Abstract
O-Acylation of proteins was known only in a few eukaryotic proteins but never in bacteria. We demonstrate, using a combination of protein chemistry and mass spectrometry, the occurrence of three O-acylated polypeptides in Corynebacterium glutamicum, PorA, PorH, and an unknown small protein. The three polypeptides are O-substituted by mycolic acids, long chain α-alkyl and β-hydroxy fatty acids specifically produced by members of the Corynebacterineae suborder. To date these acids were described only as esterifying trehalose and arabinogalactan, and less frequently glycerol, important components of the highly impermeable outer barrier of Corynebacterineae. We show that the post-translational mycoloylation of PorA occurs at Ser-15 and is necessary for the pore-forming activity of C. glutamicum.
Keywords: Cell Wall, Lipid, Lipid Structure, Lipoprotein Structure, Mass Spectrometry (MS), Corynebacteria, Mycolic Acids
Introduction
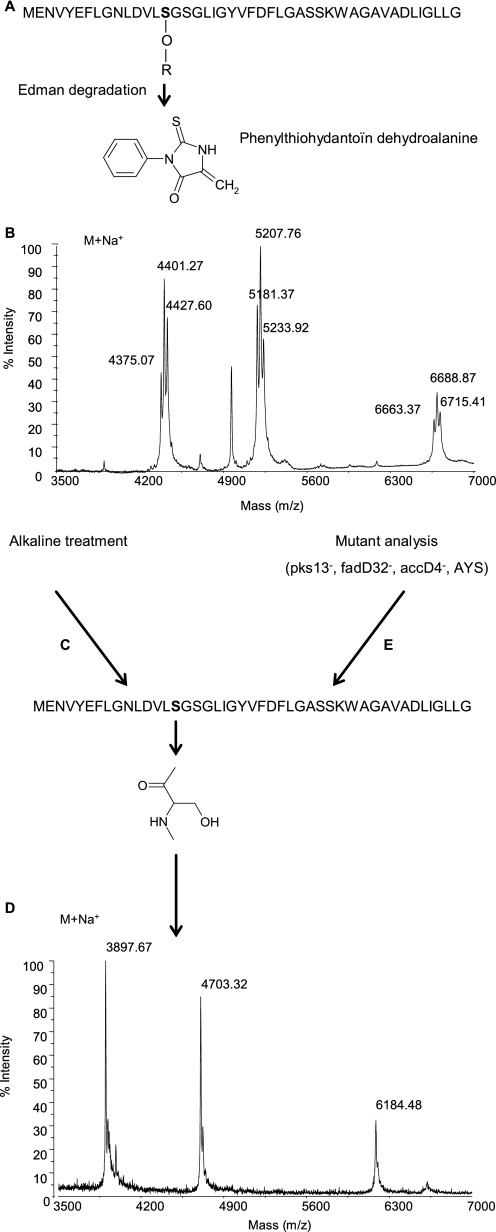
Corynebacteria and related genera that compose the Corynebacterineae suborder are unique among Gram-positive bacteria by possessing, like Gram-negative microorganisms, two membranes in their cell envelope (1, 2). The outer membrane of corynebacteria contains long chain α-alkyl and β-hydroxy fatty acids, known as mycolic acids (3, 4), and four specialized pore-forming proteins (porins), namely PorA, PorB, PorC, and PorH (5–7). We have recently shown, using recombinant PorA and PorH, that both PorA and PorH are required for the pore-forming activity of the major cell wall channels of Corynebacterium glutamicum, the prototype of amino acid producer bacteria used in industry, Corynebacterium efficiens, and Corynebacterium diphtheriae (8). The first porin characterized in corynebacteria is PorA from C. glutamicum, called CgPorA. This 45-amino acid-containing polypeptide (6) is an acidic protein with an excess of negatively charged amino acids, which explains the cation selectivity of the channel. Interestingly, a phenylthiohydantoin derivative of dehydroalanine was identified as a degradation product of serine at position 15 (see Fig. 1A) during the sequencing of CgPorA by Edman degradation (9). This observation strongly suggested a post-translational modification of this amino acid residue in CgPorA, possibly by a lipid in view of the unusual hydrophobicity of CgPorA and related porins, which are soluble in organic solvents.
FIGURE 1.
Identification of the mycoloylation of PorA. A, primary structure and Edman degradation of PorA in C. glutamicum (CgPorA) wild type (WT). R = acyl residue. B, linear MALDI-TOF mass spectrum of the hydrophobic protein extract of C. glutamicum WT. C and E, alkali-treated CgPorA (C) and PorA from the mycolate-deficient strains of C. glutamicum Pks13−, FadD32−, AccD4−, and CglΔotsAΔtreYΔtreS (E) share the primary structure. D, linear MALDI-TOF mass spectrum of the hydrophobic protein extract of C. glutamicum WT after alkaline treatment or of Pks13−, FadD32−, AccD4−, or AYS mutant strains.
The major types of lipid modification of proteins consist in the attachment of palmitoyl or myristoyl acyl chain, prenylation, cholesteroyl, and glycosylphosphatidylinositol anchors. Most acylated proteins contain amide-linked myristic acid or thioester-linked palmitic acid residues (10). Besides, eukaryotic cells may also contain, although rarely, O-acylated proteins on serine or threonine residues because only a few such proteins are known to date (11), which include the growth hormone-releasing peptide ghrelin (12) and WntA, a protein that plays a key role in numerous aspects of embryogenesis (13). The present work addresses the question of the nature of the putative post-translational modification of PorA and its possible impact on the pore-forming activity of the protein.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
C. glutamicum ATCC13032 was grown in either brain heart infusion (BHI,4 Difco) medium or minimal medium with 4% sucrose as carbon source at 30 °C. The CglΔotsAΔtreYΔtreS triple mutant, described by Wolf et al. (14), was grown only in minimal medium with 4% sucrose. The pks13::km (15), fadD32::km (16), and accD4::km (16) mutant strains of C. glutamicum were grown in BHI medium containing kanamycin at a final concentration of 10 mg/liter. The S15VPorA C. glutamicum mutant was grown in BHI supplemented with 10 mg/liter chloramphenicol. C. diphtheriae ATCC11913 and C. efficiens YS-314 were grown on BHI medium at 30 °C.
Construction of the S15VPorA Mutant
porH and porA genes are co-transcribed and are under the control of a predicted promoter located upstream porH (5). To substitute serine by valine at position 15 of the amino acid sequence of CgPorA, site-directed mutagenesis of porA was performed by overlap extension using the polymerase chain reaction (17). Two independent PCRs, PCR1a and PCR1b, were realized to generate the primary PCR products, using C. glutamicum chromosomal DNA as template. Both primary products contain the desired mutation as well as restriction sites at the beginning and end of the cloned sequence. For PCR1a and PCR1b, the primer pairs A and B and C and D were used, respectively (A, 5′-ATTAGGATCCCGGCGTGCCAAAGGGG-3′; B, 5′-GGCCGGAGCCGACAAGGACATCAAGGTTTCC-3′; C, 5′-TGATGTCCTTGTCGGCTCCGGCCTCATCGG-3′; D, 5′-ATTACCCGGGCGAGCCGTTGTTAAGTAG-3′). Mutagenic nucleotides included in the porA-coding sequence are underlined, and restriction enzyme sites introduced in primers are in bold letters, BamHI and XmaI, respectively. Then, both amplified products were hybridized and elongated to generate a 777-bp fragment (verified on agarose gel electrophoresis) consisting of porH-porA locus and 284 bp upstream porH. A second PCR was performed using primer pair AD, whose amplicons were ligated in pCGL482 using BamHI and XmaI, to generate pCGL482/S15VporA-porH. The construction was transformed in Escherichia coli DH5α strain, and transformants were selected on LB plates containing chloramphenicol. The resulting plasmid, named pCGL482/S15VporA-porH, was verified by sequencing and transformed in C. glutamicum ATCC13032 ΔporH-porA mutant strain by electroporation to yield the mutant strain ΔporH-porA(S15VporA-porH).
Protein Extraction in Organic Solvents
Cells were grown to an optical density at 600 nm of 10 and harvested by centrifugation. Then, the pellet was extracted with a mixture of chloroform:methanol (1:2, v/v) overnight at room temperature under stirring.
Alkaline Treatment
The chloroform:methanol extract (1 volume) was precipitated with diethyl ether (9 volumes) and kept overnight at −20 °C. The precipitated protein fraction was suspended in water (pH 3–4) and successively washed twice with chloroform and twice with diethyl ether until no traces of lipids (trehalose mono- and dimycolates, and free mycolic acids) were detected by thin-layer chromatography analysis. Then, the aqueous phase was de-O-acylated by 0.1 m NaOH at 37 °C during 30 min. The reaction mixture was then acidified with stoichiometric quantities of acetic acid. The resulting fatty acids were extracted with diethyl ether and converted into methyl esters with diazomethane. The aqueous phase was dialyzed to remove salts, and both the organic and the aqueous phases were analyzed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry.
Mass Spectrometry Analysis
MALDI-TOF spectra of proteins were acquired on a Voyager-DE STR mass spectrometer (PerSeptive Biosystems) equipped with a pulsed nitrogen laser emitting at 337 nm; spectra were analyzed in the linear positive ion mode using an extraction delay time set at 100 ns and an accelerating voltage of 25 kV. Native and deacylated proteins were loaded onto a metal plate with sinapinic acid as a matrix.
MALDI-TOF spectra of corynomycolic acid methyl esters were acquired in reflectron mode with a Applied Biosystems 4700 analyzer mass spectrometer (Applied Biosystems, Framingham, MA) equipped with an Nd:YAG laser (wavelength 335 nm; pulse < 500 ps; repetition rate 200 Hz). A total of 2500 shots were accumulated in positive ion mode, and mass spectrometry data were acquired with default calibration for the instrument. Lipids were loaded onto a metal plate with dihydroxybenzoic acid as a matrix.
Pore-forming Activity
The precipitated protein fraction was resuspended in 10 mm Tris-HCl, pH 8 buffer, and the channel-forming activity was measured using black lipid bilayer membranes formed as described previously (18). Membranes were formed across the hole with a surface area of about 0.5 mm2 by painting on a 1% solution of a mixture (molar ratio 4:1) of diphytanoyl phosphatidylcholine and phosphatidylserine (Avanti Polar Lipids) in n-decane.
RESULTS AND DISCUSSION
Demonstration of a Post-translational Modification of CgPorA and CgPorH by Mass Spectrometry
We first determined the masses of CgPorA and CgPorH by MALDI-TOF mass spectrometry of the porin-enriched organic extract (7). Although the predicted masses of the unmodified proteins are, respectively, 4680 Da (M+H+ = 4681; M+Na+ = 4703) and 6161 Da (M+H+ = 6162; M+Na+ = 6184), no peaks were observed at these mass values. Interestingly, the MALDI-TOF mass spectrum of the hydrophobic protein extract of C. glutamicum (Fig. 1B) showed three series of ion peaks centered at m/z 4401, 5207, and 6688. The same experiment conducted on the PorA-PorH double mutant (8) showed a complete disappearance of the two latter signals.
Nature of the Post-translational Modification
The shapes of the three series of mass peaks were suggestive of acylated compounds, with mass peaks differing by 26 atomic mass units. Accordingly, the protein extract of the wild-type C. glutamicum was treated with mild alkali using NaOH 0.1 m (Fig. 1C) and reanalyzed by MALDI-TOF mass spectrometry (Fig. 1D). Expectedly, ion peaks corresponding to the calculated molecular masses of CgPorA and CgPorH were readily observed, respectively, at m/z 4703 and 6184. The remaining series, centered at m/z 4401, also shifted at m/z 3897, indicating that the three polypeptides are alkali-labile. The latter compound, which was not assignable to any of the known polypeptides, is thereafter called protein X.
Identification of the Acyl Substituents of CgPorA, CgPorH, and Protein X
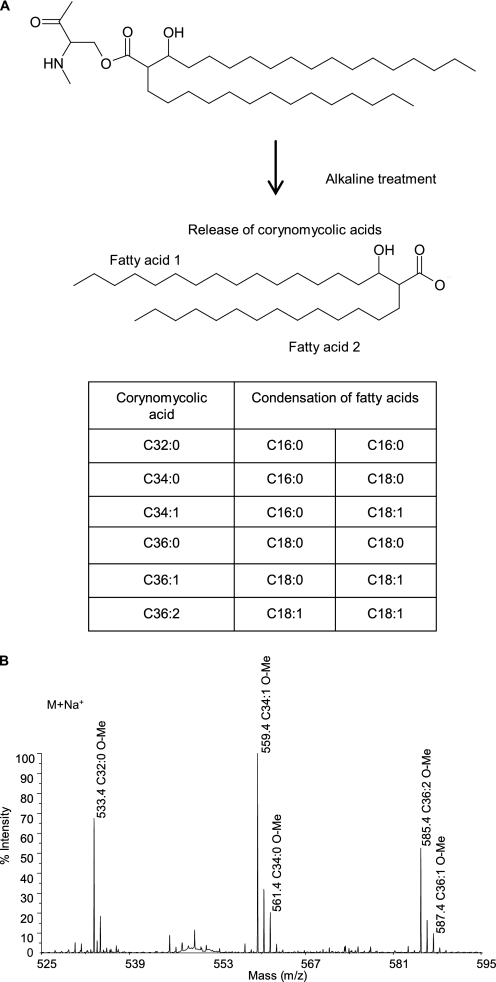
Remarkably, the mass peaks of the three series of native molecules were shifted by 478, 504, or 530 Da upon alkaline treatment, which may correspond to masses of C32:0, C34:1, or C36:2 corynomycolic acyl residues. Importantly, the 26-Da difference between the peaks of the native polypeptides, e.g. 5181, 5207, and 5233, perfectly matches the natural occurrence of the three major corynomycolic acid species in C. glutamicum, namely C32:0, C34:1, and C36:2 (15, 19) (Figs. 1B and 2A).
FIGURE 2.
Analysis of corynomycolic acids resulting from the alkaline treatment of the hydrophobic protein extract of C. glutamicum. A, structures of the corynomycoloyl residue that substitutes the serine 15 in CgPorA and of the resulting putative free corynomycolic acids upon alkaline treatment of the hydrophobic polypeptides. These fatty acids are produced through an enzymatic condensation between two regular sized (saturated or monounsaturated) fatty acids (C16 or C18). B, reflectron MALDI-TOF mass spectrum of the corynomycolic acid methyl esters obtained by alkaline treatment, followed by methylation, of the hydrophobic proteins. The observed peaks correspond to those of pseudomolecular ions (M+Na+).
To confirm these results, gas chromatography and MALDI-TOF analyses of the fatty acid derivatives released by the alkaline hydrolysis of the protein extract were performed; for this purpose, the alkali-treated protein precipitate was partitioned between diethyl ether and water and the organic phase was treated with diazomethane, to methylate any potential carboxylic acid group. Analysis of the mass spectra clearly identified a series of pseudomolecular ion (M+Na+) peaks assignable to those of corynomycolate methyl esters consisting of C32:0, C34:1, and C36:2 (Fig. 2B) and identified by gas chromatography analysis.
To further demonstrate the nature of the post-translational modification, proteins were extracted from C. glutamicum mutant strains known to be impaired in corynomycolic acid synthesis, namely CglΔotsAΔtreYΔtreS (20), pks13::km (15), fadD32::km (16), and accD4::km (16) (Fig. 1E). The protein extracts were first analyzed by MALDI-TOF mass spectrometry. Superimposable mass spectra were obtained for the different extracts and showed sharp pseudomolecular ion peaks at m/z 3897, 4703, and 6184, corresponding to whose observed in the spectrum of de-O-acylated protein extracts (Fig. 1D), reinforcing the demonstration that the three polypeptides are mycoloylated. Altogether, these data allowed us to (i) show the occurrence of an O-linkage cleaved by the alkaline conditions we used and (ii) identify corynomycolic acid as the lipid moiety that esterifies a hydroxyl group of an amino acid residue in CgPorA and at least two other proteins.
Localization of the Site of the Post-translational Modification in CgPorA
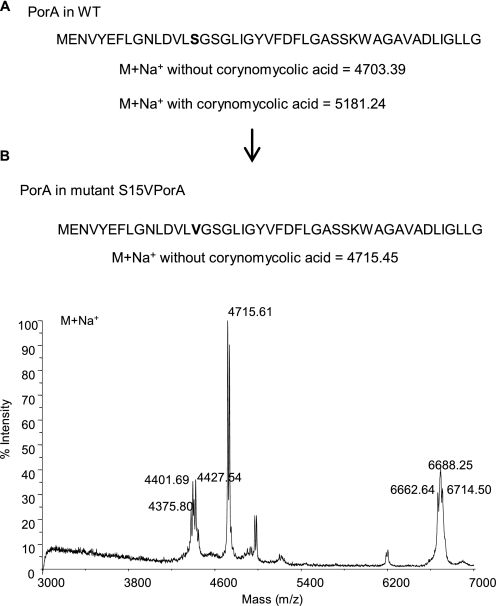
To unambiguously localize the modification in CgPorA, we first used the tandem mass spectrometry approach; all attempts in this direction were, however, unsuccessful. We then shifted to the site-directed mutagenesis strategy by constructing a S15VCgPorA mutant on the basis of the structure of the product (Fig. 1A) identified during the sequencing of CgPorA by Edman degradation (9). The MALDI-TOF mass spectrum of the S15VCgPorA hydrophobic protein extract showed the selective absence of the series at m/z 5207 and the concomitant appearance of a peak at m/z 4715 (Fig. 3), which corresponds to an up-shift of 12 Da (when compared with the calculated pseudomolecular mass of CgPorA, i.e. 4703 Da), the expected mass difference between the serine and the valine residues. Thus, these data conclusively established the modification site as Ser-15 of CgPorA.
FIGURE 3.
Identification of the position of mycoloylation in CgPorA. A and B, primary structure of CgPorA WT (A) and S15VPorA (B). The predicted pseudomolecular (M+Na+) masses of CgPorA, with and without a mycoloyl residue, are indicated. B also shows the linear MALDI-TOF mass spectrum of the hydrophobic protein extract of the C. glutamicum mutant S15VPorA.
Similar experiments were also conducted to localize the site of O-mycoloylation in CgPorH. The tandem mass spectrometry strategy was again unsuccessful. In the absence of any obvious homology between the CgPorA and CgPorH, sequence alignment of the latter protein and its orthologues from C. diphtheriae (21) and C. efficiens (8) showed that Thr-15 and Thr-48 are conserved in the proteins examined. Accordingly, T15ACgPorH and T48ACgPorH protein mutants were constructed and analyzed by MALDI-TOF mass spectrum. The polypeptides were found still O-mycoloylated, indicating that the acylation position of CgPorH differs from that of CgPorA.
O-Mycoloylated Proteins in Other Corynebacteria
We previously showed that both C. diphtheriae (21) and C. efficiens (8) contain PorA orthologues, cdPorA and cePorA, respectively. To check that mycoloylation was not restricted to C. glutamicum, the hydrophobic protein-enriched fractions of the two latter corynebacterial species were isolated and analyzed by MALDI-TOF mass spectrometry. The spectrum of the hydrophobic protein extracts from C. diphtheriae and C. efficiens showed pseudomolecular ion peaks corresponding to the predicted molecular masses of cdPorA and cePorA bearing the major corynomycoloyl residues of the species (22): 5175 (C30:0) and 5203 (C32:0) for cdPorA and 5111 (C32:0) and 5137 (C34:1) for cePorA. It was thus concluded that the cdPorA and cePorA are also acylated, likely with a corynomycoloyl residue.
Impact of the Mycoloylation of CgPorA on the Pore-forming Activity
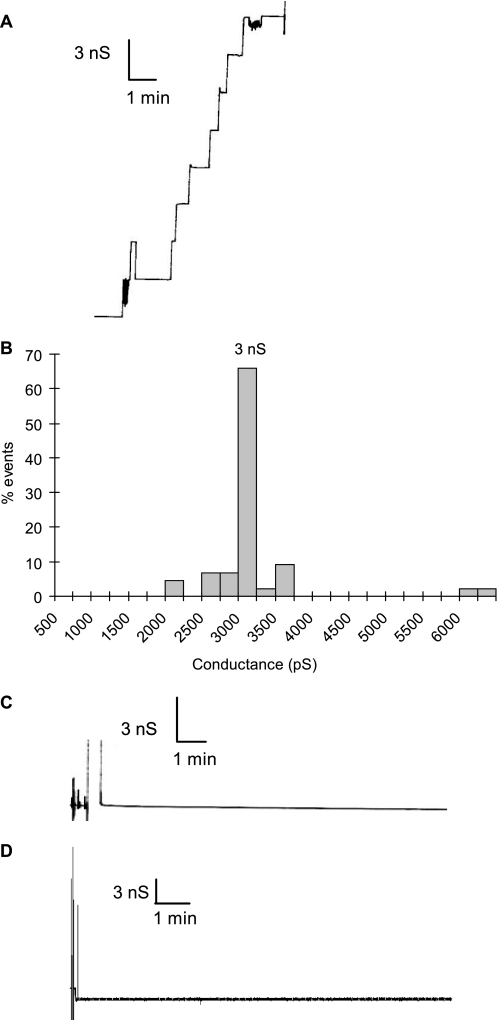
We have recently shown that both CgPorA and CgPorH are required for the pore-forming activity of the major cell wall channel of C. glutamicum (8). The protein extract of the wild-type strain formed typical channels with a single conductance (3 ns in 1 m KCl) (Fig. 4, A and B). To determine the importance of the lipid moiety on the pore-forming activity, we measured the activity of the hydrophobic protein extracts of mutant strains completely devoid of mycolates, i.e. CglΔotsAΔtreYΔtreS and pks13::km. No pore-forming activity could be detected in these extracts (Fig. 4C). Consistently, the pore-forming activity of extract from the wild-type strain was completely abolished upon de-O-acylation (Fig. 4D). To evaluate the specific role of mycoloylation of PorA, we analyzed CgPorA- and CgPorH-enriched protein extract isolated from the S15VCgPorA strain of C. glutamicum, which produced non-O-acylated PorA but O-mycoloylated PorH. Remarkably, no channel activity was observed when the standard concentration of protein extract (50 ng/ml) from the mutant was assayed; 5-fold more proteins were necessary to observe the formation of active channels. These data clearly demonstrated that the post-translational modification of CgPorA by a corynomycoloyl residue is necessary for the pore-forming activity.
FIGURE 4.
Single-channel recording of a diphytanoyl phosphatidylcholine and phosphatidylserine (molar ratio 4:1)/n-decane membrane in the presence of hydrophobic protein extract of C. glutamicum WT or ΔAYS. A, channel-forming activity of the protein extract from C. glutamicum WT. B, histogram of the percentage of events for the occurrence of a given conductivity unit, recorded for C. glutamicum WT. C and D, channel-forming activity of the CglΔotsAΔtreYΔtreS mutant cultivated on minimal medium with 4% sucrose (C) and of the alkali-treated protein extract from C. glutamicum WT (D). The aqueous phase contained 1 m KCl (pH 6). The applied membrane potential was 20 mV; T = 20 °C.
The present work is the first demonstration of O-acylation of bacterial proteins, a post-translational modification that is required for the pore-forming activity of at least one of the modified polypeptides identified, namely CgPorA. These mycoloylated proteins represent a new class of mycolate-containing molecules in corynebacteria that were so far limited to acylated carbohydrate and glycerol. Several questions remain to be addressed, among which are (i) the identification of the unknown mycoloylated protein X; (ii) the sites of mycoloylation of both PorH and protein X; (iii) the specific O-mycoloyl transferases (23) that are involved in the mycoloylation of the proteins; (iv) the consequence of the lipid modification on the structure and stability of the outer membrane of the corynebacterial cell wall; and (v) the occurrence of O-acylation in other members of the Corynebacterineae suborder. Intensive efforts are currently devoted in our laboratories to answer these questions.
Acknowledgment
We thank Dr. Françoise Laval (IPBS, Toulouse) for help in mass spectrometry.
This work was supported by grants from the CNRS, the University of Toulouse (Paul Sabatier), and the Agence Nationale de la Recherche (Grant ANR-07-BLAN-0363).
- BHI
- brain heart infusion
- MALDI-TOF
- matrix-assisted laser desorption/ionization time of flight
- WT
- wild type.
REFERENCES
- 1.Zuber B., Chami M., Houssin C., Dubochet J., Griffiths G., Daffé M. (2008) J. Bacteriol. 190, 5672–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann C., Leis A., Niederweis M., Plitzko J. M., Engelhardt H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3963–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan P. J., Nikaido H. (1995) Annu. Rev. Biochem. 64, 29–63 [DOI] [PubMed] [Google Scholar]
- 4.Daffé M., Draper P. (1998) Adv. Microb. Physiol. 39, 131–203 [DOI] [PubMed] [Google Scholar]
- 5.Hünten P., Costa-Riu N., Palm D., Lottspeich F., Benz R. (2005) Biochim. Biophys. Acta 1715, 25–36 [DOI] [PubMed] [Google Scholar]
- 6.Lichtinger T., Burkovski A., Niederweis M., Krämer R., Benz R. (1998) Biochemistry 37, 15024–15032 [DOI] [PubMed] [Google Scholar]
- 7.Costa-Riu N., Maier E., Burkovski A., Krämer R., Lottspeich F., Benz R. (2003) Mol. Microbiol. 50, 1295–1308 [DOI] [PubMed] [Google Scholar]
- 8.Barth E., Barceló M. A., Kläckta C., Benz R. (2010) J. Bacteriol. 192, 786–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtinger T., Riess F. G., Burkovski A., Engelbrecht F., Hesse D., Kratzin H. D., Krämer R., Benz R. (2001) Eur. J. Biochem. 268, 462–469 [DOI] [PubMed] [Google Scholar]
- 10.Nadolski M. J., Linder M. E. (2007) FEBS. J. 274, 5202–5210 [DOI] [PubMed] [Google Scholar]
- 11.Towler D. A., Gordon J. I., Adams S. P., Glaser L. (1988) Annu. Rev. Biochem. 57, 69–99 [DOI] [PubMed] [Google Scholar]
- 12.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. (1999) Nature 402, 656–660 [DOI] [PubMed] [Google Scholar]
- 13.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. (2006) Dev. Cell 11, 791–801 [DOI] [PubMed] [Google Scholar]
- 14.Wolf A., Krämer R., Morbach S. (2003) Mol. Microbiol. 49, 1119–1134 [DOI] [PubMed] [Google Scholar]
- 15.Portevin D., De Sousa-D'Auria C., Houssin C., Grimaldi C., Chami M., Daffé M., Guilhot C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portevin D., de Sousa-D'Auria C., Montrozier H., Houssin C., Stella A., Lanéelle M. A., Bardou F., Guilhot C., Daffé M. (2005) J. Biol. Chem. 280, 8862–8874 [DOI] [PubMed] [Google Scholar]
- 17.Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 18.Benz R., Janko K., Lauger P. (1979) Biochim. Biophys. Acta 551, 238–247 [DOI] [PubMed] [Google Scholar]
- 19.Daffe M. (2005) in Handbook of Corynebacterium glutamicum (Lothar Eggeling M. B. ed) pp. 121–148, Taylor and Francis Group A CRC Press Book, Boca Raton, FL [Google Scholar]
- 20.Tropis M., Meniche X., Wolf A., Gebhardt H., Strelkov S., Chami M., Schomburg D., Krämer R., Morbach S., Daffé M. (2005) J. Biol. Chem. 280, 26573–26585 [DOI] [PubMed] [Google Scholar]
- 21.Schiffler B., Barth E., Daffé M., Benz R. (2007) J. Bacteriol. 189, 7709–7719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puech V., Chami M., Lemassu A., Lanéelle M. A., Schiffler B., Gounon P., Bayan N., Benz R., Daffé M. (2001) Microbiology 147, 1365–1382 [DOI] [PubMed] [Google Scholar]
- 23.Kacem R., De Sousa-D'Auria C., Tropis M., Chami M., Gounon P., Leblon G., Houssin C., Daffé M. (2004) Microbiology 150, 73–84 [DOI] [PubMed] [Google Scholar]