Abstract
GSH is the major antioxidant and detoxifier of xenobiotics in mammalian cells. A strong decrease of intracellular GSH has been frequently linked to pathological conditions like ischemia/reperfusion injury and degenerative diseases including diabetes, atherosclerosis, and neurodegeneration. Although GSH is essential for survival, the deleterious effects of GSH deficiency can often be compensated by thiol-containing antioxidants. Using three genetically defined cellular systems, we show here that forced expression of xCT, the substrate-specific subunit of the cystine/glutamate antiporter, in γ-glutamylcysteine synthetase knock-out cells rescues GSH deficiency by increasing cellular cystine uptake, leading to augmented intracellular and surprisingly high extracellular cysteine levels. Moreover, we provide evidence that under GSH deprivation, the cytosolic thioredoxin/thioredoxin reductase system plays an essential role for the cells to deal with the excess amount of intracellular cystine. Our studies provide first evidence that GSH deficiency can be rescued by an intrinsic genetic mechanism to be considered when designing therapeutic rationales targeting specific redox enzymes to combat diseases linked to GSH deprivation.
Keywords: Apoptosis, Glutathione, Oxidation Reduction, Oxidative Stress, Thiol, Cystine/Cysteine Cycle, Thioredoxin Reductase
Introduction
A reduced intracytoplasmic environment is prerequisite for proper cellular function and is collectively maintained by various antioxidant systems, free thiols, and nonprotein redox-active compounds (1). The controlled reversible oxidation and reduction of cellular proteins play important roles in the redox regulation of a myriad of cellular processes, including proliferation, differentiation, and cell death. The tripeptide GSH is the predominant nonprotein sulfhydryl compound in mammalian cells (up to 10 mm) and is the major scavenger of reactive oxygen species by acting as co-factor for antioxidant enzymes like glutathione peroxidases. Oxidative stress and strongly decreased GSH levels are major causative factors in the etiology of multiple acute and chronic diseases, including Alzheimer and Parkinson diseases, diabetes, atherosclerosis, and ischemia/reperfusion-induced tissue injury (2). By using mice and cells with inducible disruption of glutathione peroxidase 4, we have recently shown that oxidative stress does not cause overall oxidation of essential cellular compounds but is rather sensed by GSH and glutathione peroxidase 4 and translated into a distinctive cell death signaling pathway, involving 12/15-lipoxygenase-derived lipid peroxides and apoptosis-inducing factor-mediated cell death (3, 4).
Beyond its antioxidant activity, GSH is involved in the redox regulation of cell signaling via reversible glutathionylation of cellular proteins (5). This modification is believed to act as a redox-sensing mechanism under conditions of oxidative stress (6). In addition, glutathione S-transferases are involved in the detoxification of xenobiotics by GSH conjugation (7, 8).
γ-Glutamylcysteine synthetase (γ-GCS)3 is the rate-limiting enzyme in the de novo synthesis of GSH. Embryonic lethality of γ-GCS knock-out mice at the gastrulation stage underlines the importance of GSH for embryo survival, growth, and development (9). Interestingly, γ-GCS−/− embryonic stem-like cells isolated from knock-out blastocysts can be cultured in vitro by provision of either GSH or the antioxidant N-acetylcysteine (NAC) in culture medium (9). This indicates that the intrinsic requirement of GSH can be bypassed at the cellular level by thiol-containing compounds.
The rate-limiting substrate for GSH biosynthesis is Cys, which is transported into the cell via neutral amino acid transporters. On the other hand, the cystine/glutamate antiporter, system xc−, facilitates the uptake of cystine (Cys)2, which is rapidly reduced to Cys presumably by GSH inside the cell, thus providing Cys for GSH synthesis (10, 11). System xc− is composed of two protein components, xCT light chain (the light chain of cystine/glutamate antiporter system xc−), which confers the substrate specificity for (Cys)2, and 4F2 heavy chain, a subunit shared with other amino acid transport systems (12). The expression of xCT appears to be regulated by oxygen, oxidative stress, electrophilic agents, and human immunodeficiency virus infection, which at least in part is mediated by the Nrf2/Keap1 system (13–15). In either case, up-regulation leads to increased (Cys)2 uptake and augmented intracellular GSH concentrations. In the brain, system xc− is predominantly expressed in regions facing the cerebrospinal fluid, suggesting that high levels of reactive oxygen species are counteracted at these sites by the supply of Cys (16). Activated neutrophils express xCT, indicating that system xc− protects neutrophils from oxidative stress during host defense (17). On the other hand, in glioma cells, xCT expression increases extracellular glutamate levels and induces glutamate toxicity, thereby contributing to neurodegeneration and brain edema formation. Hence, inhibition of xCT expression alleviates these symptoms during glioma development (18).
To address the significance of the (Cys)2/Cys redox cycle as an independent redox system and to challenge the supposedly unique role of GSH in redox homeostasis, we stably expressed xCT in γ-GCS−/− embryonic stem cell-like cells and in cells specifically lacking either mitochondrial thioredoxin reductase (Txnrd2) or cytosolic thioredoxin reductase (Txnrd1). Here, we show for the first time that GSH deficiency can be rescued by forced expression of xCT, which additionally requires the presence of functional Txnrd1, but not Txnrd2.
EXPERIMENTAL PROCEDURES
Materials and Reagents
The chemicals used for the experiments were purchased from Sigma-Aldrich unless stated otherwise. Tat-Cre fusion protein was a kind gift from Dr. W. Hammerschmidt (Department of Gene Vectors, Helmholtz Zentrum München). Anti-thioredoxin reductase 1 antibody was generously provided by Dr. V. N. Gladyshev (Lincoln, NE).
Generation of a Monoclonal Txnrd2-specific Antibody
A peptide comprising the C-terminal peptide sequence of Txnrd2 protein (VKLHISKRSGLEPTVTG) was synthesized and coupled to keyhole limpet hemocyanin and ovalbumin. The rats were immunized subcutaneously and intraperitoneally with a mixture of 50 μg of peptide-keyhole limpet hemocyanin, 5 nmol of CpG oligonucleotide (Tib Molbiol, Berlin, Germany), 500 μl of phosphate-buffered saline, and 500 μl of incomplete Freund's adjuvant. A boost without adjuvant was given 6 weeks after the primary injection. Fusion was performed using standard procedures. The supernatants were tested by a differential enzyme-linked immunosorbent assay with the Txnrd2 peptide coupled to ovalbumin and irrelevant peptides coupled to the same carrier. A monoclonal antibody (clone 1C4) that reacted specifically with the Txnrd2 peptide was used in this study.
Cell Lines
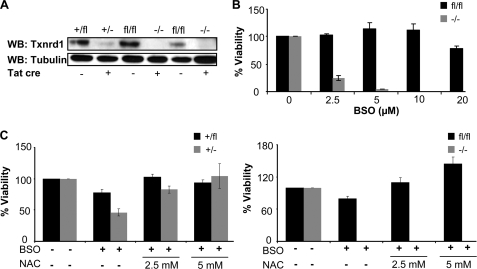
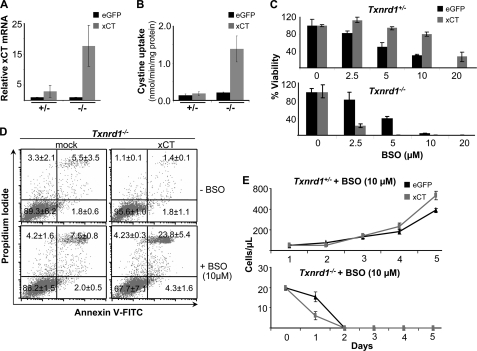
γ-GCS−/− cells were cultured as described (9). Knock-out MEF cell lines for Txnrd2 were established from Txnrd2−/− embryos as described (19). Immortalized Txnrd1−/− and Txnrd1+/− MEFs were generated from conditional transgenic mouse embryos (Txnrd1+/fl and Txnrd1fl/fl), because it was not possible to establish the MEFs cell lines directly from knock-out embryos (20). In these cell lines, disruption of Txnrd1 can be achieved by Cre recombinase leading to a loss of Txnrd1. Two independent Txnrd1fl/fl cell lines and one Txnrd1+/fl cell line were treated with Tat-Cre protein for 16 h, and clonal cell lines were generated by limiting dilution in 96-well plates. Out-growing clones were expanded, and the knock-out of Txnrd1 was confirmed by PCR for the deleted Txnrd1 allele (data not shown) and Western blotting (see Fig. 4A). Hence, after Tat-Cre treatment the cell lines derived from Txnrd1fl/fl and Txnrd1+/fl cells are Txnrd1−/− and Txnrd1+/−, respectively.
FIGURE 4.
GSH depletion-mediated cell death of Txnrd1−/− cells cannot be prevented by NAC supplementation. A, loss of Txnrd1 in Txnrd1−/− cells after Tat-Cre treatment was confirmed by Western blot (WB). B, Txnrd1fl/fl and Txnrd1−/− cells were treated with the indicated BSO concentrations for 72 h, and cell viability was determined by trypan blue exclusion. Txnrd1−/− cells were highly susceptible to BSO as compared with the parental cell line (4.14 ± 0.46% versus 113.56 ± 12.41% viability at 5 μm BSO) (means ± S.D.). The data shown here are representative of two independent sets of experiments and were confirmed with another knock-out cell line (data not shown). The heterozygous control cell line behaved in a similar manner as the respective parental cell line (data not shown). C, the thiol-containing antioxidant NAC (2.5 and 5 mm) failed to rescue Txnrd1−/− cells from cell death induced by BSO-mediated GSH depletion (10 μm BSO; right panel), whereas NAC provided resistance against BSO in wild-type (Txnrd1fl/fl and Txnrd1+/fl) and heterozygous (Txnrd1+/−) cell lines (left panel). Txnrd1+/− cells behaved similarly to Txnrd1+/fl cells, i.e. both cell lines could be efficiently rescued by NAC. The data are representative of two sets of experiments and were confirmed with another Txnrd1−/− cell line (data not shown).
Transfection of Cells
The murine xCT expression vector pCAG-3SIP-xCT, empty vector, and eGFP-expressing vector pCAG-3SIP-eGFP, obtained after cloning of eGFP (pEGFP-N1; Clontech) into vector pCAG-3SIP, were transfected in 1 × 106 cells. Electroporation (Gene Pulser II; Bio-Rad) was performed at 240 V and 500 microfarads. Stable selection was initiated 48 h after electroporation using puromycin, gradually increasing from 0.5 to 2 μg/ml over 14 days.
Northern Blot Analysis
Northern blot analysis was conducted as described (21).
Cys and GSH Detection by HPLC
The extra- and intracellular Cys and GSH levels were determined by HPLC as outlined (21).
l-Cystine Uptake Measurements
l-Cystine transport activity was measured as described (15, 22).
Determination of Extracellular Mercaptans by Ellman's Test
The cells were incubated in fetal calf serum-free Dulbecco's modified Eagle's medium ± cystine (Invitrogen catalog number 21013). 500 μl of conditioned medium was mixed with 50 μl of 5,5′-dithiobis(nitrobenzoic acid) solution (10 mm 5,5′-dithiobis(nitrobenzoic acid) in 0.2 m potassium phosphate buffer, 10 mm EDTA, pH 7.2) and incubated for 2 min. Absorbance was measured at 412 nm. Fresh Dulbecco's modified Eagle's medium was used as blank control. Standard samples of reduced GSH (10–60 μm) were prepared by serial dilution in Dulbecco's modified Eagle's medium.
Quantitative RT-PCR
Total RNA was isolated with an RNeasy mini kit (Qiagen), DNase-digested, and reverse-transcribed using a reverse transcription system (Promega, Mannheim, Germany). The transcripts were amplified and detected with a LightCycler FastStart DNA MasterPLUS SYBR Green I kit and a LightCycler 1.5 system (Roche Applied Science) using the primers xCTfor1 (5′-GGCACCGTCATCGGATCAGGCATC-3′) and xCTrev1 (5′-CACGAGCTTGATTGCAAGTTCAGG-3′) for xCT and aldolase A (5′-GGTCACAGCACTTCGTCGCACAG-3′) and aldolase B (5′-TCCTTGACAAGCGAGGCTGTTGGC-3′) for aldolase.
Western Blot
Western blot analysis was done as described earlier (3).
Co-culture Experiments
Co-culture experiments were performed by seeding xCT-overexpressing cells with eGFP-expressing cells in a 1:1 ratio in 6-well plates. The co-cultures were treated with the indicated l-buthionine sulfoximine (BSO) concentrations for 48–72 h and then analyzed by FACS (BD FACSCalibur) for the presence of eGFP-positive cells. The data were analyzed by the BD CellQuestTM Pro software (BD Biosciences).
Annexin V-Propidium Iodide (PI) Staining
Annexin V-PI staining was performed according to the manufacturer's instructions (BD Pharmingen, Heidelberg, Germany). Briefly, the cells were washed twice with cold phosphate-buffered saline and resuspended in 100 μl of annexin binding buffer. Then the cells were incubated with 5 μl of annexin V-FITC (BD Pharmingen) and 5 μl of PI (20 mg/ml stock solution; Sigma-Aldrich) for 15 min in the dark at room temperature. The cells were then analyzed by FACS (BD FACSCalibur), and the data were analyzed by the BD CellQuestTM Pro software (BD Biosciences).
RESULTS
Enforced Expression of xCT Rescues GSH Deficiency
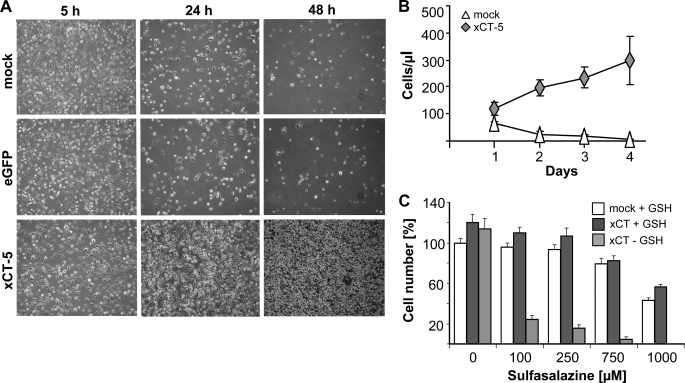
GSH is essential for the proliferation, growth, and development in mammals because the targeted disruption of γ-GCS causes early embryonic lethality (9). The embryonic stem cell-like cells derived from γ-GCS−/− blastocysts (referred to as γ-GCS−/− cells in the following) have been shown to be essentially devoid of endogenous GSH (9) (supplemental Fig. S1A); however, they can be cultured indefinitely when GSH or NAC is included in the cell culture medium (9) (supplemental Fig. S1B). Recently, we provided evidence that overexpression of xCT in Burkitt's lymphoma cells confers resistance to oxidative stress, imposed by seeding at low cell densities or by experimental GSH depletion (21). To address the question of whether enforced expression of xCT overrides the GSH requirement of cells, γ-GCS−/− cells were transfected with a bicistronic expression vector (pCAG-3SIP-xCT), allowing stable selection of transfected cells by puromycin. As controls, the cells were transfected with the empty vector (pCAG-3SIP, mock) or an eGFP expression vector (pCAG-3SIP-eGFP, eGFP). Single cell clones were isolated, and one mock transfected, one eGFP-transfected, and two xCT-transfected clones (xCT-5 and xCT-7) were used for further studies. xCT expression was confirmed by Northern blot (supplemental Fig. S1C) and quantitative RT-PCR (supplemental Fig. S1D).
We then asked whether xCT expression permits cell survival and proliferation in the absence of GSH. When cultivated in the absence of thiol-containing supplements (Fig. 1, A and B), xCT-expressing cells continued to proliferate, whereas control cells rapidly died. Of note, xCT-overexpressing cells could be maintained indefinitely in the absence of thiol-containing supplements such as GSH/NAC in the cell culture medium (data not shown). To further substantiate that this effect was due to enforced xCT expression, xCT-overexpressing cells were treated with sulfasalazine, a potent inhibitor of system xc− (23). Sulfasalazine treatment abrogated the pro-survival effect of xCT in the absence of GSH in a dose-dependent manner (Fig. 1C), showing that xCT overexpression successfully bypasses the requirement of GSH for cell survival and proliferation. To address whether xCT expression confers resistance also to oxidative stress-inducing agents, the cells were treated with pro-oxidants and genotoxic agents (hydrogen peroxide, tert-butyl hydroperoxide, antimycin A, doxorubicin), and cell viability was determined 48 h thereafter (supplemental Fig. S2, A–D). Control cells and xCT-expressing γ-GCS−/− cells died in a dose-dependent manner. No substantial difference in susceptibility toward the tested chemicals was detectable between xCT-expressing and GSH-supplemented γ-GCS−/− control cells, indicating that enforced xCT expression also substitutes for key antioxidant functions of GSH.
FIGURE 1.
Stable expression of xCT rescues GSH deficiency in γ-GCS−/− cells. A, as shown by phase contrast microscopy, control cells rapidly died within 48 h, whereas xCT-overexpressing cells proliferated in the absence of exogenous GSH or NAC. B, quantification of the results shown in A. xCT overexpression provided a growth advantage and compensated GSH deficiency. C, the system xc− inhibitor sulfasalazine abrogated the pro-survival effect of xCT in the absence of GSH. The error bars indicate the means ± S.D. of triplicate measurements.
To rule out any putative residual γ-GCS activity in γ- GCS−/− cells, NAC-supplemented eGFP-transfected or nonsupplemented xCT-transfected γ-GCS−/− cells were treated with high concentrations of the γ-GCS-specific inhibitor BSO. This was deemed necessary because the strategy for targeted disruption of γ-GCS only deleted the first exon, encoding the N terminus of γ-GCS (9). BSO treatment did not impact on cell survival or proliferation of xCT-transfected cells even at excessively high concentrations (1 mm) (supplemental Fig. S3), which are 20–100-fold higher than the toxic concentration for wild-type cells (19). These data indicate that BSO is a highly specific inhibitor of γ-GCS, and therefore it has been used in the subsequent studies for experimental GSH depletion.
System xc− Mediates an Increase of Intracellular and a Strong Increase of Extracellular Cys Levels
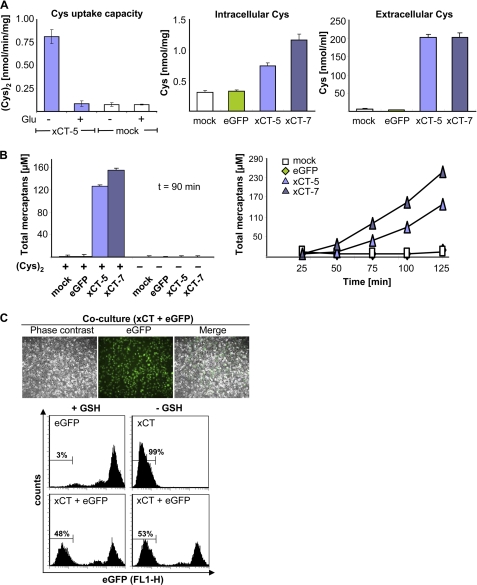
(Cys)2 uptake capacity was measured with radioactively labeled (Cys)2. Clone xCT-5 showed an 8-fold increase in (Cys)2 uptake capacity (0.82 ± 0.08 nmol/min/mg) as compared with mock transfected cells (0.08 ± 0.02 nmol/min/mg), which could be reduced to basal levels (comparable with mock) by the addition of glutamate (2.5 mm) (Fig. 2A), a specific inhibitor of system xc−. Intra- and extracellular Cys levels were quantified by HPLC to explore the fate of (Cys)2 upon uptake. Despite a 10-fold increase in (Cys)2 uptake activity, only a 2–3-fold increase of intracellular Cys was detectable in xCT-expressing cells (Fig. 2A). By stark contrast, extracellular Cys levels were increased 50-fold when compared with control cell lines. Accordingly, the levels of extracellular free thiols detected by Ellman's test increased in a time-dependent manner in xCT-overexpressing cells, whereas no increase was detected in control cells (Fig. 2B). Moreover, the levels of extracellular free thiols did not increase when cells were incubated in (Cys)2-free medium, confirming that the increase of extracellular thiols (Cys) could be fully attributed to imported (Cys)2 and subsequent efflux of Cys into the extracellular space. Because of the unexpectedly high extracellular Cys levels of xCT-overexpressing cells, we asked whether co-cultivation may allow for the proliferation of control cells. When xCT-transfected cells were co-cultured with eGFP-transfected control cells, we in fact observed that both cell lines proliferated at the same rate (Fig. 2C), indicating that xCT-overexpressing cells act in a feeder-like manner by providing Cys to eGFP-transfected control cells.
FIGURE 2.
Enforced expression of xCT in γ-GCS−/− cells causes increased intracellular and markedly augmented extracellular Cys levels. A, the (Cys)2 uptake capacity was measured by uptake of radiolabeled (Cys)2. The (Cys)2 uptake capacity was increased 8-fold in xCT-transfected cells. 2.5 mm glutamate (Glu) inhibited the activity of system xc− (left panel). HPLC analysis revealed a moderate increase of intracellular Cys levels in xCT-expressing γ-GCS−/− cells (middle panel), whereas extracellular Cys levels were strongly increased during 90 min of cultivation in fetal calf serum-free medium (right panel). B, Ellman's test showed a strong increase of extracellular free thiols in xCT-transfected cells, which could be abolished by cultivation in (Cys)2-free medium (left panel). The total mercaptans increased gradually over time in xCT-expressing cells in the extracellular medium (right panel). C, xCT-expressing cells sustained proliferation of eGFP-transfected control cells in co-culture in the absence of GSH or NAC by a feeder-like mechanism. eGFP-expressing cells were detected by fluorescence microscopy and quantified by FACS analysis 72 h after GSH removal. The error bars indicate the means ± S.D. of triplicate measurements.
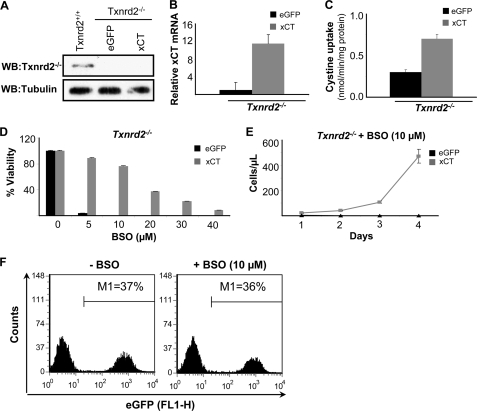
xCT Overexpression Rescues Txnrd2−/− Cells from GSH Deprivation
Contrary to the assumption that GSH is the major source for (Cys)2 reduction, our studies with xCT-expressing γ-GCS−/− cells imply that the reduction of imported (Cys)2 is still operative in the absence of GSH. This presented an open question: what is the reducing force behind (Cys)2 reduction in the cells under GSH-deprived conditions? We hypothesized that the thioredoxin/thioredoxin reductase system might be a likely candidate for exerting this function. In this context it is noteworthy that Txnrd2−/− cells have been shown to be highly susceptible to GSH depletion by BSO in contrast to wild-type cells (19). Because BSO-induced cell death of Txnrd2−/− cells can be prevented by NAC (19), we asked whether xCT overexpression abolishes the effect of BSO on Txnrd2−/− cells in a manner similar to xCT-expressing Burkitt's lymphoma cells (21). To this end, immortalized Txnrd2−/− cell lines were established from knock-out embryos. The absence of Txnrd2 was confirmed by immunoblotting (Fig. 3A). Then Txnrd2−/− cells were transfected with eGFP (control) and xCT expression plasmids, and stable cell lines were established after puromycin selection as outlined above. xCT overexpression was analyzed by quantitative RT-PCR (Fig. 3B), which was corroborated by increased (Cys)2 uptake activity (Fig. 3C). As compared with eGFP-transfected control cells, xCT-overexpressing cells showed an ∼12-fold increase in xCT expression at the transcriptional level and an ∼2-fold higher uptake of radiolabeled (Cys)2 (Fig. 3, B and C). As illustrated in Fig. 3D, xCT overexpression in Txnrd2−/− cells conferred resistance toward BSO in a dose-dependent manner and led to an increase in the number of xCT-overexpressing Txnrd2−/− cells over time (Fig. 3E). Our data reveal that BSO-induced cell death of Txnrd2 null cells can be rescued either by NAC supplementation (19) or by enforced xCT expression. Furthermore, xCT-expressing Txnrd2−/− cells were able to rescue the eGFP-expressing control cells from BSO-induced cell death in co-culture experiments (Fig. 3F), implying that reduction of (Cys)2 is still functional in Txnrd2−/− cells. Because the requirement for GSH can be bypassed by xCT overexpression in Txnrd2−/− cells, we conclude that Txnrd2 is dispensable for efficient (Cys)2 reduction.
FIGURE 3.
xCT overexpression rescues Txnrd2−/− cells from GSH depletion-induced cell death. A, loss of Txnrd2 in Txnrd2−/− cells was confirmed by Western blot (WB). B and C, stable expression of xCT in Txnrd2−/− cells was confirmed by quantitative RT-PCR and cystine uptake measurements. xCT-overexpressing cells showed a 12-fold increase in xCT transcript levels relative to eGFP control cells that resulted in a 2-fold increase in cystine uptake activity as compared with eGFP-transfected control cells. The error bars indicate the means ± S.D. D and E, overexpression of xCT rendered Txnrd2−/− cells more resistant to BSO-induced cell death as shown in a dose-response experiment (D), resulting in an increase in cell numbers over time at a given BSO concentration (10 μm) (E). The cell numbers were determined by the trypan blue dye exclusion method. The error bars indicate the means ± S.D. F, co-cultivation of xCT- and eGFP-transfected Txnrd2−/− cells rescued eGFP-expressing Txnrd2−/− cells from BSO-induced cell death.
Txnrd1−/− Cells Are Highly Susceptible to GSH Depletion-induced Cell Death
After having seen that intracellular (Cys)2 reduction is functional in γ-GCS−/− and Txnrd2−/− cells, we then asked whether Txnrd1 might be the driving force for the utilization or recycling of thiol-containing compounds and for (Cys)2 reduction, be it directly or indirectly. Txnrd1 is localized in the cytosol and displays broad substrate specificity. Because we previously failed to isolate MEF cultures directly from Txnrd1−/− knock-out embryos (20), we established MEF cell lines from conditional Txnrd1 knock-out embryos (fl, floxed). Txnrd1 knock-out (in the following referred to as Txnrd1−/−) and Txnrd1 heterozygous (Txnrd1+/−) cell lines were derived from Txnrd1fl/fl and Txnrd1+/fl cells, respectively, after treatment with Tat-Cre fusion protein and subsequent single cell cloning. The absence of Txnrd1 was confirmed by immunoblotting (Fig. 4A). Given our previous observation that we invariably failed to establish Txnrd1−/− cells directly from knock-out embryos (20), it was in fact surprising that Txnrd1 knock-out MEFs survived in culture. In line with this observation, Yoo et al. (24) reported recently that knockdown of Txnrd1 by small interfering RNA allows cell survival in vitro, although this approach does not rule out the possibility that residual amounts of Txnrd1 are still present in these cells.
Next, we analyzed the effect of GSH depletion on Txnrd1−/− cells. Like Txnrd2−/− cells, Txnrd1−/− cells were highly susceptible to BSO (Fig. 4B). But unlike Txnrd2−/− cells, Txnrd1−/− cells could not be rescued from BSO-induced cell death by NAC supplementation (Fig. 4C, right panel), whereas antioxidant supplementation efficiently reversed BSO-induced cell death in Txnrd1+/− cells (Fig. 4C, left panel, and supplemental Fig. S4). This suggests that Txnrd1−/− cells are unable to exploit the beneficial effects of antioxidants to alleviate oxidative stress induced by GSH depletion. This phenotype was unprecedented and surprising because GSH depletion-induced cell death could be prevented in γ-GCS−/− as well as in Txnrd2−/− cells by the thiol-containing antioxidant NAC.
Forced xCT Expression Does Not Rescue Txnrd1−/− Cells from GSH Depletion
Next, we established mock Txnrd1−/−, eGFP-Txnrd1−/−, and xCT-Txnrd1−/− cell lines and as controls mock Txnrd1+/−, eGFP-Txnrd1+/−, and xCT- Txnrd1+/− cell lines. Compared with eGFP-Txnrd1−/− control cells, xCT-Txnrd1−/− cells showed an 18-fold (17.73 ± 6.73) increase in xCT transcripts, translating into a 7-fold higher uptake of radiolabeled (Cys)2 (0.208 ± 0.022 versus 1.385 ± 0.347 nmol of cystine/min/mg protein) (Fig. 5, A and B). Similarly, xCT-Txnrd1+/− cells showed a 3-fold (2.9 ± 1.95) increase in xCT mRNA levels as compared with eGFP- Txnrd1+/− cells, whereas there was only a slight increase in (Cys)2 uptake activity (0.182 ± 0.05 nmol of cystine/min/mg protein) compared with eGFP control cells (0.141 ± 0.03 nmol of cystine/min/mg protein) (Fig. 5, A and B).
FIGURE 5.
Enforced expression of xCT in Txnrd1−/− cells does not rescue cells from BSO-induced cell death. A and B, xCT overexpression in Txnrd1+/− and Txnrd1−/− cells was confirmed by quantitative RT-PCR (A) and uptake measurements of radiolabeled cystine (B). A 3-fold (2.9 ± 1.95-fold) increase in xCT mRNA levels in xCT-Txnrd1+/− cells resulted in a marginal increase in cystine uptake, whereas in xCT-Txnrd1−/− cells, there was an 18-fold (17.73 ± 6.73-fold) increase in xCT mRNA levels, resulting in a 7-fold higher uptake of radiolabeled (Cys)2 (0.208 ± 0.022 versus 1.385 ± 0.347 nmol of cystine/min/mg protein]). C, despite the marginal increase in cystine uptake in xCT-Txnrd1+/− cells, ectopic xCT expression was associated with increased resistance to BSO in xCT-Txnrd1+/− cells as compared with eGFP-transfected Txnrd1+/− cells (80% versus 30% viability at 10 μm BSO) (upper panel). In stark contrast, xCT overexpression not only failed to rescue Txnrd1−/− cells from BSO-induced cell death, it rendered the cells significantly more susceptible to BSO-induced cell death (23% viability of xCT-Txnrd1−/− cells versus 81.42% viability of eGFP-Txnrd1−/− control cells at 2.5 μm BSO) (lower panel). The data are representative of two separate sets of experiments (means ± S.D.). D, the increased susceptibility of xCT-transfected Txnrd1−/− cells to BSO-induced cell death as compared with mock transfected Txnrd1−/− cells was confirmed by annexin V and propidium iodide staining. The cells were treated with 10 μm BSO for 24 h and then stained with annexin V and PI. Upon BSO treatment, the percentage of annexin V-PI-positive Txnrd1−/− cells was 4-fold higher when xCT was overexpressed as compared with control cells (23.8 ± 5.4% in xCT-Txnrd1−/− versus 7.5 ± 0.8% in mock Txnrd1−/− cells). Pooled data from two separate experiments are presented as percentages with means ± S.D. E, proliferation and viability over time of Txnrd1+/− and Txnrd1−/− cells transfected with xCT or eGFP in the presence of 10 μm BSO. The BSO treatment accelerated cell death in xCT-transfected as compared with eGFP-transfected Txnrd1−/− cells (lower panel) but only marginally affected the viability and proliferation of Txnrd1+/− cells (upper panel). The data are representative of two independent sets of experiments (means ± S.D.).
We then addressed whether xCT overexpression is able to rescue Txnrd1−/− cells from GSH depletion-induced cell death. Surprisingly, xCT-Txnrd1−/− cells not only died at a much lower concentration of BSO (2.5 μm) than eGFP- Txnrd1−/− control cells (10 μm) (Fig. 5C, lower panel), they also died at a faster rate (Fig. 5, D and E). For further quantification of cell death, mock and xCT-transfected Txnrd1−/− cells were treated with 10 μm of BSO for 24 h, stained with annexin V and PI, and subjected to FACS analysis. Compared with mock Txnrd1−/− cells (7.5 ± 0.8% annexin V-PI-positive cells), xCT-Txnrd1−/− cells revealed four times more dying cells (23.8 ± 5.4 annexin V-PI-positive cells) (Fig. 5D). Although xCT was only marginally overexpressed in the xCT-Txnrd1+/− control cell line, it conferred resistance against BSO in a dose-dependent manner (Fig. 5C, upper panel). Thus, at 10 μm BSO 79.84 ± 4.8% of xCT-Txnrd1+/− cells were viable as compared with 30.43 ± 1.45% for eGFP-transfected Txnrd1+/− cells (Fig. 5C). These results indicate that BSO treatment of xCT-overexpressing Txnrd1−/− cells causes induction of cell death at a faster rate compared with mock transfected cells. Thus, rather than conferring resistance against BSO-induced cell death, xCT overexpression in Txnrd1 knock-out cells aggravates and accelerates the induction of BSO-induced cell death.
Co-culturing of Txnrd1−/− Cells with xCT-overexpressing Cells Fails to Rescue Txnrd1−/− Cells against GSH Deprivation
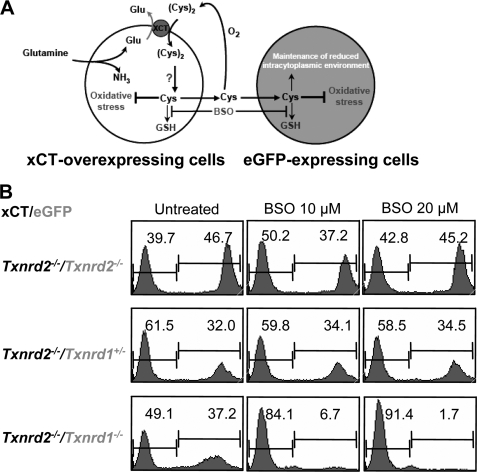
Because xCT overexpression in γ-GSC−/− and Txnrd2−/− cells was shown to provide a feeder-like effect to eGFP-transfected control cells by secreting high levels of Cys (compare Fig. 2C), we performed co-culture experiments to investigate whether GSH depletion-induced cell death of eGFP-Txnrd1−/− cells can be rescued by xCT-overexpressing cells, which do express Txnrd1. The co-culture experiment is schematically represented in Fig. 6A. We used xCT-overexpressing Txnrd2−/− cells as “feeder” cells because these cells were resistant to BSO treatment (see also Fig. 3, D and E) and rescue the eGFP control cells in co-culture experiments (Fig. 3F). As controls we used eGFP-expressing Txnrd2−/− and eGFP-expressing Txnrd1+/− cells.
FIGURE 6.
eGFP-expressing Txnrd1−/− cells cannot be rescued from BSO-induced cell death by co-culture with xCT-expressing Txnrd2−/− cells. A, schematic outline of the co-culture experiment. Under BSO-induced GSH deficiency, xCT-overexpressing cells can rescue control cells from cell death by generating a Cys-rich extracellular environment. B, in co-culture experiments, xCT-overexpressing Txnrd2−/− were able to rescue eGFP-expressing Txnrd2−/− (top panel) and Txnrd1+/− cells (middle panel) from BSO-induced cell death, but not eGFP-expressing Txnrd1−/− cells (bottom panel). Thus, even in medium conditioned by xCT-Txnrd2−/− cells and highly enriched for reducing equivalents in the form of Cys, eGFP-Txnrd1−/− cells cannot survive and proliferate when GSH synthesis is inhibited by BSO treatment.
The cells were seeded at the same cell numbers, and the co-cultures were treated with 10 and 20 μm BSO. After 48 h of treatment, the presence of eGFP-positive cells was analyzed by flow cytometry. The results of the co-culture experiment revealed that xCT-overexpressing Txnrd2−/− cells were unable to rescue eGFP-Txnrd1−/− cells from cell death induced by BSO-mediated GSH depletion (Fig. 6B, lower panel). In contrast, BSO-induced cell death was prevented in the heterozygous eGFP-Txnrd1+/− control cell line and in eGFP-transfected Txnrd2−/− cells (34 and 45% eGFP-positive cells, respectively). Hence, it was concluded from these experiments that, upon GSH depletion, Txnrd1−/− cells are unable to exploit either the beneficial effects of imported (Cys)2 or those of freely available Cys in the culture medium. Thus, Txnrd1 knock-out cells cannot be rescued from GSH depletion-induced cell death either by antioxidants, xCT overexpression, or co-culturing with xCT-overexpressing cells that condition the medium with Cys.
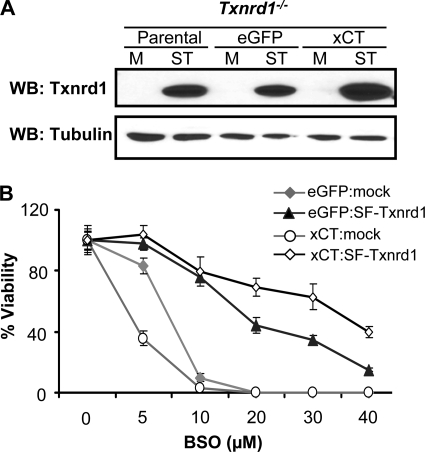
Reconstitution of Txnrd1 Expression in xCT-overexpressing Txnrd1−/− Cells Restores Resistance toward BSO
To show that the aforementioned phenotype is solely due to the loss of Txnrd1, we reconstituted Txnrd1 expression in the same cellular systems. To this end, we cloned Strep/FLAG-tagged mouse Txnrd1 (transcript variant 2 or 2ATG) (SF-Txnrd1) into a lentiviral expression vector and transduced parental Txnrd1−/− cells, eGFP-Txnrd1−/− cells, and xCT-Txnrd1−/− cells with empty lentivirus or SF-Txnrd1-expressing lentivirus. The overexpression of Txnrd1 in these cells was confirmed by Western blotting (Fig. 7A). As compared with mock transduced cells, SF-Txnrd1-overexpressing xCT-Txnrd1−/− cells showed greater resistance toward BSO (48 h of treatment) over a wide range of concentrations (Fig. 7B). Thus, we concluded that the add-back of Txnrd1 fully restored resistance toward BSO.
FIGURE 7.
Reconstitution of Txnrd1 expression in xCT-overexpressing Txnrd1−/− cells confers resistance to BSO-mediated toxicity5. A, parental Txnrd1−/− cells as well as eGFP- and xCT-overexpressing Txnrd1−/− cells were transduced either with empty virus (mock control) or with a lentivirus expressing Txnrd1 containing a Strep/FLAG tag at the N terminus (SF-Txnrd1). Overexpression of SF-Txnrd1 was confirmed by Western blotting (WB) using a thioredoxin reductase 1-specific antibody. M, mock lentivirus [442L1-puro]); ST, SF-Txnrd1-expressing lentivirus. B, xCT-overexpressing Txnrd1−/− cells were rescued from cell death over a wide range of BSO concentrations by transduction with a SF-Txnrd1-expressing lentivirus (39% viability at 40 μm BSO) but not by transduction with empty control virus (2.8% viable cells at 10 μm BSO). Note the opposing role of xCT expression depending on whether Txnrd1 is missing (more susceptible to killing by BSO than control cells) or reconstituted (more resistant to BSO than control cells).
DISCUSSION
GSH is the prevailing antioxidant in mammalian cells, being present in millimolar concentrations. Beyond its antioxidant role, emerging concepts implicated GSH in the regulation of cell signaling by reversible glutathionylation of proteins (like actin, Ras, caspase, NFκB, and thioredoxin) (25–28), yet the successful isolation of γ-GCS knock-out cells in the presence of exogenous GSH or NAC raised uncertainties about the supposedly unique role of GSH in cell physiology, even though the knock-out of γ-GCS is embryonically lethal in mice (9). Our cellular studies demonstrate for the first time that cellular GSH deficiency can be rescued by an alternative redox system, the (Cys)2/Cys redox cycle, characterized by increased cystine uptake, intracellular cystine reduction, and augmented intracellular and highly elevated extracellular Cys levels. This redox cycle, driven by the cystine-glutamate antiporter, system xc−, emerges as a powerful and independent redox mechanism, which permits cell survival and proliferation even when cells are depleted of endogenous GSH. This implies that many GSH-related functions, such as reactive oxygen species scavenging by glutathione peroxidase, protein modification by reversible oxidation (glutathionylation or cysteinylation), and cell signaling (5, 29), can apparently occur as long as a steady supply of Cys is ensured—an unexpected and surprising finding. Previously, Cys has been regarded to be merely required for protein and GSH synthesis, and import of Cys has been considered as a parameter determining the intracellular GSH levels and increasing the resistance to oxidative stress (17, 30). Our results, however, suggest that this view needs to be revised. They further highlight the complexity and redundancy between the major redox systems.
So far it has been assumed that GSH is the main reducing power for intracellular reduction of (Cys)2, which is certainly conceivable for wild-type cells, but our findings imply that under conditions of low GSH availability or even GSH deprivation, a GSH-independent system must exist that efficiently reduces (Cys)2 by a non-GSH-dependent mechanism. Although trace amounts of GSH might be provided by the serum at exceedingly low concentrations, it is very unlikely that they play a significant role in sustaining vital cellular functions. Interestingly, amitochondrial protozoans, such as Entamoeba histolytica, lack GSH metabolism and instead rely on Cys as their principal low molecular mass thiol (31). Of note, the steady-state redox potential of (Cys)2/Cys is considerably more oxidized than GSSG/GSH; thus, both redox couples must be regulated independently (32); however, the mechanism behind (Cys)2 reduction has never been dissected.
The thioredoxin/thioredoxin reductase system is a very likely candidate to exert this function. In fact, initial in vitro observations by Holmgren (33) implicated the bovine thioredoxin/thioredoxin reductase system in (Cys)2 reduction. Therefore, we stably expressed xCT in Txnrd2−/− and Txnrd1−/− cells and challenged them with BSO to address the role of thioredoxin reductases in intracellular (Cys)2 reduction. Previous work from our laboratory demonstrated that Txnrd2−/− cells rapidly die in response to GSH depletion, which can be rescued by NAC treatment (19). Likewise, overexpression of xCT in Txnrd2−/− cells provided strong rescue against BSO-mediated cell death and even prevented cell death of eGFP-expressing Txnrd2−/− control cells when co-cultured with xCT-overexpressing Txnrd2−/− cells. This is consistent with our previous observation that enforced expression of xCT rescues Burkitt's lymphoma cells from various triggers of oxidative stress (21). The finding that the (Cys)2/Cys redox cycle is operating and fully protective in Txnrd2−/− as well as γ-GCS−/− cells provides conclusive genetic evidence that neither Txnrd2 nor GSH are required for intracellular reduction of (Cys)2.
We therefore set out to address the role of Txnrd1 in this process. In fact, like Txnrd2−/− cells, Txnrd1−/− cells were highly sensitive and rapidly died in response to GSH deprivation. However, BSO-mediated cell death could be prevented neither by thiol-containing antioxidants nor by enforced expression of xCT. It rather appeared that increased (Cys)2 uptake was detrimental to Txnrd1−/− cells, because cell death was strongly induced by concomitant GSH deprivation. The fact, however, that survival of Txnrd1−/− cells relies on a functional GSH system that cannot be replaced by the (Cys)2/Cys redox cycle strongly supports the notion that the cytosolic thioredoxin/thioredoxin reductase system contributes to intracellular cystine reduction either directly or indirectly. Hence, one may infer that xCT expression aggravates oxidative stress and cellular toxicity caused by “disulfide stress” resulting from (Cys)2 overload. Additionally, even if (Cys)2 is reduced by neighboring cells and supplied to GSH-depleted Txnrd1−/− cells as Cys in co-culture experiments, the cells could not be rescued, indicating that Cys alone is not sufficient to rescue combined Txnrd1 and GSH deficiency.
The reduction of substrates other than (Cys)2 by thioredoxin/thioredoxin reductase 1 and not by thioredoxin/thioredoxin reductase 2 is apparently equally important, like, for example, the provision of electrons to ribonucleotide reductase (34). Genetic and biochemical studies in bacteria and yeast have provided evidence that the simultaneous inhibition of both disulfide-reducing pathways (the thioredoxin- and GSH-dependent pathways) is incompatible with growth and survival under aerobic conditions (35–37), but limited growth of these mutants can be restored using anaerobic conditions, suggesting that oxidative stress may play a crucial role in cell death progression. Reconstitution of Txnrd1 expression in the xCT- Txnrd1−/− cell line confirmed that Txnrd1 knock-out cells lack the essential enzymatic make-up to reduce the imported (Cys)2 and other substrates whose reduction is dependent on either Txnrd1 or the glutathione system.
In mammals, disruption of either γ-GCS (9), thioredoxin 1 (38), thioredoxin 2 (39), Txnrd1 (20), or Txnrd2 (19) is sufficient to cause embryonic lethality. At the cellular level, however, γ-GCS−/− cells can be grown in culture in the presence of GSH or NAC. Similarly, Txnrd2 (19) and Txnrd1 knock-out cells (this work) survive and proliferate in cell culture. The high susceptibility of Txnrd1 and Txnrd2 knock-out cells to GSH depletion underlines the importance of the glutathione system compensating for the deficiency of the respective thioredoxin reductases. Thus, it appears that, as in lower organisms, there is partial redundancy between the two central redox systems: the thioredoxin and the glutathione system, yet in mammalian cells, Txnrd1 is able to compensate for the loss of Txnrd2 as well as of the glutathione system, provided that the steady supply of reduced thiol compounds is maintained. However, Txnrd2 is unable to cope with combined Txnrd1 and GSH deficiency under the same conditions. The observation that antioxidants rescue Txnrd2−/− but not Txnrd1−/− cells from BSO treatment supports the fact that Txnrd1 is also required for the utilization and/or recycling of antioxidants. Several in vitro studies had shown that Txnrd1 is involved in the recycling of ascorbate (40, 41), vitamin E, l-cystine (42), and other small metabolites or compounds with antioxidant activity (43). Thus, by using three genetically defined cellular systems, we could show that Txnrd1 is essential for the utilization and recycling of small thiol-containing compounds and is the main driving force for the maintenance of the non-GSH reducing pool.
In summary, our data imply that an intrinsic genetic mechanism exists in mammalian cells that compensates for GSH deficiency. These findings also suggest that Txnrd1, but not Txnrd2, can act as a back-up system under conditions of GSH depletion when xCT expression is induced. Detailed knowledge of the redundancies and nonredundancies of the thioredoxin and the glutathione system is particularly important for the design of therapeutic strategies against many diseases linked to perturbed redox metabolism (44).
Supplementary Material
Acknowledgments
We are grateful to W. Hammerschmidt for providing Tat-Cre and to V. N. Gladyshev for providing the anti-Txnrd1 antibody. We also thank Giovanni E. Mann for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants SPP 1089 (to M. C. and G. W. B.) and CO 291/2-2 (to M. C.), funds from Deutsche Krebshilfe e.V. (to G. W. B.), a JSPS Bilateral Joint Research Project (to H. S. and G. W. B.), and a Japan Society for the Promotion of Science (JSPS) travel fellowship (to M. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- γ-GCS
- γ-glutamylcysteine synthetase
- BSO
- buthionine sulfoximine
- (Cys)2
- cystine
- NAC
- N-acetylcysteine
- Txnrd1
- cytosolic thioredoxin reductase
- Txnrd2
- mitochondrial thioredoxin reductase
- MEF
- mouse embryonic fibroblast
- HPLC
- high pressure liquid chromatography
- RT
- reverse transcription
- eGFP
- enhanced green fluorescent protein
- FACS
- fluorescence-activated cell sorter
- PI
- propidium iodide.
REFERENCES
- 1.López-Mirabal H. R., Winther J. R. (2008) Biochim. Biophys. Acta 1783, 629–640 [DOI] [PubMed] [Google Scholar]
- 2.Lin M. T., Beal M. F. (2006) Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 3.Seiler A., Schneider M., Förster H., Roth S., Wirth E. K., Culmsee C., Plesnila N., Kremmer E., Rådmark O., Wurst W., Bornkamm G. W., Schweizer U., Conrad M. (2008) Cell Metab. 8, 237–248 [DOI] [PubMed] [Google Scholar]
- 4.Loscalzo J. (2008) Cell Metab. 8, 182–183 [DOI] [PubMed] [Google Scholar]
- 5.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. (2007) Free Radic. Biol. Med. 43, 883–898 [DOI] [PubMed] [Google Scholar]
- 6.Fernandes A. P., Holmgren A. (2004) Antioxid. Redox. Signal. 6, 63–74 [DOI] [PubMed] [Google Scholar]
- 7.Hayes J. D., Pulford D. J. (1995) Crit. Rev. Biochem. Mol. Biol. 30, 445–600 [DOI] [PubMed] [Google Scholar]
- 8.Mannervik B., Danielson U. H. (1988) CRC Crit. Rev. Biochem. 23, 283–337 [DOI] [PubMed] [Google Scholar]
- 9.Shi Z. Z., Osei-Frimpong J., Kala G., Kala S. V., Barrios R. J., Habib G. M., Lukin D. J., Danney C. M., Matzuk M. M., Lieberman M. W. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5101–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannai S. (1986) J. Biol. Chem. 261, 2256–2263 [PubMed] [Google Scholar]
- 11.Bannai S., Kitamura E. (1980) J. Biol. Chem. 255, 2372–2376 [PubMed] [Google Scholar]
- 12.Sato H., Tamba M., Ishii T., Bannai S. (1999) J. Biol. Chem. 274, 11455–11458 [DOI] [PubMed] [Google Scholar]
- 13.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. (2000) J. Biol. Chem. 275, 16023–16029 [DOI] [PubMed] [Google Scholar]
- 14.Qiang W., Cahill J. M., Liu J., Kuang X., Liu N., Scofield V. L., Voorhees J. R., Reid A. J., Yan M., Lynn W. S., Wong P. K. (2004) J. Virol. 78, 11926–11938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato H., Fujiwara K., Sagara J., Bannai S. (1995) Biochem. J. 310, 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato H., Tamba M., Okuno S., Sato K., Keino-Masu K., Masu M., Bannai S. (2002) J. Neurosci. 22, 8028–8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., Takahashi S., Bannai S. (2005) J. Biol. Chem. 280, 37423–37429 [DOI] [PubMed] [Google Scholar]
- 18.Savaskan N. E., Heckel A., Hahnen E., Engelhorn T., Doerfler A., Ganslandt O., Nimsky C., Buchfelder M., Eyüpoglu I. Y. (2008) Nat. Med. 14, 629–632 [DOI] [PubMed] [Google Scholar]
- 19.Conrad M., Jakupoglu C., Moreno S. G., Lippl S., Banjac A., Schneider M., Beck H., Hatzopoulos A. K., Just U., Sinowatz F., Schmahl W., Chien K. R., Wurst W., Bornkamm G. W., Brielmeier M. (2004) Mol. Cell. Biol. 24, 9414–9423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakupoglu C., Przemeck G. K., Schneider M., Moreno S. G., Mayr N., Hatzopoulos A. K., de Angelis M. H., Wurst W., Bornkamm G. W., Brielmeier M., Conrad M. (2005) Mol. Cell. Biol. 25, 1980–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banjac A., Perisic T., Sato H., Seiler A., Bannai S., Weiss N., Kölle P., Tschoep K., Issels R. D., Daniel P. T., Conrad M., Bornkamm G. W. (2008) Oncogene 27, 1618–1628 [DOI] [PubMed] [Google Scholar]
- 22.Novogrodsky A., Nehring R. E., Jr., Meister A. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 4932–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung W. J., Lyons S. A., Nelson G. M., Hamza H., Gladson C. L., Gillespie G. Y., Sontheimer H. (2005) J. Neurosci. 25, 7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo M. H., Xu X. M., Carlson B. A., Gladyshev V. N., Hatfield D. L. (2006) J. Biol. Chem. 281, 13005–13008 [DOI] [PubMed] [Google Scholar]
- 25.Casagrande S., Bonetto V., Fratelli M., Gianazza E., Eberini I., Massignan T., Salmona M., Chang G., Holmgren A., Ghezzi P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9745–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallogly M. M., Mieyal J. J. (2007) Curr. Opin. Pharmacol. 7, 381–391 [DOI] [PubMed] [Google Scholar]
- 27.Ghezzi P. (2005) Free Radic. Res. 39, 573–580 [DOI] [PubMed] [Google Scholar]
- 28.Niwa T. (2007) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 855, 59–65 [DOI] [PubMed] [Google Scholar]
- 29.Sipos K., Lange H., Fekete Z., Ullmann P., Lill R., Kispal G. (2002) J. Biol. Chem. 277, 26944–26949 [DOI] [PubMed] [Google Scholar]
- 30.Shi S., Hudson F. N., Botta D., McGrath M. B., White C. C., Neff-LaFord H. D., Dabrowski M. J., Singh N. P., Kavanagh T. J. (2007) Cytometry A 71, 686–692 [DOI] [PubMed] [Google Scholar]
- 31.Fahey R. C., Newton G. L., Arrick B., Overdank-Bogart T., Aley S. B. (1984) Science 224, 70–72 [DOI] [PubMed] [Google Scholar]
- 32.Jones D. P., Go Y. M., Anderson C. L., Ziegler T. R., Kinkade J. M., Jr., Kirlin W. G. (2004) FASEB J. 18, 1246–1248 [DOI] [PubMed] [Google Scholar]
- 33.Holmgren A. (1977) J. Biol. Chem. 252, 4600–4606 [PubMed] [Google Scholar]
- 34.Camier S., Ma E., Leroy C., Pruvost A., Toledano M., Marsolier-Kergoat M. C. (2007) Free Radic. Biol. Med. 42, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 35.Grant C. M. (2001) Mol. Microbiol. 39, 533–541 [DOI] [PubMed] [Google Scholar]
- 36.Muller E. G. (1996) Mol. Biol. Cell 7, 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prinz W. A., Aslund F., Holmgren A., Beckwith J. (1997) J. Biol. Chem. 272, 15661–15667 [DOI] [PubMed] [Google Scholar]
- 38.Matsui M., Oshima M., Oshima H., Takaku K., Maruyama T., Yodoi J., Taketo M. M. (1996) Dev. Biol. 178, 179–185 [DOI] [PubMed] [Google Scholar]
- 39.Nonn L., Williams R. R., Erickson R. P., Powis G. (2003) Mol. Cell. Biol. 23, 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May J. M., Cobb C. E., Mendiratta S., Hill K. E., Burk R. F. (1998) J. Biol. Chem. 273, 23039–23045 [DOI] [PubMed] [Google Scholar]
- 41.May J. M. (2002) Methods Enzymol. 347, 327–332 [DOI] [PubMed] [Google Scholar]
- 42.Luthman M., Holmgren A. (1982) Biochemistry 21, 6628–6633 [DOI] [PubMed] [Google Scholar]
- 43.Nordberg J., Arnér E. S. (2001) Free Radic. Biol. Med. 31, 1287–1312 [DOI] [PubMed] [Google Scholar]
- 44.Trachootham D., Alexandre J., Huang P. (2009) Nat. Rev. Drug. Discov. 8, 579–591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.