Abstract
The heavy chain (HC) subunits of the bikunin proteins are covalently attached to a single chondroitin sulfate (CS) chain originating from bikunin and can be transferred to different hyaluronan (HA) molecules by TSG-6/HC2. In the present study, we demonstrate that HCs transferred to HA may function as HC donors in subsequent transfer reactions, and we show that the CS of bikunin may serve as an HC acceptor, analogous to HA. Our data suggest that TSG-6/HC2 link HCs randomly on the CS chain of bikunin, in contrast to the ordered attachment observed during the biosynthesis. Moreover, the results show that the transfer activity is indifferent to the new HC position, and the relocated HCs are thus prone to further TSG-6/HC2-induced transfer reactions. The data suggest that HCs may be transferred directly from HA to HA without the involvement of the bikunin CS chain. The results demonstrate reversibility of the interactions between HCs and glycosaminoglycans and suggest that a dynamic shuffling of the HCs occur in vivo.
Keywords: Extracellular Matrix/Hyaluronan, Extracellular Matrix/Glycosaminoglycan, Extracellular Matrix/Link Proteins, Protein/Protein-Protein Interactions, Proteoglycans Structure, Enzyme Mechanisms, Extracellular Matrix, Extracellular Matrix Proteins
Introduction
The bikunin proteins are proteoglycans present in plasma (1–3). They are heterodimers or -trimers composed of the serine protease inhibitor bikunin (MEROPS database; MEROPS classification, LI02-001) (4) and one or two other subunits picked from a homologous group of heavy chains (HCs)2 (1, 2). The C termini of the HCs are covalently attached to a single chondroitin sulfate (CS) chain originating from Ser-10 of bikunin (5–7) forming a protein-glycosaminoglycan-protein cross-link (5). The most abundant bikunin protein in plasma is the heterotrimer inter-α-inhibitor (IαI) composed of bikunin, HC1, and HC2 (1, 2). The two HCs are positioned in close proximity, with HC2 toward the reducing end of the CS chain (8). The CS chain is 12–18 disaccharides long, and on average, every fourth disaccharide is sulfated (C-4-S) (8, 9). The remaining disaccharides are unsulfated (C-0-S) (8, 9).
Tumor necrosis factor stimulated gene-6 protein (TSG-6) is an ∼35-kDa, secreted glycoprotein expressed during inflammation and inflammation-like conditions (10). The protein contains a glycosaminoglycan (GAG) binding Link domain (11, 12), and TSG-6 belongs to the family of hyaladherins (13). In concert with HC2, TSG-6 have a unique catalytic activity transferring HCs from bikunin proteins to hyaluronan (HA). The reaction involves two divalent cation-dependent transesterifications and a covalent intermediate composed of TSG-6 and the HC to be transferred (14–16). In the first reaction, the ester bond, between the C termini of the HCs and the CS chain in IαI is cleaved, and a new transient ester bond is formed between the C termini of the HCs and Ser-28 (numbered according to the preprotein) in TSG-6 (15, 17). In the second transesterification, the transient ester bond is cleaved and reformed between the C terminus of the HCs and HA (14). HC2 is required during both transesterification reactions, but the molecular mechanism is currently unknown (16). In addition to HA, C-0-S and the CS chain of the bikunin proteins may also function as HC acceptors during this reaction (15, 16, 18). The attachment of HCs to the bikunin CS chain generates new proteins designated HMW bikunin proteins (15, 16). The physiological importance of TSG-6 and of the unique interactions between TSG-6, bikunin proteins, and HA have been underlined in many different studies (19–24), particularly the importance during the formation of the HA-rich extracellular matrix surrounding the oocyte in mammals (25–27).
In the present study, we show that TSG-6/HC2 is able to continuously shuffle HCs to and from different glycosaminoglycan chains. This suggests that the initial transfer of HCs from the bikunin proteins to HA may be transient as the HC can be moved again to occupy another position on the same HA or to another GAG molecule. This may create a dynamic situation in the extracellular matrix where the HCs are moved around to facilitate the most favorable interactions.
EXPERIMENTAL PROCEDURES
Materials
Alexa Fluor 488 and labeling reagents were from Molecular Probes. Magnesium chloride, bovine pancreatic trypsin, CNBr-activated Sepharose, and HA sodium salt from human umbilical cord were from Sigma-Aldrich. Chondroitinase ABC (EC 4.2.2.20) was obtained from Seikagaku. Mass spectrometry grade trypsin was from Promega, and Stagetips (C18) were from Proxeon Biosystems. Brownlee C8 reverse phase column was obtained from PerkinElmer Life Sciences. Oligo-HATM 10EF was from Hyalose LLC. Human IαI was purified, with some modifications as described before (1). Human plasma was obtained from Aarhus University Hospital, Aarhus, Denmark. Polyclonal rabbit anti-sera against bikunin and against HC1 and HC2 were produced as described previously (6). Free bikunin containing the CS chain was purified from NaOH dissociated IαI as described before (28). HA oligosaccharides of distinct sizes were purified from a limited chondroitinase ABC digest of polymers of HA, as described recently (16). Human TSG-6 was expressed and purified, also as described before (23, 29). Anhydrotrypsin-Sepharose was prepared essentially as described previously (30, 31). Protein concentrations were determined spectrophotometrically at 280 nm using theoretical extinction coefficients (GPMAW software, Lighthouse Data).
SDS-PAGE and Immunoblotting
Samples were boiled shortly in sample buffer containing dithiothreitol. SDS-PAGE was performed in 5–15% gradient gels using the glycine/2-amino-2-methyl-1,3-propanediol-HCl system described previously (32). Proteins were visualized directly by Coomassie Blue staining and by i) detection of Alexa Fluor-labeled proteins, using a Typhoon Trio variable mode imager and proprietary software (GE Healthcare) or by ii) transferring the proteins to polyvinylidene difluoride membranes for immunoblotting using chemiluminescence (33). The immunoblots were developed using an ImageQuant LAS 4000 (GE Healthcare).
In-gel Digestion and Peptide Mass Fingerprinting Analysis
In-gel digestion of proteins was performed essentially as described before (15, 34). The tryptic peptides were purified on C18 stage tips (Proxeon Biosystems A/S) and eluted directly onto the matrix-assisted laser desorption/ionization target using 1 μl of 70% acetonitrile containing 4 mg/ml α-cyano-4-hydroxycinnamic acid. Mass spectra were acquired in a Micromass Q-TOF Ultima Global mass spectrometer (Waters) operated in positive ion mode (range m/z 800–3000). The instrument was calibrated over the 50–3000 m/z range using a polyethylene glycol mixture. Glu-fibrinopeptide B (m/z 1570.6774) served as an external lock-mass calibrant for each mass spectra. Micromass Masslynx 4.0 data processing software was used to generate a single Mascot-searchable peak list to query the Swiss-Prot protein database (35). The searches were performed using a peptide mass tolerance of 30 ppm and propionamide modification of cysteine residues, and no missed tryptic cleavage sites were allowed. Only significant hits as defined by Mascot probability analysis were accepted.
Assay to Determine the Transfer of HCs to the CS Chain of Bikunin
Free bikunin, containing the CS chain, was allowed to react with Alexa Flour 488 for 1 h at ambient temperature, according to the manufacturer's recommendation. The reaction was quenched by the addition of ammonium bicarbonate, and the labeled bikunin was purified by reverse phase high pressure liquid chromatography using a C8 column (Brownlee, C8, RP-300, 2.1 × 200 mm). Approximately 2 μg of the labeled protein was incubated with 0.5 μg of TSG-6 alone or in the presence of 3 μg of HC2·bikunin, Pre-α-inhibitor (PαI), or IαI. The proteins were incubated for 4 h at 37 °C in 20 mm Tris-HCl, 137 mm NaCl, pH 7.6 (TBS) containing either 1 mm MgCl2 or 5 mm EDTA. Finally, the samples were subjected to reduced SDS-PAGE.
Comparison of the Authentic HC Position on the CS and the HC Position after TSG-6/HC2-mediated HC Transfer
IαI (3 μg) and TSG-6 (0.4 μg) were incubated at a molar ratio of 3:2 in TBS at 37 °C in the presence of 1 mm MgCl2 or 1 mm EDTA. After 3 h, 0.05 unit of chondroitinase ABC was added. The samples were left incubating for an additional hour at 37 °C, and the reaction products were analyzed by reduced SDS-PAGE followed by Coomassie Blue staining. For comparison, IαI was similarly digested with chondroitinase ABC and analyzed by reduced SDS-PAGE.
Titration of IαI with TSG-6
IαI (3 μg) and TSG-6 were incubated at the following molar ratios: 1:1, 1:2, 1:3, 1:5, and 1:7 for 1 h at 37 °C in TBS containing 1 mm MgCl2 and analyzed by reduced SDS-PAGE followed by Coomassie Blue staining.
Assay to Determine TSG-6/HC2-mediated Transfer of HCs from HMW IαI to HA
IαI (3 μg) and TSG-6 (0.6 μg) were incubated in a molar ratio of 1:1 for 2 h at 37 °C in TBS containing 1 mm MgCl2 in the presence or absence of 5 μg of HA oligosaccharides (∼20 disaccharides (HA20)). Afterward, the sample containing HA20 was stored on ice, and HA20 was added to the other sample before it was left incubating for an additional 2 h at 37 °C. Finally, both samples were analyzed by reduced SDS-PAGE followed by Coomassie Blue staining.
Assays to Determine TSG-6/HC2-mediated Transfer of HCs from HA to HA
IαI (3 μg) and TSG-6 (0.6 μg) were incubated at a molar ratio of 1:1 for 2 h at 37 °C in TBS containing 1 mm MgCl2 and 3 μg of HA20, with a molecular mass of ∼3.8 kDa. Then 10 μg of a 12-mer oligosaccharide HA12, with a molecular mass of ∼ 2.3 kDa, was added, and the sample was left incubating for an additional 2 h at 37 °C. For comparison, NaOH-dissociated IαI (5) was run as control. Finally, all samples were resolved by reduced SDS-PAGE followed by Coomassie Blue staining.
The possible direct transfer of HCs from one HA to another HA molecule was examined by incubating IαI (18 μg), TSG-6 (2.4 μg) at a 3:2 molar ratio, and HA (30 μg) in TBS containing 1 mm MgCl2 (total volume 120 μl). After a 1-h incubation at 37 °C, free bikunin and bikunin·HC complexes were removed by affinity adsorption on 50 μl of anhydrotrypsin-Sepharose. The sample was incubated for 1 h at 23 °C and centrifuged, and the procedure was repeated three times to ensure complete depletion of bikunin-containing proteins. Then the final supernatant was i) analyzed directly, ii) treated with NaOH (5), iii) incubated with oligo-HA 10EF (15 μg), or iv) incubated with oligo-HA 10EF (15 μg) and subsequently treated with NaOH (5). All samples were finally analyzed by SDS-PAGE and immunoblotting using an antiserum against HC1 and HC2. In addition, the final supernatant (sample i) was subjected to immunoblotting using an anti-bikunin antibody. The incubation with oligo-HA (samples iii and iv) was done for 2 h at 37 °C in TBS containing 1 mm MgCl2 and with the addition of 1 μg of TSG-6.
RESULTS
The CS of Free Bikunin Is HC Acceptor
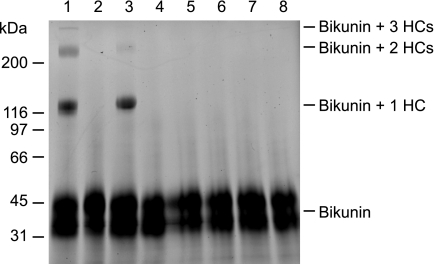
The HCs of the bikunin proteins may serve as an HC donor in the TSG-6/HC2-mediated transfer of HCs to GAGs (14, 15). As a consequence of the HC transfer, free bikunin is produced (15, 16), but it is unclear whether the generated stripped bikunin is able to act as HC acceptor. This was investigated by fluorescently labeling purified free bikunin (we use the term free bikunin for CS-substituted bikunin, which is not in complex with HCs) and incubating with TSG-6 and different bikunin proteins. Afterward, the samples were analyzed by SDS-PAGE, and the migration of the labeled bikunin was determined (Fig. 1). The results demonstrate that the CS of free bikunin is able to act as HC acceptor because HCs from IαI and from HC2·bikunin were transferred to the fluorophore-labeled bikunin (Fig. 1, lanes 1 and 3). Previously, it has been shown that the HC transfer reaction is HC2- and divalent cation-dependent (14, 16, 28). Therefore, samples without HC2 or without divalent cations were used as negative controls, and as expected, no HC transfer was observed in these samples (Fig. 1, lanes 2 and 5–7). The analyses demonstrate that the CS chain of bikunin is both HC donor (as in the bikunin proteins) and HC acceptor (as in free bikunin), demonstrating the reversibility of the HC transfer reaction.
FIGURE 1.
TSG-6/HC2 transfers HCs from bikunin proteins to the CS of free bikunin. Bikunin containing the CS chain was purified, fluorescently labeled, and incubated with TSG-6 alone (lane 4 and 8) or TSG-6, and respectively, IαI (lane 1 and 5), Pre-α-inhibitor (PαI) (lane 2 and 6), or HC2·bikunin (lane 3 and 7). The samples were incubated either in the presence of MgCl2 (lane 1–4) or in the presence of EDTA (lane 5–8). Afterward, the samples were subjected to reducing SDS-PAGE, and subsequently, the gel was imaged using a fluorescent scanner. The result demonstrated that TSG-6/HC2 transfers HCs from HC2·bikunin and from IαI to the CS chain originating from bikunin by a divalent cation-dependent mechanism.
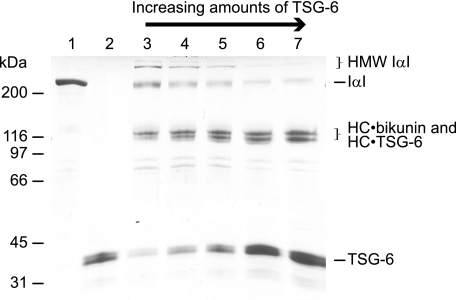
The generation of free bikunin during IαI/TSG-6 incubation (15, 36) appears to contradict that bikunin is able to act as an HC acceptor. However, our studies show that the ratio between IαI and TSG-6 during incubation determines the variant of bikunin complexes formed (Fig. 2) (15). If IαI is incubated with a molar excess of TSG-6, a high proportion of the HCs will be involved in HC·TSG-6 complexes, thus decreasing the number of HCs associated with each CS chain. Consequently, the probability of HMW IαI formation is decreased (Fig. 2, lane 7), and the amount of generated free bikunin is increased (15). In contrast, if the relative amount of TSG-6 is low, the number of HCs associated with each CS chain remains close to two (as in IαI), and the probability that TSG-6/HC2 transfers an HC to a CS chain already occupied by two HCs is higher (Fig. 2, lane 3), favoring the formation of HMW IαI. Because the HC·bikunin and HC·TSG-6 proteins co-migrate in SDS-PAGE, the amount of each protein is difficult to estimate during SDS-PAGE.
FIGURE 2.
The ratio between IαI and TSG-6 during incubation determines the variant of bikunin complexes formed. Shown is a Coomassie Blue-stained reduced SDS-PAGE gel with IαI (lane 1) and TSG-6 (lane 2) as controls. A fixed concentration of IαI was incubated with increasing amounts of TSG-6, 1:1 (lane 3), 1:2 (lane 4), 1:3 (lane 5), 1:5 (lane 6), and 1:7 (lane 7) (molar ratios). The titration demonstrates that the outcome of IαI/TSG-6 interaction is dependent on the IαI:TSG-6 ratio. This is most apparent by observing the decreasing amount of HMW IαI formed when the amount of TSG-6 is increasing.
Taken together, the experiments show that the IαI/TSG-6 interaction is dynamic and that the ratio between the products of the interaction depend on the ratio between IαI and TSG-6. The ratio determines whether the end products of the interaction primarily are HC·TSG-6 complexes and free bikunin or bikunin with one, two, or more HCs attached.
TSG-6/HC2 Change the Position of the HC on the Bikunin CS Chain
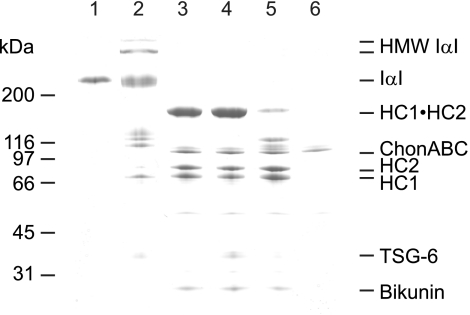
Although IαI before and after TSG-6 incubation migrates in the same position during SDS-PAGE (Figs. 2–5), we speculated that the placement of the HCs on the CS chain had been altered. It has previously been shown that the HCs of authentic IαI resist enzymatic dissociation most likely because of their close proximity, sterically preventing enzyme access (8). We exploited this property and tested whether the ability to enzymatically dissociate the HCs was affected by TSG-6 incubation, indicating that the HCs position had changes. During the experiments, the TSG-6:IαI ratio was kept low (Fig. 2) to ensure that the level of bikunin-HC·HC complexes before and after TSG-6 was the same (Fig. 3, lanes 1 and 2). Consequently, the amount of bikunin-HC·HC complexes subjected to chondroitinase ABC treatment (Fig. 3, lanes 3–5) was similar in all experiments.
FIGURE 3.
TSG-6/HC2-mediated HC transfer positions the HCs randomly on the bikunin CS. Shown is a Coomassie Blue-stained reduced SDS-PAGE gel of IαI (lane 1), IαI incubated with TSG-6 (lane 2), IαI digested with chondroitinase ABC (ChonABC, lane 3), IαI incubated with TSG-6 in the presence of EDTA and subsequently digested with chondroitinase ABC (lane 4), IαI incubated with TSG-6 and subsequently digested with chondroitinase ABC (lane 5), and chondroitinase ABC alone (lane 6). The experiment shows that after IαI has been exposed to TSG-6, chondroitinase ABC treatment generate only a faint HC1·HC2 band (lane 5), in contrast to the chondroitinase ABC treatment of authentic IαI (lane 3). This suggests that TSG-6/HC2 has rearranged the positions of the HCs on the CS chain, thus facilitating cleavage of the CS between the two HCs.
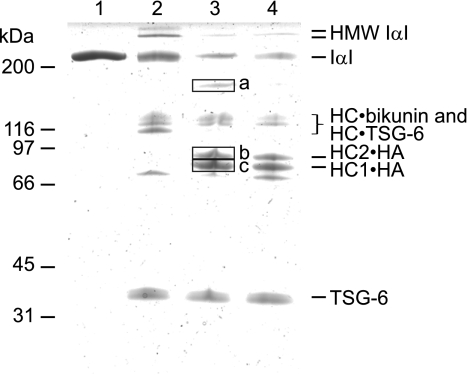
FIGURE 4.
TSG-6/HC2-generated HMW IαI proteins are HC donors. Shown is a Coomassie Blue-stained reduced SDS-PAGE gel showing samples of IαI (lane 1), IαI incubated with TSG-6 (lane 2), IαI incubated with TSG-6 and HA oligosaccharides simultaneously (lane 3), and IαI incubated with TSG-6, where HA oligosaccharides were subsequently added (lane 4). The incubation of IαI and TSG-6 generates HMW IαI, and because only trace amounts of these species are present after a subsequently incubation with HA oligosaccharides, it seems likely that the HMW IαI species serve as HC donors. To confirm the identity of the generated HC·HA complexes, the proteins of interest were excised (bands a–c) and subjected to peptide mass fingerprinting (supplemental Table S1). These analyses demonstrate that band a contains HC1 and HC2, band b contains HC2, and band c contains HC1.
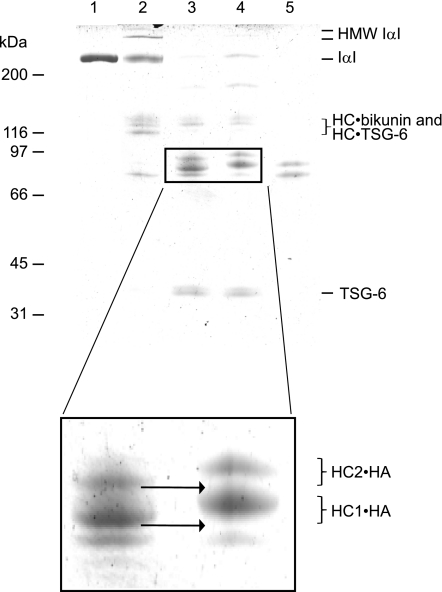
FIGURE 5.
TSG-6/HC2 transfers HCs from HA to HA. Shown is a Coomassie Blue-stained reduced SDS-PAGE gel showing samples of IαI (lane 1), IαI incubated with TSG-6 (lane 2), IαI and TSG-6 incubated with HA20 and subsequently with an excess of HA12 (lane 3), IαI and TSG-6 incubated with HA20 (lane 4), and IαI dissociated by NaOH (lane 5). Close inspection of the gel (see inset) revealed that the HC·HA molecules, generated after incubation with an excess HA12, migrated faster than the HC·HA molecules generated after incubation with HA20 only, as indicated by the arrow (see inset). The bands of interest are magnified to illustrate the band shift between HC·HA12 and HC·HA20. The experiment shows that the HCs in HC·HA20 are transferred to HA12, which demonstrates that the HC·HA complexes are HC donors in the TSG-6/HC2-mediated HC transfer reaction.
When authentic IαI was treated with chondroitinase ABC, bikunin was readily released, producing an HC·HC band composed of the two CS-linked HCs (Fig. 3, lane 3). Significantly less of the HC·HC band was apparent when IαI was first exposed to TSG-6 and then digested with chondroitinase ABC (Fig. 3, lane 5). This suggests that cleavage between the two HCs was facilitated, supporting that the placement had been altered. HMW IαI was also degraded by chondroitinase ABC, indicating that the HCs similarly had been rearranged. Moreover, inhibition of the TSG-6/HC2 transfer activity (14, 28) prevented chondroitinase ABC from cleaving and releasing the two HCs, supporting that the authentic position was maintained (Fig. 3, lane 4).
The TSG-6/HC2-generated HMW IαI and IαI Are HC Donors
The original position of the IαI HCs on the CS chain appears to be altered by TSG-6/HC2, as described above. To test whether this relocation affected the ability for further transfer, authentic IαI and TSG-6 were incubated to generate CS containing bikunin with two, three, or four attached HCs. Afterward, HA oligosaccharides were added and finally analyzed by SDS-PAGE (Fig. 4). To confirm the identity of the HC·HA complexes, the bands were excised, and the proteins were identified by peptide mass fingerprinting (supplemental Table S1). The results demonstrate that the TSG-6/HC2-generated bikunin-HC·HC and HMW IαI are HC donors. Hence, the position of the HCs on the CS chain originating from bikunin is not important for the transfer activity to be expressed.
The band in which HC1 and HC2 were identified (band a) is most likely HA oligosaccharide with two HCs attached. Indeed, incubation of IαI and TSG-6 with HA oligosaccharides of increasing length reduced the gel migration of that particular band (data not shown), indicating that HA is present in this band. The band is also observed when HA is added after IαI/TSG-6 incubation (Fig. 4, lane 4), although the appearance is weaker.
The TSG-6/HC2-generated HC·HA Complexes Are HC Donors
HA is a stronger HC acceptor than C-0-S (18), and it is not clear whether the HC·HA complexes assembled by TSG-6/HC2 are stable toward additional transfer reactions. To test this, HCs were transferred to HA20 (∼3.8 kDa) (Fig. 5, lanes 3 and 4). These complexes were then used as a substrate and incubated in the presence of TSG-6/HC2 and HA12 (∼2.3-kDa) molecule (Fig. 5, lane 3). We found that the HC·HA20 complexes disappeared when incubated with an excess of HA12 (Fig. 5, enlargement), demonstrating that HC·HA complexes are able to function as HC donors.
HCs Can Be Transferred Directly from HA to HA
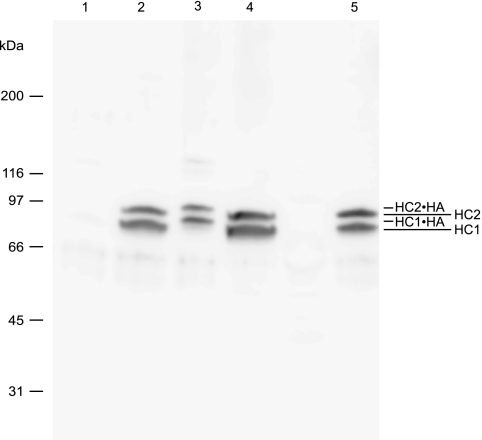
Theoretically the transfer of HCs between HA molecules (Fig. 5) can either occur directly between HA sites or require CS as a transient intermediate. To test this, we prepared HC·HA complexes and removed the bikunin-containing proteins (free bikunin and bikunin·HC(s) complexes) by anhydrotrypsin-Sepharose affinity absorption. Immunoblotting using anti-HC1/anti-HC2 antibodies confirmed that the bikunin proteins had been removed from our sample (Fig. 6, lane 1). HC·HA complexes do not migrate into the gel during SDS-PAGE because of their large size (15). The presence of HC·HA complexes was verified by NaOH treatment of the sample. This procedure released free HC1 and free HC2, strongly suggesting that HC·HA complexes were present (Fig. 6, lane 2). After HC·HA, oligo-HA, and TSG-6 incubation, HC·oligo-HA complexes were apparent (Fig. 6, lane 3). This demonstrates that HCs may be transferred directly from HA to oligo-HA. The sample did not contain free bikunin, as determined by immunoblotting using an anti-bikunin antibody. We have previously demonstrated that in addition to TSG-6, free HC2, HC2·bikunin, or IαI is required for the transfer of HCs (16). The present experiment suggests that HA-linked HC2 and TSG-6 similarly are able to mediate HC transfer.
FIGURE 6.
The transfer of HCs may occur directly between HA sites. HC·HA molecules were generated by incubation of IαI, TSG-6, and HA. The bikunin-containing proteins were subsequently removed by anhydrotrypsin-Sepharose affinity absorption. The resulting sample was analyzed directly (lane 1), treated with NaOH (lane 2), incubated with oligo-HA 10EF and TSG-6 (lane 3), or incubated with oligo-HA 10EF and TSG-6 and subsequently treated with NaOH (lane 4). For comparison, IαI treated with NaOH is also shown (lane 5). The proteins were resolved by reduced SDS-PAGE and immunoblotting using HC1/HC2 antisera. The observed weak bands around 120 kDa are likely to correspond to HC·TSG-6 complexes (lane 3). The band shift of the HCs after incubation with oligo-HA 10EF and TSG-6 (lane 3), when compared with the NaOH-treated samples (lanes 2, 4, and 5), demonstrate that HCs are transferred from HA to HA without the presence of bikunin proteins.
Taken together, our results support that TSG-6/HC2 may transfer HCs directly from one HA molecule to another. In addition, the analysis supports that HC transfer between bikunin proteins, TSG-6, and GAGs occurs in vivo in a continuous process of dismantling and regeneration.
DISCUSSION
In concert, TSG-6 and HC2 transfer HCs from bikunin proteins to HA in a reaction that involves two sequential transesterifications (14–16). Previously, the generated HC·HA was regarded as the final product of the reactions. In the present study, we show that the HC·HA complex also serves as HC donor in a new cycle of TSG-6/HC2-promoted HC-transfer reactions.
If an excess of HA is present during the incubation of IαI and TSG-6, neither HC1·bikunin nor HC2·bikunin is generated (15), suggesting that these products also serve as HC donors. The finding that HC2 derived from HC2·bikunin is transferred to HA supports this conclusion (16). The TSG-6/HC2 reaction thus strips the CS chain of the HCs (15, 36). Whether the CS chain is stripped of the HCs in vivo probably depends on the ratio between bikunin proteins and HA. In the present study, we show that the generated free bikunin also serves as HC acceptor. Interestingly, free bikunin is synthesized in large amounts by hepatocytes (37). Our results suggest that bikunin proteins could be assembled extracellularly if an HC donor, bikunin, and TSG-6 co-localize at low HA concentrations.
HA is a better HC acceptor than C-0-S (18) suggesting that transfer of HCs to HA is an irreversible event. However, in the present study, we show that TSG-6/HC2 is able to remove HA-associated HCs, revealing that HC·HA complexes also serve as HC donors, analogous to IαI. Because the HC transfer between HA molecules does not require an intermediate HC·CS complex, the HC shuffling may occur independent of the bikunin proteins in vivo, after the initial transfer of HCs from bikunin proteins to HA. The findings in the present study suggest that the TSG-6/bikunin protein interaction in HA-rich extracellular matrix is dynamic. As long as the reactants co-localize, a continuous shuffling of HCs between GAGs probably takes place.
In the present study, we have analyzed the interactions between IαI, TSG-6, and HA. The results show no significant difference between the requirements for acceptor GAG and donor GAG. The TSG-6/HC2-generated HC·GAG complexes are able to function as donor HC·GAG complexes. Contrariwise, is the TSG-6/HC2-stripped CS chain from IαI able to function as an HC acceptor GAG. Taken together, the study demonstrates the reversibility of the TSG-6/HC2-mediated transfer of HCs to GAGs.
Supplementary Material
Acknowledgment
We thank Tania Aaquist Nielsen for technical assistance.
This work was supported by grants from The Carlsberg Foundation and grants from the Danish Council for Independent Research, Medical Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
- HC
- heavy chain
- TSG-6
- tumor necrosis factor stimulated gene-6 protein
- CS
- chondroitin sulfate
- C-0-S
- unsulfated chondroitin
- GAG
- glycosaminoglycan
- HA
- hyaluronan
- HA12
- 12-mer oligosaccharide hyaluronan
- HA20
- 20-mer oligosaccharide hyaluronan
- HMW
- high molecular weight
- IαI
- inter-α-inhibitor
- TBS
- Tris-buffered saline.
REFERENCES
- 1.Enghild J. J., Thøgersen I. B., Pizzo S. V., Salvesen G. (1989) J. Biol. Chem. 264, 15975–15981 [PubMed] [Google Scholar]
- 2.Gebhard W., Hochstrasser K., Fritz H., Enghild J. J., Pizzo S. V., Salvesen G. (1990) Biol. Chem. Hoppe-Seyler 371, (suppl.) 13–22 [PubMed] [Google Scholar]
- 3.Zhuo L., Hascall V. C., Kimata K. (2004) J. Biol. Chem. 279, 38079–38082 [DOI] [PubMed] [Google Scholar]
- 4.Rawlings N. D., Morton F. R., Kok C. Y., Kong J., Barrett A. J. (2008) Nucleic Acids Res. 36, D320–D325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enghild J. J., Salvesen G., Hefta S. A., Thøgersen I. B., Rutherfurd S., Pizzo S. V. (1991) J. Biol. Chem. 266, 747–751 [PubMed] [Google Scholar]
- 6.Enghild J. J., Salvesen G., Thøgersen I. B., Valnickova Z., Pizzo S. V., Hefta S. A. (1993) J. Biol. Chem. 268, 8711–8716 [PubMed] [Google Scholar]
- 7.Morelle W., Capon C., Balduyck M., Sautiere P., Kouach M., Michalski C., Fournet B., Mizon J. (1994) Eur. J. Biochem. 221, 881–888 [DOI] [PubMed] [Google Scholar]
- 8.Enghild J. J., Thøgersen I. B., Cheng F., Fransson L. A., Roepstorff P., Rahbek-Nielsen H. (1999) Biochemistry 38, 11804–11813 [DOI] [PubMed] [Google Scholar]
- 9.Chi L., Wolff J. J., Laremore T. N., Restaino O. F., Xie J., Schiraldi C., Toida T., Amster I. J., Linhardt R. J. (2008) J. Am. Chem. Soc. 130, 2617–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milner C. M., Day A. J. (2003) J. Cell Sci. 116, 1863–1873 [DOI] [PubMed] [Google Scholar]
- 11.Day A. J. (1999) Biochem. Soc. Trans. 27, 115–121 [DOI] [PubMed] [Google Scholar]
- 12.Kohda D., Morton C. J., Parkar A. A., Hatanaka H., Inagaki F. M., Campbell I. D., Day A. J. (1996) Cell 86, 767–775 [DOI] [PubMed] [Google Scholar]
- 13.Day A. J., Prestwich G. D. (2002) J. Biol. Chem. 277, 4585–4588 [DOI] [PubMed] [Google Scholar]
- 14.Rugg M. S., Willis A. C., Mukhopadhyay D., Hascall V. C., Fries E., Fülöp C., Milner C. M., Day A. J. (2005) J. Biol. Chem. 280, 25674–25686 [DOI] [PubMed] [Google Scholar]
- 15.Sanggaard K. W., Karring H., Valnickova Z., Thøgersen I. B., Enghild J. J. (2005) J. Biol. Chem. 280, 11936–11942 [DOI] [PubMed] [Google Scholar]
- 16.Sanggaard K. W., Sonne-Schmidt C. S., Krogager T. P., Lorentzen K. A., Wisniewski H. G., Thøgersen I. B., Enghild J. J. (2008) J. Biol. Chem. 283, 18530–18537 [DOI] [PubMed] [Google Scholar]
- 17.Sanggaard K. W., Sonne-Schmidt C. S., Krogager T. P., Kristensen T., Wisniewski H. G., Thøgersen I. B., Enghild J. J. (2008) J. Biol. Chem. 283, 33919–33926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay D., Asari A., Rugg M. S., Day A. J., Fülöp C. (2004) J. Biol. Chem. 279, 11119–11128 [DOI] [PubMed] [Google Scholar]
- 19.Bárdos T., Kamath R. V., Mikecz K., Glant T. T. (2001) Am. J. Pathol. 159, 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day A. J., de la Motte C. A. (2005) Trends Immunol 26, 637–643 [DOI] [PubMed] [Google Scholar]
- 21.Milner C. M., Higman V. A., Day A. J. (2006) Biochem. Soc. Trans. 34, 446–450 [DOI] [PubMed] [Google Scholar]
- 22.Milner C. M., Tongsoongnoen W., Rugg M. S., Day A. J. (2007) Biochem. Soc Trans. 35, 672–676 [DOI] [PubMed] [Google Scholar]
- 23.Wisniewski H. G., Burgess W. H., Oppenheim J. D., Vilcek J. (1994) Biochemistry 33, 7423–7429 [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski H. G., Maier R., Lotz M., Lee S., Klampfer L., Lee T. H., Vilcek J. (1993) J. Immunol. 151, 6593–6601 [PubMed] [Google Scholar]
- 25.Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 26.Ochsner S. A., Day A. J., Rugg M. S., Breyer R. M., Gomer R. H., Richards J. S. (2003) Endocrinology 144, 4376–4384 [DOI] [PubMed] [Google Scholar]
- 27.Zhuo L., Yoneda M., Zhao M., Yingsung W., Yoshida N., Kitagawa Y., Kawamura K., Suzuki T., Kimata K. (2001) J. Biol. Chem. 276, 7693–7696 [DOI] [PubMed] [Google Scholar]
- 28.Sanggaard K. W., Sonne-Schmidt C. S., Jacobsen C., Thøgersen I. B., Valnickova Z., Wisniewski H. G., Enghild J. J. (2006) Biochemistry 45, 7661–7668 [DOI] [PubMed] [Google Scholar]
- 29.Mindrescu C., Thorbecke G. J., Klein M. J., Vilcek J., Wisniewski H. G. (2000) Arthritis Rheum 43, 2668–2677 [DOI] [PubMed] [Google Scholar]
- 30.Ako H., Foster R. J., Ryan C. A. (1972) Biochem. Biophys. Res. Commun. 47, 1402–1407 [DOI] [PubMed] [Google Scholar]
- 31.Sayers C. A., Barrett A. J. (1980) Biochem. J. 189, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bury A. F. (1981) J. Chromatogr. 213, 491–500 [Google Scholar]
- 33.Matsudaira P. (1987) J. Biol. Chem. 262, 10035–10038 [PubMed] [Google Scholar]
- 34.Shevchenko A., Tomas H., Havlis J., Olsen J. V., Mann M. (2006) Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 35.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 36.Colón E., Shytuhina A., Cowman M. K., Band P. A., Sanggaard K. W., Enghild J. J., Wisniewski H. G. (2009) J. Biol. Chem. 284, 2320–2331 [DOI] [PubMed] [Google Scholar]
- 37.Thøgersen I. B., Enghild J. J. (1995) J. Biol. Chem. 270, 18700–18709 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.