Abstract
Estrogen receptor β (ERβ) has potent antiproliferative and anti-inflammatory properties, suggesting that ERβ-selective agonists might be a new class of therapeutic and chemopreventative agents. To understand how ERβ regulates genes, we identified genes regulated by the unliganded and liganded forms of ERα and ERβ in U2OS cells. Microarray data demonstrated that virtually no gene regulation occurred with unliganded ERα, whereas many genes were regulated by estradiol (E2). These results demonstrated that ERα requires a ligand to regulate a single class of genes. In contrast, ERβ regulated three classes of genes. Class I genes were regulated primarily by unliganded ERβ. Class II genes were regulated only with E2, whereas class III genes were regulated by both unliganded ERβ and E2. There were 453 class I genes, 258 class II genes, and 83 class III genes. To explore the mechanism whereby ERβ regulates different classes of genes, chromatin immunoprecipitation-sequencing was performed to identify ERβ binding sites and adjacent transcription factor motifs in regulated genes. AP1 binding sites were more enriched in class I genes, whereas ERE, NFκB1, and SP1 sites were more enriched in class II genes. ERβ bound to all three classes of genes, demonstrating that ERβ binding is not responsible for differential regulation of genes by unliganded and liganded ERβ. The coactivator NCOA2 was differentially recruited to several target genes. Our findings indicate that the unliganded and liganded forms of ERβ regulate three classes of genes by interacting with different transcription factors and coactivators.
Keywords: Chromatin Immunoprecipitation (ChiP), Estrogen, Gene Regulation, Gene Transcription, Microarray, Nuclear Receptors, Steroid Hormone Receptor
Introduction
Estrogens are essential for the development of the reproductive system. They also exert important actions on nonreproductive tissues such as the bone (1) and cardiovascular system (2). Many biological effects of estrogens are mediated by two estrogen receptors (ERs),4 estrogen receptor α (ERα; NR3A1 and ESR1) and estrogen receptor β (ERβ; NR3A2 and ESR2) (3, 4). The binding of estrogens to ERs produces genomic effects (3, 4) that regulate gene transcription and nongenomic effects (5) that regulate ion channels and signal transduction pathways. The genomic pathway is better characterized. This pathway is initiated by the binding of the estrogen-ER complex to specific regulatory elements in target genes. The estrogen-ER complex can bind directly to DNA via an estrogen responsive element (ERE) or become tethered to a transcription factor (3, 4). Once bound to a regulatory element, the estrogen-ER complex can interact with adjacent transcription factors and recruit a variety of co-regulators that result in the activation or repression of target genes by modifying chromatin structure (6–10).
Although ERα and ERβ have a similar structure, they produce different biological effects (3, 4). The ERα and ERβ knock-out mice have different phenotypes demonstrating that the two ERs have different physiological roles (11–13). ERα is essential for the development of the reproductive tract and mammary gland because it promotes cell proliferation. In contrast, ERβ appears to have an antiproliferation role because ERβ knock-out mice develop prostate hyperplasia and a myeloproliferative disease (12, 14). In addition, the expression of ERβ in breast and colon cancer cells inhibits cancer cell proliferation and tumor formation in mouse xenograft models (15–19), which supports an antiproliferative role for ERβ. ERα and ERβ regulate different genes in response to E2 and selective estrogen receptor modulators (20, 21). For example, only ∼40% of the genes regulated by E2 in U2OS cells that express ERα are also regulated in U2OS cells that express ERβ (20).
It is unclear how ERα and ERβ produce unique physiological effects and regulate different genes, but it could be due to differences in their binding to different regulatory elements in target genes. ERα binding sites have been identified in MCF-7 breast cancer cells by using tiling arrays (6, 22, 23) and ChIP-Seq (24). In contrast to ERα, very little is known about the binding sites for ERβ in the genome. However, because of the antiproliferative properties of ERβ, a greater understanding of how it regulates genes is critical to develop ERβ-selective drugs that might be useful for chemoprevention of cancers. Although genome-wide analysis by tiling arrays and ChIP-Seq are powerful methods to identify ER binding sites and transcription factors that are associated with ER binding sites, it is clear from these studies that most of the binding sites detected by these methods are not functionally active (6, 22, 23). Furthermore, it is difficult to determine which ER binding site is associated with a particular regulated gene. For example, we used a ChIP-sequencing cloning strategy that identified 173 regulatory elements associated with ERα that were active in transfection assays (25). However, there was a poor correlation between the presence of regulatory elements and the regulation of the nearest gene by real-time PCR (25). This finding suggested that the regulatory elements regulated distant genes rather than the closest gene (26). In this study, we combined expression data with ChIP-Seq analysis to identify functional ERβ binding sites in target genes.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
Tetracycline-inducible U2OS-ERα and U2OS-ERβ cells were maintained as described previously (20). The cells were maintained continuously in phenol red-free medium with fetal bovine serum stripped of estrogens.
Microarrays and Data Analysis
Total cellular RNA was isolated utilizing the Aurum RNA isolation kit, (Bio-Rad, Hercules, CA), per the manufacturer's directions. RNA isolates were first quantified by standard spectrophotometry and then qualitatively evaluated by capillary electrophoresis employing the Bio-Rad Experion system per the manufacturer's instructions. Biotin-labeled cRNA samples were prepared with 750 ng of total RNA template. Following synthesis and purification, the biotin-labeled samples were evaluated by both 260/280 absorbance spectrophotometry and capillary electrophoresis. The final labeled cRNA samples were hybridized overnight against 48,000 transcripts of humanWG-6 BeadChip arrays (Illumina, San Diego, CA). The Illumina microarrays were processed at the University of California, San Francisco Genomics Core. All treatments were done in triplicate, and the same batch of microarrays were used for all treatments. The Illumina expression arrays were preprocessed using lumi package (27). The differential expression analysis was performed using the Limma package (28). These packages are all available in R/BioConductor. Probes were selected for further analysis if the fold change was >2 and if the multiple testing adjusted p value using Benjamini and Hochberg procedure (BH-adjusted p value) was <0.05 (29). The heat maps of log intensities of genes across different experiments were produced using Cluster and TreeView software (30). Cluster software was used to perform the hierarchical clustering based on Pearson correlation coefficients to find clusters of genes with similar expression patterns. TreeView was then used to visualize the clusters and produce the figures. All microarray data are available on the Gene Expression Omnibus (accession number GSE21790).
RNA Extraction and Reverse Transcription-PCR
Total RNA was extracted and isolated with the Aurum RNA isolation kit (Bio-Rad) per the manufacturer's directions. Reverse transcription reactions were performed using 1 μg of total RNA as described previously (31). Real-time PCR was performed with the Bio-Rad iCycler using iQ SYBR Green Supermix (Bio-Rad). The sequences of the primers used are shown in supplemental Table 1.
ChIP-Seq
Amplified ChIP DNA samples were prepared utilizing the ChIP-Seq DNA Sample prep kit per the manufacturer's protocol (Illumina) with the following modifications. Following gel purification and clean up of the adapter-ligated ChIP DNAs, PCR amplification was performed as described for 20 cycles. Final amplified ChIP DNA libraries were analyzed using the Experion capillary electrophoresis system and 1K DNA chips (Bio-Rad). Cluster preparation and subsequent single-read sequencing was performed at Vincent J. Coates' Genomics Sequencing Laboratory (University of California, Berkeley). MACS peak caller was used to identify ERβ binding sites for ChIP-seq data (32). Peaks with p values <10−20 were selected for further analysis. All ChIP-seq data have been deposited in the Gene Expression Omnibus (accession number GSE21790).
Motif Analysis
CisGenome software (33) was used to scan the peak sequences (400 bp around the peak center) with Transfac weight matrices (34). A segment was claimed as a predicted motif if its log 10 of likelihood ratio score was at least 3.6. To identify known and novel factors enriched near class I and II genes, the best 138 doxycycline peaks within 5 kb of class I genes and 132 doxycycline + E2 peaks within 5 kb of class II genes were investigated for motif enrichment. Four hundred bases centered on the peak centers were compared with the background sequences consisting of matched genomic controls, which contained sequences of the same number and length as peak sequences and were randomly chosen to match the physical distribution of the peak regions (33). Only motifs with a frequency of >8% among the investigated peaks and >1.9-fold relative enrichment compared with the background sequences were considered as enriched.
Chromatin Immunoprecipitation (ChIP)
Cells were treated as indicated in the figure legends and then were cross-linked, collected, and lysed as described previously (35). Immunoprecipitations were performed overnight at 4 °C with anti-ERβ (6A12 14C8, and 7B10; GeneTex, San Antonio, TX) or anti-NCOA2 (GRIP1; Abcam, Cambridge, MA). PCR was done with primers shown in supplemental Table 1. Experiments were done in triplicate, and the mean ± S.E. was calculated, and statistical analysis was performed using the Prism curve-fitting program (Graph Pad Software, version 3.03). Results are representative of data collected from at least three experiments.
RESULTS
ERβ Regulates Three Classes of Target Genes
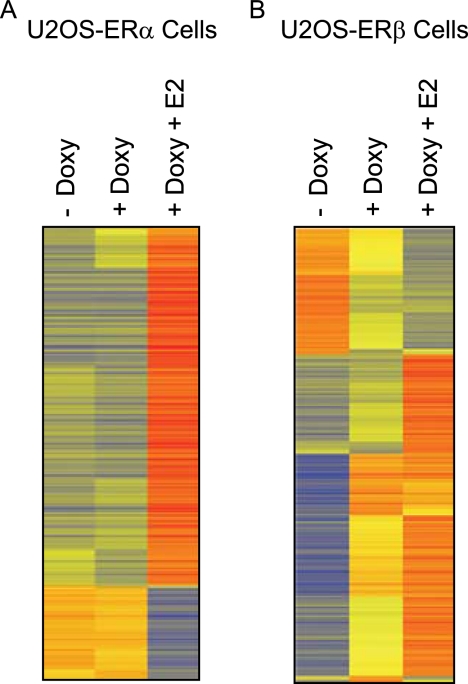
Our main objective was to identify target genes for unliganded and liganded ERβ and binding sites for ERβ in the regulated genes by ChIP-Seq. We used U2OS cell lines that were stably transfected with a doxycycline-inducible ERβ (20). This feature allowed us to measure the effects of unliganded ERβ in cells that were treated only with doxycycline and liganded ERβ when the cells were treated with both doxycycline and E2. For comparison, we also used U2OS-ERα cells for the microarray analysis. We previously reported that the U2OS-ERα and U2OS-ERβ cells express comparable levels of receptors after induction with doxycycline (20, 36). The cells were maintained in the absence or presence of doxycycline for 18 h to induce the expression of ERs. Following treatment with E2, total RNA was isolated, and then microarrays were performed. The heat maps show that a significant change in the gene expression pattern in the U2OS-ERα cells occurred only with the addition of E2 (Fig. 1A). Doxycycline produced a small up-regulation of only one gene and down-regulation of three genes (Table 1). The addition of E2 to doxycycline-treated U2OS-ERα cells resulted in the activation of 518 genes and repression of 157 genes (Table 1), demonstrating that ERα is functional in these cells. These data indicate that ERα requires the ligand to regulate gene transcription in U2OS cells. In contrast, doxycycline-induced expression of ERβ in U2OS cells produced a dramatic change in the gene expression pattern (Fig. 1B). In the absence of ligand, ERβ expression was followed by the up-regulation of 401 genes and down-regulation of 135 genes by at least 2-fold (Table 1).
FIGURE 1.
Heat map representation of the genes regulated by unliganded and liganded ERα or ERβ. U2OS-ERα (A) or U2OS-ERβ (B) cells were treated in the absence (− Doxy) or presence (+ Doxy) of doxycycline for 18 h with or without 10 nm E2. For each gene (row), the average log intensities are colored yellow, relatively higher expression are colored with reds of increasing intensity, and relatively lower expression are colored with blues of increasing intensity. Each treatment represents the average signal of triplicate samples.
TABLE 1.
Number of genes regulated in the U2OS-ERα and U2OS-ERβ cells
The cells were untreated (−Doxy), or treated with doxycycline alone (+Doxy), or doxycycline and estradiol (+Doxy + E2) for 18 h. The genes listed were significantly regulated by at least 2.0-fold (BH-adjusted p value ≤0.05).
| No. of genes regulated |
|||
|---|---|---|---|
| Up-regulated | Down-regulated | Total | |
| U2OS-ERα cells | |||
| +Doxy versus −Doxy | 1 | 3 | 4 |
| +Doxy + E2versus −Doxy | 519 | 192 | 711 |
| +Doxy + E2versus +Doxy | 518 | 157 | 675 |
| U2OS-ERβ cells | |||
| +Doxy versus −Doxy | 401 | 135 | 536 |
| +Doxy + E2versus −Doxy | 894 | 354 | 1248 |
| +Doxy + E2versus +Doxy | 308 | 33 | 341 |
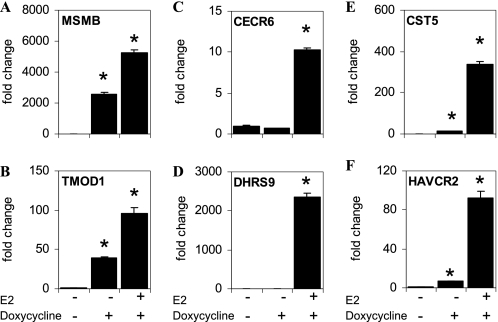
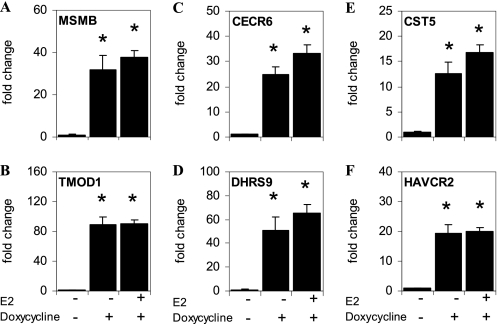
The addition of E2 to U2OS-ERβ cells treated with doxycycline led to an up-regulation of 308 genes and down-regulation of 33 genes (Table 1). Interestingly, we could distinguish between three distinct classes of regulated genes in the U2OS-ERβ cells (Table 2). There were 453 class I genes regulated by unliganded ERβ. Class II genes were 258 genes not regulated by unliganded ERβ but regulated by E2-bound ERβ. Class III genes were 83 genes regulated by unliganded ERβ and potentiated by the addition of E2 (Table 2). The three classes of regulated genes in the U2OS-ERβ cells are listed in supplemental Table 2. To verify the results of the microarray, we selected two genes in each class and studied their regulation by real-time PCR. Similar to the microarray data, doxycycline produced a strong activation of the class I genes, MSMB and TMOD1 in the absence of ligand (Fig. 2, A and B, respectively). The CECR6 and DHRS9 class II genes were not activated by doxycycline, but were stimulated by E2 (Fig. 2, C and D, respectively). The class III genes, CST5 and HAVCR2 were activated by unliganded ERβ and markedly potentiated with the addition of E2 (Fig. 2, E and F, respectively). The RT-PCR data confirm that there are three classes of genes regulated by ERβ but show that the different classes are relative, rather than a definite distinction.
TABLE 2.
Three classes of genes are regulated in U2OS-ERβ cells
Following doxycycline treatment, ERβ is expressed in the cells and regulates the expression of the class I genes in the absence of ligand. The addition of E2 induces the expression of the class II genes and potentiates the expression of the class III genes.
| No. of genes regulated | |
|---|---|
| Class I (regulated by unliganded ERβ only) | 453 |
| Class II (regulated by E2 only) | 258 |
| Class III (regulated by unliganded ERβ and E2) | 83 |
FIGURE 2.
ERβ regulates three classes of target genes in U2OS cells. U2OS-ERβ cells were not treated or treated for 18 h with 10 nm E2 in the absence or presence of doxycycline. Following treatments, mRNA levels for MSMB (A), TMOD1 (B), CECR6 (C), DHRS9 (D), CST5 (E), or HAVCR2 (F) were measured by real-time PCR (n = 6). Unliganded ERβ induces expression of class I genes (A and B), whereas class II genes are regulated following E2 treatment (C and D). Class III genes are induced by unliganded ERβ and potentiated by E2 treatment (E and F). Each data point is the average of triplicate determinations ± SEM. Asterisk denotes p value ≤0.05.
Identification of ERβ Binding Sites by ChIP-Seq
To investigate the mechanism whereby ERβ can regulate three classes of genes, we performed ChIP-Seq to identify ERβ binding sites and transcription factor motifs in each class of genes. After the addition of doxycycline, U2OS-ERβ cells were treated in the absence or presence of E2 and then DNA was immunoprecipitated with antibodies to ERβ. The DNA fragments were sequenced and mapped to the human genome (HG18 assembly) using Eland. Peaks with p values <10−20 were selected for further analysis. Using this cut-off, there were 5,768 binding sites predicted for unliganded ERβ and 11,975 binding sites predicted for liganded ERβ.
The Majority of Genome-wide ERβ Binding Sites Are Present within 50 kb of Genes
When ERβ was expressed without a ligand, the majority (76%) of ERβ binding sites were located within 50 kb of a gene. About 25% of these sites occurred within a gene, 13% were in proximal promoter region of genes, 2% at the 3′ end of a gene, 36% elsewhere in a more distal region (5–50 kb), and the remaining 24% were >50 kb from a gene (Table 3). A similar distribution of ERβ binding sites were observed when E2 was present (Table 3). The genome-wide distribution of ERβ binding sites is significantly different from that expected under a random distribution, with more enrichment within 50 kb of genes, specifically, in distal regions (5–50 kb) and proximal promoter regions of genes (p value <2 × 10−16 based on a chi-square test).
TABLE 3.
Genome-wide distribution of ERβ binding sites
ERβ-expressing U2OS cells were treated without (unliganded) and with (liganded) 10 nm E2 for 1 h. Peak regions were associated with the HG18 RefGene table downloaded from the University of California, Santa Cruz genome browser. Peaks within 5 kb upstream and 2.5 kb downstream of a RefSeq gene TSS were annotated as being in the promoter region. Peaks within 2.5 kb downstream of a TSS and 2.5 kb upstream of 3′ end of the gene were annotated as intragenic. Peaks within 2.5 kb upstream of 3′ end and 5 kb downstream of 3′ end were annotated as the 3′ end. Peaks within 50 kb of a gene but excluded from the above three categories were annotated as within 50 kb. Peaks falling outside of 50 kb window of any gene were labeled as others and considered as peaks involved in long range interactions.
| Distribution | Unliganded ERβ | Liganded ERβ |
|---|---|---|
| % | % | |
| Promoter | 13 | 12 |
| Intragenic | 25 | 25 |
| 3′ end | 2 | 2 |
| Within 50 kb | 36 | 38 |
| Others | 24 | 23 |
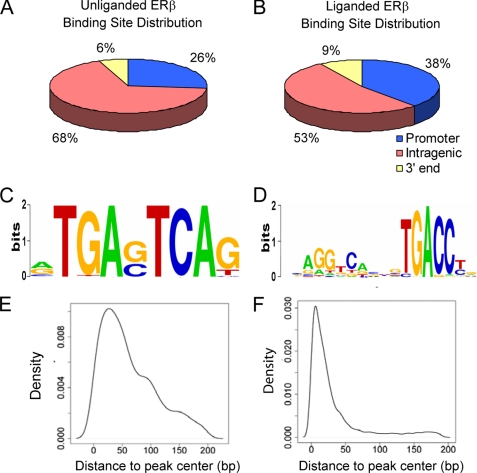
We then focused on the distribution of ERβ binding sites within 5 kb of genes because it is likely that many of these sites will be involved in regulating the activity of genes. For class I genes, when ERβ is expressed without its ligand, 26% of ERβ binding sites were located in promoters, 68% in intragenic regions, and 6% in 3′ end regions (Fig. 3A). For class II genes, with the addition of E2 more ERβ binding sites (38%) were found at promoters, whereas 53% were present in intragenic regions and 9% in 3′ end regions (Fig. 3B). These findings demonstrated that there is an enrichment of ERβ binding sites in the promoter and 3′ end regions of class II genes, (47%) compared with class I genes (32%) with a p value of 0.004 using a binomial test.
FIGURE 3.
Bioinformatic analysis of ERβ binding sites. A and B, pie diagram showing distribution of ERβ binding sites within 5 kb of class I and II genes. The peaks within 5 kb of class I and II gene distribution were analyzed with U2OS-ERβ cells (A) treated with doxycycline and ERβ expressing U2OS cells treated with 10 nm E2 (B) for 1 h. Peaks within 5 kb upstream and 2.5 kb downstream of the transcriptional start site were annotated as the “Promoter” region. Peaks within 2.5 kb downstream of a transcriptional start site and 2.5 kb upstream of the 3′ end of the gene were annotated as “Intragenic.” Peaks within 2.5 kb upstream of 3′ end and 5 kb downstream of 3′ end were annotated as “3′ end”. C, sequence logo for AP1 in doxycycline sample. D, sequence logo for ERE in doxycycline + E2 samples. E, density of distance of AP1 motifs to the center of the binding region in doxycycline sample. F, density of distance of ERE motifs to the center of the binding region in doxycycline + E2 samples.
ERβ Binding Sites Are Enriched in Target Genes
To examine whether the ERβ binding sites are likely to be functional, we focused on the ERβ binding sites that were in close proximity (5 kb) to the transcriptional start site of the three classes of regulated genes. About 11% of all genes had binding sites within 5 kb in unliganded samples, whereas 20% had sites within 5 kb in E2-treated samples (Table 4). When the analysis was restricted to regulated genes in the three classes, we found that 43% of the class I genes had unliganded ERβ sites, 76% of the class II genes had liganded ERβ binding sites, and 60 and 82% of class III genes had unliganded and liganded ERβ binding sites within 5 kb, respectively. These percentages are significantly higher than the corresponding percentages observed for all genes (p values <2.2 × 10−16 based on a binomial test).
TABLE 4.
Proportion of genes with ERβ binding site peaks within 5 kb region
ERβ-expressing U2OS cells were treated with doxycycline alone (Doxy) for 18 h, followed by vehicle or 10 nm E2 (Doxy + E2) treatment for 1 h.
| Category | Proportion of genes having peaks within 5 kb |
|---|---|
| % | |
| Class I (Doxy) | 42.9 |
| Class II (Doxy + E2) | 75.5 |
| Class III | |
| Doxy | 60.3 |
| Doxy + E2 | 82.2 |
| All genes | |
| Doxy | 11.3 |
| Doxy + E2 | 20.2 |
The average number of sites within 5 kb in these three classes of genes was also much higher compared with unregulated genes. The enrichment of ERβ binding sites within 5 kb of regulated genes indicates that there is a positive relationship between binding and regulation and that many sites near regulated genes are likely to be functional. The higher enrichment of ERβ binding sites near class II and class III genes in E2-treated samples indicates that E2 causes more ERβ recruitment to regulated genes.
AP1 Sites Are Enriched in Class I Genes Relative to Class II Genes
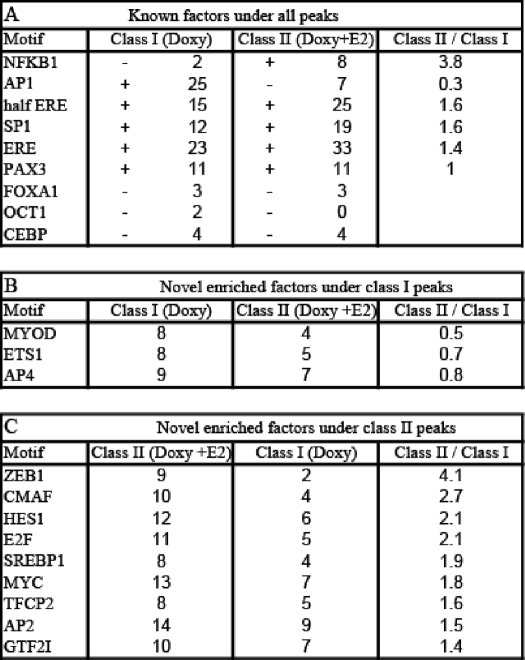
To investigate the mechanism whereby ERβ regulates different classes of genes, we next searched for the enrichment of known motifs involved in ER regulation that are adjacent to ERβ binding sites in the three classes of genes. In addition to the classical ERE, other motifs have been shown to be important for ER-mediated transcription, including AP1, SP1, FOXA1, OCT1, NFκB1, and PAX2 (6, 37–41). This suggests that differences in adjacent transcription factor motifs in the three of classes of genes might account for different pattern of gene regulation. CisGenome software (33) and Transfac (34) weight matrices were used to scan a 400-bp sequence around the peak center for ERβ binding sites. Of the known factors, we found that AP1 (Fig. 3C), ERE (Fig. 3D), SP1, NFκB1, and PAX3 were enriched in the regulated genes compared with the background sequences but not FOXA1, OCT1, and CEBP. In class I genes, only AP1 was enriched by >3-fold, whereas ERE, NFκB1, and SP1 sites were enriched in class II genes (Table 5A). We examined the location of the AP1 (Fig. 3E) and ERE (Fig. 3F) motifs in relation to the peak center and found that they were centered within the binding regions. In addition to known factors, we searched for potential novel factors that might be important for ERβ regulation of genes. MYOD, ETS1, and AP4 were enriched in class I genes (Table 5B), whereas ZEB1, CMAF, HES1, E2F, SREBP1, MYC, TFCP2, AP2, and GTF2I were enriched in class II genes (Table 5C).
TABLE 5.
Percentages of known or novel factors enriched near class I and II genes
Percentages of known ER interacting motifs, unknown motifs enriched in doxycycline peaks near class I genes and motifs enriched in doxycycline + E2 peaks near class II genes. Only motifs with a frequency of >8% among the investigated peaks and with >1.9-fold relative enrichment compared with the background sequences (matched genomic controls) were considered as enriched. A shows the percentages of known ER-interacting motifs in class I doxycycline peaks and class II doxycycline + E2 peaks and the ratios of corresponding percentages of enriched motifs. + indicates the enrichment, and − indicates the depletion. B shows the percentages of unknown motifs enriched in class I doxycycline peaks and the ratios of corresponding percentages. Motifs are ranked according to their relative abundance under class I, as compared to class II peaks. C shows the percentages of unknown motifs enriched in class II doxycycline + E2 peaks and the ratios of corresponding percentages. Motifs are ranked according to their relative abundance under class II, as compared with class I peaks.
Unliganded ERβ Binds to All Three Classes of Genes
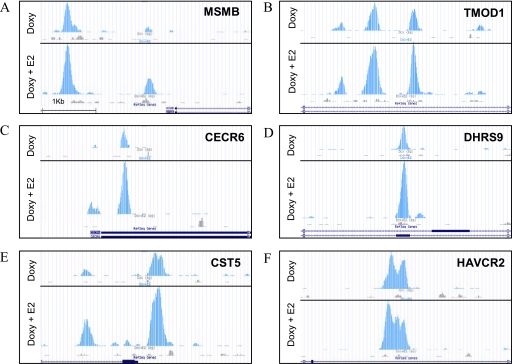
Our data indicate that the transcription factor motifs that are adjacent to ERβ binding sites are different in class I genes compared with class II genes, which could account for some differences in genes regulated by ERβ. Another possibility is that unliganded or liganded ERβ binds to different genes in three classes of genes. To explore this possibility, we examined the binding of ERβ to two members of class I (MSMB and TMOD1), class II (CECR6 and DHRS9) and class III (CST5 and HAVCR2) genes. We selected PCR primers that spanned the ERβ binding peaks from the ChIP-seq data (Fig. 4, A–F). U2OS-ERβ cells were treated with doxycycline to induce ERβ and then exposed to E2 for 1 h before ChIP was done. One h was the time of maximal ERβ binding (data not shown). For all three classes of genes, ERβ bound to the genes in the absence of ligand (Fig. 5, A–F), demonstrating that unliganded ERβ is recruited similarly to each class of target genes. The addition of the E2 did not significantly alter the binding of ERβ to the three gene classes. These data suggest that ERβ binding is necessary for all three classes of genes but is not sufficient to induce class II gene expression.
FIGURE 4.
ERβ binding sites in class I, II, and III genes. U2OS-ERβ cells were treated with or without doxycycline (Doxy) for 18 h and followed by vehicle or 10 nm E2 (doxycycline + E2) treatment for 1 h. ERβ binding sites near class I genes MSMB (A) and TMOD1 (B), class II genes CECR6 (C) and DHRS9 (D), and class III genes CST5 (E) and HAVCR2 (F) were plotted as the density of 26-bp tags mapping to the region. Figures were produced using the University of California, Santa Cruz genome browser.
FIGURE 5.
Recruitment of ERβ to class I, II, and II genes in U2OS cells. U2OS-ERβ cells were treated with or without doxycycline for 18 h, followed by vehicle or 10 nm E2 treatment for 1 h. Following treatments, ChIP for ERβ was performed. Recruitment of ERβ to the class I genes, MSMB (A) and TMOD1 (B), class II genes CECR6 (C) and DHRS9 (D), or class III genes CST5 (E) and HAVCR2 (F) were measured by real-time PCR (n = 3). Each data point is the average of triplicate determinations ± SEM. Asterisk denotes p value ≤0.05.
NCOA2 Is Differentially Recruited to Some of the ERβ Target Genes
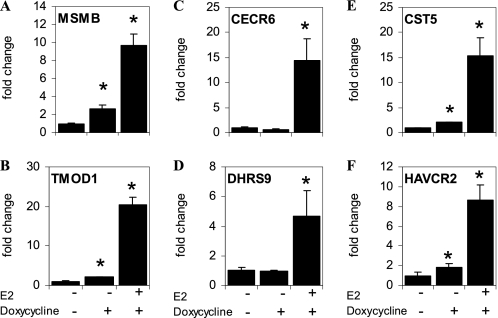
Our results show that the differential regulation of gene expression by unliganded and liganded ERβ is not due to the selective binding of ERβ to the different classes of target genes. Another possible explanation is that unliganded ERβ differentially recruits coactivators to the three classes of genes. To investigate this possibility, we performed ChIP for NCOA2, the major coactivator recruited to target genes in U2OS cells (25). NCOA2 recruitment to target genes was maximal following 2 h of treatment with E2 (25). In the presence of unliganded ERβ, NCOA2 was recruited to the class I genes MSMB and TMOD1 (Fig. 6, A and B) but not the class II genes CECR6 and DHRS9 (Fig. 6, C and D). In contrast, the addition of E2 recruited NCOA2 to the CERC6 and DHRS9 genes. Both unliganded and liganded ERβ recruited NCOA2 to the class III genes CST5 and HAVCR2 (Fig. 6, E and F). These results demonstrated that NCOA2 is differentially recruited to these genes by unliganded and liganded ERβ.
FIGURE 6.
Recruitment of NCOA2 to class I, II, and II genes in U2OS cells. U2OS-ERβ cells were treated with or without doxycycline for 18 h, followed by vehicle or 10 nm E2 treatment for 2 h. Following treatments, ChIP for NCOA2 was performed. Recruitment of NCOA2 to the class I genes, MSMB (A) and TMOD1 (B), class II genes CECR6 (C) and DHRS9 (D), or class III genes CST5 (E) and HAVCR2 (F) were measured by real-time PCR (n = 3). Each data point is the average of triplicate determinations ± SEM. Asterisk denotes p value ≤0.05.
DISCUSSION
ERβ has potent antiproliferative (15, 16) and anti-inflammatory properties (42, 43), which indicate that ERβ selective agonists could be useful therapeutic and chemopreventative agents. To exploit these properties, it is crucial to understand how ERβ regulates genes and why it has different properties than ERα, which is associated with cell proliferation and tumor formation. Our microarray data show that ERα and ERβ regulate different genes. Furthermore, we found that unliganded ERβ but not unliganded ERα regulates numerous genes in U2OS cells. The different effects by unliganded ERs were not due to differences in the levels of ERα and ERβ because we previously reported that the U2OS cell lines express comparable levels of receptors after induction with doxycycline (20, 36). These findings indicate that unliganded action is a unique property of ERβ in U2OS cells.
Whereas ERα only regulated a single class of genes that was dependent on the presence of ligand, microarray analysis revealed that there are three distinct classes of genes regulated by ERβ. Class I genes are regulated by primarily by unliganded ERβ, class II genes are regulated only by liganded ERβ, and class III genes are regulated by unliganded ERβ and potentiated with the addition of E2. Real-time PCR of multiple genes verified the microarray data, demonstrating the existence of the three classes of regulated genes. To begin to understand how ERβ regulates the different classes of genes, we performed ChIP-seq to identify genome-wide ERβ binding sites. We found that there were >5,000 ERβ binding sites for unliganded ERβ and >11,000 sites for liganded ERβ. This number of binding sites is consistent with the number of ERα binding sites identified with genomic tiling arrays (6) and ChIP-seq (24). The location of the binding sites showed that most of the unliganded and liganded ERβ binding sites are present within genes. About 13% of the ERβ binding sites were found in the proximal promoters, which suggest that these sites are involved in gene regulation.
Our findings raise several important questions regarding the action of ERs. For example, how do ERα and ERβ regulate different genes? The most likely explanation is that ERα and ERβ bind to different regulatory elements on target genes. The ERα binding sites on chromosomes 1 and 6 were previously reported using tiling arrays in U2OS cells expressing ERα (23). We compared the ERβ binding sites on these two chromosomes and found that only ∼34% of the ERβ binding sites overlapped with ERα binding sites. The ERα binding sites have also been characterized in MCF-7 breast cancer cells that express ERα. It has been shown that there is very little overlap of ERα binding sites in U2OS cells compared with MCF-7 cells, because of different ERα cistromes in these cells (23). Our comparison of the data reveals that the ERβ binding sites in U2OS cells overlapped with 11% (23) or 27% (24) ERα binding sites in MCF-7 cells. One possible explanation for the little overlap of ERα and ERβ binding sites is that ERs interact with different cis-regulatory elements. Multiple transcription factor motifs are enriched in the cistrome of ERα binding sites including, FOXA1, AP1, GATA, OCT1, and CEBP (6, 39, 44). Of these factors, only AP1 was enriched at ERβ binding sites. Taken together, our results indicate that the majority of the ERβ binding sites are distinct from ERα binding sites in both U2OS and MCF-7 cells, probably because the transcription factors adjacent ERα and ERβ binding sites are different.
Another important question raised by our findings is: how does ERβ regulate three different classes of genes? We investigated three possibilities. First, we examined if transcription factors motifs adjacent to ERβ bindings were different in class I and II genes. We found that AP1, MYOD, AP4, and ETS1 binding sites were enriched in class I genes compared with class II genes. Other motifs were enriched in class II genes, including elements known to mediate ER responses, such ERE, NFκB1, and SP1. Class II genes were also enriched in sites not known to be involved in ER regulation. These include ZEB1, CMAF, HES1, E2F, SREBP1, MYC, TFCP2, AP2, and GTF2I. Our findings indicate that some differences in gene regulation by ERβ are likely due to its interaction with different transcription factors that are adjacent to the ERβ binding site in the three classes of genes.
Another potential mechanism that we investigated is that the unliganded and liganded forms of ERβ bind differentially to the three classes of genes. However, this does not occur because we found that ERβ bound similarly to members of the three classes of genes in the absence or presence of ligand. Based on this finding, it appears that the unliganded ERβ can bind to all three classes of genes but can only regulate class II and III when the ligand is present. Another possible mechanism for the differences in the regulation of genes by ERβ is the differential recruitment of coactivators to ERβ. We found that the coactivator NCOA2 is recruited by unliganded ERβ in two of the class I and two of the class III genes, whereas only the ligand causes recruitment of NCOA2 to two of the class II genes. Our data indicate that in these genes, unliganded ERβ is bound to the regulatory elements in all three classes of genes but is activated by the recruitment of NCOA2. When ERβ is bound to class I and III genes, it is capable of recruiting NCOA2 in the absence of ligand. In contrast, in the two class II genes, the bound ERβ recruits NCOA2 only when it binds the ligand. While these findings suggest that NCOA2 recruitment is important for determining the class of genes regulated, this needs to be confirmed by studying NCOA2 recruitment to a much larger set of genes by tiling arrays or ChIP-seq. It is well established from structural studies that binding of the ligand moves helix 12 in the ligand binding domain into a position that allows the recruitment of coactivators (45, 46). It is likely that when unliganded ERβ is bound to some class I and III genes helix 12 is in a position that allows coactivator recruitment even without ligand, whereas in class II genes, the repositioning of helix 12 by the ligand is essential for the binding of coactivators. Because there are numerous co-factors involved regulating ER action (9, 47, 48) it is likely that other cofactors besides NCOA2 are also important for the differences in gene regulation observed with unliganded and liganded ERβ.
In summary, we found that ERβ has different properties than ERα in that it regulates three distinct classes of genes. Surprisingly, the unliganded form of ERβ regulated more genes than the liganded form. The differential regulation of genes by unliganded and liganded ERβ is likely due to differences in the adjacent transcription factors and cofactors that interact with ERβ at the three classes of regulated genes. Our findings raise the possibility that drugs that increase the production of the unliganded form of ERβ in cells may be valuable therapeutic agents.
Supplementary Material
Acknowledgment
We thank Jan-Åke Gustafsson for providing plasmids.
This work was supported by a grant from the American Cancer Society (to D. C. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2.
- ER
- estrogen receptor
- ERE
- estrogen responsive element
- ChIP-Seq
- chromatin immunoprecipitation-sequencing
- E2
- estradiol
- NCOA2
- nuclear receptor coactivator 2.
REFERENCES
- 1.Riggs B. L., Khosla S., Melton L. J., 3rd (2002) Endocr. Rev. 23, 279–302 [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn M. E., Karas R. H. (1999) N. Engl. J. Med. 340, 1801–1811 [DOI] [PubMed] [Google Scholar]
- 3.Katzenellenbogen B. S., Montano M. M., Ediger T. R., Sun J., Ekena K., Lazennec G., Martini P. G., McInerney E. M., Delage-Mourroux R., Weis K., Katzenellenbogen J. A. (2000) Recent Prog. Horm. Res. 55, 163–193 [PubMed] [Google Scholar]
- 4.Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Ström A., Treuter E., Warner M., Gustafsson J. A. (2007) Physiol. Rev. 87, 905–931 [DOI] [PubMed] [Google Scholar]
- 5.Levin E. R. (2005) Mol. Endocrinol. 19, 1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll J. S., Meyer C. A., Song J., Li W., Geistlinger T. R., Eeckhoute J., Brodsky A. S., Keeton E. K., Fertuck K. C., Hall G. F., Wang Q., Bekiranov S., Sementchenko V., Fox E. A., Silver P. A., Gingeras T. R., Liu X. S., Brown M. (2006) Nat. Genet. 38, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 7.Shang Y., Hu X., DiRenzo J., Lazar M. A., Brown M. (2000) Cell 103, 843–852 [DOI] [PubMed] [Google Scholar]
- 8.Métivier R., Penot G., Hübner M. R., Reid G., Brand H., Kos M., Gannon F. (2003) Cell 115, 751–763 [DOI] [PubMed] [Google Scholar]
- 9.Smith C. L., O'Malley B. W. (2004) Endocr. Rev. 25, 45–71 [DOI] [PubMed] [Google Scholar]
- 10.Lonard D. M., Lanz R. B., O'Malley B. W. (2007) Endocr. Rev. 28, 575–587 [DOI] [PubMed] [Google Scholar]
- 11.Hewitt S. C., Harrell J. C., Korach K. S. (2005) Annu. Rev. Physiol. 67, 285–308 [DOI] [PubMed] [Google Scholar]
- 12.Krege J. H., Hodgin J. B., Couse J. F., Enmark E., Warner M., Mahler J. F., Sar M., Korach K. S., Gustafsson J. A., Smithies O. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim G. J., Wang L., Andersson S., Nagy N., Kis L. L., Zhang Q., Mäkelä S., Warner M., Gustafsson J. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6694–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paruthiyil S., Parmar H., Kerekatte V., Cunha G. R., Firestone G. L., Leitman D. C. (2004) Cancer Res. 64, 423–428 [DOI] [PubMed] [Google Scholar]
- 16.Ström A., Hartman J., Foster J. S., Kietz S., Wimalasena J., Gustafsson J. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazennec G., Bresson D., Lucas A., Chauveau C., Vignon F. (2001) Endocrinology 142, 4120–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman J., Edvardsson K., Lindberg K., Zhao C., Williams C., Ström A., Gustafsson J. A. (2009) Cancer Res. 69, 6100–6106 [DOI] [PubMed] [Google Scholar]
- 19.Hartman J., Lindberg K., Morani A., Inzunza J., Ström A., Gustafsson J. A. (2006) Cancer Res. 66, 11207–11213 [DOI] [PubMed] [Google Scholar]
- 20.Kian Tee M., Rogatsky I., Tzagarakis-Foster C., Cvoro A., An J., Christy R. J., Yamamoto K. R., Leitman D. C. (2004) Mol. Biol. Cell 15, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe D. G., Getz B. J., Johnsen S. A., Riggs B. L., Khosla S., Spelsberg T. C. (2003) J. Cell. Biochem. 90, 315–326 [DOI] [PubMed] [Google Scholar]
- 22.Carroll J. S., Brown M. (2006) Mol. Endocrinol. 20, 1707–1714 [DOI] [PubMed] [Google Scholar]
- 23.Krum S. A., Miranda-Carboni G. A., Lupien M., Eeckhoute J., Carroll J. S., Brown M. (2008) Mol. Endocrinol. 22, 2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welboren W. J., van Driel M. A., Janssen-Megens E. M., van Heeringen S. J., Sweep F. C., Span P. N., Stunnenberg H. G. (2009) EMBO J. 28, 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy N., Tatomer D., Herber C. B., Zhao X., Tang H., Sargeant T., Ball L. J., Summers J., Speed T. P., Leitman D. C. (2008) Mol. Endocrinol. 22, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullwood M. J., Liu M. H., Pan Y. F., Liu J., Xu H., Mohamed Y. B., Orlov Y. L., Velkov S., Ho A., Mei P. H., Chew E. G., Huang P. Y., Welboren W. J., Han Y., Ooi H. S., Ariyaratne P. N., Vega V. B., Luo Y., Tan P. Y., Choy P. Y., Wansa K. D., Zhao B., Lim K. S., Leow S. C., Yow J. S., Joseph R., Li H., Desai K. V., Thomsen J. S., Lee Y. K., Karuturi R. K., Herve T., Bourque G., Stunnenberg H. G., Ruan X., Cacheux-Rataboul V., Sung W. K., Liu E. T., Wei C. L., Cheung E., Ruan Y. (2009) Nature 462, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du P., Kibbe W. A., Lin S. M. (2008) Bioinformatics 24, 1547–1548 [DOI] [PubMed] [Google Scholar]
- 28.Smyth G. K. (2004) Stat. Appl. Genet. Mol. Biol. 3, Article3 [DOI] [PubMed] [Google Scholar]
- 29.Dudoit S., Shaffer J. P., Boldrick J. C. (2003) Statistical Science 18, 71–103 [Google Scholar]
- 30.Eisen M. B., Spellman P. T., Brown P. O., Botstein D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivar O. I., Lin C. L., Firestone G. L., Bjeldanes L. F. (2009) Biochem. Pharmacol. 78, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., Bernstein B. E., Nussbaum C., Myers R. M., Brown M., Li W., Liu X. S. (2008) Genome Biol. 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji H., Jiang H., Ma W., Johnson D. S., Myers R. M., Wong W. H. (2008) Nat. Biotechnol. 26, 1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingender E., Chen X., Hehl R., Karas H., Liebich I., Matys V., Meinhardt T., Prüss M., Reuter I., Schacherer F. (2000) Nucleic Acids Res. 28, 316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivar O. I., Saunier E. F., Leitman D. C., Firestone G. L., Bjeldanes L. F. (2010) Endocrinology 151, 1662–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy N., Paruthiyil S., Zhao X., Vivar O. I., Saunier E. F., Griffin C., Tagliaferri M., Cohen I., Speed T. P., Leitman D. C. (2010) Mol. Cell. Endocrinol. 315, 201–207 [DOI] [PubMed] [Google Scholar]
- 37.Kushner P. J., Agard D. A., Greene G. L., Scanlan T. S., Shiau A. K., Uht R. M., Webb P. (2000) J. Steroid Biochem. Mol. Biol. 74, 311–317 [DOI] [PubMed] [Google Scholar]
- 38.Saville B., Wormke M., Wang F., Nguyen T., Enmark E., Kuiper G., Gustafsson J. A., Safe S. (2000) J. Biol. Chem. 275, 5379–5387 [DOI] [PubMed] [Google Scholar]
- 39.Carroll J. S., Liu X. S., Brodsky A. S., Li W., Meyer C. A., Szary A. J., Eeckhoute J., Shao W., Hestermann E. V., Geistlinger T. R., Fox E. A., Silver P. A., Brown M. (2005) Cell 122, 33–43 [DOI] [PubMed] [Google Scholar]
- 40.Hurtado A., Holmes K. A., Geistlinger T. R., Hutcheson I. R., Nicholson R. I., Brown M., Jiang J., Howat W. J., Ali S., Carroll J. S. (2008) Nature 456, 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cvoro A., Tzagarakis-Foster C., Tatomer D., Paruthiyil S., Fox M. S., Leitman D. C. (2006) Mol. Cell 21, 555–564 [DOI] [PubMed] [Google Scholar]
- 42.Cvoro A., Paruthiyil S., Jones J. O., Tzagarakis-Foster C., Clegg N. J., Tatomer D., Medina R. T., Tagliaferri M., Schaufele F., Scanlan T. S., Diamond M. I., Cohen I., Leitman D. C. (2007) Endocrinology 148, 538–547 [DOI] [PubMed] [Google Scholar]
- 43.Cvoro A., Tatomer D., Tee M. K., Zogovic T., Harris H. A., Leitman D. C. (2008) J. Immunol. 180, 630–636 [DOI] [PubMed] [Google Scholar]
- 44.Lupien M., Brown M. (2009) Endocr.-Relat. Cancer 16, 381–389 [DOI] [PubMed] [Google Scholar]
- 45.Shiau A. K., Barstad D., Loria P. M., Cheng L., Kushner P. J., Agard D. A., Greene G. L. (1998) Cell 95, 927–937 [DOI] [PubMed] [Google Scholar]
- 46.Nettles K. W., Greene G. L. (2005) Annu. Rev. Physiol. 67, 309–333 [DOI] [PubMed] [Google Scholar]
- 47.Westin S., Rosenfeld M. G., Glass C. K. (2000) Adv. Pharmacol. 47, 89–112 [DOI] [PubMed] [Google Scholar]
- 48.Green K. A., Carroll J. S. (2007) Nature Rev. Cancer 7, 713–722 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.