Abstract
Streptococcus pyogenes (group A streptococcus (GAS)) is a pathogen that invades non-phagocytic host cells, and causes a variety of acute infections such as pharyngitis. Our group previously reported that intracellular GAS is effectively degraded by the host-cell autophagic machinery, and that a cholesterol-dependent cytolysin, streptolysin O (SLO), is associated with bacterial escape from endosomes in epithelial cells. However, the details of both the intracellular behavior of GAS and the process leading to its autophagic degradation remain unknown. In this study, we found that two host small G proteins, Rab5 and Rab7, were associated with the pathway of autophagosome formation and the fate of intracellular GAS. Rab5 was involved in bacterial invasion and endosome fusion. Rab7 was clearly multifunctional, with roles in bacterial invasion, endosome maturation, and autophagosome formation. In addition, this study showed that the bacterial cytolysin SLO supported the escape of GAS into the cytoplasm from endosomes, and surprisingly, a SLO-deficient mutant of GAS was viable longer than the wild-type strain although it failed to escape the endosomes. This intracellular behavior of GAS is unique and distinct from that of other types of bacterial invaders. Our results provide a new picture of GAS infection and host-cell responses in epithelial cells.
Keywords: Autophagy, Bacteria, Bacterial Toxins, Confocal Microscopy, Electron Microscopy (EM), Immunology, Membrane Trafficking, Small G Proteins, Streptococcus
Introduction
Streptococcus pyogenes (group A streptococcus; GAS)2 is the causative pathogen for a diverse collection of human diseases, such as pharyngitis, bacteremia, and necrotizing fasciitis (1). GAS strains produce a variety of pathogenic factors such as streptolysin O (SLO), superantigens, and DNase (2–4). Invasive GAS diseases occur in ∼1/1000 cases, with associated mortality of 25% (5).
Autophagy is defined as “self-eating,” bulk degradation system for cytoplasmic components. During autophagy, double membrane structures are formed in the cytoplasm, in which cytoplasmic organelles and proteins are sequestered. These structures, called autophagosomes, subsequently fuse with lysosomes to degrade the components within them. This system is important for the physiological turnover of cytoplasmic components, and is involved in a number of clinical conditions and diseases (6, 7). Recently, we and others showed that GAS is captured and degraded by autophagy, not by the endosome-lysosome system (8, 9). Some species of bacteria invade host non-phagocytic cells, such as epithelial cells, and are largely degraded by the endocytosis pathway (10–12). However, some bacteria, including Listeria monocytogenes, Shigella flexneri, Mycobacterium tuberculosis, and Salmonella typhimurium, cannot be eliminated by endosomes, and replicate within the cytoplasm (13–15). The autophagic machinery is thought to act to remove these bacteria. However, it is still unclear under what circumstances the intracellular bacteria are sequestered by autophagosomes and what events in the infected cells lead to the degradation of bacteria by autophagy.
A cholesterol-dependent cytolysin of GAS, SLO, is a homolog of listeriolysin O (LLO) in L. monocytogenes. LLO is involved in the bacterial escape from phagosomes in macrophages (16, 17). It is known that SLO and LLO share 60% amino acid identity, and that their three-dimensional structures and characteristic domains are highly conserved. Although SLO was reported to be a critical factor for bacterial escape from endosomes, it has not been known whether and how SLO is involved in the subversion process of endosomes (8, 18). Thus, we examined whether SLO promotes the bacterial escape from endosomes, as well as how SLO induces autophagy in GAS-infected host cells.
When extracellular materials enter a host cell through the endocytic pathway, Rab proteins, which regulate membrane trafficking in mammalian cells, are recruited (19, 20). Rab proteins belong to the small G protein superfamily and have GTP/GDP-binding and GTPase activities. GTP-bound active Rab proteins interact with specific effectors and participate in the formation and motility of transport vesicles, as well as their fusion with endosomes (21). Among the Rab proteins Rab5 and Rab7, which are involved in the endosomal pathway, play crucial roles in the fusion of endosomes and in their maturation to lysosomes, respectively (22–24). In addition, Rab7 was reported to be associated with the maturation of autophagosomes under amino acid starvation conditions (25). Therefore, the influences of Rab5 and Rab7 should be also analyzed in detail during GAS infection.
In this study, we examined the behavior of GAS within host epithelial cells prior to bacterial elimination by the autophagic pathway. Additionally, the influences of the bacterial SLO and host Rab proteins on the fate of intracellular GAS were also studied.
EXPERIMENTAL PROCEDURES
Construction of a slo-deficient Mutant and Complemented Strains of GAS
GAS JRS4 (serotype M6) and its mutant strains were used for this study (supplemental Table S1) (12, 26). A slo-deficient mutant strain, JRS4Δslo was constructed by electroporation, as described previously (3). Briefly, JRS4 was transformed with a suicide vector pSF151-fSLO, which was pSF151 containing an internal fragment of the slo gene (27, 28). For a complemented strain of JRS4Δslo, JRS4Δslo-comp, the intact slo gene-ligated pENTR-SD-TOPO (Invitrogen) and pOGW vector, containing an isopropyl β-d-1-thiogalactopyranoside-inducible promoter and adapted for the Gateway system, were subjected to a recombination reaction using LR clonase (Invitrogen) (29). The resulting pOGW-SLO was introduced into JRS4Δslo by electroporation. Transformants were verified by PCR (supplemental Table S2) and by the determination of SLO-specific hemolytic titers (28). The expression of the SLO protein in JRS4Δslo-comp was induced by 1 mm isopropyl β-d-1-thiogalactopyranoside for 2 h and verified by Coomassie Blue staining and immunoblots using a specific anti-peptide antibody, raised using White New Zealand rabbits against a 14-amino acid peptide (ENKPDAVVTKRNPQ), corresponding to residues 190–203 of the SLO amino acid sequence.
Construction of Plasmids for Visualizing Host Cellular Proteins
Plasmids expressing proteins fused with a fluorescent protein were constructed to detect the induction of endosomes and autophagosomes (supplemental Table S1). Rat microtubule-associated protein 1 light chain 3 (LC3; a marker of autophagosomes), Rab5, and Rab7 (markers of endosomes) were fused with enhanced green fluorescence protein (EGFP) or a red fluorescent protein (mCherry) (30, 31). The dominant-active (DA) form of Rab5 (Rab5Q79L), dominant-negative (DN) form of Rab5 (Rab5S34N), DA form of Rab7 (Rab7Q67L), and DN form of Rab7 (Rab7T22N) were then constructed using the PrimeSTAR Mutagenesis Kit (Takara Shuzo) (supplemental Tables S1 and S2) (32–34). For the transfection of cultured cells without adenovirus, these expression plasmids were introduced with the FuGENE HD reagent (Roche Applied Science) for 6 h. The cells were further incubated for 48 h and then used for experimental assays.
For the transfection of cells with these genes using adenovirus vector, Gateway vectors such as pENTR11 and pAd/CMV/V5-dest were used (Invitrogen) (supplemental Table S1). Then, the linearized plasmids were transfected into human embryonic kidney-derived 293A cells according to the supplier's instructions (35). The titers of viruses were enhanced by repeated passaging until more than 90% of the cells produced fluorescence.
Bacterial Infection Assay
For GAS infection, bacteria grown to the mid-log phase were harvested and washed twice with phosphate-buffered saline (PBS; pH 7.4). Human cervical epithelial HeLa cells (ATCC CCL-2) incubated in a 24-well plate using 10% fetal calf serum-containing Dulbecco's modified Eagle's medium were infected with GAS strains at 2 × 106 colony forming units per well (multiplicity of infection = 100), except for the bacterial invasion and viability assays, in which the cells were seeded at a density of 1 × 105 cells per well and were infected with 1 × 107 colony forming units. After 1 h, the cells were washed with PBS and were further cultured with 100 μg/ml of gentamicin and 100 units/ml of penicillin G to remove extracellular bacteria. The times shown in the text and in all figures indicate the elapsed time after the infection procedure was started. Where indicated, cells were pre-infected with recombinant adenoviruses or transfected with protein-expressing plasmids for 48 h prior to their infection with GAS.
Microscopic Observations
For confocal microscopy, cells infected with GAS strains were fixed with 4% paraformaldehyde in PBS for 3 h at 4 °C. Mouse monoclonal anti-human lysosome-associated membrane protein-1 (LAMP-1; a lysosomal marker) (clone H4A3; Santa Cruz Biotechnology), anti-early endosomal antigen-1 (BD Biosciences), anti-cathepsin D (Sigma), or anti-SLO antibodies were used as the primary antibodies for immunostaining. The secondary antibody was Cy5-conjugated anti-mouse IgG or anti-rabbit IgG (Jackson ImmunoResearch). To label bacterial and cellular DNA, 0.2 μg/ml of propidium iodide (PI; Sigma) or 2 μg/ml of 4′,6-diamino-2-phenylindole (Sigma) in PBS was used. All fluorescence micrographs were confocal images acquired with a Fluoview FV1000 confocal microscope (Olympus).
For electron microscopy, cells infected with GAS were fixed with 2.5% glutaraldehyde in 0.1 m cacodylate (pH 7.4) for 2 h. Conventional electron microscopy was performed as previously described (7). Briefly, cells were pelleted, embedded in 7.5% gelatin in PBS, and infused with 2 m sucrose for 30 min. Ultrathin sections were stained by uranyl acetate plus lead citrate and were observed with an H7600 electron microscope (Hitachi).
Measurement of GAS-containing Autophagosomes and Endosomes
To estimate the induction of autophagy in GAS-infected cells, the percentage of GAS-invaded cells that contained LC3-positive autophagosomes was determined. At least 500 cells were observed to examine the formation of autophagosomes under the confocal microscope. The percentage of LC3-positive cells compared with cells invaded by GAS was determined in each experiment. In the case of endosomes, the ratio of GAS trapped within Rab5-positive compartments to total intracellular GAS was determined by area calculations of GAS using Image-J 1.41 software (Wayne Rasband, the Research Services Branch, National Institute of Mental Health), based on confocal images (8). At least 20 cells were analyzed at each time point.
Bacterial Invasion and Viability Assays
Cells in a 24-well plate were infected with GAS for 1 h and then incubated with antibiotics for an additional 10 min for bacterial invasion assays (total incubation was 1 h and 10 min after infection) or for 2 or 4 h (for bacterial viability assays; total incubation of 3 or 5 h after infection). Next, the cells were washed with PBS and disrupted in sterile distilled water, and serial dilutions of the lysates were spread on THY agar plates as described previously (36). For bacterial invasion assays, the amount of intracellular GAS at the point of cell disruption was expressed as the percentage of total bacteria added to the cell culture. For bacterial viability assays, the data are shown as the percentage of live GAS after 3 or 5 h in comparison with the viable cells detected at 1 h and 10 min after infection.
Statistics
All data are presented as the means of triplicate assays of a representative experiment ± S.E. Statistical analyses were carried out by Student's t test, Scheffe's test, and χ-square test using JMP software (SAS Institute, Cary, NC). p < 0.05 was considered to be significant.
RESULTS
Localization of Early and Late Endosomal Membrane Markers Over Time in GAS-infected Cells
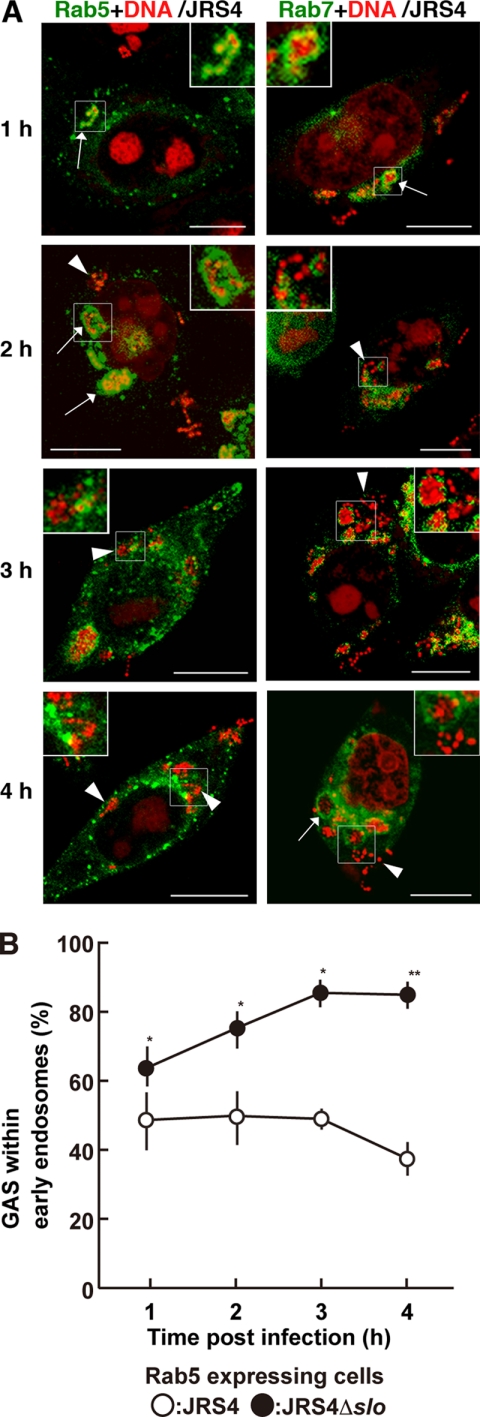
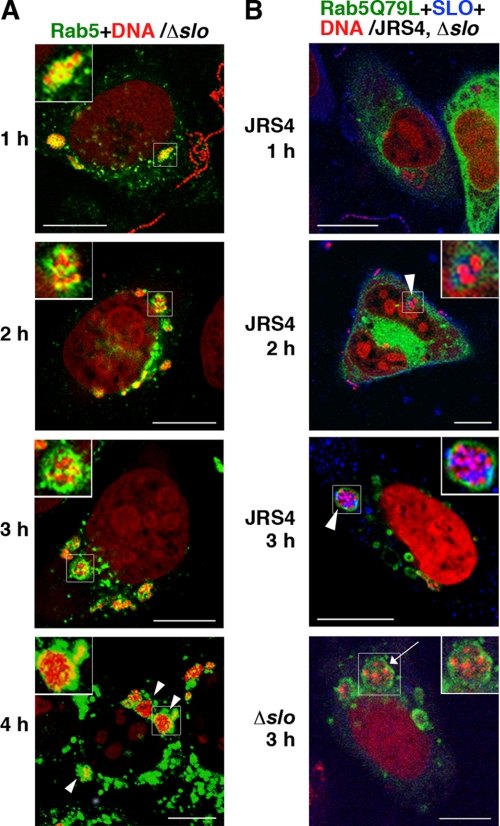
HeLa cells expressing fluorescently labeled Rab5 were infected with GAS strain JRS4, and endocytosis was monitored during the JRS4 invasion. Confocal microscopy showed that JRS4 invaded the cells and was trapped by Rab5-positive early endosomes 1 h after infection. However, JRS4 started to escape from the early endosomes 2 h after infection, and the number of bacteria in the cytoplasm increased in a time-dependent manner (Fig. 1A, left panels, and supplemental Fig. S1). The number of JRS4 cells remaining within the early endosomes did not change for up to 3 h after infection, but decreased slightly by 4 h (Fig. 1B, open circles). Although many bacteria were present in the cytoplasm at 3 h by confocal imaging, the decrease in JRS4 in the endosomes was not apparent at that time point (Fig. 1B). It should be noted that some bacteria invaded the host cells incompletely at 1 h and were not entirely trapped by the Rab5-positive vacuoles.
FIGURE 1.
Localization of intracellular GAS and Rab proteins in HeLa cells. A, HeLa cells expressing fluorescently labeled Rab5 (left panels) or Rab7 (right panels) were infected with GAS strain JRS4. The times shown indicate the elapsed time after infection. Rab proteins with EGFP are shown in green. Bacterial and host DNA was stained red with PI. Arrows indicate early endosomes (Rab5) or Rab7-positive compartments (Rab7) that contained bacteria. Arrowheads show bacteria in the cytoplasm. Insets show magnified images of bacteria trapped by endosomes or free in the cytoplasm. Bars, 10 μm. The same images divided by color are shown in supplemental Fig. S1. B, cells expressing Rab5 were infected with JRS4 (open circles) or JRS4Δslo (closed circles) for the indicated period. The ratio of trapped GAS in endosomes to total intracellular bacteria was determined from confocal microscopic images. The p value was determined by comparing cells infected with JRS4Δslo to those infected with JRS4 using Student's t test (*, p < 0.05, and **, p < 0.01).
Although Rab7 has been used as a marker of late endosomes, Rab7-positive compartments containing JRS4 could also be seen in host cells throughout the observation period, including at early time points (Fig. 1A, right panels, and supplemental Fig. S1). Intracellular JRS4 that was not sequestered by Rab7-positive compartments was also observed at 2, 3, and 4 h after infection. Because Rab7-positive compartments were found at early stages of infection, the data suggest that Rab7 presents in the cytoplasm in the absence of visible endosomes or has functions other than as a membrane protein in late endosomes in GAS-infected cells.
Autophagosomes Are Formed in the Vicinity of Early Endosomes
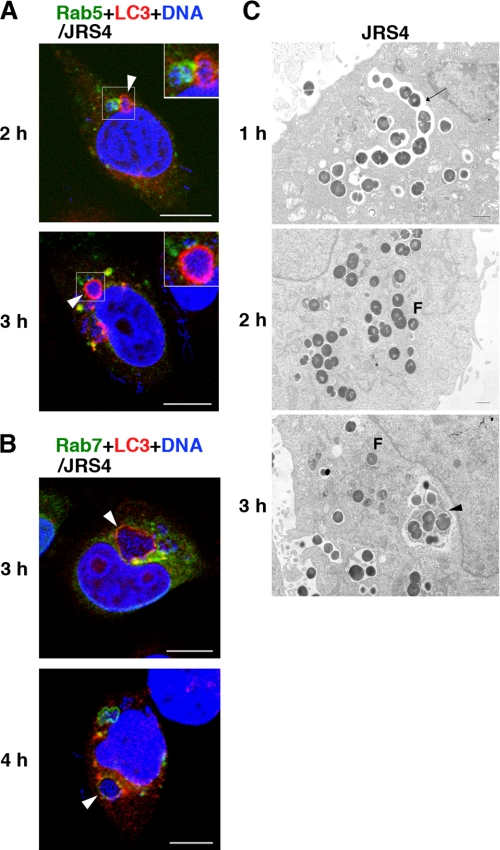
Because intracellular JRS4 is sequestered and degraded by the autophagic machinery (8), we next determined when the autophagic machinery was induced in cells infected with JRS4, and how autophagosomes were formed in these cells. In cells co-expressing Rab5 and LC3, LC3-positive autophagosomes became gradually visible by 2 h after infection and were more evident at later stages (Fig. 2A and supplemental Fig. S2A). JRS4 that was sequestered by autophagosomes was also trapped within Rab7-positive compartments, but not within early endosomes (Fig. 2, A and B, and supplemental Fig. S2, A and B). In addition, JRS4 associated with Rab7-positive compartments was not sequestered by autophagosomes, and some bacteria were found in the cytoplasm 3 or 4 h after infection (Fig. 2B). These observations suggested two possibilities regarding the autophagosome formation: (i) the autophagic machinery was induced to destroy the bacteria that had escaped from endosomes into the cytoplasm, or (ii) autophagosomes trapped the bacteria contained within endosomes that had undergone the replacement of Rab5 with Rab7.
FIGURE 2.
Localization of endosomal structures and LC3-positive compartments (indicating autophagosomes) in GAS-infected cells. A and B, cells expressing fluorescently labeled Rab5 (A), Rab7 (B), and LC3 (A and B) were infected with GAS strain JRS4. Early endosomes (A) and Rab7-positive endosomal structures (B) were labeled with EGFP (green). Autophagosomes were labeled with mCherry (red). Bacterial and host DNA was stained blue with 4′,6-diamino-2-phenylindole. Arrowheads indicate bacteria surrounded by autophagosomes that were not within early endosomes (A) or that were also trapped in Rab7-positive compartments (B). Bars, 10 μm. The same images divided by color are shown in supplemental Fig. S2, A and B. C, JRS4-infected cells were observed by electron microscopy. Arrows and arrowheads indicate endosomes and autophagosomes, respectively. F shows bacteria in the cytoplasm. Bars, 1 μm.
To see how autophagosomes sequestered bacteria, we performed electron microscopy. It showed that when JRS4 invaded the host cells, the bacteria were trapped in the characteristic single-membrane structures of endosomes (Fig. 2C). However, the JRS4 gradually escaped from the endosomes and were present in the cytoplasm, where multimembrane-bound structures containing bacteria were observed 3 h after infection (Fig. 2C). These structures were autophagosomes that contained inclusions of the cytoplasmic side multimembrane. These data indicated that autophagosomes sequestered the cytosolic GAS that escaped from endosomes, and did not surround the GAS-containing intact endosomes.
Rab5 and Rab7 Involvement in Autophagosome Formation
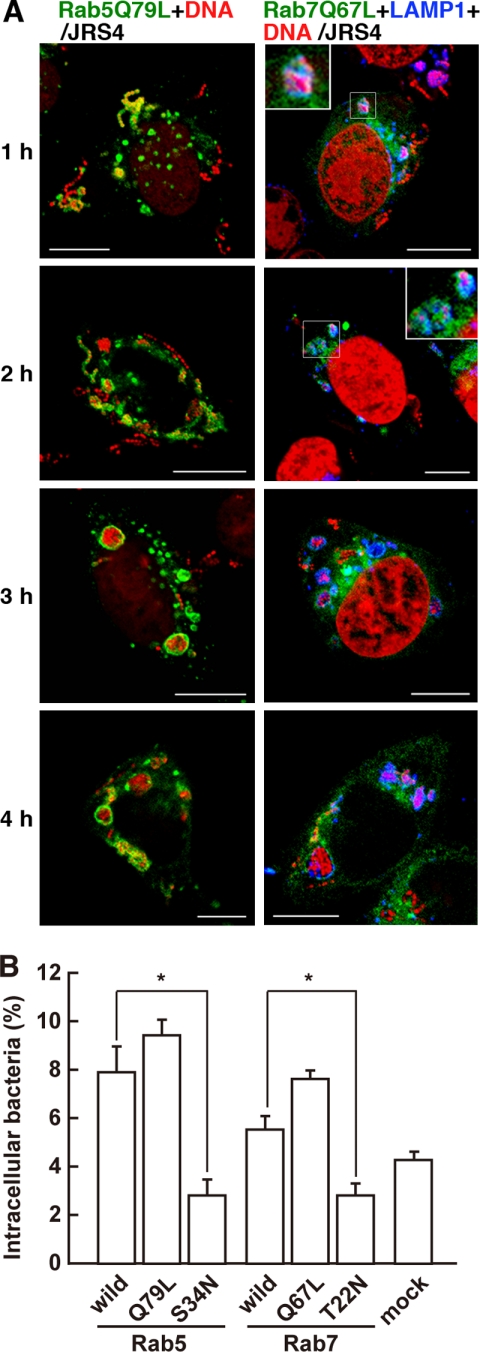
To analyze the functions of Rab5 and Rab7 in GAS-infected cells, dominant-active and dominant-negative forms of Rab proteins were constructed, i.e. plasmids expressing Rab5Q79L (DA form of Rab5), Rab5S34N (DN form of Rab5), Rab7Q67L (DA form of Rab7), and Rab7T22N (DN form of Rab7). The number and size of the early endosomes in cells expressing Rab5Q79L were significantly greater than those of cells expressing wild-type Rab5 (Fig. 3A, left panels, and supplemental Fig. S3). Cells expressing Rab5Q79L, like those expressing wild-type Rab5, contained JRS4 that had escaped from endosomes, but more bacteria remained trapped in the endosomes than in Rab5-expressing cells (Figs. 1A and 3A). There was no significant difference between the number of bacteria in cells expressing wild-type Rab5 and Rab5Q79L, but the cells expressing Rab5Q79L tended to contain more invading bacteria (Fig. 3B). Endosomal structures containing JRS4 were evident even 4 h after infection in the cells expressing Rab5Q79L (Fig. 3A). In cells expressing Rab5S34N, the bacterial invasion was remarkably reduced compared with cells expressing wild-type Rab5 (Fig. 3B). Moreover, the Rab5S34N expression was rather diffuse, and no endosomal structures containing JRS4 were seen in these cells at any point in the observation period (supplemental Fig. S4A). These findings suggested that Rab5 is involved not only in bacterial invasion, but also in the formation and fusion of endosomes in JRS4-infected cells.
FIGURE 3.
Influence of DA and DN mutants of Rab on the formation and maturation of early endosomes and bacterial invasion into GAS-infected cells. A, cells expressing Rab5Q79L (DA form of Rab5) (left panels) or Rab7Q67L (DA form of Rab7) (right panels) were infected with GAS strain JRS4. Endosomal structures were labeled with EGFP (green). Bacterial and host DNA was stained with PI (red). Lysosomes are indicated by LAMP1 stained with a specific antibody and Cy5-conjugated secondary antibody (blue, data are shown in right panels). The same images divided by color are shown in supplemental Fig. S3. Bars, 10 μm. B, cells expressing Rab5, Rab5Q79L, Rab5S34N (DN form of Rab5), Rab7, Rab7Q67L, or Rab7T22N (DN form of Rab7) were infected with JRS4 for 1 h and incubated with antibiotics for an additional 10 min. The number of intracellular live JRS4 was counted, and the ratio of intracellular JRS4 to the number of bacteria added to the cell cultures is shown. Mock cells were transfected with empty vector prior to their infection with JRS4. p values were determined by comparison with the cells expressing wild-type Rab proteins (*, p < 0.05) using Student's t test.
In cells expressing Rab7Q67L, Rab7-positive compartments that trapped intracellular JRS4 appeared 1 h after infection (Fig. 3A, right panels, and supplemental Fig. 3A), as in the cells expressing wild-type Rab7 (Fig. 1A, right panels). Moreover, most of the Rab7-positive compartments that contained bacteria fused with lysosomes, as indicated by LAMP-1 staining, at the early stage of infection. Compared with cells expressing wild-type Rab7, intracellular bacteria were slightly, but not significantly, elevated in cells expressing Rab7Q67L (Fig. 3B). By contrast, in cells expressing Rab7T22N, the Rab7-positive compartments did not form, and the aggregation of lysosomes did not occur (supplemental Fig. S4B). The amount of intracellular bacteria in cells expressing Rab7T22N was significantly less than in cells expressing wild-type Rab7 (Fig. 3B and supplemental Fig. S4B). Thus, not only Rab5 but also Rab7 appeared to be associated with bacterial invasion. In addition, Rab7 might be involved in the fusion of endosomal structures with lysosomes.
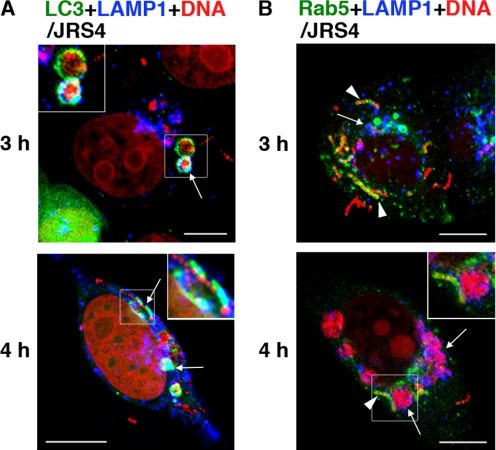
The fate of the GAS within the host cells depended on whether or not bacteria escaped from the endosomes. We studied their fate by using a specific antibody against LAMP-1. At 3 and 4 h after infection, LAMP-1 was co-localized with autophagosomes, but not with Rab5-positive endosomes (Figs. 4, A and B, and supplemental Fig. S5, A and B). We found that lysosomes accumulated in a time-dependent manner and fused with the autophagosomes rather than with the Rab5-positive endosomes. Furthermore, immunostaining for cathepsin D, a proteolytic enzyme of lysosomes, showed that cathepsin D was present in autophagosomes (supplemental Fig. S5C). These observations indicated that, although JRS4 can escape from early endosomes, its escape rapidly induces the autophagosome-lysosomal pathway.
FIGURE 4.
Localization of early endosomes, autophagosomes, and lysosomes in GAS-infected cells. A and B, cells expressing fluorescently labeled LC3 to indicate autophagosomes (A) or Rab5 to indicate early endosomes (B) were infected with JRS4 for 3 or 4 h. Autophagosomes or early endosomes were labeled with EGFP (green). Bacterial and host DNA were stained with PI (red). Cells were stained with anti-LAMP-1 to indicate lysosomes (A and B) (blue). The same images divided by color are shown in supplemental Fig. S5, A and B. A, arrows and insets indicate autophagosomes sequestering bacteria and fused with lysosomes. B, arrows indicate LAMP-1, which contained intracellular bacteria but not co-localized within early endosomes. Arrowheads indicate early endosomes surrounding bacteria but not co-localized with LAMP-1. Insets show bacteria surrounded by LAMP-1, adjacent to early endosomes. Bars, 10 μm.
Intracellular GAS Escapes from Endosomes Mediated by SLO, but the Autophagic Machinery Degrades GAS Effectively
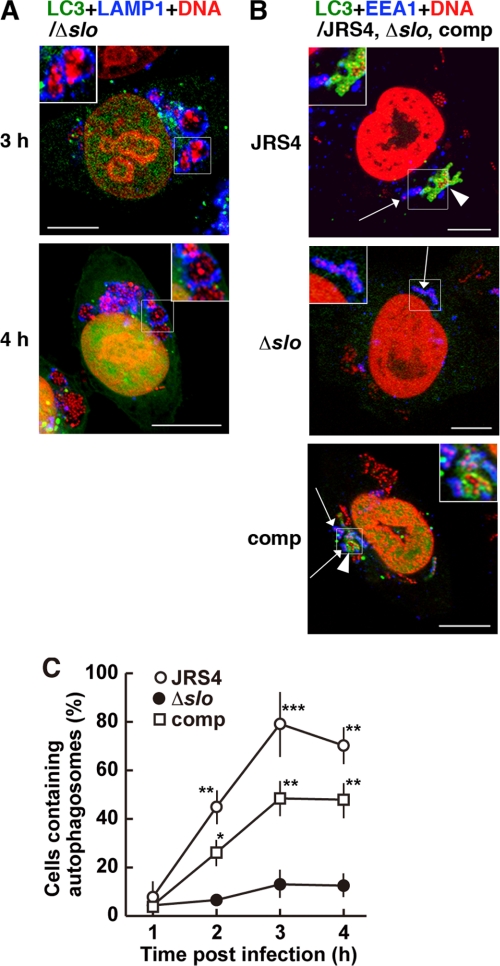
The involvement of the secretory cytolysin SLO in the induction of autophagy was examined using JRS4Δslo and JRS4Δslo-comp. The expression of a recombinant SLO protein (69 kDa) in Escherichia coli and GAS strains was detected by PCR and Western blot analyses (supplemental Fig. S6, A and B). We detected autophagosomes by expressing fluorescently labeled LC3 in the cells. When these cells were infected with JRS4Δslo, autophagosomes rarely formed, even 4 h after infection, despite a similar level of invasion as with wild-type JRS4 (Fig. 5A and supplemental Fig. S7A). Lysosomes containing intracellular JRS4Δslo were observed in some of the infected cells, but they did not co-localize with autophagosomes (Fig. 5A). Some lysosomes appeared to fuse with endosomes, but not autophagosomes, in cells infected with JRS4Δslo. Moreover, JRS4Δslo-comp induced autophagy in cells expressing LC3, just as wild-type JRS4 did (Fig. 5B, shown in green and red, and supplemental Fig. S7B). Whereas autophagy had been induced in 80% of cells infected with wild-type JRS4 by 3 h after infection, only 10% of the JRS4Δslo-infected cells formed autophagosomes (Fig. 5C), but cells infected with JRS4Δslo-comp showed considerable recovery of their ability to induce autophagy compared with those infected with JRS4Δslo (Fig. 5C). Thus, our results support the idea that GAS can escape from endosomes by expressing SLO, but the cytoplasmic GAS was then sequestered by the autophagosome-lysosomal system.
FIGURE 5.
Influence of SLO on the induction of autophagy in GAS-infected cells. A and B, cells expressing LC3 were infected with one of the GAS strains, JRS4, JRS4Δslo (Δslo), or JRS4Δslo-comp (comp). Bacterial and host DNA was stained with PI (red). Autophagosomes were visualized with EGFP (green). The same images divided by color are shown in supplemental Fig. S7, A and B. Bars, 10 μm. A, cells infected with JRS4Δslo were stained with an anti-LAMP-1 antibody to indicate lysosomes (blue). The insets show that bacteria were surrounded by lysosomes but not by autophagosomes. B, cells were infected with GAS for 2 h. Early endosomal antigen-1 was stained with a specific antibody to indicate early endosomes (blue). Arrows indicate early endosomes surrounding bacteria. Arrowheads indicate bacteria sequestered by autophagosomes. Insets show autophagosomes containing bacteria adjacent to endosomes (JRS4, comp) or bacteria only within endosomes (Δslo). C, cells expressing fluorescently labeled LC3 to indicate autophagosomes were infected with JRS4 (open circles), JRS4Δslo (Δslo; closed circles), or JRS4Δslo-comp (comp; open squares). Data are shown as the percentage of GAS-infected cells forming autophagosomes. p values were determined by comparison with the percentage of cells infected with JRS4Δslo using the χ-square test (*, p < 0.05; **, p < 0.01; and ***, p < 0.005).
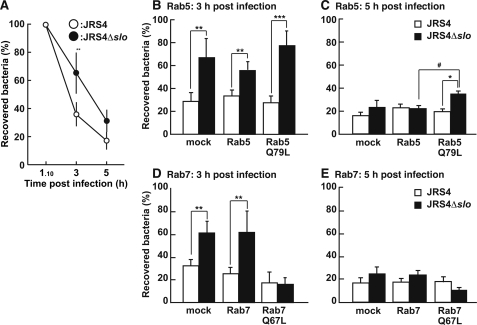
To assess whether intracellular GAS trapped within endosomes was degraded by the endosome-lysosomal system at early stages of infection, the number of viable intracellular bacteria was determined. Wild-type JRS4 was degraded more rapidly than JRS4Δslo at 3 h after infection (Fig. 6A). More than 60% of the JRS4Δslo was degraded at 5 h after infection, which was later than when JRS4 reached the same degree of degradation (Fig. 6A). In host cells expressing Rab5 or Rab7, JRS4 was degraded more rapidly than JRS4Δslo, and the degradation rates were not significantly altered by the expression of intact Rab5 or Rab7 (Fig. 6, B–E). However, cells expressing Rab5Q79L did not degrade JRS4Δslo effectively, even at 5 h (Fig. 6C). This might have been because gradual inactivation and replacement of Rab7 did not occur in these cells. On the other hand, both JRS4 and JRS4Δslo were eliminated effectively in cells expressing Rab7Q67L (Fig. 6D). These findings indicate that both Rab5 and Rab7 modulate the progression of endosome maturation in GAS-infected cells.
FIGURE 6.
Influence of SLO expression on bacterial viability in infected cells. A–E, cells were infected with JRS4 (open circles and bars) or JRS4Δslo (closed circles and bars) for 1 h and 10 min, 3 h (A, B, and D), or 5 h (A, C, and E). Data are shown as the percentage of GAS at 3 or 5 h after infection in comparison with that 10 min after the replacement of medium (1 h and 10 min after infection). B–E, cells were transfected with plasmids expressing wild-type Rab5 (B and C), Rab5Q79L (B and C), wild-type Rab7 (D and E), or Rab7Q67L (D and E) prior to infection. Mock cells were transfected with empty vector. p values were determined by comparison with the percentage of live JRS4 using Student's t test (*, p < 0.05; **, p < 0.01; and ***, p < 0.005) or live JRS4Δslo in cells expressing wild-type Rab5 using Scheffe's test (#, p < 0.05).
We next examined the effects of SLO expression on early endosomes. In cells expressing Rab5, JRS4Δslo was contained in early endosomes like the wild-type JRS4, but the bacteria did not escape the endosomes even 3 or 4 h after infection (Fig. 7A and supplemental Fig. S8A). Similar results were observed in the Rab5Q79L-expressing cells (supplemental Fig. S9A). The proportion of GAS within the early endosomes among the total number of intracellular bacteria was significantly different between JRS4 and JRS4Δslo (Fig. 1B). In cells expressing Rab7, JRS4Δslo was trapped by Rab7-positive compartments throughout the observation period (supplemental Fig. S9B). Electron microscopy showed that the intracellular JRS4Δslo was enclosed by single membrane structures (indicating endosomes), and free bacteria were not found in the cytoplasm (supplemental Fig. S9C). Furthermore, no autophagosomes that sequestered JRS4Δslo was observed.
FIGURE 7.
The lack of SLO accumulation in endosomes influences the behavior of intracellular GAS. A and B, cells expressing Rab5 (A) or Rab5Q79L (B) were infected with JRS4 (B) or JRS4Δslo (Δslo) (A and B). Early endosomal structures were visualized with EGFP (green). Bacterial and cellular DNA was stained with PI (red). The same images divided by color are shown in supplemental Fig. S8, A and B. Bars, 10 μm. A, arrowheads and insets indicate bacteria located in early endosomes. B, SLO was stained with a specific antibody (blue). Arrowheads and insets (JRS4) indicate the localization of SLO within endosomes. Arrow and inset in the image of JRS4Δslo-infected cells indicate endosomes without accumulated SLO.
The positional relationship between the early endosomes and autophagosomes in the cells was investigated using an antibody against early endosomal antigen-1. At 2 h after infection, JRS4 and JRS4Δslo-comp were found in autophagosomes adjacent to early endosomes, whereas almost all JRS4Δslo still resided in endosomes and not in autophagosomes (Fig. 5B). Subsequently, SLO was detected within endosomes in JRS4-infected cells at 2 h after infection, but not in JRS4Δslo-infected cells (Fig. 7B and supplemental Fig. S8B). These results indicated that the expression and accumulation of SLO acts directly on the pore formation of early endosomes in JRS4-infected cells and facilitates bacterial escape, leading to bacterial entry into the cytoplasm.
DISCUSSION
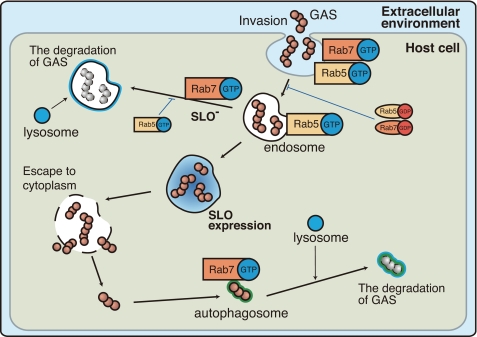
From these results, we obtained a comprehensive view of the pathway leading to autophagosome formation after GAS invasion, which involves endosome formation, GAS escape into the cytoplasm, and GAS degradation by the autophagosome-lysosomal pathway. This overall pathway is summarized in Fig. 8. The involvement of both GDP- and GTP-bound forms of Rab5 and Rab7 in this pathway is also described. SLO promotes the escape of GAS from endosomes, however, bacteria are sequestered.
FIGURE 8.
Schematic diagram of the endosomal and autophagic degradation pathway of intracellular GAS in HeLa cells. The illustration shows the intracellular behavior of GAS, including their invasion and escape into the cytoplasm (with SLO expression), in relation to the endosomal and autophagic degradation of GAS by the host cell. The involvement of both GDP- and GTP-bound forms of Rab5 and Rab7 in each event is also indicated. The endosomal and autophagic pathways of GAS are dependent on SLO expression and shown as thick lines. Blue arrows indicate the inhibition of these pathways by active or inactive Rab proteins.
GAS is reported to be capable of invading host cells via endocytosis, a process involving several bacterial fibronectin-binding proteins (e.g. F protein and FbaB) (11, 12, 37). The main purpose of this study was to elucidate the fate of GAS after its invasion into host cells. To achieve this, Rab5 and Rab7 were used as markers of early and late endosomes, respectively (23, 38–41). Rab5 was reported to localize to the membranes of early endosomes, activating Rab7 and promoting the Rab5-Rab7 exchange (24, 42). Rab7 is presumed to promote the fusion of endosomes with lysosomes (23).
First, the movement of the invading JRS4 in cells expressing Rab5 or Rab7 was analyzed using confocal microscopy. Although Rab7 is known to be a specific marker of late endosomes, our study showed that Rab7-positive compartments were seen throughout the observation period (Fig. 1A). If Rab7 associated only with the membranes of late endosomes, the results suggest that the emergence of Rab7-positive compartments occurred in conjunction with early endosomes. Alternatively, Rab7 might function in roles besides the promotion of endosome-lysosome fusion, such as the uptake of GAS (40). Under the electron microscope, intracellular JRS4 was seen in endosomes, however, the bacteria gradually escaped into the cytoplasm. Next, autophagosomes, which are characterized as containing cytoplasm inside multimembrane structures, began to emerge and sequester JRS4 (Fig. 2C) (30, 43). These observations confirmed that autophagosomes formed within cells in response to the bacterial infection.
It was reported that intracellularly invading M. tuberculosis is sequestered by autophagosomes, which surround not only the bacteria but also endosomal membranes (44). L. monocytogenes possessed a cytolysin LLO, a homolog of SLO of GAS. This bacterium was reported to escape from phagosomes into the cytoplasm using LLO and to evade autophagy by using another bacterial protein named ActA (45, 46). The intracellular behavior of L. monocytogenes is different from that of GAS, because the ActA facilitates its motility and cell-to-cell spread by polymerizing the host cell actin (47). Furthermore, L. monocytogenes can form Listeria-containing phagosomes in host macrophages and replicate within these vacuoles under conditions of low LLO expression (48). An intracellular obligate parasite, Coxiella burnetii, has an ability to replicate within intracellular vacuoles that are altered phagosomes and recruits Rab5 and Rab7 quickly to facilitate its uptake and vacuole development (49). Therefore, the behavior of GAS in host cells found in the present study was different from the strategies reported previously for other bacteria.
We also studied the functions of Rab proteins in GAS-infected cells using the DN mutant forms of Rab5 and Rab7 (33, 34). The number of early endosomes in cells expressing the DA form of Rab5 (Rab5Q79L) was significantly greater than in cells expressing wild-type Rab5 (Fig. 3A, left panels). This finding was consistent with a previous report that examined the uptake of transferrin in cells expressing Rab5Q79L (22). The inhibition of Rab5 GTPase activity may prevent the dissociation of Rab5 and its downstream events, including the Rab5-Rab7 exchange (22). In contrast, Rab5Q79L was reported to induce the rapid recruitment of Rab7, but failed to displace Rab5 during low-density lipoprotein uptake (24). Others have reported that Rab5Q79L restricted the viability of L. monocytogenes within phagosomes (50). In this study, although the elimination of JRS4Δslo occurred more slowly than that of JRS4, Rab5Q79L delayed its elimination even further (Fig. 6C). If Rab5Q79L promotes the Rab7 recruitment and lysosome fusion with endosomes, the intracellular GAS should have degraded rapidly, but this is not what we observed.
In the case of Rab7, our result using the DA form of Rab7 (Rab7Q67L) was consistent with a previous study showing that Rab7Q67L up-regulated the fusion of endosomes with lysosomes (Fig. 6D) (23). It is likely that GAS was degraded not only by the autophagic machinery, but also by the endosome-lysosomal pathway in cells expressing Rab7Q67L. Furthermore, DN mutants of Rab5 and Rab7 were reported to reduce the invasion of Trypanosoma cruzi in Chinese hamster ovary cells (20). This study also showed that both Rab5S34N and Rab7T22N decreased the invasion of GAS (Fig. 3B). On the other hand, the overexpression of Rab7T22N as reported does not influence the internalization of horseradish peroxidase or paramyxovirus SV5 protein (34).
Whereas our findings indicated that Rab5 was involved in both the fusion of endosomes and the uptake of GAS when cells were infected, involvement of Rab7 in the induction of endosomes may depend on the proteins or specific cellular receptors involved in the bacterial infection. Rab7 is also thought to be involved in the maturation of autophagosomes (25, 51). The present data suggested that Rab7 is localized to both endosomes and autophagosomes and is involved in the fusion of membrane structures with lysosomes (Figs. 2B and 6D). Thus, Rab7 showed multiple functions when the cells were infected with GAS strains. Furthermore, until GAS was eliminated by the autophagic machinery, the behavior of the intracellular bacteria was strongly influenced by Rab5 and Rab7 expression.
Bacterial cytolysin SLO is known to trigger multiple cellular responses, such as the induction of inflammatory cytokines and apoptosis (52–54). Although SLO is clearly a pathogenic factor of GAS, it seems counterintuitive that GAS expressing SLO are degraded more quickly than SLO-deficient mutants (Fig. 6A). However, we found that JRS4Δslo remained within endosomes for long periods and could not be eliminated efficiently by the endosome-lysosomal pathway (Fig. 6A). It seems that the autophagic machinery is more effective than the endosome-lysosomal pathway against intracellular GAS. Although it is not clear whether GAS can elude autophagy like S. flexneri (55), the behavior of GAS within host cells appears to be unique. In contrast to GAS, it was also reported that autophagy did not affect the fate of intracellular L. monocytogenes in macrophages (49, 56). The ability of GAS that provokes the autophagosome formation leads to degradation of itself after all. This phenomenon indicates that GAS has only an insufficient system for the evasion of intracellular host defense. However, a bacterial viability assay showed that small numbers of GAS were recovered from cells even 24 and 48 h after infection even under the effective bacterial killing (data not shown). In this regard, it is reported that the in vivo entry of GAS into host cells contributes to a bacterial persistence despite antibiotic therapy for a long term (12, 57). There might be another unique strategy of GAS to survive in host cells. In any case, our data support the idea that the autophagosome formation is effective for eliminating intracellular GAS and is influenced by SLO-dependent bacterial escape from endosomes.
GAS-induced autophagy is likely to be a mechanism derived specifically and selectively against intracellular bacteria, although autophagy has been identified as a nonspecific and bulk degradation pathway for cytoplasmic components (58, 59). If autophagy is controlled by specific molecules, such as hormones or cytokines, medical treatments against infectious diseases could be designed to take advantage of this regulation. Therefore, further studies on the mechanisms leading to autophagosome formation in bacteria-infected epithelial cells and the corresponding host responses should be pursued.
Supplementary Material
Acknowledgments
GAS strain JRS4 was kindly provided by Dr. E. Hanski (The Hebrew University, Jerusalem, Israel). The expression plasmids pOGW, pEGFP-LC3, and pmcherry-1 were given by Dr. S. Ishikawa (Nara Institute of Science and Technology, Nara, Japan), Dr. T. Yoshimori (National Institute for Basic Biology, Okazaki, Japan), and Dr. R. Y. Tsien (University of California), respectively.
This work was supported in part by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (B) 22390394, for Scientific Research (C) 22592032, for young scientists (B) 21792069, the Uehara Memorial Foundation, the Takeda Foundation, and the Mitsubishi Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9 and Tables S1 and S2.
- GAS
- group A streptococcus
- SLO
- streptolysin O
- LLO
- listeriolysin O
- EGFP
- enhanced green fluorescent protein
- DA
- dominant-active
- DN
- dominant-negative
- LC3
- microtubule-associated protein 1 light chain 3
- LAMP-1
- lysosome associated membrane protein-1
- PI
- propidium iodide
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Bisno A. L., Brito M. O., Collins C. M. (2003) Lancet Infect. Dis. 3, 191–200 [DOI] [PubMed] [Google Scholar]
- 2.Lukomski S., Sreevatsan S., Amberg C., Reichardt W., Woischnik M., Podbielski A., Musser J. M. (1997) J. Clin. Invest. 99, 2574–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madden J. C., Ruiz N., Caparon M. (2001) Cell 104, 143–152 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan J. T., Simpson A. J., Aziz R. K., Liu G. Y., Kristian S. A., Kotb M., Feramisco J., Nizet V. (2006) Curr. Biol. 16, 396–400 [DOI] [PubMed] [Google Scholar]
- 5.Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. (2005) Lancet Infect. Dis. 5, 685–694 [DOI] [PubMed] [Google Scholar]
- 6.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. (2004) Nature 432, 1032–1036 [DOI] [PubMed] [Google Scholar]
- 7.Yoshimori T., Yamagata F., Yamamoto A., Mizushima N., Kabeya Y., Nara A., Miwako I., Ohashi M., Ohsumi M., Ohsumi Y. (2000) Mol. Biol. Cell 11, 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., Hamada S., Yoshimori T. (2004) Science 306, 1037–1040 [DOI] [PubMed] [Google Scholar]
- 9.Ogawa M., Sasakawa C. (2006) Cell. Microbiol. 8, 177–184 [DOI] [PubMed] [Google Scholar]
- 10.Kornfeld S., Mellman I. (1989) Annu. Rev. Cell Biol. 5, 483–525 [DOI] [PubMed] [Google Scholar]
- 11.Terao Y., Kawabata S., Kunitomo E., Murakami J., Nakagawa I., Hamada S. (2001) Mol. Microbiol. 42, 75–86 [DOI] [PubMed] [Google Scholar]
- 12.Sakurai A., Okahashi N., Nakagawa I., Kawabata S., Amano A., Ooshima T., Hamada S. (2003) Infect. Immun. 71, 6019–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falkow S. (1991) Cell 65, 1099–1102 [DOI] [PubMed] [Google Scholar]
- 14.Gruenheid S., Finlay B. B. (2003) Nature 422, 775–781 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen L., Pieters J. (2005) Trends Cell Biol. 15, 269–276 [DOI] [PubMed] [Google Scholar]
- 16.Beauregard K. E., Lee K. D., Collier R. J., Swanson J. A. (1997) J. Exp. Med. 186, 1159–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara H., Tsuchiya K., Nomura T., Kawamura I., Shoma S., Mitsuyama M. (2008) J. Immunol. 180, 7859–7868 [DOI] [PubMed] [Google Scholar]
- 18.Amano A., Nakagawa I., Yoshimori T. (2006) J. Biochem. 140, 161–166 [DOI] [PubMed] [Google Scholar]
- 19.Novick P., Zerial M. (1997) Curr. Opin. Cell Biol. 9, 496–504 [DOI] [PubMed] [Google Scholar]
- 20.Wilkowsky S. E., Barbieri M. A., Stahl P. D., Isola E. L. (2002) Biochem. Biophys. Res. Commun. 291, 516–521 [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer S. (2003) Cell 112, 507–517 [DOI] [PubMed] [Google Scholar]
- 22.Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. (1994) EMBO J. 13, 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. (2000) Mol. Biol. Cell 11, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rink J., Ghigo E., Kalaidzidis Y., Zerial M. (2005) Cell 122, 735–749 [DOI] [PubMed] [Google Scholar]
- 25.Jäger S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. L. (2004) J. Cell Sci. 117, 4837–4848 [DOI] [PubMed] [Google Scholar]
- 26.Jadoun J., Ozeri V., Burstein E., Skutelsky E., Hanski E., Sela S. (1998) J. Infect. Dis. 178, 147–158 [DOI] [PubMed] [Google Scholar]
- 27.Tao L., LeBlanc D. J., Ferretti J. J. (1992) Gene 120, 105–110 [DOI] [PubMed] [Google Scholar]
- 28.Ruiz N., Wang B., Pentland A., Caparon M. (1998) Mol. Microbiol. 27, 337–346 [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa S., Kawai Y., Hiramatsu K., Kuwano M., Ogasawara N. (2006) Mol. Microbiol. 60, 1364–1380 [DOI] [PubMed] [Google Scholar]
- 30.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) EMBO J. 19, 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 32.Ridley A. J., Hall A. (1992) Cell 70, 389–399 [DOI] [PubMed] [Google Scholar]
- 33.Li G., Stahl P. D. (1993) J. Biol. Chem. 268, 24475–24480 [PubMed] [Google Scholar]
- 34.Feng Y., Press B., Wandinger-Ness A. (1995) J. Cell Biol. 131, 1435–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake S., Makimura M., Kanegae Y., Harada S., Sato Y., Takamori K., Tokuda C., Saito I. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai A., Okahashi N., Maruyama F., Ooshima T., Hamada S., Nakagawa I. (2008) Biochem. Biophys. Res. Commun. 373, 450–454 [DOI] [PubMed] [Google Scholar]
- 37.Terao Y., Kawabata S., Nakata M., Nakagawa I., Hamada S. (2002) J. Biol. Chem. 277, 47428–47435 [DOI] [PubMed] [Google Scholar]
- 38.Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. (1990) Cell 62, 317–329 [DOI] [PubMed] [Google Scholar]
- 39.Méresse S., Gorvel J. P., Chavrier P. (1995) J. Cell Sci. 108, 3349–3358 [DOI] [PubMed] [Google Scholar]
- 40.Press B., Feng Y., Hoflack B., Wandinger-Ness A. (1998) J. Cell Biol. 140, 1075–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceresa B. P., Lotscher M., Schmid S. L. (2001) J. Biol. Chem. 276, 9649–9654 [DOI] [PubMed] [Google Scholar]
- 42.Grosshans B. L., Ortiz D., Novick P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskelinen E. L., Schmidt C. K., Neu S., Willenborg M., Fuertes G., Salvador N., Tanaka Y., Lüllmann-Rauch R., Hartmann D., Heeren J., von Figura K., Knecht E., Saftig P. (2004) Mol. Biol. Cell 15, 3132–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine B. (2005) Cell 120, 159–162 [DOI] [PubMed] [Google Scholar]
- 45.Portnoy D. A., Jacks P. S., Hinrichs D. J. (1988) J. Exp. Med. 167, 1459–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pust S., Morrison H., Wehland J., Sechi A. S., Herrlich P. (2005) EMBO J. 24, 1287–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilney L. G., Portnoy D. A. (1989) J. Cell Biol. 109, 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birmingham C. L., Canadien V., Kaniuk N. A., Steinberg B. E., Higgins D. E., Brumell J. H. (2008) Nature 451, 350–354 [DOI] [PubMed] [Google Scholar]
- 49.Romano P. S., Gutierrez M. G., Berón W., Rabinovitch M., Colombo M. I. (2007) Cell Microbiol. 9, 891–909 [DOI] [PubMed] [Google Scholar]
- 50.Prada-Delgado A., Carrasco-Marín E., Peña-Macarro C., Del Cerro-Vadillo E., Fresno-Escudero M., Leyva-Cobián F., Alvarez-Dominguez C. (2005) Traffic 6, 252–265 [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi H., Nakagawa I., Yamamoto A., Amano A., Noda T., Yoshimori T. (2009) PLoS Pathog. 5, e1000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limbago B., Penumalli V., Weinrick B., Scott J. R. (2000) Infect. Immun. 68, 6384–6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stassen M., Müller C., Richter C., Neudörfl C., Hültner L., Bhakdi S., Walev I., Schmitt E. (2003) Infect. Immun. 71, 6171–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmer A. M., Timmer J. C., Pence M. A., Hsu L. C., Ghochani M., Frey T. G., Karin M., Salvesen G. S., Nizet V. (2009) J. Biol. Chem. 284, 862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa M., Yoshimori T., Suzuki T., Sagara H., Mizushima N., Sasakawa C. (2005) Science 307, 727–731 [DOI] [PubMed] [Google Scholar]
- 56.Meyer-Morse N., Robbins J. R., Rae C. S., Mochegova S. N., Swanson M. S., Zhao Z., Virgin H. W., Portnoy D. (2010) PLoS One 5, e8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neeman R., Keller N., Barzilai A., Korenman Z., Sela S. (1998) Lancet 352, 1974–1977 [DOI] [PubMed] [Google Scholar]
- 58.Mizushima N., Ohsumi Y., Yoshimori T. (2002) Cell Struct. Funct. 27, 421–429 [DOI] [PubMed] [Google Scholar]
- 59.Wang C. W., Klionsky D. J. (2003) Mol. Med. 9, 65–76 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.