where animals either ingest an ethanol supplemented liquid diet or are pair-fed an isocaloric diet for the stated times. Two days prior to the start of the experiment, animals in the ethanol-fed arm are habituated to the diet with lesser amounts of ethanol.
Immunohistochemistry
TNFα
Formalin-fixed paraffin-embedded rat liver sections were deparaffinized in Safeclear II xylene substitute and consecutively hydrated in 100, 95, and 70% followed by one wash in phosphate-buffered saline. Sections were treated with proteinase K (20 μg/ml) at 24 °C for 15 min for antigen retrieval. Sections were blocked with 10% rabbit serum and 0.1% Triton X-100 in phosphate-buffered saline and incubated overnight with 1:200 polyclonal goat anti-rat TNFα (R&D Systems, Minneapolis, MN). Washed sections were incubated with 1:1000 Alexa Fluor 488-conjugated donkey-anti-goat IgG. Sections were washed and mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA). Images acquired with a ×40 objective were quantified using ImagePro Plus software (Media Cybernetics Inc., Bethesda, MD).
4-Hydroxynonenal
Formalin-fixed paraffin-embedded rat liver sections were deparaffinized and hydrated as described for TNFα immunostaining. Sections were treated with proteinase K (20 μg/ml) at room temperature for 5 min for antigen retrieval. Sections were blocked with peroxidase block (DAKO North America. Inc., Carpinteria, CA) for 30 min, and then blocked with Biotin block (DAKO North America. Inc.) for 20 min (10 min for avidin block and 10 min for biotin block). Liver sections were incubated with rabbit anti-rat 4-hydroxynonenal antibody (Alpha Diagnostic International, 1:200 dilution) overnight at 4 °C. Liver sections were then incubated with biotinylated link (DAKO North America. Inc.) for 30 min, streptavidin peroxidase for 30 min, and then substrate-chromogen solution for 2 min at room temperature. Slices were counterstained with hematoxylin for 2 min and then mounted with DAKO Ultramount aqueous permanent mounting medium (DAKO North America. Inc.). Images were taken at ×40 by bright light field.
Histopathology
Individual features including degree of steatosis, inflammation, and ballooning were assessed in ethanol-fed and pair-fed animals by an experienced pathologist in a blinded fashion. Steatosis and inflammation based on inflammatory cell infiltrate were scored from 0 to 3, whereas ballooning was assessed as being either present or not. Circulating alanine transaminase (ALT) and aspartate aminotransaminase were quantified as described previously (17).
RNA Isolation and Quantitative Reverse Transcription-PCR for TNF-α in Liver
Total RNA was extracted from liver tissues preserved in RNAlater (Qiagen, Germantown, MD) using RNeasy mini kit (Qiagen, Germantown, MD). RNA content was measured using NanoDrop ND-1000 spectrophotometer. Messenger RNA expression was quantified by SYBR Green one-step reverse transcription-PCR for TNFα and S18 with the Bio-Rad MyiQ real-time PCR detection system. The primers were: sense, 5′-CTA TGT GCT CCT CAC CCA CA-3′; antisense, 5′-TGG AAG ACT CCT CCC AGG TA-3′. The expression of TNFα was normalized to S18 mRNA content and calculated relative to normal control.
Mass Spectrometry Analysis of Intact Oxidized Phospholipids in Rat Plasma
Lipids were extracted with [2H]PAF as an internal standard and quantified by liquid chromatography/electrospray ionization/tandem mass spectrometry. PAF was purified by silica normal phase HPLC before reversed phase LC/MS/MS (18). Each sample in 85% methanol was injected onto a reverse phase C18 HPLC column (2 × 150 mm, 5-μm ODS(2) Phenomenex) equilibrated with 85% methanol containing 0.2% formic acid at a flow rate of 0.2 ml/min. Oxidized phospholipids were resolved using a linear gradient from 85 to 100% methanol containing 0.2% formic acid over 5 min, the solvent composition was held at 100% methanol for 17 min, and then a linear gradient was formed from 100 to 85% methanol in 0.5 min and held for 6.5 min. Mass spectrometric analyses were performed with a Quattro Ultima triple-quadrupole mass spectrometer (Micromass, Wythenshawe, UK) configured with the capillary voltage at 5 kV, the cone voltage at 60 V, the source temperature at 120 °C, and a desolvation temperature at 250 °C. The flow rates for the nitrogen in the cone and desolvation gas were 90 and 811 liters/h, respectively. Collision-induced dissociation was obtained using argon gas. Analyses were performed using electrospray ionization in the positive-ion mode with multiple reaction monitoring. The transitions used to detect the choline phospholipids were the mass-to-charge ratio (m/z) for the molecular ion [M+H]+ and the m/z 184 phosphocholine daughter ion.
Mass Spectrometry of Esterified H(p)ODE Stereo- and Regioisomers
Plasma was collected from rats (n = 4) maintained for 4 weeks on the Lieber-DeCarli ethanol diet or their paired isocaloric controls. Phospholipids were purified over an amino solid phase extract column, reduced by NaBH4, and saponified by NaOH to recover reduced free fatty acids. 13R-HODE and 13S-HODE from the total phospholipid pool were separated by chiral HPLC with 13S-[2H4]HODE as an internal standard. 13R-HODE and 13S-HODE were quantified by LC/MS/MS, whereas 9-HODE was directly quantified by LC/MS/MS. Extracts were reconstituted in 50% methanol, injected into a reverse phase HPLC column (2 × 150 mm, 5-μm ODS(2) Phenomenex) at a flow rate of 0.2 ml/min. The gradient was started with methanol/water (1:1, v:v) containing 0.2% formic acid and held for 1 min. The solvent was increased to 85% methanol in 1 min and held for 4 min and then linearly increased to 100% methanol in 4 min and held for 8 min. The gradient was dropped to 50% and held for 6.5 min. The mass spectrometer parameters were: source capillary, 3.5 kV; cone voltage, 40 V; source temperature, 120 °C; desolvation temperature, 250 °C; collision energy, 10 eV; multiplier, 650 V. Negative-ion mode with multiple reaction monitoring of parent (19) and individual daughter ions of oxidized and unoxidized fatty acids used the m/z transitions: 5-HETE (319→115); 8-HETE (319→155); 9-HETE (319→151); 11-HETE (319→167); 12-HETE (319→179); 15-HETE (319→175); 9-HODE (295→171); 13-HODE (295→195); arachidonate (303→259); linoleate (279→261).
PAF Acetylhydrolase Activity
Hydrolysis of [acetyl-3H-]PAF by plasma was assessed by separation and solvent recovery of [3H]acetate (20). PAF hydrolysis was linear with the amount of plasma added.
Data Analysis
All data are presented as mean ± S.E. Independent t test (two groups) or one way analysis of variance (multiple groups) was performed by SPSS statistics software. Statistical significance was considered to be p < 0.05.
RESULTS
Plasma Peroxidized Phospholipids Are Increased in Subjects with Alcoholic Steatohepatitis
We used tandem mass spectrometry to quantify circulating phospholipid hydroperoxides in several consecutive patients undergoing a liver biopsy at our institution for clinical suspicion of alcoholic steatohepatitis, whose diagnosis was then confirmed by liver biopsy. We found a significant increase in circulating peroxidized phosphatidylcholine in these patients when compared with healthy control individuals (Fig. 1A). The cognate HODE-PC, formed by chemical or enzymatic reduction of the HpODE-PC hydroperoxide, was not different between the two groups. This unambiguous structural identification and quantitation show that increased phospholipid hydroperoxides described by Adachi et al. (3), using a problematic assay, do increase during chronic alcoholic use.
We determined whether an experimental model of chronic alcoholic liver injury, the Lieber-DeCarli diet, mirrored the increase in circulating hydroperoxy-containing phosphatidylcholines that has been proposed as a new marker of oxidative stress in alcoholic patients (3). Rats chronically ingesting a diet where ethanol provides 35% of their caloric intake were compared with rats where dextrin maltose was isocalorically substituted for ethanol. We found the content of HpODE-PC and HODE-PC was increased 3- and 10-fold, respectively, in animals ingesting ethanol for 4 weeks when compared with their pair-fed controls (Fig. 1B). We determined that the circulating hydroperoxy- and hydroxy-phospholipids were non-enzymatic oxidation products because they were composed of an equal mixture of R- and S-stereoisomers (Fig. 1C) rather than the single 13S-HpODE stereochemical product formed by 12/15-lipoxygenase. We also found that neutral lipids did not accumulate peroxidized fatty acyl residues during chronic ethanol ingestion (Fig. 1D), so phospholipid polyunsaturated fatty acyl residues were oxidized in place because otherwise both classes of complex lipids would have accumulated peroxidized free fatty acids.
Circulating Oxidatively Fragmented Phospholipids Are Increased by Chronic Ethanol Ingestion
Phospholipid hydroperoxides are precursors for a host of truncated phospholipids formed by further oxidation, with associated bond rearrangement and scission. We found that the amount of truncated acyl and alkyl (phospholipids with an sn-1 ether-linked lysoPAF backbone) glutaroyl choline phospholipids, which initiate endothelial cell interaction with inflammatory cells (21), was increased 2-fold by prolonged ethanol ingestion (Fig. 2A). We also found (Fig. 2B) a 2-fold increase in acyl and alkyl choline phospholipids containing the cytotoxic (14, 22) and pro-inflammatory (11, 23) nine-carbon azelaoyl fragment derived from cleavage of esterified 9-HpODE residues. The 2-fold increase in oxidatively fragmented phospholipids with either glutaroyl or azelaoyl sn-2 residues occurred for both alkyl and acyl phospholipids, although as expected from the relative abundance of their precursors, the content of the diacyl truncated phospholipid was far greater than that of the corresponding alkyl acyl species.
FIGURE 2.
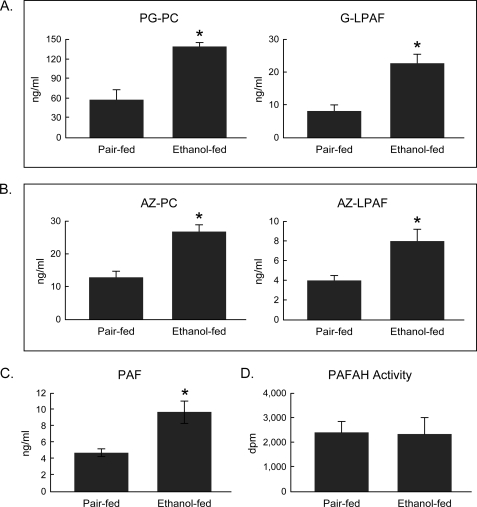
Circulating oxidatively truncated phospholipids increase during chronic ethanol ingestion. A, sn-2 glutaroyl choline phospholipids. PG-PC, palmitoyl glutaroyl phosphatidylcholine; G-LPAF, glutaroyl lysoPAF (LPAF, 1-O-hexadecyl-sn-glycero-3-phosphocholine). B, sn-2 azelaoyl choline phospholipids. AZ-PC, azelaoyl phosphatidylcholine; AZ-LPAF, azelaoyl lysoPAF. C, platelet-activating factor. D, plasma PAF acetylhydrolase (PAFAH) activity. Plasma was from rats (n = 5) maintained on the Lieber-DeCarli ethanol diet for 4 weeks or their pair-fed controls. PAF and the truncated phospholipids were quantified by LC/MS/MS using [2H4]PAF as the internal standard. PAF acetylhydrolase activity was quantified using [acetyl-3H-]PAF. Alkyl phospholipids contain a lysoPAF, rather than a lysophosphatidylcholine, backbone. *, p < 0.05 comparing ethanol-fed to pair control.
Chronic ethanol ingestion also doubled the amount of PAF in the circulation (Fig. 2C), providing de facto evidence of an inflammatory response to this insult. The presence of both PAF and alkyl species of oxidatively truncated phospholipids indicates that the site of the oxidative attack on polyunsaturated phospholipids was not hepatocytes because rat liver does not contain the alkyl phospholipid precursors (24) required for their production. In contrast, phospholipid-rich high density lipoprotein particles oxidize in preference to neutral lipid-rich low density lipoprotein, and high density lipoprotein particles are the primary carriers of lipid hydroperoxides (25). This suggests that both phospholipid hydroperoxides and their truncated phospholipid products were primarily derived from oxidation of lipoproteins in the circulation.
The enzyme PAF acetylhydrolase hydrolyzes PAF, phospholipid hydroperoxides, esterified isoprostane residues, and oxidatively truncated phospholipids (26, 27). However, accumulation of all these substrates of plasma PAF acetylhydrolase was not due to diminished circulating PAF acetylhydrolase activity after 4 weeks of chronic ethanol ingestion (Fig. 2D).
Accumulation of Circulating Oxidized Phospholipids Is Temporally Dissociated from Ethanol Catabolism
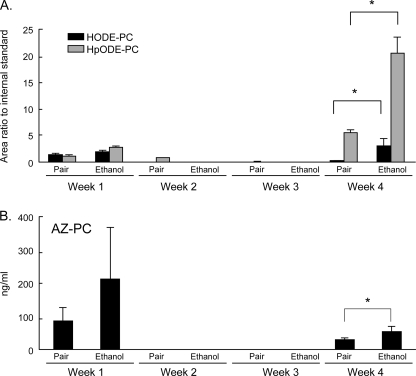
If circulating HpODE-PC and HODE-PC arise from ethanol catabolism and reactive oxygen specie production, then the increase in these oxidized phospholipids should be contemporaneous with the onset of ethanol consumption. We collected plasma weekly from animals ingesting ethanol or the isocaloric control diet and found that both HpODE-PC and HODE-PC were present in the circulation after 1 week on the diet but that ethanol ingestion did not significantly increase these oxidized phospholipids when compared with the isocaloric pair-fed control (Fig. 3A). Remarkably, these oxidized phospholipids then disappeared from the circulation during the subsequent 2 weeks of the ethanol supplemented dietary regimen, although ethanol continued to provide 35% of their caloric intake. This low background of oxidized phospholipids during weeks two and three of the diet then accentuates a sharp increase in the level of both oxidized phospholipids at the 4th week of the protocol, which was particularly prominent in ethanol-fed animals. Similarly, the circulating oxidatively truncated phospholipid palmitoyl azelaoyl phosphatidylcholine was also present at week one, with no significant difference between the control and ethanol-fed groups, but then this fragmented phospholipid largely disappeared from the circulation over the subsequent 2 weeks (Fig. 3B). As with its precursor, oxidized phospholipid HpODE-PC, the oxidatively truncated phospholipid azelaoyl phosphatidylcholine (AZ-PC) returned to the circulation after 4 weeks on the liquid diets with a significantly larger amount in the circulation of animals fed the ethanol-supplemented diet.
FIGURE 3.
The increase in circulating oxidized phospholipids during chronic alcohol consumption is biphasic. A, HODE-PC and HpODE-PC plasma levels in rats (n = 4) maintained on the Lieber-DeCarli liquid ethanol diet and in their isocaloric pair-fed controls for the stated period of time. The concentration of these phospholipids was determined as in Fig. 1. B, oxidatively truncated phospholipid palmitoyl azelaoyl phosphatidylcholine (AZ-PC) was collected and analyzed after the stated time on the dietary regimen as described in the legend for Fig. 2. Week two and three lipids were below a quantifiable level. *, p < 0.05 comparing ethanol-fed to pair-fed.
Diet and Ethanol Exposure Increase Hepatic Oxidative Stress
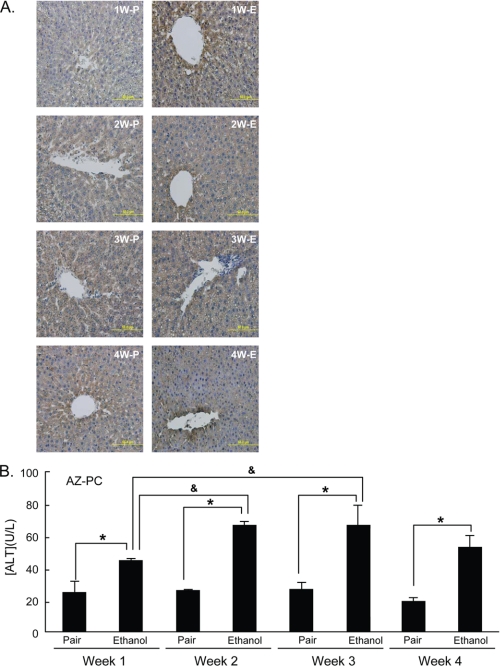
Hepatic oxidative stress was increased in animals ingesting the ethanol diet for a single week when compared with the pair-fed controls as determined by immunohistochemical detection of 4-hydroxynonenal protein adducts (Fig. 4A). Hydroxynonenal adduct accumulation developed throughout the ethanol feeding protocol, primarily with a centrilobular distribution. Hepatic oxidative stress developed over time in ethanol-fed animals, but oxidative stress was also increased in a more uniform fashion in pair-fed animals over time, showing that the control diet itself is not benign. Circulating alanine transaminase (Fig. 4B) was significantly increased after a single week of ethanol ingestion, with a small increase thereafter. Pair-fed animals displayed no change in this circulating marker of liver damage despite a generalized increase in hepatic 4-hydroxynonenal immunostaining.
FIGURE 4.
Chronic ethanol feeding induces hepatic oxidative stress and hepatocyte damage. A, 4-hydroxynonenol immunohistochemistry. Sections of livers from rats treated for the stated time and conditions were sectioned and stained as described under “Experimental Procedures.” W, weeks; E, ethanol; P, pair-fed. B, circulating alanine transaminase levels. ALT was assessed in blood drawn from rats at the stated times and conditions by the Cleveland Clinic medical laboratory. *, p < 0.05 comparing ethanol-fed to pair-fed controls, &, p < 0.05 comparing animals fed ethanol for a different period.
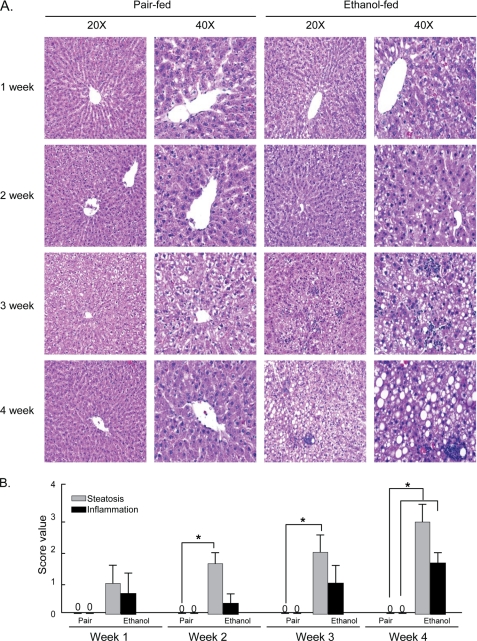
Steatohepatitis Develops over Time in Animals Ingesting Ethanol
Hepatic lipid accumulation, steatosis, was apparent and significant after 2 weeks of ethanol ingestion, and steatohepatitis, marked by foci of inflammatory cells, was apparent and significant by week three in hepatic sections of animals on the ethanol diet (Fig. 5, A and B). The extent of these conditions was most marked in animals that had spent 4 weeks on the ethanol diet. Frank hepatocyte nuclear condensation and necrotic lesions were only observed in animals exposed to ethanol for 4 weeks, and hepatocyte ballooning was not observed in these slices. Neither steatosis nor steatohepatitis developed in pair-fed animals. The developing hepatic oxidative stress, steatosis, and steatohepatitis in ethanol-fed animals were distinct from the initial increase in circulating ALT (Fig. 4B) and were more closely, although not perfectly, allied with the increase in circulating oxidized and truncated phospholipids.
FIGURE 5.
Rats chronically ingesting ethanol develop steatohepatitis. A, hepatic histology as a function of time on the Lieber-DeCarli ethanol diet. Images are at ×20 and ×40 of hematoxylin and eosin-stained sections from rats ingesting the ethanol liquid diet or their pair-fed controls for the stated times. B, score values of hepatic steatosis and hepatic inflammation over time. Livers from rats treated for the stated time and conditions were sectioned and stained with hematoxylin and eosin. Steatosis and steatohepatitis were scored by an experienced pathologist in a blinded fashion with scores from 0 to 3. Values are mean ± S.E., n = 3. *, p < 0.05 comparing ethanol-fed to pair-fed controls.
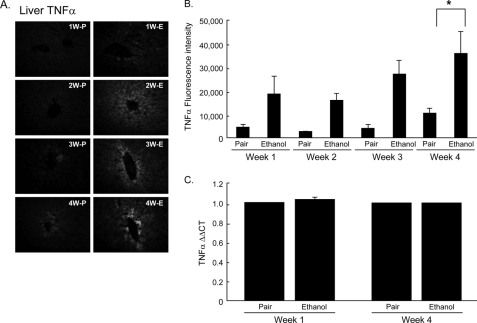
TNFα Accumulates in Liver over Time during Chronic Ethanol Ingestion
We investigated whether chronic ethanol ingestion initiated a progressive inflammatory reaction as a source of reactive oxygen species distinct from that from ethanol catabolism. TNFα promotes an inflammatory response with oxidant generation (28) and PAF synthesis (29), prompting us to analyze circulating TNFα by enzyme-linked immunosorbent assay. We did not find this cytokine circulating in ethanol-fed animals (not shown), but then TNFα is cleared from the circulation with a t½ of ∼3 min through uptake by liver and other organs (30, 31). Furthermore, its clearance is sharply increased by acute ethanol exposure (31). Accordingly, TNFα immunohistochemistry (Fig. 6, A and B) showed that TNFα accumulated in the livers of animals ingesting ethanol that reached statistical significance when compared with their pair-fed controls by weeks 3 and 4. The staining pattern did not correspond to Kupffer cells, the primary cellular source of this cytokine, but rather was enriched around the central vein. The increase in hepatic TNFα was not accompanied by changes in hepatic mRNA for this inflammatory cytokine (Fig. 6C), suggesting that its production is not local and that circulating TNFα is not an appropriate biomarker of hepatic inflammation.
FIGURE 6.
TNFα accumulates in liver over time during chronic alcohol consumption. A, TNFα in rat livers increased in a time-dependent manner after 1–4 weeks (W) of ethanol (E) feeding. Paraffin-embedded livers were deparaffinized followed by immunodetection of TNFα. B, fluorescence intensity of anti-TNFα was quantified using the ImagePro software. Values represent mean ± S.E., n = 5–8, *, p < 0.05 comparing ethanol-fed to pair-fed (P) animals. C, quantitative PCR analysis of hepatic TNFα mRNA. RNA from animals maintained on ethanol or pair-fed for 1 or 4 weeks was quantified as described under “Experimental Procedures.”
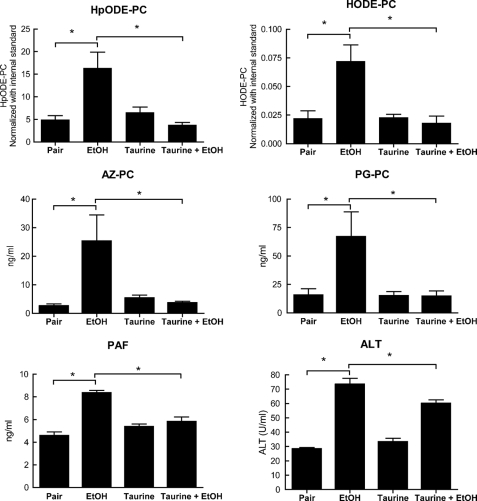
The Organic Osmolyte Taurine Reduces Circulating Short Chained Phospholipids
The osmolyte taurine is not itself an antioxidant or free radical scavenger (32), but rather, alters cell and organelle volume, reducing endoplasmic reticulum stress and its associated radical production (33). We found (Fig. 7) that dietary taurine alone did not increase the level of circulating HpODE-PC or HODE-PC but that it did completely suppress the increase of these oxidized phospholipids after 4 weeks of ethanol ingestion. Similarly, taurine supplementation alone had no effect on circulating oxidatively truncated phospholipids phospholipid azelaoyl phosphatidylcholine (AZ-PC) or palmitoyl glutaroyl phosphatidylcholine (PG-PC) derived from oxidized phospholipids but again abolished the increase associated with chronic ethanol ingestion. Taurine had a modest, but significant, effect on plasma ALT (Fig. 7), and it abolished the increase in serum aspartate aminotransaminase (supplemental Fig. 3), so taurine has interceded in events promoting an inflammatory response and liver damage. Circulating oxidized and truncated phospholipids correlate to this event and are informative markers of a developing hepatic, and potentially systemic, inflammatory reaction.
FIGURE 7.
Oral taurine supplementation reduces plasma oxidatively truncated phospholipids and PAF from chronic ethanol consumption. Circulating PAF, oxidized phospholipids, and truncated phospholipids were quantified by LC/MS/MS in rats ingesting ethanol or pair-fed for 4 weeks as described in the legend for Fig. 2 after taurine supplementation for 4 weeks. Plasma ALT was measured as in Fig. 4. n = 4 *, p < 0.05.
DISCUSSION
The main observations of this work are that pro-inflammatory and pro-apoptotic agents accumulate in the circulation of animals chronically ingesting alcohol. These arise from free radical, versus enzymatic, attack on polyunsaturated phospholipids. Circulating phospholipid hydroperoxides have been proposed as new markers of oxidative stress in alcoholic patients (3), but our data show that this measurement of oxidative stress does not directly correlate with the hepatic oxidative stress imposed by liver catabolism of ethanol. Instead, these lipids reflect an unappreciated, temporally distinct, hepatic and non-hepatic response to chronic ethanol exposure.
Enhanced amounts of peroxidized phospholipids and their truncation products in the circulation have previously been observed only in chronic models of atherosclerosis (34) and in the presence of oxidative stressors such as aging (35) or tobacco smoke exposure (35, 36). Ethanol is nearly exclusively metabolized in liver, a process that increases liver production of reactive oxygen species when metabolized through the ethanol-inducible cytochrome P450 pathway (1). We therefore postulated that ethanol catabolism might overwhelm the endogenous antioxidant capacity of liver, allowing free and esterified lipid hydroperoxides to escape the liver and appear in the systemic circulation.
Animals ingesting alcohol for 4 weeks displayed an increase in circulating HpODE-PC, similar to the increase in these oxidized phospholipids that we (Fig. 1A) and others (3) detect in human patients, so the Lieber-DeCarli model reproduces this aspect of human disease. These peroxidized phospholipids are markers of oxidative stress, but they themselves have only limited direct biologic effects. They are, however, precursors of other phospholipids that can fulfill pathologic roles, including the initiation of an inflammatory reaction (11) or cell death (14, 15), and so we additionally hypothesized that biologically active agents such as these oxidative truncation products of phospholipid hydroperoxides would appear in the circulation of animals chronically ingesting ethanol.
We did find that truncated phospholipid oxidation products were significantly increased in the plasma of human and rat model after long term ethanol metabolism. However, in contrast to our expectations, we also found that these circulating lipid oxidation products were not the direct consequence of liver catabolism of ethanol by Cyp2E1. Rather, the sharp increase in circulating oxidatively modified phospholipids between 3 and 4 weeks of ethanol ingestion marked the transition from simple excess fatty infiltration of liver to inflammatory steatohepatitis. Currently, the sole diagnostic tool to distinguish steatosis from the more problematic steatohepatitis is through invasive liver biopsy, and even this technique suffers from high variability from the small sample that does not fully represent overall liver health (37). Identification of circulating markers of this medically important transition may prove to be a useful new diagnostic tool.
Some phospholipids derived from further oxidation and/or fragmentation of these phospholipid hydroperoxides are biologically active because they interact with TLR4 (38), CD36 (39), or PAF (40) receptors, because they chemically derivatize proteins (41–44), or because they enter cells and initiate the process of mitochondria-dependent apoptosis (14, 15). The presence of these types of phospholipid oxidation products in the circulation will have systemic consequences that would extend the oxidative stress of ethanol exposure to distal organs.
The most potent of the biologically active oxidatively truncated phospholipids are those that interact with the PAF receptor (11). High affinity, submicromolar recognition by this inflammatory receptor requires the ether bond of alkyl phospholipids. Circulating oxidized alkyl choline phospholipids containing this ether bond were increased by just more than 2-fold after 4 weeks of ethanol feeding. The modified alkyl phospholipids we analyzed, e.g. glutaroyl lysoPAF and azelaoyl lysoPAF, display PAF-like bioactivity (11) and would be expected to promote pro-inflammatory changes during ethanol feeding. Oxidative truncation of HpODE-PC to phospholipids containing an azelaoyl residue also has important consequences for mitochondrial function in numerous types of cells because alkyl and acyl azelaoyl choline phospholipids rapidly enter cells to preferentially associate with mitochondria (14). These oxidatively truncated phospholipids depolarize mitochondria, induce swelling, open the permeability transition pore, and activate the intrinsic apoptotic cascade (15). The presence of such bioactive phospholipids in the circulation after chronic ethanol exposure will promote systemic metabolic stress through impaired mitochondrial function.
Rats (45) and mice (46) maintained on the Lieber-DeCarli ethanol diet for 4 weeks display hallmarks of chronic inflammatory disease, and accordingly, animals in this study displayed marked pathologic changes in liver oxidant stress including steatosis, inflammation, and necrosis. We were unable to detect increased circulating levels of TNFα, but this cytokine is rapidly cleared from the circulation (30, 31) and disappears even more rapidly after ethanol exposure (31), so it is a poor biomarker of systemic inflammation. Still, immunohistochemistry showed that TNFα accumulated in the liver after 3 and 4 weeks of ethanol feeding. The lack of staining of non-parenchymal cells in the inflammatory foci or sinusoids shows that production of this inflammatory cytokine by resident or infiltrating inflammatory cells is not the primary driver of the developing steatohepatitis. Because the TNFα increase occurred without a corresponding increase in TNFα mRNA levels and because the protein was localized just around hepatic vessels, this inflammatory cytokine may reflect a developing systemic innate immune response.
Additional support for the presence of a developing systemic inflammatory response is the increase in circulating PAF in animals chronically ingesting alcohol. PAF is the product of activated inflammatory cells, with macrophages being the primary source of secreted PAF (47). The enzyme PAF acetylhydrolase (lipoprotein-associated phospholipase A2) hydrolyzes phospholipid hydroperoxides (27), esterified isoprostane residues (48), and oxidatively truncated phospholipids (49) in addition to PAF. However, accumulation of all these substrates during chronic ethanol feeding was not due to decreased circulating activity, indicating instead that plasma PAF acetylhydrolase does not afford complete protection from circulating oxidized phospholipids under these conditions.
Taurine is an abundant intracellular osmolyte (50) that suppresses conditions favoring endoplasmic reticulum stress and radical production (33) and so reduces ethanol-initiated decoration of plasma (51) and hepatocyte (17) proteins with reactive lipid fragments. Taurine as an oxidized sulfonic acid is not a radical scavenger (32), so this reduction in markers of oxidative attack reflects a reduction of cellular events that impose an oxidative stress in the first place rather than elimination of radicals after their formation. We found that manipulation of these underlying events by taurine supplementation reduced circulating oxidized phospholipids and their oxidatively truncated phospholipid products. The accompanying decrease in circulating PAF levels indicates that this taurine supplementation reduced the inflammation caused by chronic ethanol consumption, a postulate supported by decreased hepatic TNFα accumulation (17). Plasma oxidized phospholipids, truncated phospholipids, and PAF reflect events other than the oxidative stress of liver ethanol catabolism or hepatocyte damage allowing ALT escape. These mediators have the capacity to disseminate oxidative stress beyond the site of ethanol catabolism, and monitoring them in the circulation provides a new way to mark the transition from steatosis to steatohepatitis.
Supplementary Material
Acknowledgments
We thank Sanjoy Roychowdhury, Becky Sebastian, Michael Berk, John Peterson, Eric Diskin, and Judy Drazba in the Cleveland Clinic Imaging Core for aid with the immunohistochemistry and ImagePro analysis, Mark Calabro (Cleveland Clinic) for facilitating the mass spectrometry work, Michael Berk for aid with ALT sample processing, and Dr. Robert G. Salomon (Case Western Reserve) for phospholipid hydroperoxide mass spectrometry standards.
This work was supported, in whole or in part, by National Institutes of Health Grants AA017748 (to T. M. M.), AA011876 (to L. E. N.), AA011975 (to L. E. N.), 1 P20 AA017837 (to L. E. N.), DK076852 (to A. E. F.), and CTSA 1UL1RR024989 (to Pamela Davis).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- HpODE
- hydroperoxyoctadecadienoic acid
- HpETE
- hydroperoxyeicosatetraenoic acid
- HODE
- hydroxyoctadecadiene
- HETE
- hydroxyeicosatetraenoic acid
- PC
- phosphatidylcholine
- PAF
- platelet-activating factor
- TNFα
- tumor necrosis factor-α
- taurine
- 2-aminoethylsulfonic acid
- ALT
- alanine transaminase
- LC/MS/MS
- liquid chromatography-tandem mass spectrometry
- HPLC
- high pressure liquid chromatography
- lysoPAF
- lysoplatelet-activating factor.
REFERENCES
- 1.Dey A., Cederbaum A. I. (2006) Hepatology 43, S63–S74 [DOI] [PubMed] [Google Scholar]
- 2.Meagher E. A., Barry O. P., Burke A., Lucey M. R., Lawson J. A., Rokach J., FitzGerald G. A. (1999) J. Clin. Invest. 104, 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi J., Matsushita S., Yoshioka N., Funae R., Fujita T., Higuchi S., Ueno Y. (2004) J. Lipid Res. 45, 967–971 [DOI] [PubMed] [Google Scholar]
- 4.Schroder H., Marrugat J., Fíto M., Weinbrenner T., Covas M. I. (2006) Free Radic. Biol. Med. 40, 1474–1481 [DOI] [PubMed] [Google Scholar]
- 5.Bailey S. M., Cunningham C. C. (2002) Free Radic. Biol. Med. 32, 11–16 [DOI] [PubMed] [Google Scholar]
- 6.Cahill A., Cunningham C. C., Adachi M., Ishii H., Bailey S. M., Fromenty B., Davies A. (2002) Alcohol. Clin. Exp. Res. 26, 907–915 [PMC free article] [PubMed] [Google Scholar]
- 7.Nordmann R., Ribière C., Rouach H. (1990) Alcohol Alcohol. 25, 231–237 [DOI] [PubMed] [Google Scholar]
- 8.Smith W. L., Murphy R. C. (2008) J. Biol. Chem. 283, 15513–15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas C. P., Morgan L. T., Maskrey B. H., Murphy R. C., Kühn H., Hazen S. L., Goodall A. H., Hamali H. A., Collins P. W., O'Donnell V. B. (2010) J. Biol. Chem. 285, 6891–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruhwirth G. O., Loidl A., Hermetter A. (2007) Biochim. Biophys. Acta 1772, 718–736 [DOI] [PubMed] [Google Scholar]
- 11.Chen R., Chen X., Salomon R. G., McIntyre T. M. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moumtzi A., Trenker M., Flicker K., Zenzmaier E., Saf R., Hermetter A. (2007) J. Lipid Res. 48, 565–582 [DOI] [PubMed] [Google Scholar]
- 13.Rhode S., Grurl R., Brameshuber M., Hermetter A., Schütz G. J. (2009) J. Biol. Chem. 284, 2258–2265 [DOI] [PubMed] [Google Scholar]
- 14.Chen R., Yang L., McIntyre T. M. (2007) J. Biol. Chem. 282, 24842–24850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R., Feldstein A. E., McIntyre T. M. (2009) J. Biol. Chem. 284, 26297–26308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur V., Pritchard M. T., McMullen M. R., Wang Q., Nagy L. E. (2006) J. Leukoc. Biol. 79, 1348–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Sebastian B. M., Tang H., McMullen M. M., Axhemi A., Jacobsen D. W., Nagy L. E. (2009) Hepatology 49, 1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen J. S., Wykle R. L., Samuel M. P., Thomas M. J. (2005) J. Lipid Res. 46, 373–382 [DOI] [PubMed] [Google Scholar]
- 19.Johnson E. F., Palmer C. N., Hsu M. H. (1996) Ann. N.Y. Acad. Sci. 804, 373–386 [DOI] [PubMed] [Google Scholar]
- 20.Marathe G. K., Zimmerman G. A., McIntyre T. M. (2003) J. Biol. Chem. 278, 3937–3947 [DOI] [PubMed] [Google Scholar]
- 21.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., Berliner J. A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2248–2254 [DOI] [PubMed] [Google Scholar]
- 22.Itabe H., Kushi Y., Handa S., Inoue K. (1988) Biochim. Biophys. Acta 962, 8–15 [PubMed] [Google Scholar]
- 23.Subbanagounder G., Leitinger N., Shih P. T., Faull K. F., Berliner J. A. (1999) Circ. Res. 85, 311–318 [DOI] [PubMed] [Google Scholar]
- 24.Diagne A., Fauvel J., Record M., Chap H., Douste-Blazy L. (1984) Biochim. Biophys. Acta 793, 221–231 [DOI] [PubMed] [Google Scholar]
- 25.Bowry V. W., Stanley K. K., Stocker R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 10316–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stafforini D. M. (2009) Cardiovasc. Drugs Ther. 23, 73–83 [DOI] [PubMed] [Google Scholar]
- 27.Kriska T., Marathe G. K., Schmidt J. C., McIntyre T. M., Girotti A. W. (2007) J. Biol. Chem. 282, 100–108 [DOI] [PubMed] [Google Scholar]
- 28.Czaja M. J. (2004) Hepatology 40, 19–22 [DOI] [PubMed] [Google Scholar]
- 29.Camussi G., Bussolino F., Salvidio G., Baglioni C. (1987) J. Exp. Med. 166, 1390–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beutler B. A., Milsark I. W., Cerami A. (1985) J. Immunol. 135, 3972–3977 [PubMed] [Google Scholar]
- 31.Deaciuc I. V., Alappat J. M., McDonough K. H., D'Souza N. B. (1996) Alcohol. Clin. Exp. Res. 20, 293–301 [DOI] [PubMed] [Google Scholar]
- 32.Shi X., Flynn D. C., Porter D. W., Leonard S. S., Vallyathan V., Castranova V. (1997) Ann. Clin. Lab. Sci. 27, 365–374 [PubMed] [Google Scholar]
- 33.Nonaka H., Tsujino T., Watari Y., Emoto N., Yokoyama M. (2001) Circulation 104, 1165–1170 [DOI] [PubMed] [Google Scholar]
- 34.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., Silverstein R. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38517–38523 [DOI] [PubMed] [Google Scholar]
- 35.Frey B., Haupt R., Alms S., Holzmann G., König T., Kern H., Kox W., Rüstow B., Schlame M. (2000) J. Lipid Res. 41, 1145–1153 [PubMed] [Google Scholar]
- 36.Lehr H. A., Weyrich A. S., Saetzler R. K., Jurek A., Arfors K. E., Zimmerman G. A., Prescott S. M., McIntyre T. M. (1997) J. Clin. Invest. 99, 2358–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., Grimaldi A., Capron F., Poynard T. (2005) Gastroenterology 128, 1898–1906 [DOI] [PubMed] [Google Scholar]
- 38.Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C. J., Penninger J. M. (2008) Cell 133, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. (2006) Cell Metab. 4, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marathe G. K., Davies S. S., Harrison K. A., Silva A. R., Murphy R. C., Castro-Faria-Neto H., Prescott S. M., Zimmerman G. A., McIntyre T. M. (1999) J. Biol. Chem. 274, 28395–28404 [DOI] [PubMed] [Google Scholar]
- 41.Itabe H., Yamamoto H., Suzuki M., Kawai Y., Nakagawa Y., Suzuki A., Imanaka T., Takano T. (1996) J. Biol. Chem. 271, 33208–33217 [DOI] [PubMed] [Google Scholar]
- 42.Hörkkö S., Miller E., Branch D. W., Palinski W., Witztum J. L. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato Y., Osawa T. (1998) Arch. Biochem. Biophys. 351, 106–114 [DOI] [PubMed] [Google Scholar]
- 44.Hoff H. F., O'Neil J., Wu Z., Hoppe G., Salomon R. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 275–282 [DOI] [PubMed] [Google Scholar]
- 45.Thakur V., Pritchard M. T., McMullen M. R., Nagy L. E. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roychowdhury S., McMullen M. R., Pritchard M. T., Hise A. G., van Rooijen N., Medof M. E., Stavitsky A. B., Nagy L. E. (2009) Hepatology 49, 1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elstad M. R., Prescott S. M., McIntyre T. M., Zimmerman G. A. (1988) J. Immunol. 140, 1618–1624 [PubMed] [Google Scholar]
- 48.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., 2nd. (2006) J. Biol. Chem. 281, 4616–4623 [DOI] [PubMed] [Google Scholar]
- 49.Stremler K. E., Stafforini D. M., Prescott S. M., McIntyre T. M. (1991) J. Biol. Chem. 266, 11095–11103 [PubMed] [Google Scholar]
- 50.Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009) Physiol. Rev. 89, 193–277 [DOI] [PubMed] [Google Scholar]
- 51.Harada H., Kitazaki K., Tsujino T., Watari Y., Iwata S., Nonaka H., Hayashi T., Takeshita T., Morimoto K., Yokoyama M. (2000) Hypertens. Res. 23, 277–284 [DOI] [PubMed] [Google Scholar]
- 52.National Research Council (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press, National Institutes of Health, Bethesda, MD [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.