Abstract
Acid-sensing ion channels (ASICs) are proton-activated channels expressed in neurons of the central and peripheral nervous systems where they modulate neuronal activity in response to external increases in proton concentration. The size of ASIC1 currents evoked by a given local acidification is determined by the number of channels in the plasma membrane and by the apparent proton affinities for activation and steady-state desensitization of the channel. Thus, the magnitude of the pH drop and the value of the baseline pH both are functionally important. Recent characterization of ASIC1s from an increasing number of species has made evident that proton affinities of these channels vary across vertebrates. We found that in species with high baseline plasma pH, e.g. frog, shark, and fish, ASIC1 has high proton affinity compared with the mammalian channel. The β1-β2 linker in the extracellular domain, specifically by the substitution M85L, determines the interspecies differences in proton affinities and also the time course of ASIC1 macroscopic currents. The mechanism underlying these observations is a delay in channel opening after application of protons, most likely by stabilizing a closed conformation that decreases the apparent affinity to protons and also slows the rise and decay phases of the current. Together, the results suggest evolutionary adaptation of ASIC1 to match the value of the species-specific plasma pH. At the molecular level, adaptation is achieved by substitutions of nonionizable residues rather than by modification of the channel proton sensor.
Keywords: Acid-sensing Ion Channels (ASIC), Ion Channels, Methionine, Oocyte, Xenopus, Channel Gating, Proton Affinity
Introduction
Acid-sensing ion channels (ASICs)2 are proton-activated sodium channels (1) expressed in the nervous system of species from the vertebrate lineage. ASIC1 modulates synaptic transmission (2) and other functions by depolarizing the plasma membrane and subsequently allowing influx of calcium into neurons (3).
Three identical or homologous subunits associate to form functional channels (4). Each subunit has a large extracellular domain that contains the proton sensor. The identity and location of the proton sensor remain elusive, but it is generally accepted that ionizable residues, most likely negatively charged, constitute the proton binding sites that control opening of the channel.
Two properties define the magnitude of the ASIC1 response to changes in the concentration of external protons. These are the proton affinity for half-maximal activation or pH50A, and the proton affinity for half-maximal steady-state desensitization or pH50D. The value of the former determines the size of the peak current evoked by a rapid increase in the concentration of external protons, whereas the latter determines the fraction of channels available for activation at any given resting pH. In mammals, the pH50A of ASIC1 is in the range of 6.4 to 6.6, and the pH50D is ∼7.2 (5). The steady-state desensitization of the mammalian ASIC1 exhibits a high degree of cooperativity to protons with a Hill coefficient ≥6; thus, a small decrease in external pH such as 0.1 units can lead to inactivation of as much as 80% of channels. These observations underscore the importance of baseline pH in determining the magnitude of the ASIC1 response to local acidification. However, physiological values of baseline external pH are not the same in all vertebrates but spread over a wide range from 7.38 to 8.0, raising the question of whether species with high plasma pH control ASIC1 responses in the same way as mammals. Although, in general, species with high plasma pH also have lower temperature, lower partial pressure of CO2, and lower sodium concentration than mammals (6), all of which change the degree of ionization of water and of charged groups in proteins, it remains to be shown whether the compound effect of those factors is sufficient to render the ASIC1 response to equivalent levels across species.
In this work, we examined the proton response of ASIC1 from two species with high arterial pH: frog and elephant shark. We found indeed that ASIC1s from these species have higher proton affinities than the mammalian channel and that residues underlying the differences in proton affinities are in the β1-β2 linker of the extracellular domain rather than in the proton sensor. The mechanism consists in stabilization of a closed conformation by the β1-β2 linker specifically; the substitution M85L decreases the apparent proton affinity and slows the rise and decay phases of the currents by delaying channel openings.
EXPERIMENTAL PROCEDURES
Cloning of ASIC cDNAs
Cloning of ASICs were conducted by a combination of RT-PCR and 5′ and 3′ extension as described previously (7). Total RNA was extracted from frog brain with TRIzol reagent and used for synthesis of single strand cDNA with SuperReverse transcriptase III (Invitrogen) and oligo(dT) priming. The full-length cDNAs were ligated into pCDNA3.1 vector (Invitrogen), which added a V5 epitope tag at the C terminus. High fidelity Taq polymerase (Invitrogen) was used in all experiments. DNA of eight independent clones from two different RT-PCR reactions were sequenced in both directions by the Keck facility at Yale University. Elephant shark ASIC was cloned by RT-PCR from a brain cDNA library provided by Dr. Byrappa Venkatesh (Institute of Molecular and Cell Biology, Biopolis). We used specific N and C termini DNA primers designed to match sequences from the DNA genomic data bank of elephant shark (8). The use of frogs was in accordance to guidelines approved by Institutional Animal Care and Use Committee of Yale University.
Mutagenesis
Point mutations were introduced into cDNAs using QuikChange (Agilent). All products were sequenced to confirm the presence of mutations.
Biotinylation of Proteins and Western Blotting
Fifteen oocytes per conditions were biotinylated with sulfo-NHS-SS-biotin (Pierce) on ice. After lysis of cells with 1% Triton X-100, the biotinylated proteins were recovered with streptavidin beads (Pierce). The beads were washed with 1% Triton X-100 and 300 mm NaCl. Biotinylated proteins were eluted from beads, resolved in 10% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Immobilon). ASIC was detected with anti-V5 monoclonal antibody (Invitrogen). Signals were developed with ECL+ (Amersham Biosciences) and exposed to BioMax MR film (Eastman Kodak).
Two-electrode Voltage Clamp
Xenopus laevis oocytes were injected with 5 ng cRNA in a volume of 50 nl and were incubated at 16 °C for 2–3 days. For two-electrode voltage clamp experiments, oocytes were placed in a recording chamber (400 μl) perfused by gravity at a rate of 4 ml/min. Oocytes were impaled with two glass microelectrodes filled with 3 m KCl having resistance <1 megohm. Membrane potential was held at −60 mV, and whole-cell currents were recorded with a clamp OC-725B (Warner Instrument Corp., Hamden, CT) and digitized at a sampling rate of 2 kHz (PowerLab 4/30, ADInstruments). Composition of the standard bath solution was as follows: 120 mm NaCl, 2 mm KCl, 1.5 mm CaCl2, and 15 mm HEPES-MES. The pH was adjusted to obtain a series of solutions differing by 0.1 pH units from pH 8.0 to 5.0. The same set of solutions was used for all experiments to minimize variations owing to calibration of pH. All experiments were conducted at room temperature.
Apparent proton affinities were calculated with Equation 1 as shown below.
Time constants for the rise and decay phases were obtained by fitting the time course of currents to the function (Equation 2),
where Io is a scaling factor.
Patch Clamp Recordings in the Outside-out Configuration
Patch pipettes were pulled from PG150T glass (Warner Instruments) to a tip diameter of 2–5 μm after heat polishing. Pipette solution was as follows: 120 mm KCl, 5 mm EDTA, and 10 mm HEPES titrated with KOH to pH 7.4. Bath and activating solutions were as follows: 120 mm NaCl, 2 mm KCl, 1.5 mm CaCl2, and 15 mm HEPES-MES titrated from pH 8.0 to 5.0. Activating solutions were applied using a modified Perfusion Fast Step device SF-77B (Warner Instruments) onto which eight perfusion pipettes were mounted. The data acquisition program Pulse in turn controlled the SF-77B. Time resolution of the solution exchange device is 0.5 ms as described previously (9). Recordings were made using a EPC-9 amplifier and the Pulse acquisition program (HEKA Electronic). Membrane potential was held at −60 mV. Experiments were conducted at room temperature.
RESULTS
Cloning of X. laevis ASIC1
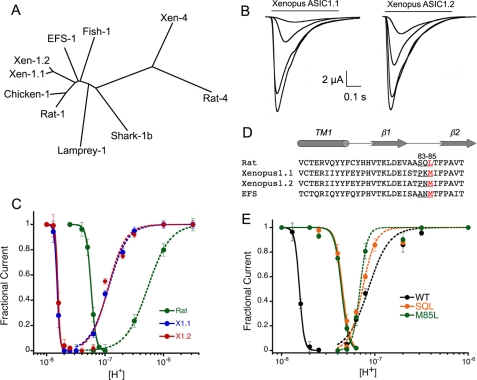
We cloned two cDNAs from brain X. laevis that are 94% identical and encode proteins that differ only in 17 out of 525 amino acids, including one deletion. The relation of the frog proteins to other ASICs is shown in the tree of Fig. 1A, and the amino acid sequences are presented in supplemental Fig. 1A. We refer to the frog proteins as xASIC1.1 and xASIC1.2. They are products of different genes and not the result of differential splicing because the amino acid differences are scattered in the whole cDNA rather than being restricted to one exon. The presence of two copies of the same gene and expression of the corresponding proteins in brain of X. laevis is the consequence of a previous tetraploidization event dated ∼30 million years ago (10).
FIGURE 1.
Properties of Xenopus xASIC1.1 and xASIC1.2 and effect of mutations in the β1-β2 linker in proton affinity. A, cladogram generated by Clustal alignment of ASIC proteins. Xen, Xenopus. B, whole-cell currents of xASIC1.1 and xASIC1.2 evoked by sequential application of solutions of pH 7.3, 7.1, 6.8, and 6.5. Between stimuli, the external solution was returned to pH 8.0 for 30 s. C, dose response curves to protons of xASIC1.1 (X.1), xASIC1.2 (X.2), and rat ASIC1a (Rat). D, amino acid sequence alignment of transmembrane domain 1 and the β1-β2 linker of rat, Xenopus, and EFS ASIC1. E, dose response curves to protons of xASIC1.1 (WT) with a pH50D of 7.8, n = 10, pH50A of 7.0 (n = 3.2), and the mutant xASIC1-P83S-K84Q-M85L (SQL) with a pH50D of 7.35 (n = 9.4) and a pH50A of 7.1 (n = 3.7). Symbols represent the mean of 8–10 independent cells ± S.D. Lines are the fit of the data to Equation 1. Error bars are ± standard deviation (S.D.).
The protein cloned from elephant shark, Callorhinchus milii, (8) is 79% identical to frog and 77% identical to rat ASIC1a and corresponds also to ASIC1 as indicated in Fig. 1A. The amino acid sequence is shown in supplemental Fig. 1A.
Xenopus ASIC1 Has High Proton Affinity for Steady-state Desensitization
Expression of xASIC1.1 and xASIC1.2 in oocytes produced inward currents evoked by pH 6.0 of mean peak amplitude 20 ± 7 μA/cell (n = 44). The magnitude and time course of currents from the two channels were indistinguishable. Fig. 1B shows representative examples of whole-cell currents of xASIC1.1 and xASIC1.2 evoked by increasing concentrations of protons from pH 7.2 to 6.0. The apparent proton affinity for activation (pH50A) was measured by preconditioning cells with pH 8.0 followed by stimuli of pH 7.2 to 5.5. The apparent proton affinity for activation was calculated with the peak currents giving values for pH50A of 7.0 and a Hill coefficient of 3. (Fig. 1C). The apparent affinity of protons for steady-state desensitization pH50D, i.e. desensitization from the closed state, was measured by lowering the preconditioning pH by 0.1 units starting from pH 8.0 to 6.8. The calculated pH50D had values of 7.8 for both xASIC1.1 and xASIC1.2. To contrast the differences with rat ASIC1a, the graph in Fig. 1C also shows the corresponding proton affinities of rat ASIC1a measured under identical conditions: pH50D 7.2 and pH50A 6.3. Thus, frog ASIC1 has higher affinity for protons than the mammalian ASIC1 channel.
Amino Acid Composition of the β1-β2 Linker Changes ASIC1 Apparent Affinity to Protons
The determinants of proton sensitivity of ASIC1 currently are not known, but it usually is assumed that differences in charged residues in the extracellular domain underlie the variations in proton affinities. Comparison of negatively and positively charged amino acids of frog and rat ASIC1 proteins showed a high degree of conservation, suggesting that ionizable residues do not account for the differences in proton sensitivity. We examined instead the β1-β2 linker that is a short segment in the extracellular domain that connects the β1 with the β2 strands. We have shown previously that the β1-β2 linker plays an essential role in conferring proton sensitivity to lamprey ASIC1 (11) and also modifies the rate of decay of fish ASIC1 currents (12). The amino acid composition of the β1-β2 linker of xASIC1.1 is Pro83-Lys84-Met85, whereas that of rat ASIC1a is Ser83-Gln84-Leu85 (Fig. 1D). These three residues were substituted in frog to generate the triple mutant xASIC1.1-P83S-K84Q-M85L (SQL). The mutations decreased proton affinity for steady-state desensitization; the apparent pH50D was shifted from 7.8 to 7.35, whereas the pH50A changed from 7.0 to 7.1 (Fig. 1E). These results were duplicated by the single substitution M85L, indicating that position 85 is the main contributor of the three residues in the β1-β2 linker.
Residues in the β1-β2 Linker Slow the Time Course of xASIC1 Currents
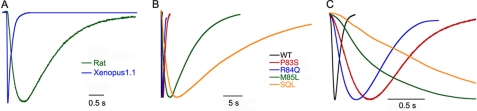
Macroscopic currents of xASIC1.1 rapidly increase and decay upon proton stimulation. Fig. 2A contrasts the time course of normalized frog and rat ASIC1a (rASIC1a) currents evoked by pH 6.8 recorded under identical conditions. Frog and rat channels completely desensitize in the continuous presence of external protons, but both the rise and the decay phases are faster in xASIC1.1 than in rASIC1.
FIGURE 2.
Time course of the current of Xenopus ASIC1.1 wild type and mutants. A, normalized whole-cell currents of wild type Xenopus ASIC1.1 (blue) and rat ASIC1a (green). Currents were evoked by pH 6.8 in the presence of 120 mm Na+ in the bath and a holding potential −60 mV. B, normalized currents of xASIC1.1 wild type (WT) and mutant channels bearing single or three mutations in the β1-β2 linker evoked by a change in pH from 8.0 to 7.1. C, same current traces as in B but the time scale is expanded 10-fold. Bars below traces indicate the time scale.
Analysis of xASIC1.1 mutants showed that the substitutions in the β1-β2 linker also slow channel kinetics. Traces in Fig. 2B are normalized currents evoked by pH 7.1 in xASIC1.1 wild type and channels bearing single substitutions: P83S, K84Q, M85L, or three substitutions together: P83S-K84Q-M85L. To discern the effect of individual mutations on the rise phase of the currents, the same traces are presented in an expanded time scale (Fig. 2C). Of the three single substitutions, M85L has the largest effect, but together, they produce a further small additive effect. To examine whether other residues have a similar effect in position 85, we tested the amino acids indicated in Table 1. Currents of single mutants were examined at pH 7.1. The time constants for the rise (τa) and the decay phases (τd) were derived from the fit of whole-cell currents to Equation 2. Only leucine slowed the currents; all other residues either did not change significantly the time constants or decreased the values, threonine and phenylalanine. Currents produced by M85R were too small to be examined.
TABLE 1.
Time constants of xASIC1.1 mutants
Whole-cell currents evoked by pH 7.1 in oocytes expressing xASIC1.1 wild type, single mutant, and triple mutant. The values of the activation τa and desensitization τd time constants were obtained by fitting the time course of the macroscopic currents to Equation 2 (see “Experimental Procedures”). ND, not determined.
| Current (mean ± S.D.) | No. of cells | τa (mean ± S.D.) | τd (mean ± S.D.) | |
|---|---|---|---|---|
| μA/cell | n | ms | ms | |
| Wild type Xenopus | 3 ± 0.7 | 24 | 40 ± 8 | 180 ± 30 |
| S83Q84L85 | 10 ± 4 | 26 | 230 ± 28 | 3100 ± 350 |
| P83S | 4 ± 0.8 | 9 | 52 ± 3 | 260 ± 40 |
| K84Q | 1.8 ± 0.4 | 8 | 51 ± 4 | 270 ± 30 |
| M85L | 9 ± 0.11 | 25 | 220 ± 20 | 2920 ± 180 |
| M85T | 0.8 ± 0.4 | 11 | 12 ± 2 | 70 ± 13 |
| M85F | 1.1 ± 0.2 | 5 | 11 ± 3 | 65 ± 20 |
| M85C | 2.8 + 0.4 | 6 | 42 ± 82 | 222 ± 40 |
| M85A | 1.5 ± 0.3 | 5 | 40 ± 3 | 190 ± 30 |
| M85R | 0.2 ± 0.1 | 12 | ND | ND |
Delay of the First Opening Underlies the Slow Time Course xASIC1-SQL Channels
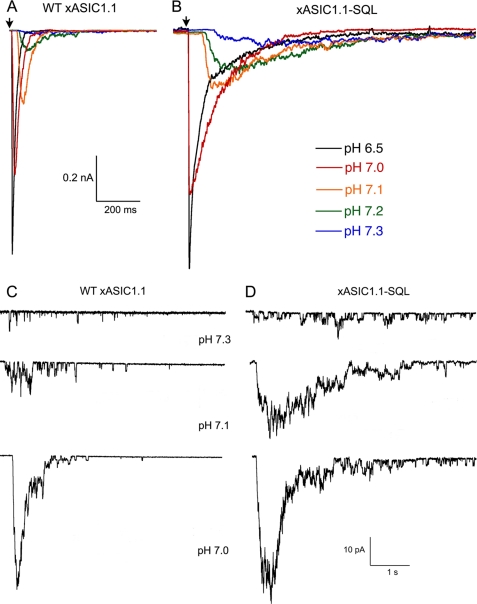
To investigate the mechanism underlying the functional changes produced by substitutions in the β1-β2 linker, we examined xASIC1.1 wild type and the mutant SQL in outside-out patches exposed sequentially to a series of activating solutions with increasing concentrations of protons (pH 7.4, 7.3, 7.2, 7.1, 7.0, 6.8, and 6.0). From wild type xASIC1.1, we recorded 10 to 20 sweeps of 4-s exposure to the activating pH and 8-s recovery at pH 8.0. For mutant channels, owing to the slow time course of the currents, the duration of the sweeps was lengthened to a 20-s exposure to activating pH, followed by a 10-s recovery. Representative examples of xASIC1.1 wild type (Fig. 3A) and xASIC1.1-SQL (Fig. 3B) currents evoked by increasing concentration of protons show the slow time course of the mutant currents compared with wild type particularly in the pH range from 7.3 to 7.1. Time constants of activation τa and desensitization τd calculated at several proton concentrations are presented in Table 2. The constants exhibit proton dependence in the pH range from 7.3 to 6.0. At pH ≤ 6.0, the τa reached a maximal value without further decrease, suggesting that at very high concentrations of protons, when diffusion rate is not a factor, the activation rate saturates in both channels.
FIGURE 3.
Patch clamp recordings of xASIC1 and mutant xASIC1-SQL. A, currents from wild type xASIC1.1 evoked by the indicated concentrations of protons recorded from a patch in the outside configuration. Protocol was as follows: 4 s of pH 8.0 followed by 8 s of activating pH. The arrow marks the initiation of stimulus. The patch contains ∼400 channels. B, currents from xASIC1.1-SQL. Protocol was as follows: 8 s of pH 8.0 and 12 s activating pH. C, currents from patches containing xASIC1 channels activated by low concentrations of protons to observe single channel events. At pH 7.3, individual openings are evident. D, currents from patches containing xASIC1-SQL. WT, wild type.
TABLE 2.
Proton dependence of time constants of wild type xASIC1.1, SQL mutant, and rat ASIC1a
Activation τa and desensitization τd time constants of xASIC1.1 wild type and the triple mutant xASIC1.1-SQL derived from outside-out patches activated by various concentrations of external protons. For comparison, rat ASIC1 rates are shown on the right columns. Owing to lower apparent proton affinity of rat ASIC1 compared to frog, currents evoked by pH 7.2 and 7.1 were too small to calculate the rates. Each value represent the mean of 10 to 20 activations recorded from two to four independent patches.
| pH | xASIC1.1 |
xASIC1.1-SQL |
rASIC1a |
|||
|---|---|---|---|---|---|---|
| τa | τd | τa | τd | τa | τd | |
| ms | Ms | ms | ||||
| 7.2 | 52 | 250 | 215 | 10,200 | ||
| 7.1 | 31 | 130 | 178 | 2500 | ||
| 6.8 | 12 | 65 | 60 | 800 | 180 | 2480 |
| 6.0 | 2 | 25 | 2 | 100 | 10 | 1700 |
| 5.5 | 1.5 | 18 | 1.5 | 50 | 6 | 670 |
Analysis of patches indicates that the fraction of channels opening late after application of the stimulus and the length of the delay decreased as the concentration of protons increased (Fig. 3, C and D). At very high proton concentrations, pH ≤6.5, almost all channels open immediately after the stimulus producing a sharp increase in the inward current and reducing the duration of the sweep, black traces in Fig. 3, A and B. The main difference observed in the mutant ASIC1.1-SQL is a larger delay in first openings (Fig. 3D) that markedly broadens the peak current and slows the time course of the current produced by the ensemble of channels. Consequently, it increases the values of both τa and τd constants.
The Substitution M85L Stabilizes a Closed Protonated Conformation
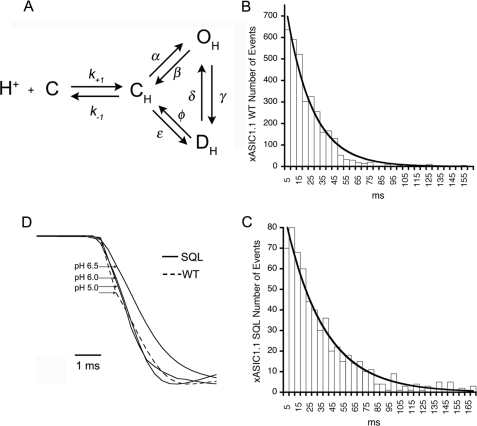
To understand the effect of the β1-β2 linker in the reaction cycle of ASIC1, we considered the simplest scheme with two shut states: closed protonated (CH) and desensitized (DH) and one open state (OH) (Fig. 4A). Because desensitization occurs without channel opening, the reaction is written in a cyclical form. The arrows represent the rate constants that lead to or leave the state. Frog and rat ASIC1 completely desensitize in the continuous presence of protons; thus, the rate constant δ is assumed to be negligible as channels move irreversibly from the open to an absorbing desensitized state (9).
FIGURE 4.
The β1-β2 linker stabilizes a closed conformation of ASIC1. A, scheme of the simplest mechanism of ASIC1, where C is the closed nonprotonated state, CH represents the ensemble of different conformations the channel undergoes as it binds protons prior to opening. At least three protons are needed to open the channel, and nine protons are needed for steady-state desensitization according to the Hill coefficients of obtained in Fig. 1C. OH is the open protonated state, and DH is the desensitized state. The rate constants are denoted by the symbols on the arrows, and k−1/k+1 is the equilibrium constant for the initial binding step of protons. B and C, histograms of open times of wild type and mutant SQL xASIC1.1. The lines are fits of the data to single exponentials. D, rising phase of averaged current responses from patches with wild type (WT) or SQL xASIC channels. Currents are scaled to the same peak current value. The region of the onset curve >20% of maximum is well described by a single exponential function. This part of the curve is determined primarily by the channel opening rate rather than agonist binding so that the time constant of the exponential function approximates the reciprocal of the opening rate. Onset times were as follows: 2 ms at pH 6.5, 1.5 ms at pH 6.0, and 1.4 ms at pH 5.0.
Mean open times τo of wild type and mutant channels were calculated by fitting histograms of the duration of OH to single exponentials (Fig. 4, B and C). Values of τo were not significantly different, 6.8 versus 4.5 ms for wild type and mutant; thus, we conclude that stability of the open state and therefore the rate constants that leave OH (β and γ) are not significantly modified by mutations in the β1-β2 linker.
We next examined whether the mutation M85L decreases the value of the transition rate α. The opening rate of the ensemble of channels in the patch (1/τa) is a function of both α and of the fraction of channels in the CH state, which, in turn, increase with the concentration of protons. Table 2 shows steep proton dependence of τa in the range of pH ≥6.0 and larger values of τa for the mutant than for wild type. However, at high concentration of protons, τa reached the similar minimum value in both channels ∼2 ms. Fig. 4D shows the rising phase of normalized currents (average of 10 sweeps) of patches containing mutant channels (continuous lines) activated by pH 6.5, 6.0, and 5.0 and wild type (dashed line) activated by pH 5.0. Activation of mutant channels by pH 6.0 significantly decreases the onset time compared with pH 6.5, whereas pH 5.0 does not produce a further decrease. Although pH 6.0 nearly saturates the channel binding sites (same peak current), binding of protons is still the rate-limiting process. In contrast, there is only a small decrease in onset time as the concentration is raised to pH 5.0, suggesting that at this high proton concentration, the onset approximates the value of α in the scheme of Fig. 4A. Because the onset time at pH 5.0 does not differ in wild type and mutant SQL, the mutation does not significantly change the opening rate α. This conclusion holds even if the values obtained for the opening time are underestimated by our perfusion system because such a small value cannot explain the more than one order of magnitude difference in time course of wild type and mutant currents.
Together, these observations suggest that the β1-β2 linker most likely modifies a step before channel opening. This is represented in the scheme by the transition C to CH, which describes the sum of several events including the binding of protons and subsequent conformational changes of closed protonated channels. Thus, the substitution M85L in the β1-β2 linker by decreasing the number of channels in the CH state delays channel openings and slows the time course of the macroscopic currents. The same mechanism decreases the number of channels entering the desensitized state, leading to a decrease in the apparent affinity for steady-state desensitization (transition CH to DH in the scheme of Fig. 4A).
The Effect of M85L Applies to ASIC1 of Other Species
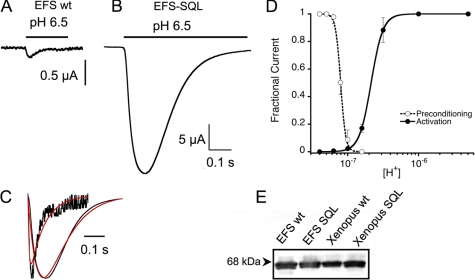
To ascertain the role of residue 85 in modulating the function of ASIC1 and whether it works also in other species, we examined channels with Met85 in the β1-β2 linker. The previously cloned fish ASIC1 and shark ASIC1b both have Met85 and have been shown to have fast kinetics and high sensitivity to protons (12, 13). ASIC1 cloned here from EFS also has Met85 and produces fast and small proton-activated currents with an average magnitude of 0.3 ± 0.15 μA/cell. Further analysis of the properties of wild type EFS was not possible owing to the small currents. However, after substitution of the three residues in the β1-β2 linker Ala83-Asn84-Met85 to Ser83-Gln84-Leu85 or M85L alone the currents became ∼100-fold larger, with an average 35 ± 7 μA/cell at pH 6.5 (n = 20) (Fig. 5, A and B). Representative examples of wild type and mutant EFS ASIC1 shown in the same time scale but a 10-fold expanded current scale make evident the fast time course of currents from the wild type channel (Fig. 5C). Calculated time constants of the rise and decay phases of the currents were 3 and 17 ms, whereas time constants for the triple mutant were 26 and 140 ms, respectively. The fast kinetics, above the limits of the two-electrode voltage clamp perfusion system, result in small values for the peak currents of wild type EFS channels. Despite this limitation, the important result is the mark slowing of channel kinetics induced by the mutations. Additional evidence that the small current of wild type EFS results from fast kinetics and not from low expression level is provided by the equal amount of protein found at the cell surface of cells expressing wild type or mutant EFS-SQL channels, whereas the difference in current in the same cells was 100-fold (Fig. 5E). The apparent affinities for protons of EFS-SQL channels were pH50D 7.1 and pH50A 6.7 (Fig. 5D) similar to those of rat.
FIGURE 5.
Elephant shark ASIC1. A, wild type (wt) EFS ASIC1 produces small and fast currents. B, substitutions of residues in the β1-β2 linker for the corresponding SQL of rat results in large currents. C, comparison of normalized currents of wild type and SQL mutant EFS channels. Red lines are fits of the currents to Equation 2. Wild type versus SQL mutant: τa 3 versus 16 ms, and τd 17 versus 140 ms. E, apparent proton affinities of EFS-SQL. E, Western blot of surface biotinylated proteins from 15 oocytes expressing the corresponding channels. The blot was probed with a V5 antibody.
DISCUSSION
In summary, the results of this work show that inter species differences in the response to protons of ASIC1 stem, at least in part, from variations in amino acid composition of the β1-β2 linker. Subtle substitutions of neutral residues but not of negatively charged residues in the proton-sensor are a strategy used by nature to achieve diversity in pH sensitivity of ASIC1. From an evolutionary point of view, this approach must be more cost effective than changing the structure of the proton-sensor. Differences in proton affinities of ASIC1 may represent an adaptation to maintain similar levels of ASIC1 response to a given change in pH across vertebrates thereby compensate for the high baseline plasma pH of amphibian and aquatic species.
We determined that delay of channel openings upon proton stimulation is the mechanism whereby the apparent proton affinity is reduced. A decrease in affinity of any of the proton binding sites could slow the opening rate not because the β1-β2 linker directly binds protons but because the M85L substitution could perturb the structure of the proton sensor indirectly decreasing the affinity of the proton bind site(s). Alternatively, the β1-β2 linker stabilizes a closed conformation through the interaction of residue 85 with other yet to be identified amino acids. Among the residues tested in position 85, leucine was the only one that delayed the progression of C to CH, whereas other amino acids increased the rate of the transition (Table 1), suggesting that Leu85 forms the strongest contact. The crystal structure of chicken ASIC1 (4, 14), which is most likely in the desensitized state, shows Leu85 exposed to water, but the side chain points toward the interior of the protein consistent with our proposal of interacting with other residue. Taken together, the results from this work strengthen the functional importance of the segment following transmembrane domain 1 (β1 strand and β1-β2 linker) in channel gating and provide the first evidence that it participates in channel opening.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- ASIC
- acid-sensing ion channel
- RT
- reverse transcription
- MES
- 4-morpholineethanesulfonic acid
- EFS
- elephant shark.
REFERENCES
- 1.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. (1997) Nature 386, 173–717 [DOI] [PubMed] [Google Scholar]
- 2.Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) Neuron 34, 463–477 [DOI] [PubMed] [Google Scholar]
- 3.Yermolaieva O., Leonard A. S., Schnizler M. K., Abboud F. M., Welsh M. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez de la Rosa D., Zhang P., Shao D., White F., Canessa C. M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2326–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton R. F. (2002) J. Exp. Biol. 205, 641–650 [DOI] [PubMed] [Google Scholar]
- 7.Coric T., Zheng D., Gerstein M., Canessa C. M. (2005) J. Physiol. 568, 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesh B., Kirkness E. F., Loh Y. H., Halpern A. L., Lee A. P., Johnson J., Dandona N., Viswanathan L. D., Tay A., Venter J. C., Strausberg R. L., Brenner S. (2007) PLoS Biol. 5, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P., Sigworth F. J., Canessa C. M. (2006) J. Gen. Physiol. 127, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes M. K., Hughes A. L. (1993) Mol. Biol. Evol. 10, 1360–1369 [DOI] [PubMed] [Google Scholar]
- 11.Li T., Yang Y., Canessa C. M. (2010) Am. J. Physiol., in press [Google Scholar]
- 12.Coric T., Zhang P., Todorovic N., Canessa C. M. (2003) J. Biol. Chem. 278, 45240–45247 [DOI] [PubMed] [Google Scholar]
- 13.Springauf A., Gründer S. (2010) J. Physiol. 588, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales E. B., Kawate T., Gouaux E. (2009) Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.