Abstract
We identified a novel prostaglandin (PG)-specific organic anion transporter (OAT) in the OAT group of the SLC22 family. The transporter designated OAT-PG from mouse kidney exhibited Na+-independent and saturable transport of PGE2 when expressed in a proximal tubule cell line (S2). Unusual for OAT members, OAT-PG showed narrow substrate selectivity and high affinity for a specific subset of PGs, including PGE2, PGF2α, and PGD2. Similar to PGE2 receptor and PGT, a structurally distinct PG transporter, OAT-PG requires for its substrates an α-carboxyl group, with a double bond between C13 and C14 as well as a (S)-hydroxyl group at C15. Unlike the PGE2 receptor, however, the hydroxyl group at C11 in a cyclopentane ring is not essential for OAT-PG substrates. Addition of a hydroxyl group at C19 or C20 impairs the interaction with OAT-PG, whereas an ethyl group at C20 enhances the interaction, suggesting the importance of hydrophobicity around the ω-tail tip forming a “hydrophobic core” accompanied by a negative charge, which is essential for substrates of OAT members. OAT-PG-mediated transport is concentrative in nature, although OAT-PG mediates both facilitative and exchange transport. OAT-PG is kidney-specific and localized on the basolateral membrane of proximal tubules where a PG-inactivating enzyme, 15-hydroxyprostaglandin dehydrogenase, is expressed. Because of the fact that 15-keto-PGE2, the metabolite of PGE2 produced by 15-hydroxyprostaglandin dehydrogenase, is not a substrate of OAT-PG, the transport-metabolism coupling would make unidirectional PGE2 transport more efficient. By removing extracellular PGE2, OAT-PG is proposed to be involved in the local PGE2 clearance and metabolism for the inactivation of PG signals in the kidney cortex.
Keywords: Anion Transport, Kidney, Membrane Proteins, Prostaglandins, Transport Drugs, SLC Transporter, Organic Anion
Introduction
Prostaglandins (PGs)3 compose a diverse family of autacoids generated by cyclooxygenase-mediated metabolism of arachidonic acid. Four PGs (PGD2, PGE2, PGF2α, and PGI2) and another cyclooxygenase metabolite thromboxane A2 are the primary bioactive derivatives (1). These primary bioactive prostanoids exert complex and diverse functions via their own selective receptors (2). Because no enzymes inactivates PGD2, PGE2, and PGF2α in the plasma, these PGs could potentially activate receptors at a substantial distance from their sites of release (3). However, such a long distance action does not occur because PGs are cleared in a single passage through any of several vascular beds, including those in the lung (3). PG-specific transporter PGT (prostaglandin transporter) and other organic anion transporting polypeptide members of the SLC21/SLCO family that transport PGs have been found widely in various tissues, and thus, these transporters may play a primary role in the removal of bioactive PGs from the circulation (4).
In the kidney, PGE2 is a predominant arachidonic acid metabolite and plays an important role in renal functions, including regulation of vascular smooth muscle tonus, glomerular filtration, renin release, and tubular sodium and water transport (5). In the renal medulla, PGE2 inhibits Na+ reabsorption at the medullary thick ascending limbs of Henle and antagonizes anti-diuretic hormone action in collecting ducts (5). To limit or terminate PG actions on the target receptors, some mechanisms must exist for the clearance of PGs in the proximity of the sites of action in the kidney. In line with this notion, the aforementioned PG transporter PGT that is expressed mainly in interstitial cells in renal medulla has been suggested to affect tubular sodium and water reabsorption through regulating local PGE2 concentrations in the renal medulla (6, 7). However, there have been no reports on the renal cortex PG clearance. PGs are most likely metabolized in the proximal tubular cells, where PG-inactivating enzyme activity has been abundantly detected (8, 9). Recently, immunohistochemical studies demonstrated the massive proximal-tubular localization of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) that catalyzes the first and rate-limiting step in the inactivation and degradation of prostaglandins (9). For PGs to be metabolized by 15-PGDH, they must be taken up by the proximal-tubular epithelial cells. Because PGs are negatively charged organic compounds that require transporters for membrane permeation, it is assumed that such transporters should exist in proximal tubular cells. Among organic anion transporters, transporting PGs, PGT and other organic anion transporting polypeptide members of the SLC21/SLCO family that favor PGs as substrates, have not been detected in the proximal tubules. Although OAT1, OAT2, OAT3, and OAT4 of the SLC22 family and an ATP-binding cassette transporter MRP4 are present in the proximal tubules and transport PGs (10–16), they are not appropriate for the PG clearance transporters because of their broad substrate selectivity and lower affinity for PGs (10–16). Therefore, other unknown transporters would be involved in the local PG disposition in renal cortex. We propose, based on the substrate selectivity and localization, that a newly identified OAT member of the SLC22 family, OAT-PG, is the transporter for the PG clearance in renal cortex.
The SLC22 family was first defined by the finding of an organic cation transporter OCT1 (organic cation transporter 1) in 1994 (17). In 1997, we and others identified the first member of the multispecific organic anion transporter of renal proximal tubules OAT1 (organic anion transporter 1) structurally related to OCT1 and established the OAT group of the SLC22 family (10, 18). Discovery of other OATs followed, and the genes of these OATs were included in the largest subgroup of SLC22 (10–14, 16–19). Additional branches of the SLC22 family were added following the discovery of a zwitterion transporter OCTN1 (20). Interestingly, most of the SLC22 members are largely expressed in the proximal tubules of the kidney (19). Therefore, we have searched for the aforementioned renal cortex PG clearance transporters among orphan members of the SLC22 family. We identified a novel member of the SLC22 family designated OAT-PG (organic anion transporter for prostaglandins) in mouse kidney that specifically transports PGs and is localized on the basolateral membrane of proximal tubules.
EXPERIMENTAL PROCEDURES
Materials
[3H]PGE2 (7400 GBq/mmol), [3H] PGF2α (6808 GBq/mmol), and [3H]PGD2 (6290 GBq/mmol) were obtained from PerkinElmer Life Sciences. [3H]PGE1 (1702 GBq/mmol) was purchased from GE Healthcare. [3H]H2O (100 mCi/ml) and [14C]inulin-carboxyl (1 μCi/mg) were from MP Biomedicals, Inc. PGE2, PGE1, PGF2α, PGD2, PGI2, PGJ2, 15-HPETE, thromboxane B2, LTA4, LTB4, and LTC4 were purchased from Sigma. PGA2, PGB2, 6-keto-PGF1α, PGG2, PGK2, 5-HPETE, 12-HPETE, 12-HETE, 15-HETE, LTE4, and all of the PGE2 analogues were obtained from Cayman Chemical (Ann Arbor, MI). PGH2, LTD4, and lipoxin A4 were acquired from Calbiochem. Fetal bovine serum, trypsin, and geneticin were purchased from Invitrogen. Recombinant epidermal growth factor and insulin were obtained from Wakunaga (Hiroshima, Japan) and Shimizu (Shizuoka, Japan), respectively. RITC 80-7 culture medium was acquired from Iwaki Co. (Tokyo, Japan). All other chemicals and reagents were of analytical grade and were readily available from commercial sources.
Cloning and Sequence Analysis of OAT-PG cDNA
An expressed sequence tag (EST) from mouse kidney cDNA library (mouse kidney cDNA clone IMAGE:2331833, GenBankTM/EMBL/DDBJ accession numbers AW319237 (5′) and AW261558 (3′)) was found to exhibit nucleotide sequence similar to human OAT1. For PCR amplification of full-length cDNA, total RNA was extracted from an adult mouse (ICR) kidney using Isogen reagent (Nippon Gene, Tokyo, Japan), and first-strand cDNA was synthesized using SuperScript III first-strand synthesis kit (Invitrogen). PCR amplification followed using a set of primers that include sequences for restriction enzyme cleavage sites and additional sequences for the efficient restriction enzyme digests at the 5′ end (BamHI for sense primer, XhoI for antisense primer) for directional cloning as follows: sense primer, CGG GAT CCG GCT GTG TGC AAC AGA AGT CCT AT (1–24 of nucleotide sequence of AW319237); antisense primer, CCG CTC GAG CTA TAT AAC GGA TTG GCT AAG AGA (50–73 of nucleotide sequence of AW261558). The PCR fragment was inserted into the BamHI and XhoI sites for pcDNA3.1(+) (OAT-PG-pcDNA3.1(+)), and the full sequence was confirmed by DNA sequencing. The obtained nucleotide sequence has been submitted to GenBankTM/EMBL/DDBJ Data Bank with accession number AB520670.
Multiple sequence alignment with other members of the SLC22 family, mouse OAT1 (10, 11, 18), mouse OAT2 (12), mouse OAT3 (13), human OAT4 (14), mouse OAT5 (21), mouse OAT6 (22), human OAT7 (23), rat OAT8 (24), and mouse URAT1 (25)), was computed by GENETYX programs (GENETYX, Tokyo, Japan) and ClustalW. Topology prediction was performed with TopPred. The phylogenetic tree was based on amino acid sequence alignment and bootstrap analysis using ClustalW and was drawn using NJplot software (26).
Establishment of a Cell Line Stably Expressing OAT-PG
S2 cell line derived from renal proximal tubules of mice transgenic for temperature-sensitive SV40 large T antigen was used as a host cell line (27). The procedure to establish S2 cell lines stably expressing exogenous genes has been described elsewhere (28). Empty vectors (i.e. pcDNA3.1(+)) and OAT-PG-pcDNA3.1(+) were transfected into S2 cells. The cells were grown at 33 °C under 5% CO2 in RITC 80-7 medium containing 10% fetal bovine serum, 10 μg/ml transferrin, 0.08 unit/ml insulin, 10 ng/ml recombinant epidermal growth factor, and 400 μg/ml geneticin (27, 28). The cells with less than 25 passages were used for experiments.
Uptake Experiments
Uptake experiments were performed as described previously (16, 28). The S2 cells were seeded in 24-well tissue culture plates at a density of 1 × 105 cells/well. After 2 days of culture, the cells were washed with Dulbecco's modified phosphate-buffered saline (D-PBS) containing 137 mm NaCl, 3 mm KCl, 8 mm Na2HPO4, 1 mm KH2PO4, 1 mm CaCl2, and 0.5 mm MgCl2 (pH 7.4), followed by incubation in 0.5 ml of D-PBS containing either [3H]PGE2, [3H]PGF2α, [3H]PGD2, or [3H]PGE1 at 37 °C for 30 s or period otherwise indicated. The cells in each well were washed five times in ice-cold D-PBS and lysed with 0.5 ml of 0.1 n sodium hydroxide (NaOH). The radioactivity was measured using a β-scintillation counter (LSC-3100; Aloka, Tokyo, Japan). To examine pH dependence of the transport, uptake was measured in various solutions (125 mm NaCl, 1.2 mm MgSO4, 4.8 mm KCl, 1.2 mm KH2PO4, 1.3 mm CaCl2, and 5.6 mm glucose, and 10 mm MES for pH 5.0, 5.5, and 6.5, HEPES for pH 7.4, or Tris for pH 8.5) at 37 °C for 30 s. In Na+ substitution experiments, NaCl in the uptake solution was replaced with equimolar choline chloride.
Determination of Intracellular Volume of S2 Cells and Estimation of Intracellular PGE2 Concentration
Three independent methods were adopted to estimate the intracellular volume of S2 cells.
First, intracellular water volume was estimated following the modified method described by Rottenberg (29). Briefly, [3H]H2O or [14C]inulin-carboxyl dissolved in HBSS (125 mm NaCl, 4.8 mm KCl, 1.2 mm MgCl2, 1.3 mm CaCl2, 5.6 mm glucose, and 25 mm HEPES, pH 7.4) was added to S2 cells seeded on a 24-well plate (2.721–3.544 (×105 cells)/well). The cells were incubated in the medium at 25 °C for 10 min. The medium (“extracellular medium”) was then collected, and the cells containing [3H]H2O were lysed with 0.1 n NaOH. The extracellular medium and the cell lysate were mixed with a liquid scintillation mixture (EMULSIFIER-SAFE, PerkinElmer Life Sciences), and the radioactivity was counted in a liquid scintillation counter (LSC-5100, Aloka, Tokyo, Japan). [3H]H2O-distributed volume (Vc) in the cells was calculated based on the radioactivity of the cells (Ac3H) and of the medium (Am3H) as follows: Vc = Vm × Ac3H/Am3H (Vm = volume of extracellular medium).
The radioactivity conferred by 14C yielded the extracellular water space Ve of S2 cells as follows: Ve = Vm × Ac14C/Am14C. The difference Vc − Ve is equal to the intracellular water volume (Vi).
Second, digitonin permeabilization assay was developed. Cells, prepared in a 24-well plate as described above, were incubated for 10 min in the presence or absence of 0.005% digitonin, which permeabilizes plasma membranes selectively (30), and then further incubated with 5 mg/ml [14C]inulin-carboxyl in HBSS for 10 min. This incubation period was determined in the preliminary experiment in which equilibration of [14C]inulin-carboxyl was completed within 10 min. After the equilibration period, extracellular medium was promptly removed. The radioactivity of the cells and the extracellular medium was measured with a liquid scintillation counter. Intracellular volume of S2 cells was calculated from the radioactivity of intracellular [14C]inulin-carboxyl (Ac14C) against extracellular [14C]inulin-carboxyl counts (Am14C) as follows: V− or V+ = Vm × Ac14C/Am14C (Vm = volume of extracellular medium).
The intracellular volume (Vi) was calculated from the volumes with the digitonin treatment (V+) and without the treatment (V−) as follows: Vi = V+ − V−.
Third, the diameter of trypsinized S2 cells was measured by Countess (Invitrogen), and the single cell volume was calculated by using the equation V = 4/3πr3. The intracellular volume was estimated as 80–90% of the total cell volume (31, 32).
Intracellular PGE2 concentrations were calculated based on the intracellular volume that was measured in the [3H]H2O/[14C]inulin-carboxyl partition experiment. The amount of [3H]PGE2 associated with S2-mock or S2-OAT-PG cells was measured after the 30-s uptake reaction, and [3H]PGE2 concentration in the intracellular space was calculated based on the results from [3H]H2O/[14C]inulin-carboxyl partition experiments. The intracellular PGE2 concentration was calculated by subtracting the value of S2-mock from S2-OAT-PG.
Kinetic Analyses
The kinetic parameters for the uptake of PGE1, PGE2, PGF2α, and PGD2 via OAT-PG were estimated from the following equation: ν = Vmax × S/(Km + S), where ν is the uptake rate of the substrate (femtomoles/mg of protein/min); S is the substrate concentration in the medium (nanomolar); Km is the Michaelis-Menten constant (nanomolar), and Vmax is the maximum uptake rate (femtomoles/mg of protein/min). To obtain the kinetic parameters, the linear range of uptake was determined for each substrate. The uptake was measured at a time point within this linear range. The net uptake values used for the calculation were obtained by subtracting the uptake values for vector-transfected S2 cells (S2-mock) from those for OAT-PG-expressing S2 cells (S2-OAT-PG). The input data were weighted as the reciprocal of the observed values, and the Damping Gauss Newton Methods algorithm was used for fitting. The fitted line was converted to the v/s versus v form (Eadie-Hofstee plot).
Efflux Study
Efflux study was performed as described previously (33). S2-OAT-PG and S2-mock cells were briefly seeded on 24-well tissue culture plates at a cell density of 1 × 105 cells/well. After the cells were cultured for 2 days, they were preincubated with [3H]PGE2 (0.4 nm for S2-OAT-PG cells and 2.5 nm for S2-mock cells in the medium, respectively) for 5 min. Afterward, the cells in each well were washed three times with D-PBS at 37 °C and incubated with 0.25 ml of D-PBS with or without unlabeled eicosanoids or other compounds. The supernatant was collected after a 30-s efflux period, and the cells were lysed by 0.5 ml of 0.1 n NaOH. The radioactivity of each solution was measured. The efflux value was expressed as percent radioactivity calculated from the radioactivity in the supernatant × 100%/(the radioactivity in cells plus the radioactivity in the supernatant) (34).
Inhibition Study
S2-OAT-PG cells were incubated in D-PBS containing 1 nm [3H]PGE2 in the absence or presence of various inhibitors (1 μm) for 30 s at 37 °C. Eicosanoids and PGE2-related compounds were dissolved in ethanol, and other compounds were dissolved in dimethyl sulfoxide (DMSO). Each of the compounds was diluted with the incubation medium. The final concentration of ethanol and DMSO was less than 0.1%, which did not affect the OAT-PG-mediated transport of prostaglandins in our experimental conditions.
Northern Blot Analysis and RT-PCR Analysis
To prepare the probe for Northern blot, a fragment of OAT-PG cDNA was amplified with RT-PCR using ICR mouse kidney poly(A)+ RNA as a template. The sense and antisense primers were 5′-TAA AGA GAG TCA AGA TCC ACA GGG-3′ and 5′-CTA TAT AAC GGA TTG GCT AAG AGA-3′, respectively, which were designed to amplify a fragment in the 3′-untranslated region of OAT-PG. The PCR product of expected size (154 bp) was subcloned into the EcoRV site of pBluescript II SK2 (Stratagene) and verified by sequencing. For [α-32P]dCTP labeling, the cDNA fragment was cleaved out by EcoRI and HindIII and labeled with [α-32P]dCTP (3000 Ci/mmol, GE Healthcare) by Ready-to-Go DNA Labeling Beads (-dCTP) kit (GE Healthcare). Total RNA was isolated from various tissues of 8-week-old ICR mice using Isogen reagent according to the manufacturer's instruction (Nippon Gene, Tokyo, Japan). Twenty micrograms of total RNA was electrophoresed and transferred to a Hybond-N+ membrane (GE Healthcare) and hybridized with the [α-32P]dCTP-labeled cDNA probe in PerfectHyb solution (TOYOBO, Osaka, Japan) at 68 °C for 20 h as described elsewhere (35). The final stringent wash of the membrane was in 0.1× SSC and 0.1% SDS at 65 °C three times for 1 h. The membrane was then washed and exposed to an imaging plate to be processed with a Fujix BAS 2000 bioimaging analyzer (FUJIFILM, Tokyo, Japan).
For RT-PCR analysis, first strand cDNA was synthesized from 1 μg of total RNA of each tissue in 20 μl of reaction mixture using SuperScript III first-strand synthesis kit (Invitrogen). Each cDNA (1 μl out of 20 μl) was amplified in 50 μl of the PCR mixture consisting of 2.5 units of ExTaq polymerase (Takara, Japan) under the following conditions: 30 cycles of 30 s at 95 °C, 30 s at 58 °C, and 1 min at 72 °C. The PCR products (3 μl) were resolved on a 1.5% agarose gel. The primers used for PCR amplification are as follows: sense primer, CGT ACT ATT ATT TTC ATT TTG (1345–1365 of nucleotide sequence of AW261558); antisense primer, CTA TAT AAC GGA TTG GCT AAG (1844–1864 of nucleotide sequence of AW261558).
Antibody Generation
Antibodies were generated in rabbits immunized with a recombinant 40-residue COOH-terminal putative intracellular domain of OAT-PG fused to the glutathione S-transferase tag. To produce this glutathione S-transferase-tagged antigen protein, a cDNA encoding the COOH-terminal peptide (residues 515–554) was cloned into BamHI and XhoI sites of pGEX6p-1 (GE Healthcare). Recombinant protein was expressed in BL21 (DE3) (Invitrogen) and then purified with glutathione-Sepharose 4B (GE Healthcare) in accordance with the manufacturer's instructions and dialyzed against saline (0.9% (w/v) NaCl) at 4 °C. Japanese White rabbits (Saitama Experimental Animals Supply Co., Ltd., Japan) were immunized by intramuscular injection of ∼200 μg of purified recombinant protein emulsified with the adjuvant TiterMax Gold (CytRx, Norcross, GA) (1:1) at 1-month intervals. The rabbits were bled 7 days after the third immunization.
Immunohistochemistry
Immunohistochemistry was performed following our previous reports (36, 37). Briefly, mouse kidney was fixed in 0.1 m PBS, pH 7.4, containing 4% (w/v) paraformaldehyde. For OAT-PG immunostaining, the fixed kidney was immersed in PBS containing 30% (w/v) sucrose overnight at 4 °C and frozen in Tissue-Tek OCT compound. Frozen sections (6 μm) were prepared in a cryostat at −20 °C, immersed in PBS containing 1% H2O2, exposed to 2% normal goat serum, and incubated with primary antibodies at 1:3000 dilution. After washing, the slides were incubated with peroxide-conjugated secondary antibodies (Dako, Glostrup, Denmark), and the peroxidase was detected with diaminobenzidine. For 15-PGDH immunostaining, the fixed kidney was dehydrated through a graded series of ethanol, washed, followed by embedding in paraffin, and then sectioned (6 μm) by a sliding microtome (Leica, Wetzlar, Germany). After deparaffinization and rehydration, the sections were blocked with 2% normal goat serum and then incubated with primary antibodies against 15-PGDH (CAY160615, Cayman Chemical) at 1:1000 dilution. The sections were counterstained with hematoxylin.
Immunofluorescence Double Staining of OAT-PG and 15-PGDH
Zenon IgG labeling technologies (Molecular Probes) were applied for the immunofluorescence double staining of OAT-PG and 15-PGDH. Frozen sections of mouse kidney were prepared and immersed in PBS containing 1% BSA and 2% normal donkey serum and incubated with anti-OAT-PG primary antibody at 1:3000 dilutions. After washing, the sections were incubated with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG for 1 h, washed with PBS, and postfixed with 4% paraformaldehyde. Alexa Fluor 594-labeled rabbit anti-15-PGDH antibody was prepared by using Zenon IgG labeling technologies following the manufacturer's recommendations. Anti-15-PGDH antibody was briefly mixed and incubated with Alexa Fluor 594-labeled Fab fragments that specifically bind to the Fc portion of rabbit IgG antibodies. After the incubation, the reactions were blocked with excess rabbit IgG, which interacts with and absorbs unbound Fab fragments. The labeled antibody was applied to the tissue sections prelabeled with anti-OAT-PG antibody and incubated for 1 h at room temperature. The tissue sections were further washed and fixed with 4% paraformaldehyde. These sections were viewed and imaged with a confocal microscope Zeiss LSM-510 META (Carl Zeiss, Heidelberg, Germany).
Statistical Analysis
Data are expressed as means ± S.E. Statistical differences were determined using the Student's unpaired t test. Differences were considered significant at p < 0.05.
RESULTS
Deduced Amino Acid Sequence of OAT-PG
A full-length cDNA with 2029 bp was obtained by RT-PCR of mouse kidney total RNA. The cDNA contained an open reading frame from nucleotides 86 to 1747 encoding a putative 554-amino acid protein (supplemental Fig. S1). The deduced amino acid sequence exhibited remarkable homology to those of organic anion transporters of the SLC22 family (30–40% identity) (supplemental Figs. S1 and S2). We determined that this protein was a novel prostaglandin-specific transporter as described below and designated the protein as OAT-PG (organic anion transporter for prostaglandins). Similar to other organic anion transporters of the SLC22 family, 12 transmembrane regions were predicted on the deduced amino acid sequence (supplemental Fig. S1). N-Glycosylation sites (residues 39, 65, 101, 106, and 194) and protein kinase C-dependent phosphorylation sites (residues 13, 321, 329, 403, 454, 459, 466, 537, and 541) were also predicted on OAT-PG (supplemental Fig. S1).
Transport Activity
The functional properties of OAT-PG were examined in the S2 cells stably expressing OAT-PG (S2-OAT-PG). Various radiolabeled organic ions were first tested in uptake assays. The compounds examined included typical substrates of transporters of the SLC22 family such as para-aminohippurate, estrone sulfate, dehydroepiandrosterone sulfate, urate, ochratoxin A, taurocholate, lactate, α-ketoglutarate, glutarate, succinate, tetraethylammonium, choline, carnitine, and PGE2 (19). Among them, only PGE2 was taken up by S2-OAT-PG cells (data not shown).
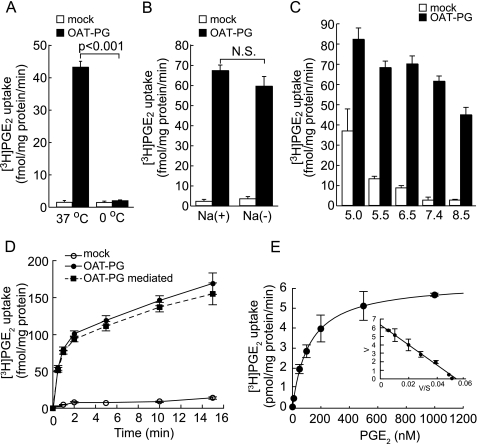
As shown in Fig. 1A, [3H]PGE2 uptake was highly sensitive to temperature. [3H]PGE2 uptake detected at 37 °C was strongly suppressed in ice-cold D-PBS (0 °C), consistent with transporter-mediated uptake. The uptake rate of [3H]PGE2 was not affected by the replacement of extracellular sodium with choline (Fig. 1B). An external pH change in the range of 5.5–7.4 did not affect the OAT-PG-mediated uptake (uptake rate of S2-OAT-PG cells − uptake rate of S2-mock cells), whereas the uptake rate was slightly lowered at pH 5.0 and 8.5 (Fig. 1C). Time and concentration dependence of PGE2 uptake are shown in Fig. 1, D and E. The uptake of [3H]PGE2 increased linearly during the 1st min of incubation (Fig. 1D), so that all the following uptake measurements were performed for 30 s. The OAT-PG-mediated [3H]PGE2 uptake was saturable and followed Michaelis-Menten kinetics (Fig. 1E). Km and Vmax values are shown in Table 1.
FIGURE 1.
OAT-PG-mediated uptake of PGE2. A, PGE2 transport via OAT-PG. Uptake of [3H]PGE2 (1 nm) was measured in S2-mock cells (open columns) and S2-OAT-PG cells (closed columns). The uptake rate of [3H]PGE2 was measured at 37 or 0 °C for 30 s. B, Na+-dependence of the transport mediated by OAT-PG. The uptake rate of [3H]PGE2 (1 nm) by S2-mock (open columns) and S2-OAT-PG cells (closed columns) was measured in the presence or absence of extracellular Na+. N.S., not significant. C, effects of extracellular pH on [3H]PGE2 uptake. Uptake rate of PGE2 (1 nm) was measured in S2-mock (open columns) and S2-OAT-PG cells (closed columns) in the media with varied pH values (from 5.0 to 8.5). D, time-dependent uptake of PGE2. The time course of uptake of [3H]PGE2 (1 nm) in S2-mock (open circles) and S2-OAT-PG cells (closed circles) was measured up to 15 min. OAT-PG-mediated transport (closed squares) was calculated by subtracting the uptake in S2-mock cells from that in S2-OAT-PG cells. E, concentration dependence of OAT-PG-mediated [3H]PGE2 uptake (1, 10, 50, 100, 200, 500, and 1000 nm). OAT-PG-mediated transport was calculated by subtracting the uptake of [3H]PGE2 in S2-mock cells from that in S2-OAT-PG cells. The [3H]PGE2 uptake was saturable and was fitted to the Michaelis-Menten curve. Inset shows the Eadie-Hofstee plots of the [3H]PGE2 uptake. V, velocity; S, concentration of PGE2.
TABLE 1.
Kinetics parameters of OAT-PG substrates
| Substrate | Kma | Vmaxa |
|---|---|---|
| nm | pmol/mg of protein/min | |
| PGE2 | 118.3 ± 3.0 | 6.32 ± 0.05 |
| PGF2α | 158.2 ± 18.7 | 4.61 ± 0.20 |
| PGE1 | 156.8 ± 18.9 | 2.71 ± 0.11 |
| PGD2 | 371.6 ± 58.1 | 8.95 ± 0.51 |
a Km and Vmax values were determined as described under “Experimental Procedures.” The table was constructed based on three separate experiments. Each value represents the mean ± S.E.
To distinguish whether OAT-PG-mediated PGE2 transport is concentrative or not, the intracellular volume of S2 cells was measured to estimate the concentration of intracellular PGE2. The intracellular volume of S2 cells was 2.05 ± 0.38 pl/cell by means of the [3H]H2O/[14C]inulin-carboxyl partition assay. In addition, the digitonin permeabilization assay showed a similar value (1.86 ± 0.51 pl/cell). These results were consistent with 80-90% of the whole cell volume as reported in previous studies (31, 32). The whole cell volume of S2 cells we used was calculated from the diameter of the trypsinized cell (cell volume, 2.43 ± 0.10 pl/cell; diameter, 16.66 ± 0.22 μm), and thus the calculated intracellular volume was 1.94–2.19 pl/cell. Based on these results, the intracellular concentration of [3H]PGE2 was estimated to reach to 5.85 ± 0.21 nm after 30 s of incubation with 1 nm PGE2. The intracellular concentration of [3H]PGE2 indicates that OAT-PG accumulated about 6-fold of PGE2 into the cells from the extracellular space.
Transport Mode of OAT-PG
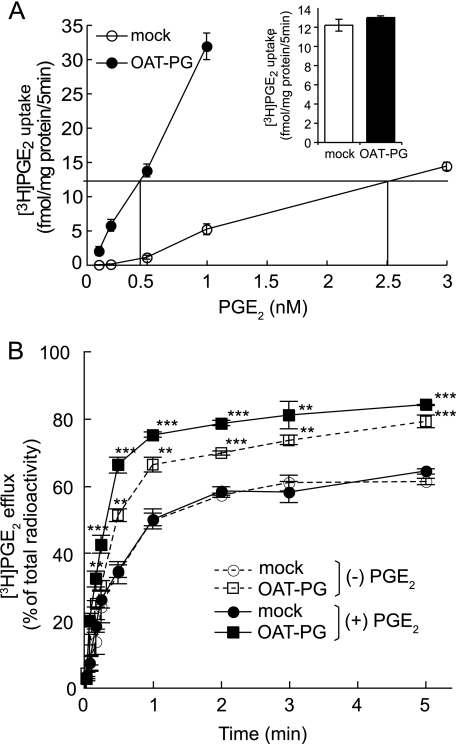
To determine the transport mode of OAT-PG, we compared the efflux of preloaded [3H]PGE2 in the presence and absence of extracellular PGE2. To load the equivalent amount of [3H]PGE2 into S2-mock and S2-OAT-PG cells, the accumulation of [3H]PGE2 at varied concentrations was determined for 5 min on each cell (Fig. 2A). The equivalent amount of [3H]PGE2 was able to be preloaded at 2.5 and 0.4 nm [3H]PGE2 into S2-mock and S2-OAT-PG cells, respectively (Fig. 2A). At these concentrations, nearly identical levels of [3H]PGE2 were loaded in both S2-mock and S2-OAT-PG cells (Fig. 2A, inset).
FIGURE 2.
Efflux of [3H]PGE2 mediated by OAT-PG. A, concentration-dependent uptake of [3H]PGE2 by S2-OAT-PG cells and S2-mock cells. The uptake of 0.1, 0.2, 0.5, 1, and 3 nm [3H]PGE2 in S2-mock cells (open circles) and the uptake of 0.1, 0.2, 0.5, and 1 nm [3H]PGE2 in S2-OAT-PG cells (closed circles) were measured for 5 min. The concentration of [3H]PGE2 was determined as 2.5 nm for S2-mock cells and 0.4 nm for S2-OAT-PG cells to obtain equivalent loading of [3H]PGE2 for both S2-mock and S2-OAT-PG cells. Inset depicts the amount of [3H]PGE2 loaded in S2-mock (open column) and S2-OAT-PG cells (closed column) incubated for 5 min in [3H]PGE2 at the concentration determined above. B, time course of the efflux of [3H]PGE2 from S2-mock cells and S2-OAT-PG cells (2, 5, 10, 15, and 30 s and 1, 2, 3, and 5 min). S2-OAT-PG cells and S2-mock cells were preloaded with [3H]PGE2 as described above for equivalent loading of [3H]PGE2. After washing with PBS, the cells were incubated in the absence ((−)PBS) or presence ((+)PBS) of 1 μm nonradiolabeled PGE2. The efflux value was expressed as percent of total radioactivity calculated from the radioactivity in the supernatant × 100%/(the radioactivity in cells plus the radioactivity in the supernatant). Circles and squares depict the levels of [3H]PGE2 efflux measured in the media with (closed symbols) or without (open symbols) 1 μm nonradiolabeled PGE2, respectively. **, p < 0.01; ***, p < 0.001 (S2-OAT-PG cells versus S2-mock cells).
For the efflux experiments, the [3H]PGE2-preloaded cells were incubated up to 5 min in the medium with or without nonradiolabeled PGE2 (1 μm). The value of the efflux of PGE2 from S2-OAT-PG cells was significantly higher than that from S2-mock cells at each time point even in the absence of extracellular PGE2 (Fig. 2B). In the presence of extracellular PGE2, the efflux of [3H]PGE2 from S2-OAT-PG cells was further increased (Fig. 2B), whereas [3H]PGE2 efflux from S2-mock cells was not affected by extracellular PGE2. These findings suggest that OAT-PG is able to mediate both facilitative transport and substrate exchange.
Substrate Selectivity of OAT-PG
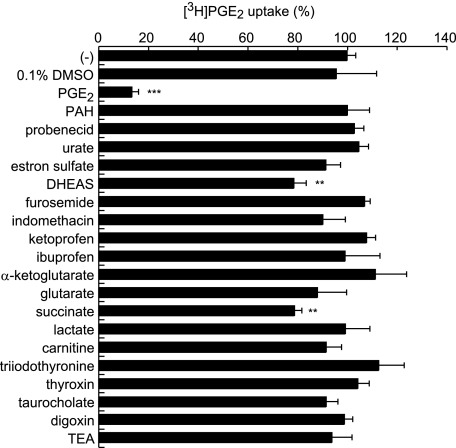
To determine the substrate selectivity of OAT-PG, we examined the inhibitory effects of prototypical substrates for SLC22 family transporters on PGE2 uptake in S2-OAT-PG cells. The cis-inhibitory effects of various compounds (1 μm) on OAT-PG-mediated uptake of [3H]PGE2 (1 nm) are shown in Fig. 3. Unlabeled PGE2 strongly suppressed [3H]PGE2 uptake, whereas the other compounds showed little to no suppression. The compounds examined included organic anions (para-aminohippurate, probenecid, urate, estrone sulfate, dehydroepiandrosterone sulfate, furosemide, indomethacin, ketoprofen, ibuprofen, α-ketoglutarate, glutarate, succinate, lactate, and taurocholate), a zwitterion (carnitine), organic cations (tetraethylammonium), and others (triiodothyronine, thyroxine, and digoxin) (19). Although high concentrations of indomethacin and furosemide (100 μm) exhibited significant inhibition on the PG uptake (supplemental Fig. S3), OAT-PG-mediated uptake of [3H]PGE2 (1 nm) was not inhibited by indomethacin, furosemide, and probenecid at 1 μm (Fig. 3). These results indicate that OAT-PG has narrow substrate selectivity, which is unusual for organic anion transporters of the SLC22 family (19).
FIGURE 3.
Inhibition of OAT-PG-mediated [3H]PGE2 uptake by various organic compounds. The OAT-PG-mediated uptake of [3H]PGE2 (1 nm) was measured in the absence or presence of nonlabeled various organic compounds at 1 μm. The values were expressed as percent of the control [3H]PGE2 uptake measured in the absence of the inhibitors (−). **, p < 0.01; ***, p < 0.001 (versus control [3H]PGE2 uptake in the absence of inhibitors). PAH, para-aminohippurate; DHEAS, dehydroepiandrosterone sulfate; TEA, tetraethylammonium.
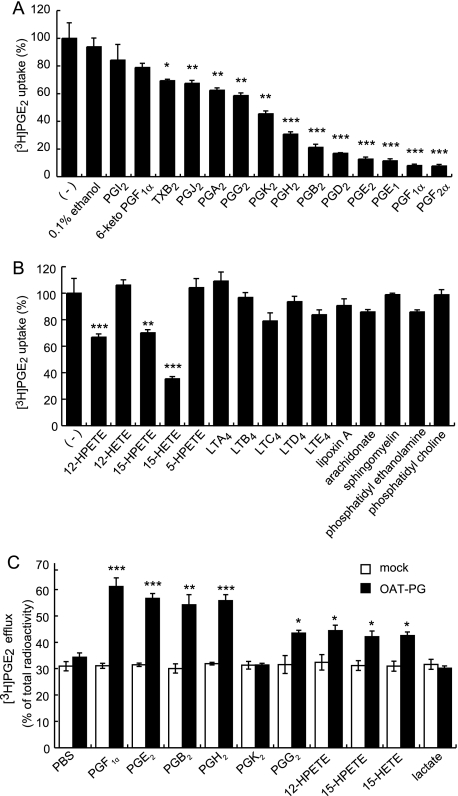
We further investigated the selectivity of OAT-PG for eicosanoids by examining the inhibitory effects of various prostanoids and leukotrienes (LTs) (1 μm) on the uptake of [3H]PGE2 (1 nm) in S2-OAT-PG cells (Fig. 4, A and B). The chemical structures of these compounds are shown in supplemental Fig. S4. PGF2α, PGF1α, PGE1, PGD2, PGB2, PGH2, and PGE2 showed strong inhibition (<40% of control uptake in Fig. 4A) on OAT-PG-mediated [3H]PGE2 uptake. PGK2, PGG2, PGA2, PGJ2, and TXB2 exhibited mild inhibition (>50% of control uptake in Fig. 4A). In contrast, 6-keto-PGF1α and PGI2 did not affect [3H]PGE2 uptake (Fig. 4A). Among the leukotrienes tested, 12-hydroperoxyeicosatetraenoate (12-HPETE), 15-hydroperoxyeicosatetraenoate (15-HPETE), and 15-hydroxyeicosatetraenoate (15-HETE) exhibited moderate to weak inhibitory effects on [3H]PGE2 uptake, whereas 12-hydroxyeicosatetraenoate (12-HETE), 5-hydroperoxyeicosatetraenoate (5-HPETE), LTA4, LTB4, LTC4, LTD4, and LTE4 did not inhibit OAT-PG-mediated [3H]PGE2 uptake (Fig. 4B). An atypical eicosanoid lipoxin A, arachidonate, and phospholipids did not inhibit [3H]PGE2 uptake (Fig. 4B). These results indicate that OAT-PG has higher affinity for a particular group of PGs among eicosanoids.
FIGURE 4.
Inhibitory effects of various eicosanoids, arachidonate, and phospholipids on OAT-PG-mediated [3H]PGE2 uptake. The OAT-PG-mediated uptake of [3H]PGE2 (1 nm) was measured in the absence or presence of inhibitors (A, prostanoids, B, leukotrienes, arachidonate, and phospholipids) at 1 μm. The values were expressed as percent of OAT-PG-mediated [3H]PGE2 uptake in the absence of the inhibitors (−). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (versus control [3H]PGE2 uptake in the absence of inhibitors). C, effects of eicosanoids to induce the efflux of [3H]PGE2 via OAT-PG. The level of [3H]PGE2 efflux from S2-OAT-PG cells and S2-mock cells was determined in the absence or presence of eicosanoids at 1 μm in the medium with 30 s of incubation and expressed as percent of efflux. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (S2-OAT-PG cells versus S2-mock cells).
The uptake of PGF2α, PGD2, and PGE1 as well as PGE2, whose radiolabeled compounds were available, was measured in S2-OAT-PG cells, and the kinetics of their OAT-PG-mediated transport were examined. The transport of PGF2α, PGD2, and PGE1 as well as PGE2 was saturable and followed Michaelis-Menten kinetics. The calculated Km and Vmax values for these substrates are listed in Table 1.
Transport of Eicosanoids by OAT-PG
By performing efflux experiments, one can evaluate whether nonradiolabeled compounds are transported by OAT-PG. Among prostanoids, similar to PGE2, extracellularly applied PGF1α, PGB2, PGH2, and PGG2 (1 μm) induced higher efflux of [3H]PGE2 from S2-OAT-PG cells than S2-mock cells, whereas PGK2 did not induce OAT-PG-mediated [3H]PGE2 efflux (Fig. 4C). 12-HPETE, 15-HPETE, and 15-HETE mildly but significantly increased the efflux of [3H]PGE2 (Fig. 4C). Lactate as a negative control had no effect on [3H]PGE2 efflux.
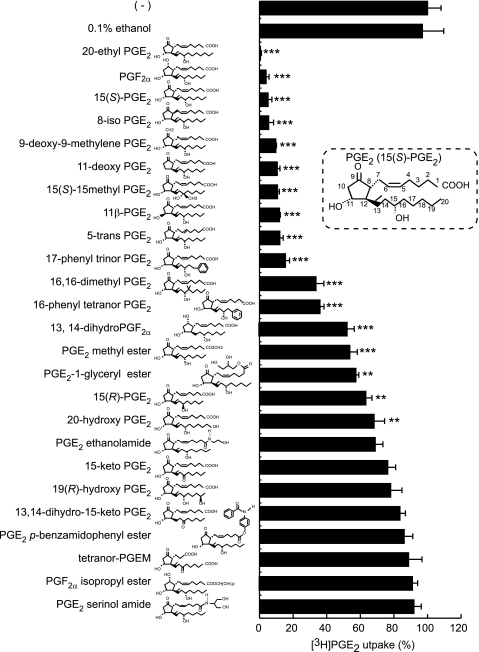
Structure-Activity Relationship of Analogues of PGE2 and PGF2α
To determine which structural features of the compounds are important for interactions with OAT-PG, we examined 22 PGE2 analogues and 3 PGF2α analogues (1 μm) on OAT-PG-mediated PGE2 uptake (Fig. 5). Compounds with modified cyclopentane rings, including 8-iso-PGE2, 9-deoxy-9-methylene PGE2, 11-deoxy-PGE2, and 11β-PGE2, exhibited strong inhibition of [3H]PGE2 uptake similar to unmodified PGE2 (15(S)-PGE2). On the contrary, nearly all analogues with modification of an α-carboxyl group (PGE2 methyl ester, PGE2-1-glyceryl ester, PGE2 ethanolamide, PGE2 serinol amide, PGE2 p-benzamidophenyl ester, and PGF2α isopropyl ester) failed to suppress the uptake of [3H]PGE2 or showed weak suppression. In the modification of the α-tail (C1–C7 of PGE2 and PGF2α), 5-trans-PGE2 strongly inhibited [3H]PGE2 uptake, whereas tetranor PGEM (tetranor-prostaglandin E metabolite) with its shorter α-tail did not inhibit [3H]PGE2 uptake. Additionally, nearly all modifications in the ω-tail (positions C13–C20) influenced the inhibitory effect on the uptake of [3H]PGE2. In particular, the lack of the double bond between positions C13 and C14 (13,14-dihydro PGF2α) and the lack of or altered configuration of the hydroxyl group at the C15 position (15-keto-PGE2, 13,14-dihydro-15-keto-PGE2, and 15(R)-PGE2) resulted in decreased suppression of [3H]PGE2 uptake. Adding an extra hydroxyl group at the C19 position (19(R)-hydroxy PGE2) or at the C20 position (20-hydroxy PGE2) also reduced the inhibitory effect on OAT-PG-mediated [3H]PGE2 uptake.
FIGURE 5.
Effects of PGE2, PGF2α, and their derivatives on OAT-PG-mediated [3H]PGE2 uptake. The OAT-PG-mediated uptake of [3H]PGE2 (1 nm) was measured in the absence or presence of indicated inhibitors (1 μm). The values are expressed as percent of OAT-PG-mediated [3H]PGE2 uptake in the absence of inhibitors (−). 0.1% ethanol, a solvent for each compound, had no significant effect on [3H]PGE2 uptake. The structure of PGE2 is shown in the inset for reference, where the numbers indicate the carbon positions from the carbon at the carboxyl group. Chemical structure of each compound is attached next to the name of the compound. **, p < 0.01; ***, p < 0.001 (versus control [3H]PGE2 uptake in the absence of inhibitors). Tetranor-PGEM, tetranor prostaglandin E metabolite.
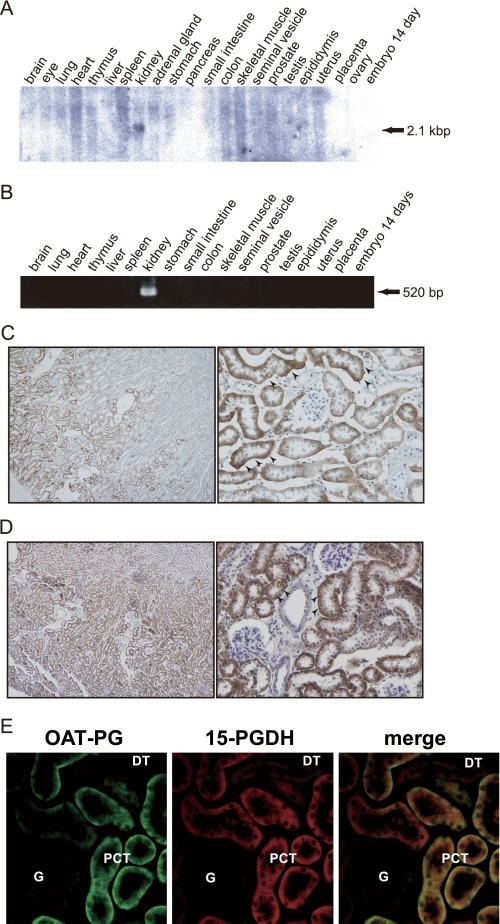
Tissue Distribution of OAT-PG in Mice
The expression of OAT-PG in mouse tissues was first examined by Northern blot (Fig. 6A). OAT-PG mRNA signal was detected in the kidney as a 2.1-kb transcript. No signals were detected in other tissues examined (i.e. brain, eye, lung, heart, thymus, liver, spleen, adrenal gland, stomach, pancreas, small intestine, colon, skeletal muscle, seminal vesicle, prostate, testis, epididymis, uterus, placenta, ovary, and embryo day 14). This specific expression in the kidney was confirmed by RT-PCR (Fig. 6B).
FIGURE 6.
Expression of OAT-PG in mouse tissues. A, Northern blot analysis. Total RNA (20 μg/lane) isolated from adult mouse tissues was analyzed by Northern blot using a 32P-labeled OAT-PG cDNA probe under high stringency conditions. The 2.1-kb OAT-PG mRNA band was only detected in the kidney. B, RT-PCR analysis. Kidney-specific expression of OAT-PG was confirmed by RT-PCR. C, immunolocalization of OAT-PG in mouse kidney. OAT-PG was detected at the basolateral membrane of proximal tubules (arrowheads) in the cortex and the outer stripe of outer medulla. Original magnifications, ×100 (left) and ×400 (right). D, immunolocalization of 15-PGDH in mouse kidney. Strong 15-PGDH immunoreactivity was found in proximal convoluted tubules (arrowheads). Original magnifications, ×100 (left) and ×400 (right). E, immunofluorescence double staining of OAT-PG (green, left) and 15-PGDH (red, middle). The co-localization of OAT-PG and 15-PGDH was found in the epithelial cells of proximal tubules (PCT) as shown with yellow color (merged image, right). DT, distal tubule; G, glomerulus. Original magnifications, ×400.
To determine the localization of OAT-PG in the kidney, a rabbit polyclonal antibody against the COOH-terminal cytoplasmic region of OAT-PG was generated. The specificity of the antibody was confirmed by Western blot using mouse kidney lysate, S2-mock cell, and S2-OAT-PG cell lysates as shown in supplemental Fig. S5. Immunostaining using the antibody demonstrated the strong signals in the basolateral membrane of proximal convoluted tubules (Fig. 6C). Staining signals were not detected in glomeruli, distal tubules, thick ascending limbs, or collecting ducts. Thus, OAT-PG showed very limited localization, only present in the basolateral membrane of the proximal tubules in the kidney.
The localization of 15-PGDH in mouse kidney was also examined. An intense signal for 15-PGDH was detected in the proximal convoluted tubules (Fig. 6D). Weak staining signals were also detected in distal tubules in the renal cortex, whereas no signals were observed in the renal medulla and papilla (data not shown).
In addition, immunofluorescence double staining confirmed the co-localization of OAT-PG and 15-PGDH in the proximal tubule (Fig. 6E). Although positive staining of 15-PGDH was detected in distal tubules, as shown in Fig. 6D, the staining did not merge with OAT-PG.
DISCUSSION
In this study, we report the function and localization of an orphan member of the SLC22 organic cation/anion/zwitterion transporter family. Functional analyses using a stably transfected cell line revealed that this novel transporter OAT-PG mediates transport of PGs selectively. Although other OAT members, such as OAT1–4, also transport PGs, they possess broad substrate selectivity (10–14, 16, 19). In contrast, OAT-PG showed little interaction with organic anions other than eicosanoids. OAT-PG was less inhibited by indomethacin, furosemide, and probenecid (Fig. 3), distinct from many OAT members that are highly inhibited by such organic anions. These characteristics suggest that OAT-PG has a unique substrate-binding site among OAT members (21, 38).
OAT-PG recognizes PGs such as PGH2, PGB2, PGD2, PGE1, PGE2, PGF1α, and PGF2α and some of the LTs. The tracer uptake and efflux assay revealed that prostanoids, PGE2, PGE2α, PGE1, PGD2, PGF1α, PGB2, PGH2, and PGG2, and leukotrienes, 15-HETE, 12-HPETE, and 15-HPETE, are transported by OAT-PG, although the affinity may be varied among them (Fig. 4C). An interesting exception was PGK2, which did not induce the efflux of [3H]PGE2 yet inhibited [3H]PGE2 uptake (Fig. 4, A and C). The reason for this exception is not clear at the moment; however, the existence of two keto groups on the cyclopentane ring might interfere with the conformational changes associated with the translocation of PGK2 after binding to the binding site.
Among PGE2 and PGF2α analogues that we examined to further obtain clues to the mechanisms of substrate recognition (Fig. 5), the compounds with structural modification on the cyclopentane ring exhibited strong inhibition on PGE2 uptake, suggesting that structural variations on the cyclopentane ring do little to influence substrate recognition of OAT-PG. This is consistent with the high affinity of OAT-PG for PGEs, PGFs, PGB2, and PGD2 because the structural differences among these prostanoids lie in their cyclopentane rings.
Most analogues with structural modification at the side chains lacked or showed significantly less inhibition. In particular, deletion of a double bond between C13 and C14, replacement, or S-to-R conversion of a hydroxyl group at C15, replacement of the carboxyl group (C1), and a hydroxylation at C19 or C20 strongly affected the inhibitory effects. The double bond between C13 and C14 likely supports the orientation of the hydroxyl group at C15 (Fig. 5). The preference of an S- rather than R-configuration of the hydroxyl group at C15 is consistent with the importance of the conformation of the ω-tail in close proximity to the cyclopentane ring. In agreement with this notion, 12-HPETE, 15-HPETE, and 15-HETE that have the (S)-hydroxyl group at C15 and the double bond between C13 and C14 exhibited weak but significant interaction with OAT-PG, even though each lacks the cyclopentane ring (Fig. 4B).
Another remarkable feature regarding the structure-activity relationship of PGE2 and PGF2α analogues is the importance of the carboxyl group in the α-tail and a hydrophobic ω-tail (Fig. 5). The substrate recognition of OAT members of the SLC22 family relies on the recognition of a negatively charged moiety such as carboxyl group and an accompanied “hydrophobic core” intrinsic to the compounds (39–42). The required negative charge is probably the one at the carboxyl group in the α-tail, whereas the hydrophobic core of PGE2 is proposed to be associated with the hydrophobicity around the ω-tail tip. This is because the presence of a hydroxyl group at C19 or C20 strongly impaired the interaction with OAT-PG, whereas the presence of an ethyl group at C20 enhanced the interaction (Fig. 5).
Schuster and co-workers (44) previously identified a PG-specific transporter PGT from the SLC21/SLCO family. Although OAT-PG belongs to the transporter family (SLC22) structurally distinct from PGT, substrate selectivity of OAT-PG mentioned above is quite similar to that of PGT (43). Previous studies investigating mechanisms of substrate recognition by PGT demonstrated that the modifications on cyclopentane rings do little to influence substrate recognition. On the other hand, modifications at the carboxyl group (C1), hydroxyl group at C15, and double bond between C13 and C14 strongly impair the interaction with PGT (43). Most of the members of the SLC21/SLCO have multispecific substrate selectivity similar to most of the OAT members of the SLC22 family, although PGT has selective affinity for PGs (4). The SLC21/SLCO family members transport relatively larger molecules (Mr 350–900). In contrast, OAT members of the SLC22 family transport molecules with lower molecular mass (Mr 100–400) (19). Molecular weight of PGs is around 350 which is within the range transported by both families. PGT and OAT-PG exhibit quite high affinity to PGs with similar Km values (94 nm for PGT (44) versus 118.3 nm for OAT-PG for PGE2(Table 1)). Thus, PGT and OAT-PG are unique members of individual families, which may have developed to enhance interaction with PGs based on the different structural traits of two distinct transporter families.
The structure-activity relationship of PGE2 and PGE2 analogues for the PGE2 receptor (EP receptor) has been well documented (45). PGE2 receptor ligands require the presence of hydroxyl groups at C11 and C15 with specific configuration (enantiomer) of PGE2, double bond between C13 and C14, and a carboxyl group at the C1 position (45). Surprisingly, these properties are quite similar to those of OAT-PG with the exception of the hydroxyl group at the C11 position of importance. The removal of a hydroxyl group at the C11 position (11-deoxy-PGE2), which is important for PGE2 receptor binding, did not strongly influence the interaction with OAT-PG (Fig. 5). This finding is also consistent with the observation that OAT-PG recognized PGD2 and PGB2 that do not have a hydroxyl group at the C11 position in the cyclopentane ring (Fig. 4A). Because PG receptors DP, EP, FP, and IP are highly specific to PGD2, PGE2, PGF2α, and PGI2, respectively (2), it is reasonable that PG receptors may recognize structural differences on the cyclopentane ring characteristic to each PG.
OAT members of the SLC22 family transport substrates by both exchange and facilitative modes (19), unlike heterodimeric amino acid transporters of the SLC7 family that, in general, mediate an obligatory exchange of the substrates (46). Our data suggest that OAT-PG is able to mediate both facilitative and exchange transport (Fig. 2B). Furthermore, we found that OAT-PG-mediated PGE2 transport is fairly concentrative. To concentrate such an anionic substrate in the cells against an electrical gradient would not be possible by facilitative transport. Therefore, it is likely that OAT-PG uses intracellular compounds as counter substrates to drive the exchange mode, although the intracellular counter substrates of OAT-PG remain to be identified.
OAT-PG is exclusively expressed in the kidney. The protein is localized on the basolateral membrane of proximal tubules in the renal cortex (Fig. 6, C and E). In the renal cortex, a constitutive type of PGE2-producing enzyme COX-1 is expressed in the collecting duct and glomerular mesangial cells, whereas the inducible type COX-2 is expressed predominantly in epithelial cells associated with the macula densa and the cortical thick ascending limb (5). PGE2 released from macula densa cells regulates renin release and renal vascular resistance and maintains glomerular hemodynamics (5). In the renal medulla, PGT is suggested to be involved in the regulation of interstitial PGE2 concentration influencing sodium and/or water reabsorption in the collecting ducts (5). Based on the localization and function, we propose that OAT-PG plays a role to remove PGE2 from the extracellular space and to terminate or limit PGE2 action in renal cortex. Multispecific organic anion transporters 1 and 3 that transport PG are also present in the basolateral membrane of proximal tubules (16, 19). Among these PG-transporting transporters, OAT-PG is beneficial due to its high selectivity for PGs without competition from the other anionic compounds.
PGE2-metabolizing enzyme 15-PGDH, which inactivates PGs, including PGE2, is present in proximal tubular cells (8, 9) where OAT-PG is located. A recent in situ hybridization and immunohistochemistry study confirmed that 15-PGDH is mainly localized in the proximal tubules in renal cortex and outer medulla of rats and mice (9). Our results confirmed the localization of 15-PGDH in the proximal tubules (Fig. 6, D and E). The fact that OAT-PG does not interact with 15-keto-PGE2 (Fig. 5) provides additional physiological significance. 15-Keto-PGE2 is the metabolite of PGE2 generated by 15-PGDH (6, 8). We suggest that once PGE2 is taken up into proximal tubule cells by OAT-PG, the PGE2 is converted to 15-keto-PGE2 by 15-PGDH, which is abundant in the proximal tubule cells. The generated 15-keto-PGE2 leaves the proximal tubule cells via unknown apical membrane transporters and is excreted into urine (8). Such a transport-metabolism coupling would allow more efficient unidirectional PGE2 transport. Therefore, specific localization of OAT-PG at the basolateral membrane of proximal tubules suggests that this concentrative PG transporter is involved in the clearance of extracellular PGE2 and local PGE2 regulation. There are some examples that the functional coupling of transporters and metabolic enzymes plays an important role in removing extracellular signaling molecules. For example, plasma membrane glutamate transporters and glutamine synthetase in glial cells in the brain work in concert to remove extracellular excitatory neurotransmitter glutamate (47, 48). The metabolic conversion of glutamate to glutamine by glutamine synthetase maintains the capacity of glial cells to absorb glutamate so that the glutamate transporters continue transporting extracellular glutamate into glial cells. The similar transport-metabolism coupling mechanism may also be applicable to renal cortex for the clearance of extracellular PGE2.
In summary, we identified a kidney-specific novel prostaglandin transporter OAT-PG as an OAT member of the SLC22 family. Its high affinity and selectivity for PGE2 and co-localization with 15-PGDH in proximal tubules of the kidney suggest that OAT-PG mediates PGE2 uptake into proximal tubule cells and is involved in the local clearance and metabolism of PGE2 in the renal cortex.
Supplementary Material
Acknowledgments
We thank Akihiro Tojo and Maristela Lika Onozato for critical discussions and Keiko Sakama-Yamazaki and Michiko Minobe for technical assistance. We also thank Tomoko Muto, Ellappan Babu, and Kanokporn Phetdee for help with preliminary experiments and Elyse M. Scileppi for proofreading.
This work was supported in part by a grant-in-aid for scientific research on priority areas of “transportsomes” from the Ministry of Education, Culture, Sports, Science and Technology of Japan and grants-in-aid for scientific research from the Japan Society for the Promotion of Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods, Table 1, and Figs. S1–S5.
The nucleotide sequence(s) reported in this paper has been submitted to the DDBJ/GenBankTM/EBI Data Bank with accession number(s)AB520670.
- PG
- prostaglandin
- 15-PGDH
- 15-hydroxyprostaglandin dehydrogenase
- LT
- leukotriene
- HETE
- hydroxyeicosatetraenoic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- OAT
- organic anion transporter
- OCT
- organic cation transporter
- SLC
- solute carrier family
- RT
- reverse transcription
- MES
- 2-(N-morpholino)ethanesulfonic acid.
REFERENCES
- 1.Vane J. R., Bakhle Y. S., Botting R. M. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 97–120 [DOI] [PubMed] [Google Scholar]
- 2.Narumiya S., Sugimoto Y., Ushikubi F. (1999) Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 3.Piper P. J., Vane J. R., Wyllie J. H. (1970) Nature 225, 600–604 [DOI] [PubMed] [Google Scholar]
- 4.Hagenbuch B., Meier P. J. (2004) Pflugers Arch. 447, 653–665 [DOI] [PubMed] [Google Scholar]
- 5.Hao C. M., Breyer M. D. (2008) Annu. Rev. Physiol. 70, 357–377 [DOI] [PubMed] [Google Scholar]
- 6.Nomura T., Lu R., Pucci M. L., Schuster V. L. (2004) Mol. Pharmacol. 65, 973–978 [DOI] [PubMed] [Google Scholar]
- 7.Pucci M. L., Endo S., Nomura T., Lu R., Khine C., Chan B. S., Bao Y., Schuster V. L. (2006) Am. J. Physiol. Renal. Physiol. 290, F641–F649 [DOI] [PubMed] [Google Scholar]
- 8.Uchida S., Nonoguchi H., Endou H. (1985) Pflugers Arch. 404, 278–284 [DOI] [PubMed] [Google Scholar]
- 9.Yao B., Xu J., Harris R. C., Zhang M. Z. (2008) Am. J. Physiol. Renal. Physiol. 294, F433–F439 [DOI] [PubMed] [Google Scholar]
- 10.Sekine T., Watanabe N., Hosoyamada M., Kanai Y., Endou H. (1997) J. Biol. Chem. 272, 18526–18529 [DOI] [PubMed] [Google Scholar]
- 11.Wolff N. A., Werner A., Burkhardt S., Burckhardt G. (1997) FEBS Lett. 417, 287–291 [DOI] [PubMed] [Google Scholar]
- 12.Sekine T., Cha S. H., Tsuda M., Apiwattanakul N., Nakajima N., Kanai Y., Endou H. (1998) FEBS Lett. 429, 179–182 [DOI] [PubMed] [Google Scholar]
- 13.Kusuhara H., Sekine T., Utsunomiya-Tate N., Tsuda M., Kojima R., Cha S. H., Sugiyama Y., Kanai Y., Endou H. (1999) J. Biol. Chem. 274, 13675–13680 [DOI] [PubMed] [Google Scholar]
- 14.Cha S. H., Sekine T., Kusuhara H., Yu E., Kim J. Y., Kim D. K., Sugiyama Y., Kanai Y., Endou H. (2000) J. Biol. Chem. 275, 4507–4512 [DOI] [PubMed] [Google Scholar]
- 15.Reid G., Wielinga P., Zelcer N., van der Heijden I., Kuil A., de Haas M., Wijnholds J., Borst P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9244–9249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura H., Takeda M., Narikawa S., Enomoto A., Ichida K., Endou H. (2002) J. Pharmacol. Exp. Ther. 301, 293–298 [DOI] [PubMed] [Google Scholar]
- 17.Gründemann D., Gorboulev V., Gambaryan S., Veyhl M., Koepsell H. (1994) Nature 372, 549–552 [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Nieto C. E., You G., Bush K. T., Barros E. J., Beier D. R., Nigam S. K. (1997) J. Biol. Chem. 272, 6471–6478 [DOI] [PubMed] [Google Scholar]
- 19.Koepsell H., Endou H. (2004) Pflugers Arch. 447, 666–676 [DOI] [PubMed] [Google Scholar]
- 20.Tamai I., Yabuuchi H., Nezu J., Sai Y., Oku A., Shimane M., Tsuji A. (1997) FEBS Lett. 419, 107–111 [DOI] [PubMed] [Google Scholar]
- 21.Anzai N., Jutabha P., Enomoto A., Yokoyama H., Nonoguchi H., Hirata T., Shiraya K., He X., Cha S. H., Takeda M., Miyazaki H., Sakata T., Tomita K., Igarashi T., Kanai Y., Endou H. (2005) J. Pharmacol. Exp. Ther. 315, 534–544 [DOI] [PubMed] [Google Scholar]
- 22.Kaler G., Truong D. M., Sweeney D. E., Logan D. W., Nagle M., Wu W., Eraly S. A., Nigam S. K. (2006) Biochem. Biophys. Res. Commun. 351, 872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin H. J., Anzai N., Enomoto A., He X., Kim, Do K., Endou H., Kanai Y. (2007) Hepatology 45, 1046–1055 [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama H., Anzai N., Ljubojevic M., Ohtsu N., Sakata T., Miyazaki H., Nonoguchi H., Islam R., Onozato M., Tojo A., Tomita K., Kanai Y., Igarashi T., Sabolic I., Endou H. (2008) Cell. Physiol. Biochem. 21, 269–278 [DOI] [PubMed] [Google Scholar]
- 25.Hosoyamada M., Ichida K., Enomoto A., Hosoya T., Endou H. (2004) J. Am. Soc. Nephrol. 15, 261–268 [DOI] [PubMed] [Google Scholar]
- 26.Perrière G., Gouy M. (1996) Biochimie 78, 364–369 [DOI] [PubMed] [Google Scholar]
- 27.Hosoyamada M., Obinata M., Suzuki M., Endou H. (1996) Arch. Toxicol. 70, 284–292 [DOI] [PubMed] [Google Scholar]
- 28.Takeda M., Khamdang S., Narikawa S., Kimura H., Hosoyamada M., Cha S. H., Sekine T., Endou H. (2002) J. Pharmacol. Exp. Ther. 302, 666–671 [DOI] [PubMed] [Google Scholar]
- 29.Rottenberg H. (1979) Methods Enzymol. 55, 547–569 [DOI] [PubMed] [Google Scholar]
- 30.Adam S. A., Sterne-Marr R., Gerace L. (1991) Methods Cell Biol. 35, 469–482 [DOI] [PubMed] [Google Scholar]
- 31.Adebodun F., Post J. F. (1993) J. Cell. Physiol. 154, 199–206 [DOI] [PubMed] [Google Scholar]
- 32.Vasquez B., Ishibashi F., Howard B. V. (1982) In Vitro 18, 643–649 [DOI] [PubMed] [Google Scholar]
- 33.Takeda M., Tojo A., Sekine T., Hosoyamada M., Kanai Y., Endou H. (1999) Kidney Int. 56, 2128–2136 [DOI] [PubMed] [Google Scholar]
- 34.Segawa H., Fukasawa Y., Miyamoto K., Takeda E., Endou H., Kanai Y. (1999) J. Biol. Chem. 274, 19745–19751 [DOI] [PubMed] [Google Scholar]
- 35.Utsunomiya-Tate N., Endou H., Kanai Y. (1996) J. Biol. Chem. 271, 14883–14890 [DOI] [PubMed] [Google Scholar]
- 36.Kanai Y., Stelzner M. G., Lee W. S., Wells R. G., Brown D., Hediger M. A. (1992) Am. J. Physiol. 263, F1087–F1092 [DOI] [PubMed] [Google Scholar]
- 37.Chairoungdua A., Segawa H., Kim J. Y., Miyamoto K., Haga H., Fukui Y., Mizoguchi K., Ito H., Takeda E., Endou H., Kanai Y. (1999) J. Biol. Chem. 274, 28845–28848 [DOI] [PubMed] [Google Scholar]
- 38.Morita N., Kusuhara H., Nozaki Y., Endou H., Sugiyama Y. (2005) Drug Metab. Dispos. 33, 1151–1157 [DOI] [PubMed] [Google Scholar]
- 39.Burckhardt G., Wolff N. A. (2000) Am. J. Physiol. Renal Physiol. 278, F853–F866 [DOI] [PubMed] [Google Scholar]
- 40.Apiwattanakul N., Sekine T., Chairoungdua A., Kanai Y., Nakajima N., Sophasan S., Endou H. (1999) Mol. Pharmacol. 55, 847–854 [PubMed] [Google Scholar]
- 41.Uchino H., Kanai Y., Kim D. K., Wempe M. F., Chairoungdua A., Morimoto E., Anders M. W., Endou H. (2002) Mol. Pharmacol. 61, 729–737 [DOI] [PubMed] [Google Scholar]
- 42.Sekine T., Cha S. H., Endou H. (2000) Pflugers Arch. 440, 337–350 [DOI] [PubMed] [Google Scholar]
- 43.Itoh S., Lu R., Bao Y., Morrow J. D., Roberts L. J., Schuster V. L. (1996) Mol. Pharmacol. 50, 738–742 [PubMed] [Google Scholar]
- 44.Kanai N., Lu R., Satriano J. A., Bao Y., Wolkoff A. W., Schuster V. L. (1995) Science 268, 866–869 [DOI] [PubMed] [Google Scholar]
- 45.Ungrin M. D., Carrière M. C., Denis D., Lamontagne S., Sawyer N., Stocco R., Tremblay N., Metters K. M., Abramovitz M. (2001) Mol. Pharmacol. 59, 1446–1456 [DOI] [PubMed] [Google Scholar]
- 46.Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. (2004) Pflugers Arch. 447, 532–542 [DOI] [PubMed] [Google Scholar]
- 47.Kanai Y., Hediger M. A. (2003) Eur. J. Pharmacol. 479, 237–247 [DOI] [PubMed] [Google Scholar]
- 48.Kanai Y., Hediger M. A. (2004) Pflugers Arch. 447, 469–479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.