Abstract
Interleukin-2 (IL-2) regulates different functions of various lymphoid cell subsets. These are mediated by its binding to the IL-2 receptor (IL-2R) composed of three subunits (IL2-Rα, -β, and -γc). IL-2Rβ is responsible for the activation of several signaling pathways. Ectodomain shedding of membrane receptors is thought to be an important mechanism for down-regulation of cell surface receptor abundance but is also emerging as a mechanism that cell membrane-associated molecules require for proper action in vivo. Here, we demonstrate that IL-2Rβ is cleaved in cell lines of different origin, including T cells, generating an intracellular 37-kDa fragment (37βic) that comprises the full intracellular C-terminal and transmembrane domains. Ectodomain shedding of IL-2Rβ decreases in a mutant deleted of the juxtamembrane region, where cleavage is predicted to occur, and is inhibited by tissue inhibitor of metalloproteases-3. 37βic is tyrosine-phosphorylated and associates with STAT-5, a canonic signal transducer of IL-2R. Finally, lymphoid cell transfection with a truncated form of IL-2Rβ mimicking 37βic increases their proliferation. These data indicate that IL-2Rβ is subject to ectodomain shedding generating an intracellular fragment biologically functional, because (i) it is phosphorylated, (ii) it associates with STAT5A, and (iii) it increases cell proliferation.
Keywords: Metalloprotease, Receptor Modification, Shedding, Signal Transduction, Tissue Inhibitors Metalloprotease (TIMPS), IL-2 Receptor β
Introduction
IL-23 is a cytokine secreted by antigen-activated T cells. It regulates proliferation, differentiation, and survival of T, B, and NK cells (1, 2). It exerts its pleiotropic activities, as other growth factors, through binding to its receptors at the cell surface. The interleukin-2 receptor (IL-2R) is a complex that may be composed of different associated subunits that confer different affinity and functional characteristics to the receptor complex (2). The high affinity (Kd ≈ 10–100 pm) IL-2R is a heterotrimer constituted of the α chain (IL-2Rα, CD25), the β chain (IL-2Rβ, CD122), and the common cytokine receptor γ chain (γc, CD132). IL-2Rβ and γc may associate and bind IL-2 with intermediate affinity (Kd ≈ 1 nm), whereas IL-2Rα itself may also bind IL-2 with low affinity (Kd ≈ 10 nm)(3, 4). IL-2Rα is usually considered to be an affinity converter, because it cannot transduce signals into the cytoplasm due to its short intracellular tail (5). IL-2Rβ is shared with IL-15R, and γc with receptors for IL-4, -7, -9, -15, and -21. They belong to type I cytokine receptor family and are devoid of intrinsic kinase activity (5). Hence, signaling events that are elicited in response to IL-2 binding are primarily due to cytosolic signaling molecules associated to IL-2Rβ and γc. IL-2-induced signaling pathways have intensively been studied, these include among others, the well known Jak/STAT, phosphatidylinositol 3-kinase, and Ras/mitogen-activated protein kinase pathways (3, 5). The tyrosine phosphorylation of IL-2Rβ and γc allows the recruitment of adaptor molecules that are in turn themselves phosphorylated and thus play their role as anchor proteins for downstream signaling molecules.
IL-2R is endocytosed through clathrin-independent process (3, 6, 7). After internalization, the three receptor subunits are sorted independently; IL-2Rα recycles back to the plasma membrane, whereas the IL-2Rβ and γc receptors may be recycled back to the membrane, but may also be targeted to late endosomes and lysosomes where they are degraded (8). Endocytosis and degradation of cell surface receptors is one mechanism that regulates their accessibility and functionality. However, ectodomain shedding is also involved in such regulation (9, 10). This mechanism regulates membrane-bound cell surface molecules, including cytokines and their receptors, growth factors, adhesion molecules, and others (9, 11). It is well established that IL-2Rα may be subject to this kind of proteolysis that delivers its extracellular domain as a soluble receptor capable of regulating IL-2 biological functions (12–14). Also, it is known that shedding of γc occurs (15). Concerning IL-2Rβ, to our knowledge, only one study has reported the existence of a 50-kDa soluble IL-2Rβ, but its origin was not addressed (13). Shedding proteases belong to two different metalloprotease families, the matrix metalloproteases (MMPs) or the disintegrins and metalloproteases (ADAMs) (9). They cleave cell surface molecules in the extracellular region proximal to the transmembrane domain (the stem region) (9, 10). For instance, it has been shown that transmembrane proteins such as Notch and its ligand Delta-like 1, growth hormone receptor, epidermal growth factor receptor, p75 neurotrophin receptor, colony-stimulating factor-1 receptor, and others are subject to ectodomain shedding (16–21). Furthermore, it has been found that ectodomain shedding of cell surface receptors enables further intracellular cleavage by the γ-secretase complex, releasing soluble intracellular fragments that may translocate to the nucleus where they may act as transcriptional regulators (16–18, 22). Thus, it is now evident that ectodomain shedding is not only important, as initially thought, for down-regulation of intracellular signaling and receptor abundance in the cell membrane, but also as an important mechanism that generates functional intracellular fragments that some membrane molecules require to carry out particular functions (10).
In this study, we report for the first time that IL-2Rβ is subject to ectodomain shedding in human T cells and different cell lines. We also demonstrate that this shedding mechanism is mediated by a TIMP-3-sensitive metalloprotease that generates a C-terminal 37-kDa fragment (abbreviated as 37βic), including the transmembrane and intracellular domains. Finally, we show that this intracellular fragment is subject to tyrosine phosphorylation, associates with STAT5A, a canonical signal transducer of the IL-2R pathway, and enhances cell proliferation, indicating that it is biologically functional.
EXPERIMENTAL PROCEDURES
Cells, Antibodies, and Reagents
IL-2Rβ stably transfected cell lines Lαβγ and Hep2β as well as HeLa cells were grown in Dulbecco's modified Eagle's medium. Human NK-like cell line NKL (23) and T cell-derived cell line Kit-225 (24) were grown in RPMI 1640 supplemented with 200 pm IL-2. In some experiments Kit-225 and NKL cells were deprived of IL-2 by washing three times and resuspending them in culture media without IL-2 at 5 × 105 cells/ml for 48 h. For IL-2 stimulation, cells were resuspended in culture medium at 2 × 107cells/ml and incubated at 37 °C with or without 200 pm IL-2 for the indicated periods of time. The BAF-B03 cell line is a murine IL-3-dependent pre-B cell line. This cell line was maintained in RPMI 1640 medium, supplemented with WEHI cell culture supernatant medium (RPMI 1640+5%FBS) as a source of IL-3. Baf-β cells were generated by stably transfecting BAF-B03 cells with a pCDNA3-IL-2Rβ construct and maintaining them in IL-2-supplemented medium (2 nm) instead of IL-3. Transfected cells then showed a proliferative response to IL-2. In some experiments Baf-β cells were deprived of IL-2 for 18 h and then stimulated with 2 nm IL-2 for the indicated times. Human peripheral blood mononuclear cells were obtained from volunteer anonymous healthy donors from the “Etablissement Francais du Sang.” Mononuclear cells were separated by Ficoll-Hypaque gradient centrifugation and treated as described elsewhere (25). All media were supplemented with 10% heat-inactivated fetal bovine serum and 2 mm l-glutamine. Cell culture reagents and carboxyfluorescein diacetate succinimidyl ester (CFSE) were from Invitrogen (Carlsbad, CA), chemicals were from Sigma, and restriction enzymes were from New England Biolabs (Ipswich, MA). FuGENE reagent was from Roche Applied Science, and Cell line Nucleofector kit V reagent was from Amaxa (Gaithersburg, MD). Rabbit polyclonal Ab C-20 against the C-terminal domain of IL-2Rβ was from Santa Cruz Biotechnology (Santa Cruz, CA). Ab anti-STAT5A and monoclonal Ab anti-phosphotyrosine 4G10 were from Upstate Biotechnologies (Lake Placid, NY). Mouse monoclonal Ab 561 against the extracellular domain of the IL-2Rβ used in cytofluorometry experiments was given by Dr. R. Robb (Dupont Merck Pharmaceutical Co., Wilmington, DE). Goat anti-mouse and anti-rabbit horseradish peroxidase-conjugated Abs were purchased from Amersham Biosciences, and ECL was from Pierce. TIMP-3 was from Biomol International (Plymouth, PA). On-target plus smart pool of siRNA against the IL-2Rβ was purchased from Dharmacon (Lafayette, CO). TAPI and GM6001, protease inhibitors, were from Calbiochem (San Diego, CA).
Cell Transfection
HeLa cells were transfected with FuGENE reagent according to the manufacturer's conditions. Cells were harvested 48 h after transfection, and treatments were added for the indicated times. NKL cells were transfected with the Amaxa (Lonza Cologne, Germany) system as elsewhere described (26). Cells were used for the appropriate protocol 48 h after transfection. Baf-β and BAF-B03 cells were transfected using a Bio-Rad (Hercules, CA) electroporator at 280 V and 950 microfarads. Cells were used for the appropriate protocol 48 h after transfection.
Plasmids and Construction
Truncated forms of IL-2Rβ were generated by PCR using the forward primers: β-TM&IC, 5′-GGA TCC ATG CAC CTC CTC GTG GGC CTC AGC GG-3′; β-TM&IC2, 5′-GGA TCC ATG TTA GTG TAC TTG CTG ATC AAC TG-3′; β-IC, 5′-GGA TCCA TGAA CTGC AGGA ACAC CGG GCC-3′, and the reverse primer, 5′-GAA TTC CTA CACC AAG TGA GTT GGG TCC TGA CC-3′. PCR products were cloned into pCR-BluntII-TOPO vector (Invitrogen) and verified by DNA sequencing. For eukaryotic expression, all constructs were subcloned into pCDNA3.1 expression vector. For β-ΔP226-D239 the QuikChange II-E site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used with the primer 5′-CC TGG AGC CCC TGG AGC CAG ACC ATT CCG TGG CTC GGC CAC-3′. Expression plasmid containing mouse STAT5A was a kind gift from Dr. Isabelle Dusanter (Institut Cochin, Paris, France).
Immunoprecipitation and Western Blotting
Cells were lysed with ice-cold lysis buffer (50 mm Tris (pH 8), 150 mm NaCl, 10 mm NaF, 1 mm EDTA, 1 mm EGTA, and 0.5% Triton X-100) containing 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm iodoacetamide, 10 mm N-ethylmaleimide, and 1/100 Protease Mixture Inhibitors. Nuclear debris was removed by centrifugation at 15 × 103 × g 20 min at 4 °C. 15–25 μg of total protein from cell lysates were mixed with an equal volume of reducing sample buffer 2× and boiled for 5 min. For immunoprecipitation experiments, an equivalent amount of protein from cell lysates was incubated overnight at 4 °C with Ab. Protein-G beads were added to precipitate the protein-Ab complexes, washed four times with ice-cold lysis buffer. Immunoprecipitates were mixed with 15 μl of reducing sample buffer 2× and boiled for 5 min. Whole cell lysates and immunoprecipitates were resolved on 10% SDS-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes that were blocked for 2 h in TBS-T (TBS/0.5% Tween 20) with 3% bovine serum albumin. Blots were probed 1 h with the proper primary Ab in TBS-T. After washing they were incubated 1 h with the proper secondary Ab in TBS-T. The membranes were washed, and protein detection was conducted using ECL reagents.
Mass Spectrometry
Large scale immunoprecipitation experiments were performed to obtain enough material for mass spectrometry analysis. Briefly, Lαβγ cells were plated on fifty 15-cm dishes. At 80–90% confluence, dishes were lysed in lysis buffer, in a total volume of 75 ml, corresponding to 2 × 107 cells/ml. The lysate was cleared and divided in two equal aliquots. One aliquot was used for precipitation with a serum from non-immunized rabbit, the second aliquot was precipitated with 20 μg anti-IL-2Rβ antibody. As described above, Abs were recovered on 50-μl protein-G beads and washed in lysis buffer. Complexes were eluted from the beads in boiling sample buffer and separated by SDS-PAGE. The gel was silver-stained according to Blum et al. (27). Proteins present within the gel, were eluted and trypsin-digested, and the peptides were identified by a combined analysis using MS and MS/MS data (4800 MALDI TOF/TOF Analyzer, Applied Biosystems, MDS-SCIEX). The number of precursor peaks for MS/MS acquisition per band were set at 12. In MS/MS positive ion mode, 4000 spectra were averaged. For searching the human species within the NCBInr database a local copy of Mascot version 2.1 (Matrix Science, London, UK) was used with a mass tolerance of 50 ppm for MS and 0.3 Da for MS/MS. Protein hits with a Mascot Protein Score > 64 were used for further manual validation.
Flow Cytometry
Cells were washed with cold phosphate-buffered saline and surface staining was performed at 4 °C for 1 h using Ab 561 coupled to Alexa-fluor 647 in phosphate-buffered saline (1×)/1% bovine serum albumin. After washing with cold phosphate-buffered saline, cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences) with CellQuest software.
Cell Proliferation Assays
Cells were labeled with CFSE 48 h after transfection and seeded into flasks at 2.5 × 105 cells/ml with or without IL-2. 48–72 h after, proliferation was evaluated by flow cytometry according to CFSE manufacturer's instructions in a FACSCalibur with CellQuest software. For methylthiazolyldiphenyl-tetrazolium bromide assays, transfected cells were seeded 48 h after into 96-well dishes at 2.5 × 105 cells/ml with or without IL-2. 48 h after proliferation was evaluated with an enzyme-linked immunosorbent assay plate reader Bio-Rad.
RESULTS
IL-2Rβ Is Cleaved and Generates an Intracellular Fragment of ∼37 kDa
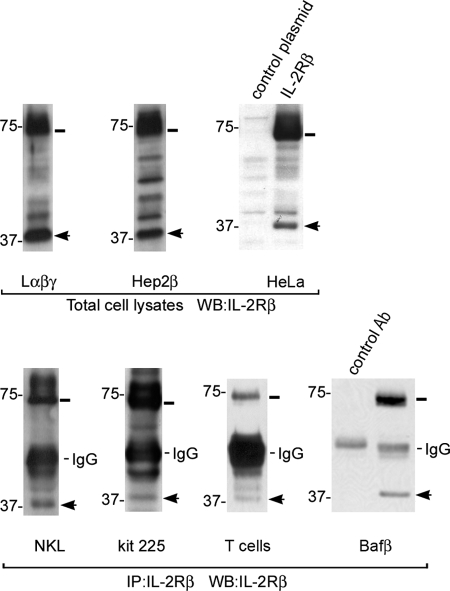
We wanted to investigate the shedding of the IL-2Rβ and the functionality of the intracellular product. We analyzed the fragment pattern of different cell lines by Western blot using an antibody directed against the C-terminal domain of IL-2Rβ. In cell lines stably transfected with IL-2Rβ (Hep2β) or with IL-2Rα, -β, and -γ chains (Lαβγ, Fig. 1), a band of ∼37 kDa was reproducibly observed (Fig. 1). A similar band was also found in HeLa cells transiently transfected with IL-2Rβ. More importantly, this band was also observed in NKL and Kit-225 cells, two human IL-2-dependent lymphocytic cell lines that endogenously express the IL-2 receptor, as well as in human T cells and Baf-β cells (Fig. 1). However, because expression of IL-2Rβ in these cells is lower than in non-lymphoid transfected cell lines, the 37-kDa band was observable only after immunoprecipitating IL-2Rβ. These data indicate that IL-2Rβ may undergo proteolytic processing in cells of different origin, including lymphoid cells, and this may represent a general mechanism of receptor processing.
FIGURE 1.
IL-2Rβ is proteolytically processed in different cell lines and yields a fragment of 37 kDa. Whole cell lysates from stably or transiently transfected cell lines expressing the IL-2Rβ (top panel) or anti-IL-2Rβ Ab immunoprecipitates (IP) from lymphoid cell lines NKL and Kit-225, T cells, or Bafβ cells (bottom panel) were resolved by gel electrophoresis and analyzed by Western blot using the anti-IL-2Rβ Ab. In all cells tested a band of ∼37 kDa was detected (arrow). Full-length IL-2Rβ migrates at 75 kDa (line). Empty pcDNA3 was used as a transfection control plasmid in HeLa cells. One representative experiment of three is shown.
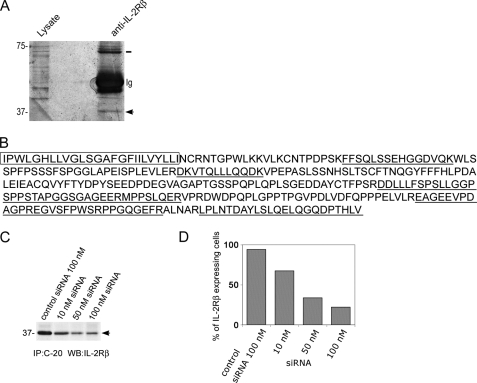
We next investigated whether the 37-kDa band was originated from IL-2Rβ. A large-scale immunoprecipitation was performed and separated by SDS-PAGE from Lαβγ cell lysates. This stably transfected cell line was used, because it expresses large amounts of IL-2Rβ that render this type of experiment feasible. Proteins were silver-stained, and the 37-kDa band was cut from the gel, digested, and analyzed by mass spectrometry (Fig. 2A). This analysis enabled the identification of eight peptides that were all found to belong to the intracytoplasmic tail of IL-2Rβ, including all amino acids at the C-terminal end (Fig. 2B).
FIGURE 2.
MS identification of 37βic and siRNA silencing. A, gel section with a silver-stained band (arrow) corresponding to 37βic from IL-2Rβ. Proteins from whole cell lysates of Lαβγ cells were immunoprecipitated using an anti-IL-2Rβ Ab or control Ab and resolved in polyacrylamide gel. After fixation and silver staining as described under “Experimental Procedures,” the excised protein was analyzed by MS. The three bands at 75 kDa correspond to full IL-2R. B, amino acid sequence of transmembrane and C-terminal domains of the IL-2Rβ. The underlined sequences are those identified by MS of protein purified from the excised 37-kDa band. Amino acids of the transmembrane domain are boxed. C, siRNA silencing of 37βic produced in NKL cells. 1 × 107 NKL cells were transfected with the indicated concentrations of siRNA against IL-2Rβ or control siRNA using the Amaxa system. 48 h after transfection, immunoprecipitates were performed using anti-IL-2Rβ Ab and analyzed by Western blot. D, siRNA silencing of cell surface IL-2Rβ in NKL. Cells were transfected with the indicated concentrations of siRNA against the IL-2Rβ or control siRNA. 48 h after transfection, cell surface IL-2Rβ was analyzed by flow cytometry using monoclonal Ab 561. The percentage of cells expressing IL-2Rβ on their surface is presented. In A the arrow shows 37βic, and the line shows full-length IL-2Rβ.
The identity of the 37-kDa fragment (named hereafter 37βic for 37-kDa IL-2Rβ intracellular) was also verified in lymphoid cells NKL, an NK-derived cell line that endogenously expresses IL-2 receptor. This identification was performed by knocking down IL-2Rβ using a pool of IL-2Rβ directed siRNA (Fig. 2C). In these experiments we show that, after transfection of cells with different concentrations of siRNA, the 37βic immunoprecipitated from equivalent protein amounts decreased, whereas a control siRNA did not have this effect (Fig. 2C). The specific silencing of the IL-2Rβ gene by the siRNA was confirmed by NKL surface labeling of IL-2Rβ with an Ab directed against its extracellular region followed by flow cytometry analysis (Fig. 2D). These experiments confirmed that the 37-kDa band observed in the human lymphoid cell line NKL results from IL-2Rβ processing. Similar results were also obtained in Lαβγ cell line (not shown). Altogether, these experiments show that 37βic is a proteolytic product of the IL-2Rβ receptor containing the whole C-terminal intracytosolic part and possibly the transmembrane domain, considering its apparent molecular weight.
Work from our laboratory has shown that, after endocytosis, the full-length IL-2Rβ is sorted toward lysosomes where it is degraded (8). Therefore, we investigated if 37βic is generated in the lysosomal compartment. To test this possibility, HeLa cells were transiently transfected with IL-2Rβ or control plasmid, and 48 h later, they were treated for 3 h with 200 μm chloroquine, an inhibitor of lysosomal activity (28). As shown in Fig. 3, this treatment did not prevent the presence of 37βic and also, as previously reported, increased the amount of full-length IL-2Rβ (8). This result indicates that 37βic is not generated during the proteolytic processing of the IL-2Rβ in the lysosome. Hence, its increased amount implies that, after being generated, 37βic may follow the same route as the full-length IL-2Rβ toward the lysosomal degradation compartment.
FIGURE 3.
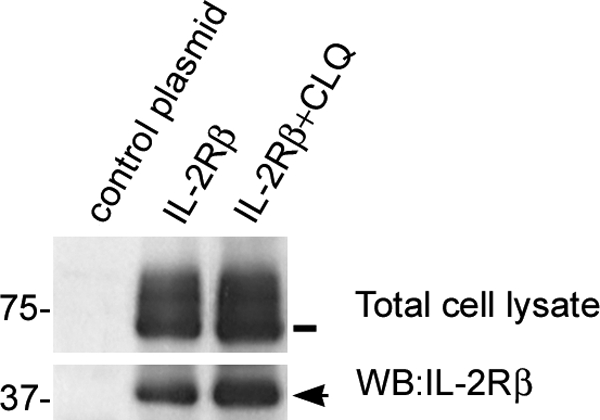
Degradation of 37βic is inhibited by chloroquine. HeLa cells were transfected with IL-2Rβ or control plasmid and treated or not with 200 μm chloroquine (CLQ) for 3 h. Whole cell lysates were prepared, resolved by gel electrophoresis, and analyzed by Western blot, loading the same amount of protein per lane. The arrow shows 37βic, and the line shows full-length IL-2Rβ. One representative experiment is shown.
The 37βic Is Generated by a TIMP-3-sensitive Metalloprotease That Cleaves IL-2Rβ in the Stem Region
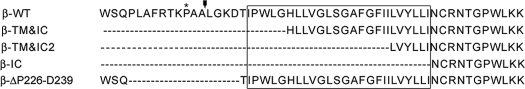
We attempted to identify the cleavage site of the IL-2Rβ by an Edman N-terminal sequencing of 37βic. Unfortunately, and probably due to an N-terminal blockade, this approach did not provide us with any conclusive result. This important question was then addressed by a different strategy. Several reports have shown that some membrane proteins undergo ectodomain shedding mediated by members of the MMP or ADAM families (9). The shedding process releases the membrane-protein extracellular domain by cleaving at an extracellular site proximal to the cell membrane, the so-called “stem region” (9, 10, 16). Therefore, taking this into consideration and the previous report of a soluble IL-2Rβ (13), we hypothesized that the cleavage site of IL-2Rβ could be proximal to the transmembrane domain. Thus, we generated three different constructs of truncated IL-2Rβ, two lacking the extracellular region and including different number of amino acids from the transmembrane domain (β-TM&IC and β-TM&IC2), and one including only its intracellular domain (β-IC) (Table 1). These constructs were transfected into HeLa cells. These cells were used because they do not endogenously express IL-2Rβ, therefore avoiding any misleading interpretation due to the presence of an already existing fragment from the native receptor. The migration pattern of the expressed truncated receptors was compared by Western blot with that of 37βic generated by cells transfected with the full-length IL-2Rβ construct. The three constructs migrated accordingly to their expected size, and 37βic migrated slightly above all three constructs (Fig. 4A). This result suggests that (a) 37βic includes the transmembrane domain and (b) the cleavage site resides in the stem region. Intriguingly, we repeatedly observed that a second upper band of ≈40 kDa appeared with the β-TM&IC and β-IC constructs (see “Discussion”).
TABLE 1.
Amino acid sequences surrounding the transmembrane region of wild-type IL-2Rβ and deleted constructs
The box indicates amino acids of the transmembrane domain. In the truncated forms a Met was inserted before the first amino acid.
* Pro-233.
The solid square with line indicates the putative clevage site.
FIGURE 4.
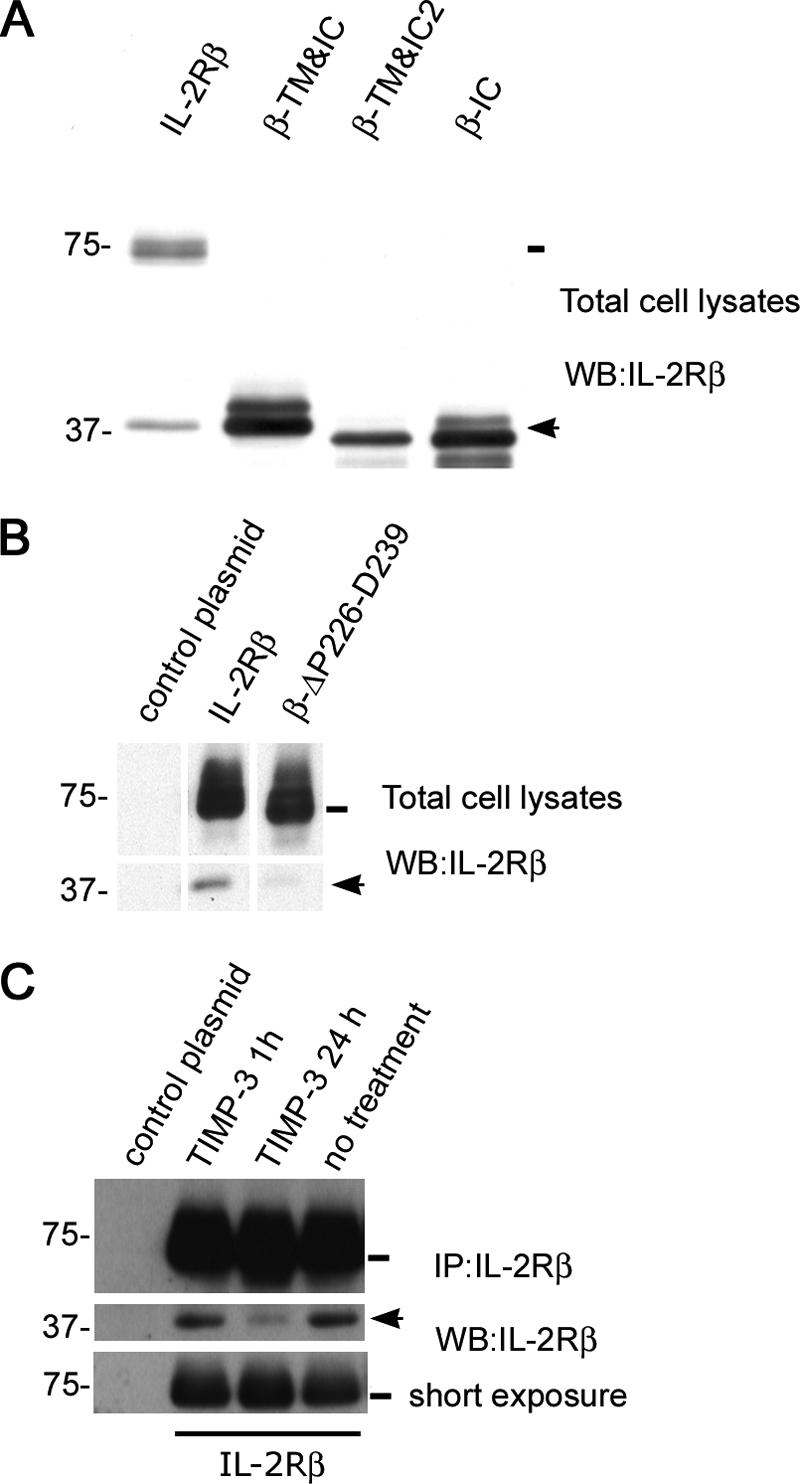
Cleavage site analysis of the IL-2Rβ and inhibition by TIMP-3. A, HeLa cells were transfected with full-length IL-2Rβ, the truncated constructs, or a control plasmid, 24 h after being seeded. 48 h after transfection cells were harvested and whole cell lysates were prepared and resolved by gel electrophoresis loading the same amount of total cell protein into each lane. Western blot was performed using the anti-IL-2Rβ Ab. B, HeLa cells were transfected with full-length IL-2Rβ, the β-ΔP226-D239 construct, or a control plasmid, 24 h after being seeded. Cells were harvested 48 h after transfection and treated with 200 μm chloroquine for the last 3 h. Whole cell lysates were prepared and resolved by gel electrophoresis, loading the same amount of total cell protein into each lane. Western blot was performed using the anti-IL-2Rβ Ab. C, HeLa cells were transfected with full-length IL-2Rβ or a control plasmid 24 h after being seeded. Transfected cells were treated or not for 24 or 1 h with TIMP-3 (1 μg/ml) and harvested 48 h after transfection. Immunoprecipitates were prepared with anti-IL-2Rβ Ab and resolved by gel electrophoresis. Western blot was performed using the anti-IL-2Rβ Ab. The lower panel shows the same blot with shorter exposure to compare total amounts of IL-2Rβ. In all panels, arrows and lines indicate the position of 37βic and of full-length IL-2Rβ, respectively. In each case one representative experiment is shown.
To further investigate the notion that cleavage site is located in the stem region, we generated a mutated form of IL-2Rβ with a deletion of 14 amino acids in this region (β-ΔP226-D239) (Table 1). HeLa cells were transiently transfected with either IL-2Rβ WT or βΔP226-D239 constructs. We found that, when these constructs were expressed in HeLa cells treated with chloroquine, the amount of 37βic was less abundant in β-ΔP226-D239-transfected cells (Fig. 4B). This result together with the size comparison experiment supports the idea that the cleavage site is located in the stem region and hence, that the proteolytic process that gives rise to 37βic is an ectodomain-shedding mechanism.
We next tried to identify the type of protease involved in ectodomain shedding of IL-2Rβ. Members of the MMP or ADAM families mediate this kind of cleavage. For this reason, we initially investigated the effect of hydroxamate-based metalloprotease inhibitors GM6001 and TAPI. However, these inhibitors did not significantly reduce the amount of 37βic generated (not shown). Therefore, we used the glycopeptide tissue inhibitor of metalloproteinases-3 (TIMP-3), a well known inhibitor of several metalloproteases. We found that in HeLa cells transiently transfected with IL-2Rβ, a 24-h treatment with TIMP-3 (1 μg/ml) substantially decreased the amount of 37βic produced, whereas 1 h of treatment had no effect (Fig. 4C). HeLa cells were chosen for this type of experiment due to the small amounts of media used for TIMP-3 incubation. Taken together, these results indicate that IL-2Rβ undergoes ectodomain shedding by a TIMP-3-sensitive and GM6001- and TAPI-insensitive metalloprotease that yields a C-terminal 37βic that contains the transmembrane domain.
37βic Is Tyrosine-phosphorylated and Associates with Signal Transducers of the IL-2R Pathway
Because 37βic contains the region of the receptor that is responsible for the association with Jak-1 and the recruitment of signaling molecules activated in response to IL-2, we investigated whether this fragment has biological functional significance in lymphoid cells using the Bafβ cell line and human T cells, testing whether 37βic could be phosphorylated. Human T cells were isolated, starved overnight, and then treated with 200 pm IL-2 for the indicated times. Cell lysates were prepared, and immunoprecipitation was performed using anti-IL-2Rβ Ab. Immunoprecipitates were separated by SDS-PAGE, transferred, and probed with 4G10 Ab or anti-IL-2Rβ Ab. We found that 37βic is produced in human T cells independently of IL-2 treatment and that IL-2 induces its tyrosine phosphorylation (Fig. 5A). Similar experiments were also performed with Baf-β cells. These cells were obtained by stably transfecting a pCDNA3-β construct in the BAF-BO3 parental cell line, a murine pre-B cell that endogenously expresses the IL-2Rα and γc chains. The transfected cells were then selected through their ability to grow in response to IL-2. Similar cells were generated and characterized in the past (31). Western blot with anti-IL-2Rβ showed that these cells also generated the 37βic independently of IL-2, but in contrast to T cells, we reproducibly observed that 37βic was constitutively phosphorylated, even in the absence of IL-2 stimulation (Fig. 5B). These experiments indicate that 37βic is subject to tyrosine phosphorylation in lymphoid cells and thus suggest that it may be functional.
FIGURE 5.
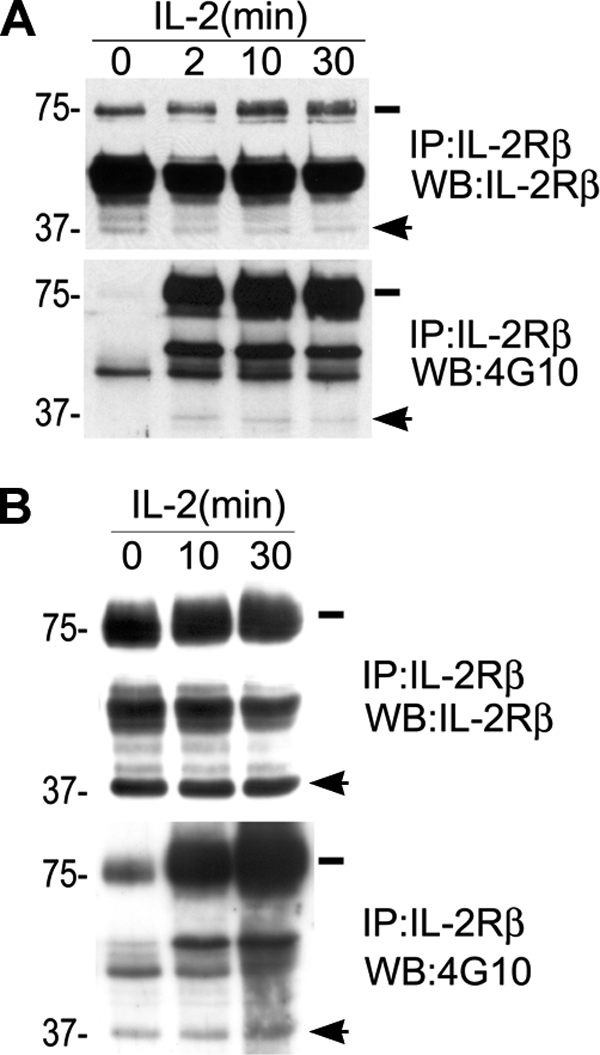
37βic generation and its tyrosine phosphorylation in T and Baf-β cells. A, T cells were isolated, and after overnight starvation they were treated with 200 pm IL-2. Immunoprecipitates were prepared with anti-IL-2Rβ Ab and resolved by gel electrophoresis. Tyrosine phosphorylation was analyzed by Western blot using 4G10 Ab (lower panel), and the same blot was reprobed using the anti-IL-2Rβ Ab (upper panel). B, Baf-β cells were treated with 2 nm IL-2 after overnight starvation. Immunoprecipitates were prepared with anti-IL-2Rβ Ab and resolved by gel electrophoresis. Tyrosine phosphorylation was analyzed by Western blot using 4G10 Ab (lower panel), and the same blot was reprobed using the anti-IL-2Rβ Ab (upper panel). In all panels, arrows and lines indicate the position of 37βic and of full-length IL-2Rβ, respectively. In each case one representative experiment is shown.
Considering these results we further investigated whether 37βic could participate in some IL-2 signaling process by associating with signal transduction molecules. For this purpose, we transfected HeLa cells with IL-2Rβ or the β-TM&IC construct and treated them with pervanadate (PVD). PVD has been shown to mimic IL-2R activation through inhibition of tyrosine phosphatase activity (29). Here again, we use HeLa cells instead of lymphoid cells, because we wanted to dissect the mechanisms affecting full IL-2Rβ from those exclusively affecting 37βic. First we confirmed tyrosine phosphorylation of 37βic by Western blot with 4G10 Ab in these cells (Fig. 6A). To test this further, we studied the β-TM&IC construct, which is nearly the size of 37βic. PVD treatment of β-TM&IC-transfected HeLa cells let us observe also its tyrosine phosphorylation (Fig. 6A). It is noteworthy that, for reasons that remain unclear, the total amount of β-TM&IC was significantly decreased by PVD treatment. These results confirmed that the IL-2Rβ C-terminal fragment originated after IL-2Rβ shedding may be subject to tyrosine phosphorylation. We then investigated whether 37βic could also associate with STAT5 by co-transfecting HeLa cells with full-length IL-2Rβ or the β-TM&IC construct and an expression vector containing murine STAT5A cDNA. STAT5A is a 95-kDa signal transducer that associates only with phosphorylated IL-2Rβ (30). 48 h after transfection cells were treated with PVD, and IL-2Rβ or β-TM&IC were immunoprecipitated using anti-IL-2Rβ Ab. As shown in Fig. 6B, we found by Western blot that β-TM&IC fragment co-immunoprecipitated STAT5A, as did the full-length IL-2Rβ. These results confirm that 37βic is subject to tyrosine phosphorylation, associates with STAT5A (30), and suggest that 37βic may itself be functional, although it lacks the extracellular domain after shedding.
FIGURE 6.
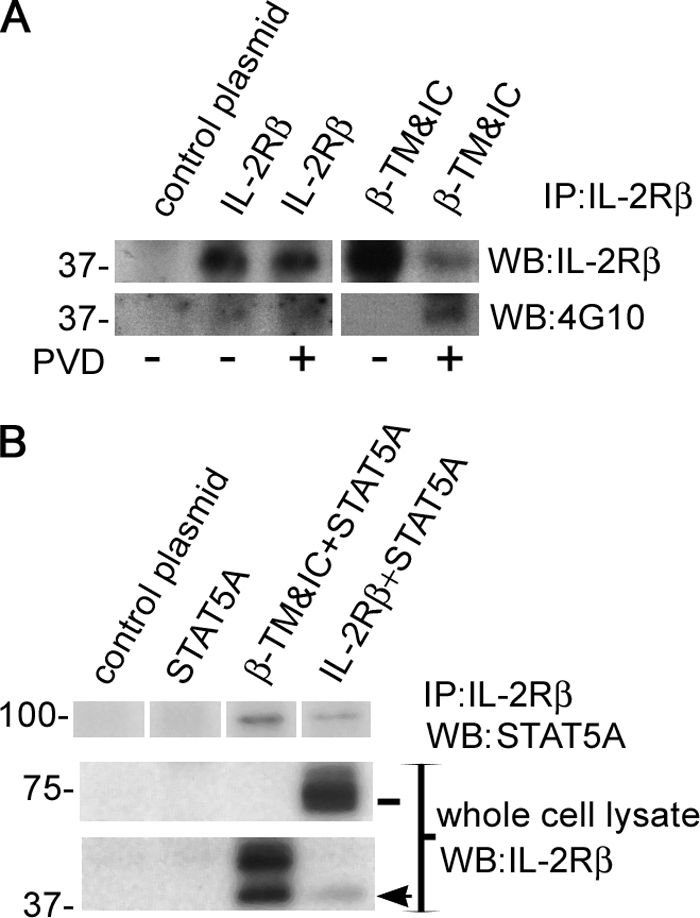
Tyrosine phosphorylation of 37βic and STAT5A recruitment in HeLa cells. A, HeLa cells were transfected with full-length IL-2Rβ, β-TM&IC, or control plasmid 24 h after being seeded. 48 h after transfection, cells were treated with 25 μm pervanadate (PVD) for 20 min as indicated and harvested. Immunoprecipitates were prepared with anti-IL-2Rβ Ab and resolved by gel electrophoresis. Tyrosine phosphorylation was analyzed by Western blot using 4G10 Ab, and the same blot was reprobed using the anti-IL-2Rβ Ab. B, HeLa cells were transfected with mouse STAT5A and full-length IL-2Rβ, β-TM&IC, or control plasmid alone. 48 h after cells were treated with PVD (25 μm) for 30 min and harvested. Immunoprecipitates were prepared with anti-IL-2Rβ Ab and resolved by gel electrophoresis. Co-immunoprecipitation of STAT5 was analyzed by Western blot. Construct expression was verified in whole cell lysates using anti-IL-2Rβ Ab (lower panels). In each case one representative experiment is shown.
Transfection of Baf-β, NKL, and BAF-B03 Cells with β-TM&IC Increases Cell Proliferation Rate
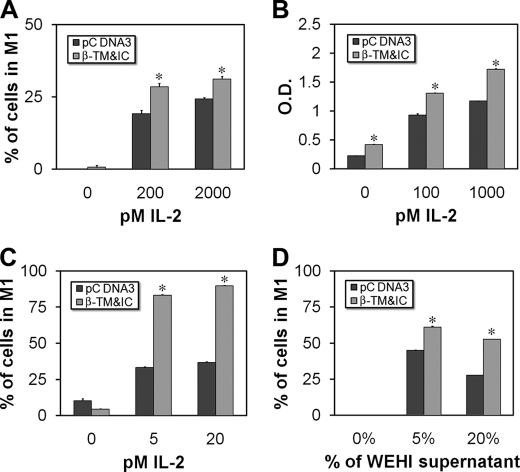
Considering our results suggesting the functionality of 37βic, we wanted to test whether it could play a biological role in lymphoid cells, where IL-2 signaling occurs. For this aim we transfected Baf-β cells with β-TM&IC, which mimics the shed IL-2Rβ, and incubated them 48 h with different picomolar concentrations of IL-2. We found that Baf-β cells transfected with β-TM&IC had increased proliferation rate as measured by CFSE and methylthiazolyldiphenyl-tetrazolium bromide (MTT) assays in comparison with empty pCDNA3-transfected cells (Fig. 7, A and B). Similarly, when NKL cells were transfected with β-TM&IC and treated 72 h with IL-2, proliferation rate was augmented as determined by CFSE assay (Fig. 7C). Surprisingly, when parental BAF-B03 cells were transfected with β-TM&IC, these cells also showed an increased proliferation rate in response to different amounts of WEHI supernatant as source of IL-3 (Fig. 7D). In all these experiments, empty vector-transfected cells also proliferated in response to increasing concentrations of IL-2 or WEHI supernatant as expected, because these are IL-2- or IL-3-dependent cell lines.
FIGURE 7.
Proliferation of Baf-β, NKL, and BAF-B03 transfected with β-TM&IC. A, Baf-β cells were transfected with β-TM&IC, labeled with CFSE, and treated with the indicated concentrations of IL-2. 48 h later cell proliferation was evaluated by cell fluorometry. B, Baf-β cells were transfected with β-TM&IC and treated with indicated concentrations of IL-2. 48 h later cell proliferation was evaluated by methylthiazolyldiphenyl-tetrazolium bromide assay. C, NKL cells were transfected with β-TM&IC, labeled with CFSE, and treated with the indicated concentrations of IL-2. 72 h later cell proliferation was evaluated by cell fluorometry. D, BAF-B03 cells were transfected with β-TM&IC, labeled with CFSE, and treated with the indicated amounts of WEHI supernatant as source of IL-3. 48 h later cell proliferation was evaluated by cell fluorometry. Each bar is mean of triplicates ± S.E. *, p < 0.01 versus pcDNA-transfected cells (Student's t-test). In each case one representative experiment is shown of at least three performed.
Altogether, these findings showed that the IL-2Rβ intracellular fragment generated after shedding is biologically functional, as suggested by its tyrosine phosphorylation and association with STAT5, playing a role in the regulation of cell proliferation.
DISCUSSION
Ectodomain shedding is believed to be important for down-regulation of receptor abundance in the cell membrane but is also emerging as a mechanism that cell surface molecules require for proper action in vivo (9, 10). IL-2 regulates proliferation, differentiation, function, and survival of different lymphoid cells. In this work we demonstrate that IL-2Rβ undergoes a proteolytic cleavage mechanism that is well conserved in cells of different origin, including human lymphoid cell lines, and more importantly, isolated human T cells. These results also indicate that IL-2Rβ cleavage is not prevented by presence of IL-2Rα and γc chains, because the amount of 37βic generated, relative to full IL-2Rβ, did not dramatically change in stably transfected cells expressing IL-2Rα and -γc subunits (Lαβγ) in comparison with stably transfected cells expressing only IL-2Rβ (Hep2β). This observation is also supported by Baf-β cells that endogenously express IL-2Rα and -γc (31). Moreover, to test further the possible γc interference, we also analyzed shedding of IL-2Rβ in HeLa cells co-expressing IL-2Rβ and increasing amounts of γc. In these experiments we observed a reduction in 37βic generation, but only at high γc/IL-2Rβ expression levels ratio (not shown), whereas a mild effect was found at physiological relevant ratios. These observations could be of importance during T cell activation, when expression of IL-2Rβ increases ≈10 times, whereas γc expression is maintained.
Mass spectrometry analysis as well as siRNA experiments showed that 37βic is not unspecific but a proteolysis product derived from IL-2Rβ chain. Furthermore, the possibility that this fragment is an unspecific product was completely ruled out by our experiments, because it is not detected in cells that do not express IL-2Rβ receptor and neither was immunoprecipitated by an irrelevant Ab in Bafβ cells. Moreover, we could observe 37βic in whole cell lysates (i.e. without immunoprecipitation) of highly expressing transfected cells. In these experiments the proportion of 37βic compared with IL-2Rβ did not dramatically change even after overnight immunoprecipitation (Figs. 1 or 6B versus Fig. 4C). This clearly argues against the possibility that this proteolytic cleavage occurs in the cell lysate during long immunoprecipitation experiments.
We further determined that treatment of cells with chloroquine increased the amount of 37βic. This result indicates that 37βic is generated before it reaches the lysosomal compartment where it is probably degraded, as has been shown for the full IL-2Rβ (8).
We tried to determine precisely the cleavage site on IL-2Rβ chain. Several attempts were made to perform N-terminal sequencing by an Edman degradation experiment on a radiolabeled 37βic. Unfortunately, our attempts remained unsuccessful, probably due to an N-terminal blockade of the polypeptide that rendered the Edman degradation unproductive. However, and to go further with this valuable question, we decided to express three truncated forms of IL-2Rβ to help determine the regions of IL-2Rβ that are contained in 37βic. The migration patterns of 37βic and the truncated forms suggested that the cleavage site is ∼10 to 20 amino acids upstream of the His-289 residue included in the β-TM&IC construct. Therefore, these results along with the mass spectrometry data indicate that 37βic contains all amino acids of the cytoplasmic domain of IL-2Rβ, as well as the transmembrane domain. Considering this, we assumed that 37βic remains associated with the cell membrane. This assumption was supported by immunofluorescence experiments using the β-TM&IC construct, in which we observed that it distributes in the cell membrane and intracellular vesicles resembling full-length IL-2Rβ (not shown). Taken together, these results support the idea that IL-2Rβ might be cleaved in the “stem region,” similarly to what has been shown for other cell membrane receptors (16–21). In fact, it has been shown that the cleavage site for metalloproteases in other receptors resides between 6 to10 amino acids upstream their transmembrane domain where, in most cases, there is a Pro residue (18, 20, 32, 33). Also, it is known that most MMPs cleave within motifs containing a Pro residue at P3 position (3 amino acids before the cleavage site) (34). Therefore, considering IL-2Rβ Pro-233 located 8 amino acids upstream of the transmembrane domain within the stem region (Table 1), the cleavage probably occurs between Ala-235 and Leu-236. Consistent with this notion, we found that the metalloprotease inhibitor TIMP-3 blocks IL-2Rβ cleavage and that deletion of 14 amino acids in the stem region of IL-2Rβ (β-ΔP226-D239) reduced the production of 37βic. Altogether, these results indicate that the proteolytic cleavage of IL-2Rβ that yields 37βic is an ectodomain-shedding mechanism mediated by a TIMP-3-sensitive metalloprotease that cleaves the receptor in its stem region. This conclusion is supported by previous findings made by Honda et al. (13) who identified a soluble IL-2Rβ in culture supernatants of lymphoid cell lines.
It would be attractive to explore the responsible metalloprotease involved in IL-2Rβ ectodomain shedding. The most appealing candidates are metalloproteases induced in T and B lymphocytes or NK cells after activation (35), when IL-2R signaling becomes biologically important for cell proliferation and function. An attractive candidate may be MMP-14, whose cleavage site motif may be KPAALK (34), very similar to the KPAALGK sequence, including Pro-233 in the stem region of IL-2Rβ. Nevertheless, it is possible that several other metalloproteases are capable of processing the IL-2Rβ, because it is well known that redundancy is the rule and not the exception in these enzymes (10). It is noteworthy that GM6001 and TAPI did not inhibit IL-2Rβ shedding. This observation may be due to the narrower inhibition spectra of these hydroxamate-based molecules in comparison to TIMP-3.
Ectodomain shedding of IL-2Rβ opens the possibility that the intracellular fragment may have an additional resultant function, as has been shown for other membrane receptors (17, 18). For instance, it could be substrate of the γ-secretase complex, because cleavage by metalloproteases precedes γ-secretase activity on certain cell membrane receptors (36). Indeed, it has been proposed that the γ-secretase component presenilin may cleave type I transmembrane proteins in the transmembrane domain, provided that they have a small extracellular domain (36, 37). However, we did not find evidence for a shorter fragment than the 37 kDa, which would result from a γ-secretase cleavage. This may be because the proteolysis product of γ-secretase activity may be rapidly degraded, as has been demonstrated for p75 neurotrophin receptor, Notch1, and others (38–40). Nevertheless, we observed an approximately ≈3 kDa higher band in IL-2Rβ-, β-TM&IC-, and β-IC-transfected cells (Fig. 4A). This band may be a posttranslationally modified product of 37βic, however, this remains to be clarified.
Finally, we asked whether the proteolysis product of IL-2Rβ may play a role in signal transduction, as has been proposed for other membrane receptors that undergo ectodomain shedding (10, 16, 17, 41–43). Our experimental data show that 37βic is tyrosine-phosphorylated. Strikingly, we observed that, in activated T cells, tyrosine phosphorylation of 37βic is dependent upon IL-2 treatment, whereas it is constitutively phosphorylated in Baf-β cells. This observation may be related to differential activity of intracellular signaling kinases in Baf-β cells in comparison with T cells. It is conceivable that, due to the pro-B origin of parental BAF-B03 cells, some kinases are basally activated, whereas in T cells they depend upon IL-2R activity. Also, it is possible that in both cells the kinases involved in tyrosine phosphorylation of 37βic are different. However, this observation indicates that some phosphorylation events may occur on the fragment once it has been shed. Otherwise, we would not observe a phosphorylated fragment when the full-length receptor is not, as shown in Fig. 5B. Furthermore, considering that β-TM&IC construct mimics 37βic, we show that it can be phosphorylated, corroborating the idea that it can be subject to phosphorylation on its own, further enabling the recruitment of STAT5. This result has high biological significance considering that 37βic is also generated in isolated human T cells. Moreover, the fact that 37βic is functional, even after its ectodomain shedding, is in agreement with the idea that the shedding mechanism is important, as has been suggested, not only to down-regulate intracellular signaling and receptor abundance in the cell membrane (9). In this regard, it will be of interest to further study regulation of intracellular signaling by 37bic, using point-mutated forms of the receptor.
More importantly, the increased proliferation in β-TM&IC-transfected lymphoid cells (Baf-β, NKL, and BAF-B03) also demonstrates the functionality of the shed receptor in the lymphoid cellular context, consistently with its phosphorylation and STAT5A association. Interestingly, our proliferation experiments indicate that 37βic does not signal by itself but instead it requires signaling activated by IL-2 (Baf-β and NKL cells) or even IL-3 (BAF-B03 cells) to potentiate the cellular response. In this regard, the fact that BAF-BO3 cells have an increased proliferation rate when β-TM&IC is expressed is not due to the presence of IL-2 in the WEHI medium, because these cells do not express IL-2Rβ, they are not able to proliferate in response to IL-2, and their requirement for IL-3 has been previously tested (31). Moreover, strictly IL-2-dependent T-cell lines do not proliferate in the presence of WEHI medium, thus excluding the possibility that some IL-2 present in the WEHI medium is responsible of this effect. Also, the possibility that increased proliferation of β-TM&IC-transfected cells was due to higher expression of IL-2Rβ was ruled out by cytofluorometry experiments (not shown). Together, these findings point to the existence of cross-talk mechanisms between 37βic and full IL-2Rβ or IL-3R, probably occurring in particular membrane domains, or alternatively between signal transduction pathways. More experiments are needed to dissect the mechanisms involved in this phenomenon.
In addition, the requirement by 37βic of activated signaling by IL-2 or IL-3 to potentiate cellular proliferation seems to be the regulatory step of its function. This is meaningful, because our experiments show that, in human T lymphoblasts or Baf-β cells, the amount of 37βic generated is independent of IL-2 (Fig. 5), in clear contrast with ectodomain shedding described with other receptors that depend upon ligand binding (16, 18).
The ectodomain-shedding process of IL-2Rβ may have implications for development and persistence of adult T-cell leukemia/lymphoma that is due to infection with human T-cell leukemia virus, type I. It is known that adult T-cell leukemia/lymphoma cells often require IL-2 for their proliferation and survival and that they produce a soluble IL-2Rβ (13, 44). Also, it has been reported that human T-cell leukemia virus, type I-infected cells augment the expression of IL-2Rβ and MMP-9 (45, 46).
In summary, our data show that IL-2Rβ undergoes ectodomain shedding, yielding a 37-kDa fragment that is biologically functional in signal transduction and cell function. Further analysis may provide new insight concerning the role of such events in the regulation of cytokine signaling mechanisms in normal or pathological conditions.
Acknowledgments
We are grateful to Prof. Paul Lazarow for his advice and comments on the manuscript. We thank M. Namane Abdelkader and Pascal Lenormand from the Proteomics Platform at Institut Pasteur for their invaluable technical support for MS analysis. We also thank Dr. Isabelle Dusanter from the Hematology Department at Institute Cochin who kindly gave us the STAT5 plasmid.
This work was supported in part by Action Concertée, Iniciative Biologie Cellulaire, Moléculaire et Structurale.
- IL-2
- interleukin-2
- IL-2R
- IL-2 receptor
- STAT
- signal transducers and activators of transcription
- MMP
- matrix metalloprotease
- ADAM
- disintegrins and metalloproteases
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- Ab
- antibody
- TIMP-3
- tissue inhibitor of metalloproteinases-3
- siRNA
- small interference RNA
- MS
- mass spectrometry
- MS/MS
- tandem MS
- PVD
- pervanadate
- TAPI
- TNF-α protease inhibitor.
REFERENCES
- 1.Nelson B. H., Willerford D. M. (1998) Adv. Immunol. 70, 1–81 [DOI] [PubMed] [Google Scholar]
- 2.Wang X., Rickert M., Garcia K. C. (2005) Science 310, 1159–1163 [DOI] [PubMed] [Google Scholar]
- 3.Gesbert F., Sauvonnet N., Dautry-Varsat A. (2004) Curr. Top. Microbiol. Immunol. 286, 119–148 [PubMed] [Google Scholar]
- 4.Ma A., Koka R., Burkett P. (2006) Annu. Rev. Immunol. 24, 657–679 [DOI] [PubMed] [Google Scholar]
- 5.Gaffen S. L. (2001) Cytokine 14, 63–77 [DOI] [PubMed] [Google Scholar]
- 6.Lamaze C., Dujeancourt A., Baba T., Lo C. G., Benmerah A., Dautry-Varsat A. (2001) Mol. Cell 7, 661–671 [DOI] [PubMed] [Google Scholar]
- 7.Sauvonnet N., Dujeancourt A., Dautry-Varsat A. (2005) J. Cell Biol. 168, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hémar A., Subtil A., Lieb M., Morelon E., Hellio R., Dautry-Varsat A. (1995) J. Cell Biol. 129, 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arribas J., Borroto A. (2002) Chem. Rev. 102, 4627–4638 [DOI] [PubMed] [Google Scholar]
- 10.Kheradmand F., Werb Z. (2002) BioEssays 24, 8–12 [DOI] [PubMed] [Google Scholar]
- 11.Tsakadze N. L., Sithu S. D., Sen U., English W. R., Murphy G., D'Souza S. E. (2006) J. Biol. Chem. 281, 3157–3164 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Botran R., Chilton P. M., Ma Y. (1996) Adv. Immunol. 63, 269–336 [DOI] [PubMed] [Google Scholar]
- 13.Honda M., Kitamura K., Takeshita T., Sugamura K., Tokunaga T. (1990) J. Immunol. 145, 4131–4135 [PubMed] [Google Scholar]
- 14.Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. (1985) J. Immunol. 135, 3172–3177 [PubMed] [Google Scholar]
- 15.Meissner U., Blum H., Schnare M., Röllinghoff M., Gessner A. (2001) Blood 97, 183–191 [DOI] [PubMed] [Google Scholar]
- 16.Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J. R., Cumano A., Roux P., Black R. A., Israël A. (2000) Mol. Cell 5, 207–216 [DOI] [PubMed] [Google Scholar]
- 17.Lin S. Y., Makino K., Xia W., Matin A., Wen Y., Kwong K. Y., Bourguignon L., Hung M. C. (2001) Nat. Cell Biol. 3, 802–808 [DOI] [PubMed] [Google Scholar]
- 18.Six E., Ndiaye D., Laabi Y., Brou C., Gupta-Rossi N., Israel A., Logeat F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7638–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urra S., Escudero C. A., Ramos P., Lisbona F., Allende E., Covarrubias P., Parraguez J. I., Zampieri N., Chao M. V., Annaert W., Bronfman F. C. (2007) J. Biol. Chem. 282, 7606–7615 [DOI] [PubMed] [Google Scholar]
- 20.Wang X., He K., Gerhart M., Huang Y., Jiang J., Paxton R. J., Yang S., Lu C., Menon R. K., Black R. A., Baumann G., Frank S. J. (2002) J. Biol. Chem. 277, 50510–50519 [DOI] [PubMed] [Google Scholar]
- 21.Wilhelmsen K., van der Geer P. (2004) Mol. Cell Biol. 24, 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta-Rossi N., Six E., LeBail O., Logeat F., Chastagner P., Olry A., Israël A., Brou C. (2004) J. Cell Biol. 166, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson M. J., Cochran K. J., Cameron C., Le J. M., Tantravahi R., Ritz J. (1996) Exp. Hematol. 24, 406–415 [PubMed] [Google Scholar]
- 24.Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. (1987) Blood 70, 1069–1072 [PubMed] [Google Scholar]
- 25.Gesbert F., Guenzi C., Bertoglio J. (1998) J. Biol. Chem. 273, 18273–18281 [DOI] [PubMed] [Google Scholar]
- 26.Maasho K., Marusina A., Reynolds N. M., Coligan J. E., Borrego F. (2004) J. Immunol. Methods 284, 133–140 [DOI] [PubMed] [Google Scholar]
- 27.Helmut Blum H. B. H. J. G. (1987) Electrophoresis 8, 93–99 [Google Scholar]
- 28.Michihara A., Toda K., Kubo T., Fujiwara Y., Akasaki K., Tsuji H. (2005) Biol. Pharm. Bull. 28, 947–951 [DOI] [PubMed] [Google Scholar]
- 29.Evans G. A., Garcia G. G., Erwin R., Howard O. M., Farrar W. L. (1994) J. Biol. Chem. 269, 23407–23412 [PubMed] [Google Scholar]
- 30.Delespine-Carmagnat M., Bouvier G., Bertoglio J. (2000) Eur. J. Immunol. 30, 59–68 [DOI] [PubMed] [Google Scholar]
- 31.Kawahara A., Minami Y., Taniguchi T. (1994) Mol. Cell Biol. 14, 5433–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman C., Chernajovsky Y. (1998) J. Immunol. 160, 2478–2487 [PubMed] [Google Scholar]
- 33.Zampieri N., Xu C. F., Neubert T. A., Chao M. V. (2005) J. Biol. Chem. 280, 14563–14571 [DOI] [PubMed] [Google Scholar]
- 34.Turk B. E., Huang L. L., Piro E. T., Cantley L. C. (2001) Nat. Biotechnol. 19, 661–667 [DOI] [PubMed] [Google Scholar]
- 35.Bar-Or A., Nuttall R. K., Duddy M., Alter A., Kim H. J., Ifergan I., Pennington C. J., Bourgoin P., Edwards D. R., Yong V. W. (2003) Brain 126, 2738–2749 [DOI] [PubMed] [Google Scholar]
- 36.Struhl G., Adachi A. (2000) Mol. Cell 6, 625–636 [DOI] [PubMed] [Google Scholar]
- 37.Parks A. L., Curtis D. (2007) Trends Genet. 23, 140–150 [DOI] [PubMed] [Google Scholar]
- 38.Galichet A., Weibel M., Heizmann C. W. (2008) Biochem. Biophys. Res. Commun. 370, 1–5 [DOI] [PubMed] [Google Scholar]
- 39.Gupta-Rossi N., Le Bail O., Gonen H., Brou C., Logeat F., Six E., Ciechanover A., Israël A. (2001) J. Biol. Chem. 276, 34371–34378 [DOI] [PubMed] [Google Scholar]
- 40.Kanning K. C., Hudson M., Amieux P. S., Wiley J. C., Bothwell M., Schecterson L. C. (2003) J. Neurosci. 23, 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Määttä J. A., Sundvall M., Junttila T. T., Peri L., Laine V. J., Isola J., Egeblad M., Elenius K. (2006) Mol. Biol. Cell 17, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polavarapu R., An J., Zhang C., Yepes M. (2008) Am. J. Pathol. 172, 1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Díaz-Rodríguez E., Cabrera N., Esparís-Ogando A., Montero J. C., Pandiella A. (1999) Eur. J. Neurosci. 11, 1421–1430 [DOI] [PubMed] [Google Scholar]
- 44.Fujimura S., Arakawa F., Yamada Y., Liao S., Khare P. D., Kuroki M., Ono J. (2004) Int. J. Oncol. 25, 437–443 [PubMed] [Google Scholar]
- 45.Mori N., Sato H., Hayashibara T., Senba M., Hayashi T., Yamada Y., Kamihira S., Ikeda S., Yamasaki Y., Morikawa S., Tomonaga M., Geleziunas R., Yamamoto N. (2002) Blood 99, 1341–1349 [DOI] [PubMed] [Google Scholar]
- 46.Pise-Masison C. A., Radonovich M., Mahieux R., Chatterjee P., Whiteford C., Duvall J., Guillerm C., Gessain A., Brady J. N. (2002) Cancer Res. 62, 3562–3571 [PubMed] [Google Scholar]