Abstract
Antibiotics can induce cell death via a variety of action modes, including the inhibition of transcription, ribosomal function, and cell wall biosynthesis. In this study, we demonstrated directly that iron availability is important to the action of antibiotics, and the ferric reductases of Pseudomonas putida and Pseudomonas aeruginosa could accelerate antibiotic-mediated cell death by promoting the Fenton reaction. The modulation of reduced nicotinamide-adenine dinucleotide (NADH) levels and iron chelation affected the actions of antibiotics. Interestingly, the deletion of the ferric reductase gene confers more antibiotic resistance upon cells, and its overexpression accelerates antibiotic-mediated cell death. The results of transcriptome analysis showed that both Pseudomonas species induce many oxidative stress genes under antibiotic conditions, which could not be observed in ferric reductase mutants. Our results indicate that iron homeostasis is crucial for bacterial cell survival under antibiotics and should constitute a significant target for boosting the action of antibiotics.
Keywords: Antibiotics, Antioxidant, Bacteria, Bacterial Genetics, Flavoproteins, Iron, Oxidative Stress, Oxygen Radicals, Bacterial Cell Death, Fenton Reaction
Introduction
Pseudomonas species perform key roles in the environment, including the degradation of natural and man-made chemicals and the establishment of important interactions with plants and animals (1–3). One pseudomonad, Pseudomonas aeruginosa, is a focus of particular study because it is the predominant opportunistic pathogen of cystic fibrosis patients (1, 4). Clearance is difficult because of its unusually low susceptibility to antibiotic treatment (2, 5). Several general factors contribute to this resistance, including the low permeability of the cellular envelope and the actions of multidrug efflux pumps (6, 7). In addition, quinolone treatment can be confounded by the outgrowth of subpopulations that have an altered lipid composition that is poorly permeable to hydrophilic quinolones (8–11). Ominously, integrons that harbor genes encoding metallo-β-lactamase have also been found in the transposons and plasmids of Pseudomonas putida isolates (12, 13), suggesting that these genetic elements might be transferred to P. aeruginosa and the Enterobacteriaceae (3). As many pseudomonads inhabit natural environments in which antibiotic exposure in possible, and because P. aeruginosa in particular comprises a public health hazard, Pseudomonas species are model strains for studying the development of antibiotic resistance.
Until recently, bactericidal antibiotics were believed to kill cells by several well established mechanisms, typically involving the disruption of cell wall biosynthesis (ampicillin), the interruption of DNA replication (norfloxacin), or the overwhelming inhibition of protein synthesis (gentamicin and kanamycin) (14, 15). However, a system-analysis investigation conducted with Escherichia coli by Kohanski, Collins, and co-workers (14–16) raised the possibility that in these organisms, bacteriocidal antimicrobials might additionally create oxidative stress, and that a part of their toxicity in aerobic habitats might be due to the accumulation of reactive oxygen species. Reactive oxygen species such as superoxide and hydrogen peroxide can block growth by inactivating key enzymes; additionally, they also are precursors of the hydroxyl radical (17). The latter species is formed through the Fenton reaction (17, 18), in which unincorporated intracellular iron transfers an electron to hydrogen peroxide (Reaction 1).
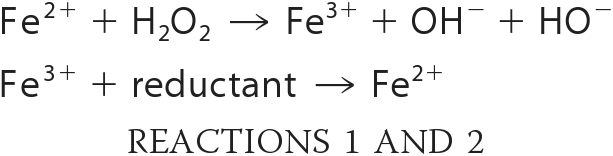 |
The process is cyclical in vivo because intracellular reductants, including cysteine and reduced flavins, can reduce the oxidized iron back to its ferrous form (Reaction 2). The hydroxyl radical is powerful oxidant enough to react with either the base or sugar residues of DNA, leading to base modification and strand breakage. Because iron associates easily with nucleic acids, DNA is a common target, and, in fact, DNA damage is the cause of cell death when cells are stressed with either exogenous or endogenous H2O2 (18, 19). Strikingly, E. coli was substantially protected against the lethal effects of antibiotics by cell-permeable iron chelators that inhibit the Fenton reaction (19).
In this study, we tested whether oxidative stress is a significant component of antibiotic action against two Pseudomonas species, P. putida KT2440 and P. aeruginosa PAO1. Transcriptional profiling data confirmed that the expression of antioxidant enzymes was induced during exposure to a variety of antibiotics, and fluorescent probes indicated an increase in intracellular oxidants. DNA damage was detected, and cell death depended upon unincorporated iron and was facilitated by ferredoxin reductase, an iron-reducing enzyme that catalyzes reaction 2 above. Collectively, these data provide further support to the notion that antibiotics create oxidative stress, and they demonstrate that this phenomenon is not limited to enteric bacteria.
EXPERIMENTAL PROCEDURES
Antibiotics, Media, and Bacterial Strains
P. putida and P. aeruginosa are cultured in M9 minimal medium containing Na2HPO4·7H2O (6.8 g), KH2PO4 (3 g), NaCl (0.5 g), NH4Cl (1 g), MgSO4 (2 mm), and CaCl2 (0.1 mm) with glucose (2 g/liter) as a carbon source and ferric citrate 100 μm at 30 °C and 37 °C under vigorous aeration. For antibiotic stress experiments, we used the antibiotics (ampicillin (Fluka); kanamycin (Sigma); norfloxacin (Sigma); gentamycin (Invitrogen). The mutant strains of P. aeruginosa were purchased from the Washington University Genome Center.
Gene Expression Analysis
The cells were grown to mid-log phase (A600 of ∼0.5) at 30 °C or 37 °C, with aeration. Then, the cells were treated by adding antibiotics over a period of 15 min. Total RNA was isolated from 5 ml of exponentially growing cells, using the RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Gene expression analysis was performed as described previously (20). RNA concentrations were estimated using absorbance at 260 nm. Samples of total RNA (10 μg) were loaded on denaturing agarose gels containing 0.25 m formaldehyde and electrophoresed, and the gels were stained with ethidium bromide to visualize 23 S and 16 S rRNA. The fractionated RNA was transferred to nylon membranes (Schleicher & Schuell) using a TurboBlotter (Schleicher & Schuell). The amounts of ahpC, gor, and recA mRNA were determined by hybridizing the membrane with a ahpC, gor, and recA-specific, 32P-labeled probe (Takara Bio, Inc.) prepared by PCR amplification with primer pairs, ahpC F/ahpC R, gor F/gor R, and recA F/recA R (where R is reverse and F is forward), respectively. The sequence of ahpC F/R are 5′-CTT CAA CGC CAC CGC CTA CCA-3′/5′-CGA CGG CGC CAG AGT GGC TTC-3′ and gor F/R are 5′-TCC GGT GGT GTG CGC GCA GCG C-3′/5′-ATG GTC ACT GGC C-3′. The recA F/R primers are 5′-CGG CAA GCT GGG CGT CAA CGT CGA TGA CCT-3′/5′-GCA GTT CGC GTT CTT GAT GTT ACC GGT GAT-3′.
Enzyme Activity Assay and Staining
All cells were harvested at exponential phase (A600 of ∼0.5). Harvested cells were suspended in Tris/Cl buffer (pH 7.2) and then disrupted via sonication (Sonics and Materials, Inc.). The suspensions were clarified via centrifugation (8,000 × g) at 4 °C. The amounts of total protein in the cell extracts were quantified via a protein assay (Bio-Rad). The total catalase activity levels in the cell extracts were spectrophotometrically measured as described by Beers and Sizer (21). The peroxide activity method was previously described by Heym and Cole (22). The catalase activity measures the decrease in absorbance at 240 nm, and an activity unit is defined as the enzyme activity required to decompose 1 mol of H2O2 per min at room temperature. To determine the concentration of a hydrogen peroxide solution, the absorbance was measured at 240 nm and a molar extinction coefficient of 43.6 m−1 cm−1 was used. The peroxide activity was then assayed by continuously measuring the increase in absorbance at 500 nm and using a molar extinction coefficient of 12.2 mm−1 cm−1. For catalase and peroxidase activity staining, aliquots of cell extracts containing 50 μg of proteins were electrophoresed on a 7.5% (v/v) native polyacrylamide gel and stained for catalase and peroxidase activity as described elsewhere (22). The band was analyzed using ProXPRESS 2D (Perkin Elmer) and Total Lab 2.0 software (Nonlinear Dynamics, BioSystematica).
Cell Survival and Oxidative Stress Detection Using Flow Cytometry
The cells were grown to mid-log phase (A600 of ∼0.5) at 30 °C or 37 °C, with aeration. Then, the cells were treated by adding antibiotics over a period of 1 h. The treated cells were washed in phosphate-buffered saline buffer and aliquot ∼1 ml to flow cytometry tubes. For the viability test, cells were stained with the LIVE/DEAD BacLight RedoxSensorTM Green Viability kit (Molecular Probes, Invitrogen) for 10 min at 37 °C in the dark, in accordance with the manufacturer's instructions. The oxidative stress probe dihydrorhodamine 123 (Sigma) was used to measure intracellular oxidation levels in bacteria. Antibiotics were added to the culture as indicated, and the samples were incubated for an additional 1 h. The cells were washed in phosphate-buffered saline, and dihydrorhodamine was added to the cells at 5.0 μg/ml from a 2.5 mg/ml stock solution in ethanol, after which the cells were incubated for 1 h. The cells were then washed, resuspended in phosphate-buffered saline, and transferred to flow cytometry tubes. Fluorescence intensity was measured on a FACSCalibur (Becton Dickinson), and the mean fluorescence channel value of 20,000 cells was determined upon analysis of the live or dead cell population, which was defined by forward and side scatter using BD CellQuest Pro software (BD Biosciences). The data were analyzed in Forward Scatter (FSC)/FL-1, FSC/FL-3, and FL-1/FL-3 dot plots or histograms.
Survival and Sensitivity of P. putida and P. aeruginosa under Antibiotics
Different concentrations of antibiotics (ampicillin, norfloxacin, and gentamycin) and hydrogen peroxide were employed to determine the survival rates of the wild-type and mutant strains under a variety of antibiotic stress conditions. The M9 with ferric citrate cultured all cells of P. putida and P. aeruginosa were collected at the exponential phases, respectively, and then washed three times with autoclaved phosphate-buffered saline (pH 7.4). Approximately 107 colony-forming units/ml were inoculated into phosphate-buffered saline (2 ml) containing different antibiotics, and the cultures were incubated at room temperature with constant agitation (220 rpm). At each time point (0, 3, 5, 10, and 15 min), the cells were harvested and washed in phosphate-buffered saline. The number of viable cells was determined by dilution in LB and by plating them on LB plates. In the sensitivity test, all cells were collected at the exponential phases and then washed three times with autoclaved phosphate buffer saline (pH 7.4). All samples counted the number of cells and diluted. Diluted samples were spotted on M9 with ferric citrate plates containing a variety of antibiotics.
Detection of DNA Damage by Antibiotics
DNA damage was detected by the hOGG1 FLARETM assay kit (Trevigen). Slide preparation and electrophoresis condition was performed according to the manufacturer's instructions. All cells were treated by 1 mm hydrogen peroxide and 150 μg/ml ampicillin for 1 h. For the lysis of bacteria cells, a high concentration (0.5 mg/ml) of lysozyme was required for the complete destruction of the bacterial cell wall, which was rich in peptidoglycan lipopolysaccharide. RNase digestion was also essential as bacterial cells, because bacteria were also rich in RNA, which was involved in the maintenance of the tertiary structure of the chromosome. The disrupted cells were electrophoresed for 1 h at 12 V in 1× TAE buffer and visualized using SYBR® Green I dye. All pictures were imaged by the fluorescence microscope (Axio Imager M1, Carl-Zeiss).
Gene Expression Analysis
The integrity of the bacterial total RNA was assessed by capillary electrophoresis with an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA) and further purified with an RNeasy mini kit (Qiagen). cDNA probes for cDNA microarray analysis were prepared via the reverse transcription of total RNA (50 μg) in the presence of aminoallyl-dUTP and 6 μg of random primers (Invitrogen) for 3 h. The cDNA probes were cleaned up using a Microcon YM-30 column (Millipore, Bedford, MA) followed by coupling to Cy3 dye (for reference) or Cy5 dye (for test sample) (Amersham Biosciences Pharmacia). The dried Cy3- or Cy5-labeled cDNA probes were then resuspended in hybridization buffer containing 30% formamide (v/v), 5× SSC, 0.1% SDS (w/v), and 0.1 mg/ml salmon sperm DNA. The Cy3- or Cy5-labeled cDNA probes were mixed together and hybridized to a microarray slide. The hybridization images on the slides were scanned by Axon 4000B (Axon Instrument, Union City, CA). Hybridization image was analyzed by GenePix Pro 3.0 software (Axon Instruments, Union City, CA) to determine the gene expression ratios (control versus test sample). Gene expression ratios were normalized using GenePix Pro 3.0 software (Axon Instrument, Union City, CA). Clustering images were obtained from hierarchical clustering, which involves computing “distances” between data elements.
RESULTS
Several Markers Indicate That Antibiotics Create Oxidative Stress in Pseudomonas
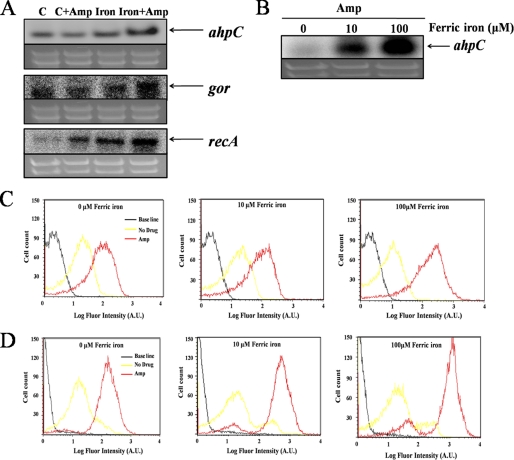
Recent studies indicated that antibiotics generate oxidative stress in enteric bacteria (15). To test whether a similar phenomenon occurs in pseudomonads, we monitored the expression levels of ahp and gor, encoding NADH peroxidase and glutathione reductase. These genes are regulated positively by the OxyR transcription factor, which, in turn, can be activated by intracellular hydrogen peroxide (23). We observed that both genes were induced by antibiotic exposure (Fig. 1A). Antibiotic-driven induction was especially strong when iron was included in the growth medium (Fig. 1, A and B). These data indicate that H2O2 levels are elevated during antibiotic stress.
FIGURE 1.
Iron concentration-dependent oxidative stress response in P. putida. A, expression of oxidative stress-related genes (ahpC, gor, and recA) was assessed at the transcriptional level with ampicillin (Amp, 100 μg/ml) and iron (ferric-citrate, 10 μm) for 15 min. B, iron concentration-dependent gene expression under ampicillin (100 μg/ml). C, direct detection of intracellular oxidative stress is conducted with dihydrorhodamine 123 (DHR) using flow cytometry (1 h under 150 μg/ml ampicillin). D, cell death was confirmed using flow cytometry with LIVE/DEAD BacLight RedoxSensorTM Green Viability Kit. A.U., absorbance unit.
Dihydrorhodamine 123 dye is commonly used as an indicator of intracellular oxidative stress (24, 25). This uncharged, nonfluorescent compound can diffuse passively across most uncharged membranes. Oxidation converts it to the fluorescent product rhodamine 123 (24, 25). We observed that ampicillin-treated cells accumulated the fluorescent product, especially when iron was provided (Fig. 1C). This result resembles the effect that was observed in antibiotic-treated E. coli with a different dye probe (15). Neither dye is absolutely selective, so the identity of the oxidant in these experiments is uncertain. Elevated concentrations of H2O2 typically lead to an increased rate of hydroxyl radical formation and, as a consequence, oxidative DNA damage. Indeed, the recA gene was strongly induced during antibiotic treatment, indicating the presence of DNA lesions (Fig. 1A). This effect also was more pronounced when iron was included in the growth medium. Finally, measurements of colony formation showed that cell viability declined only moderately when ampicillin alone was added to cells but that cell death was much greater when iron was present (see supplemental Fig. 1). A similar result was observed when a LIVE/DEAD BacLight RedoxSensor Green Viability probe, which indicates cell survival and death with high specificity, was used to quantify live cells (Fig. 1D). These data support the conclusion that antibiotics create severe oxidative stress when iron is available.
Other Antibiotics Similarly Activate Oxidative Stress Genes
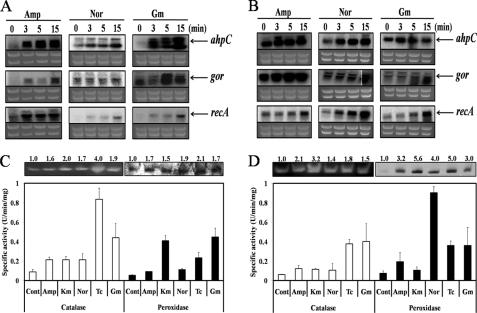
Gene expression analysis demonstrated that ahpC, gor, and recA also were induced abundantly when cells were treated with norfloxacin and gentamycin (Figs. 2, A and B) as well as tetracycline (see supplemental Fig. 2). Interestingly, we were able to observe that tetracycline, a bateriostatic antibiotic, also can generate oxidative stress in bacterial cells (supplemental Fig. 2). This is partially supported by the facts that superoxide dismutase is required for cell survival under the antibiotic class of tetracycline, and hydroxyl radical generation by bacteriostatic tetracycline antibiotics can damage DNA, lipids, and carbohydrates in the presence of iron and copper (26). Therefore, we confirmed that both bacteriostatic and bacteriocidal antibiotics can generate oxidative stress. Enzyme assays confirmed that a variety of antibiotics stimulate synthesis of catalase, another OxyR-controlled protein (Fig. 2, C and D). These effects were observed in both P. putida and P. aeruginosa. Thus, both bacteriostatic and bacteriocidal antibiotics can generate oxidative stress.
FIGURE 2.
Various antibiotics (Amp, ampicillin; Nor, norfloxacin; Gm, gentamycin) can increase expression of oxidative stress-related genes (ahpC, gor, and recA). Expression was assessed for 15 min with 100 μm ferric citrate in P. putida (A) and in P. aeruginosa (B). Catalase and peroxidase activity assays in P. putida (C) and in P. aeruginosa (D) are shown. Cont, control (no antibiotics); Amp, ampicillin (100 μg/ml); Km, kanamycin (2 μg/ml); Nor, norfloxacin (0.2 μg/ml); Tc, tetracycline (10 μg/ml); Gm, gentamicin (2 μg/ml).
Intracellular Ferrous Iron Is Involved in Antibiotic-induced Cell Death
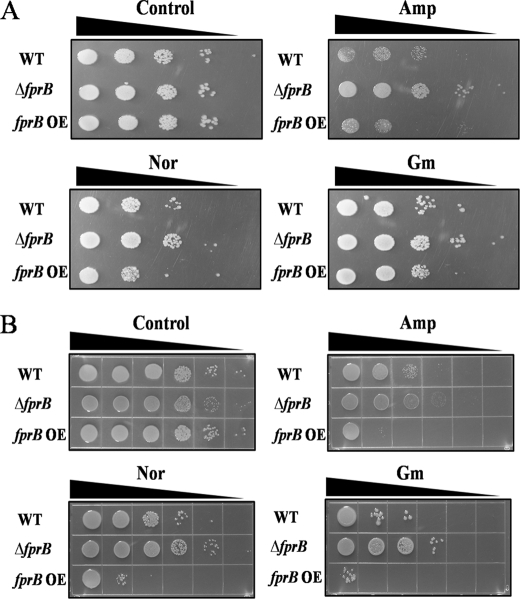
When cells are stressed by H2O2, the rate of hydroxyl radical formation depends upon the rate at which oxidized iron is recycled to the reduced to the ferrous form. We discovered previously that ferredoxin-NADP+ reductase (FprB)3 is a ferric reductase in P. putida (20, 27). Thus, we hypothesized that if antibiotic exposure creates H2O2 stress, FprB might promote Fenton chemistry and contribute to cell death. Indeed, an fprB mutant (PA4615 in P. aeruginosa) was more resistant to ampicillin, norfloxacin, and gentamycin than its wild-type parent (Fig. 3, A and B). Conversely, a strain engineered to overproduce FprB was more sensitive. The same outcome was observed by the LIVE/DEAD BacLight assay (supplemental Fig. 3).
FIGURE 3.
The sensitivity tests were conducted under antibiotic condition using the wild-type, the fprB mutant strain (ΔfprB) and the fprB overexpressed strain (fprB OE) of P. putida (A) and P. aeruginosa (B). Amp, ampicillin (100 μg/ml); Nor, norfloxacin (0.2 μg/ml); Gm, gentamicin (2 μg/ml); WT, wild-type.
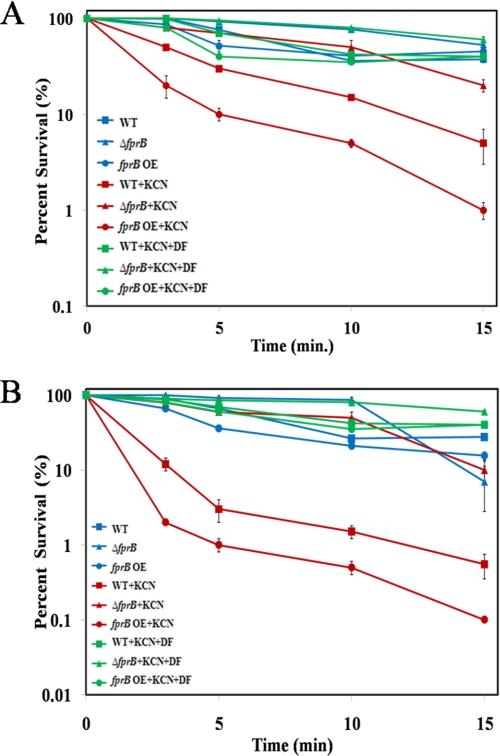
Cyanide treatment accelerates the rate of Fenton chemistry in E. coli by diverting electrons from the respiratory chain to its ferric reductase (Fre) (19). We observed that cyanide also enhanced the toxicity of antibiotics in both Pseudomonas species (Fig. 4, A and B, and supplemental Fig. 4), and this potentiation depended upon FprB. (An equivalent 15-min exposure to cyanide alone did not kill these strains (supplemental Fig. 5).) Importantly, desferrioxamine, a cell-permeable iron chelator that blocks intracellular Fenton chemistry, prevented cell death. When dihydrorhodamine 123 was used to measure antibiotic-generated oxidative stress, its conversion to intracellular rhodamine also was diminished in strains that lacked FprB, and it was elevated in strains that overproduced it (Fig. 5). Furthermore, desferrioxamine also inhibited its oxidation (supplemental Fig. 6). These data suggest that the oxidant of dihydrorhodamine is likely to be either the ferryl or hydroxyl radicals that are generated by the Fenton reaction. Collectively, these data indicate that antibiotic stress drives intracellular Fenton chemistry and that this process is an important contributor to the death of Pseudomonas strains.
FIGURE 4.
The survival test was conducted with 100 μm ferric citrate in liquid conditions under ampicillin (200 μg/ml) in P. putida (A) and in P. aeruginosa (B). Potassium cyanide (KCN, 3 mm) and desferoxamin (DF, 5 mm) were added prior to antibiotics treatment. WT, wild-type; fprB OE, fprB overexpressed strain.
FIGURE 5.
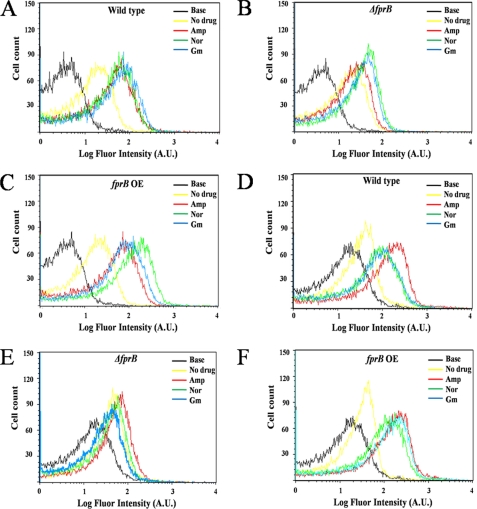
Intracellular oxidative stress was detected by dihydrorhodamine dye (5. 0 μg/ml) using flow cytometry under antibiotics: the wild-type, the fprB mutant strain (ΔfprB), and the fprB overexpressed strain (fprB OE) of P. putida (A, B, and C, respectively) and P. aeruginosa (D, E, and F, respectively). The concentrations of antibiotics were as follows: ampicillin (Amp, 150 μg/ml, red color), norfloxacin (Nor, 0.5 μg/ml, green color), and gentamicin (Gm, 5 μg/ml, blue color). A.U., absorbance unit.
Antibiotic Stress Causes Oxidative DNA Damage
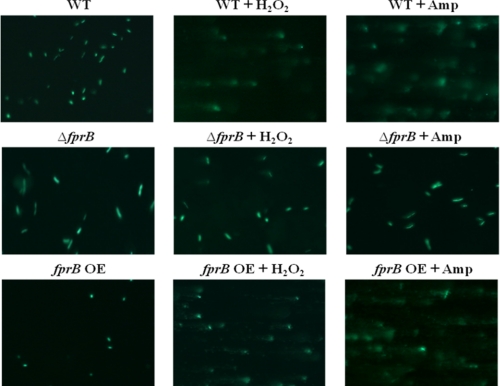
In well studied bacterial systems, the primary cause of cell death during Fenton chemistry is damage to DNA. The observation that recA was induced during antibiotic stress (Fig. 1A) suggested that this would be true of pseudomonads as well. To appraise DNA damage more directly, we used an assay that detects 8-oxoguanine, one of the most common base modifications that arise from DNA oxidation (28). 8-Oxoguanine is one of the most common DNA lesions resulting from reactive oxygen species and can result in a mismatched pairing with adenine. Glycosylase OGG1 enzyme can detect 8-oxoguanine and cut it out. In the 8-oxoguanine assay, cleaved DNA fragments migrate out of the cell under electrophoresis condition, whereas undamaged supercoiled DNA remains within the cell. If antibiotics cause DNA damage, long comet tails are shown in the 8-oxoguanine assay (comet assay). Treated or control cells were embedded into agar, disrupted with lysozyme and RNase, incubated with OGG1 glycosylase, and then electrophoresed. DNA was then visualized by SYBR® Green I dye. Cells that were treated with H2O2, as a control, or with ampicillin displayed longer DNA tails than did untreated cells (Fig. 6 and supplemental Fig. 7). The DNA of fprB mutants was substantially less damaged by either H2O2 or ampicillin.
FIGURE 6.
A photomicrograph of damaged DNA molecules under ampicillin (Amp, 150 μg/ml) in P. putida. Cells were treated by hydrogen peroxide (H2O2, 1 mm) as a positive control. All cells were disrupted by lysozymes, and bacterial RNAs were removed by RNase. And then, samples were electrophoresed for 1 h at 12 V in 1× TAE buffer. The SYBR® GREEN I dye was used for visualizing damaged DNA molecules with 400× magnification. The long tails in photomicrograph indicates DNA fragments and damaged DNAs. fprB OE, fprB overexpressed strain; WT, wild-type.
Transcriptome Analysis of P. putida and P. aeruginosa during Antibiotic Stress
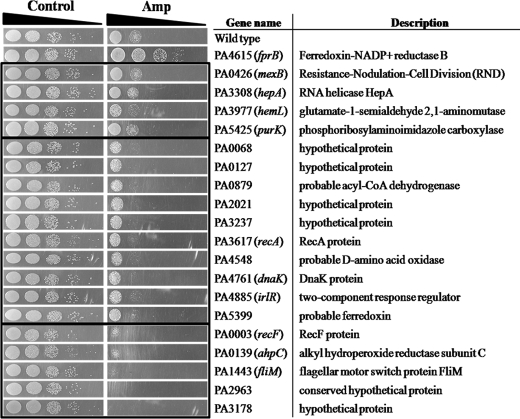
Microarray experiments were conducted after ampicillin was added to P. putida (supplemental Table 1) and P. aeruginosa (supplemental Table 2). The genes that were induced or repressed by at least 2-fold were divided into eight categories (supplemental Fig. 8A). The induced genes included oxidative defense genes such as ahpC and the DNA repair genes such as ung (encoding uracil DNA glycosylase); genes in these categories are displayed in supplemental Fig. 8, B (P. putida) and C (P. aeruginosa). However, many other genes were induced as well (supplemental Tables 1 and 2), and no simple picture emerged. To identify possible cellular targets for antibiotics, we purchased mutants of twenty genes that were induced in P. aeruginosa, including several with likely roles in oxidative defense or DNA metabolism. The ampicillin sensitivity of four of these mutants was unchanged from that of their wild-type parent, whereas ten were slightly sensitive and five were very sensitive (Fig. 7). Among the latter were mutants in ahpC and recF, which would have a reduced capacity for H2O2 scavenging and for repair of DNA damage, respectively.
FIGURE 7.
The sensitivity test was conducted on plates with 100 μm ferric citrate under ampicillin (Amp, 150 μg/ml) in P. aeruginosa mutants. All mutant cells are chosen by P. aeruginosa microarray results (supplemental Fig. 8C). Serial dilutions of all mutants were spotted. Cells grew at 37 °C.
DISCUSSION
Although antibiotics remain potent in anaerobic habitats, our data extend the observation that in aerobic habitats, they may impose an additional, oxidative stress upon target bacteria. Generally, bacteria require iron to survive as well as pseudomonads (19). To acquire iron from the soil environment, bacteria make biosynthetic iron chelators, which are known as siderophores. However, under conditions of excessive intracellular iron concentration, iron can cause bacterial cell death via the Fenton reaction (19). In such cases, the intracellular iron concentration must be adjusted clearly. Our study provides evidence to suggest that antibiotics can boost oxidative stress in the presence of specific iron concentrations. Our data directly showed that i) antibiotics generate oxidative stress, ii) iron concentration is an important factor in the mode of antibiotic action, iii) ferric reductase might accelerate bacterial cell death under antibiotic conditions, and iv) many oxidative stress defense and DNA repair genes are highly up-regulated in microarray analysis. These conclusions pertained both to the soil environment model strain, P. putida, and to the human pathogen, P. aeruginosa. Thus, oxidative stress appears to be a common mechanism of antibiotic-induced cellular death (15, 29, 30). It is far from clear why this is the case. One notion is that antibiotics somehow stimulate the intracellular formation of superoxide and/or hydrogen peroxide (15, 29). Because Pseudomonas strains synthesize pyocyanin (31, 32), a redox-cycling antibiotic that can generate reactive oxygen species, we tested whether it might be involved in the oxidative stress phenomena that are triggered by antibiotics. However, antibiotics continued to stimulate dihydrorhodamine oxidation in phzM and phzS mutants that cannot synthesize pyocyanin (supplemental Fig. 9) and antibiotics did not induce synthesis of pyocyanin (data not shown).
So how might antibiotics stimulate intracellular oxidant formation? Oxidative stress must be secondary to the primary modes of action of antibiotics because mutations that circumvent the action of aminoglycosides upon ribosomes or of ampicillin upon penicillin-binding proteins also block cell death (33). One clue might be the observation that oxidative injuries appear to be created by treatments that inhibit DNA replication or that activate addiction proteins, which causes programmed cell death in prokaryote cells (16, 33, 34). A common element to these stresses may be that they, like ribosomal inhibitors, cause incomplete protein peptides to be formed. It seems possible that these peptides might associate with metabolic redox enzymes in a way that disrupts their function, perhaps causing them to transfer electrons to oxygen rather than to their physiological acceptor (34). However, it is important to note that the methods that have been used to document oxidative stress under these conditions have thus far been indirect, so the idea that H2O2 production is accelerated should not be regarded as proven (35). Further, given that ampicillin is known only to act directly as an inhibitor of cell wall biosynthesis, its effects do not easily fit the small peptide model. Thus, the immediate challenge in the field remains the discovery of a molecular connection between antibiotics and oxidative stress. Strikingly, our data indicate that antibiotic efficiency can be modulated by altering the iron concentration. The ferric iron reductase, FprB, was important for this effect. In principle, this opens the door to combination therapies that maximize antibiotic efficacy by elevating the levels of intracellular ferrous iron; conversely, mutants that are deficient in iron import and/or reduction might be expected to be outgrowers when populations are exposed to antibiotics that owe some of their potency to oxidative toxicity.
Supplementary Material
This work was supported by Grant 20090091491 from the MEST/NRF to the Environment Biotechnology National Core Research Center and Grant 2009-0076488from the MEST/NRF program, South Korea.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–9.
- FprB
- ferredoxin-NADP+ reductase.
REFERENCES
- 1.Gómez M. I., Prince A. (2007) Curr. Opin. Pharmacol. 7, 244–251 [DOI] [PubMed] [Google Scholar]
- 2.Gooderham W. J., Hancock R. E. (2009) FEMS Microbiol. Rev. 33, 279–294 [DOI] [PubMed] [Google Scholar]
- 3.Cornelis P. (ed) (2008) Pseudomonas: Genomics and Molecular Biology, pp. 177–236, Caister Academic Press, Norfolk, UK [Google Scholar]
- 4.Thomas C. M. (1998) Pseudomonas, pp. 133–134, Plenum Press, New York [Google Scholar]
- 5.Page M. G., Heim J. (2009) Curr. Opin. Pharmacol. 9, 558–565 [DOI] [PubMed] [Google Scholar]
- 6.Jeannot K., Elsen S., Köhler T., Attree I., van Delden C., Plésiat P. (2008) Antimicrob. Agents Chemother. 52, 2455–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lister P. D., Wolter D. J., Hanson N. D. (2009) Clin. Microbiol. Rev. 22, 582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michéa-Hamzehpour M., Furet Y. X., Pechère J. C. (1991) Antimicrob. Agents Chemother. 35, 2091–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris M. J., Rogers D. T., Russell A. D. (1985) Lett. Appl. Microbial. 1, 3–6 [Google Scholar]
- 10.Perz J. F., Craig A. S., Stratton C. W., Bodner S. J., Phillips W. E., Jr., Schaffner W. (2005) J. Clin. Microbiol. 43, 5316–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolston K. V., Kontoyiannis D. P., Yadegarynia D., Raad I. I. (2005) Diagn. Microbiol Infect. Dis. 51, 215–218 [DOI] [PubMed] [Google Scholar]
- 12.Docquier J. D., Riccio M. L., Mugnaioli C., Luzzaro F., Endimiani A., Toniolo A., Amicosante G., Rossolini G. M. (2003) Antimicrob. Agents Chemother. 47, 1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel L., Cabanne L., Collet L., Nordmann P. (2006) Antimicrob. Agents Chemother. 50, 2889–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer D. J., Kohanski M. A., Collins J. J. (2009) Curr. Opin. Microbiol. 12, 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohanski M. A., Dwyer D. J., Hayete B., Lawrence C. A., Collins J. J. (2007) Cell 130, 797–810 [DOI] [PubMed] [Google Scholar]
- 16.Kohanski M. A., Dwyer D. J., Wierzbowski J., Cottarel G., Collins J. J. (2008) Cell 135, 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imlay J. A., Linn S. (1988) Science 240, 1302–1309 [DOI] [PubMed] [Google Scholar]
- 18.Touati D. (2000) Arch. Biochem. Biophys. 373, 1–6 [DOI] [PubMed] [Google Scholar]
- 19.Woodmansee A. N., Imlay J. A. (2002) J. Biol. Chem. 277, 34055–34066 [DOI] [PubMed] [Google Scholar]
- 20.Yeom J., Jeon C. O., Madsen E. L., Park W. (2009) J Bacteriol 191, 1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beers R. F., Sizer I. W., Jr (1952) J. Biol. Chem. 195, 133–140 [PubMed] [Google Scholar]
- 22.Heym B., Cole S. T. (1992) Res. Microbiol. 143, 721–730 [DOI] [PubMed] [Google Scholar]
- 23.Banerjee R. (2008) Redox Biochemistry, Wiley Interscience, New Jersey [Google Scholar]
- 24.Cabiscol E., Bellí G., Tamarit J., Echave P., Herrero E., Ros J. (2002) J. Biol. Chem. 277, 44531–44538 [DOI] [PubMed] [Google Scholar]
- 25.Semenza G. L. (1999) Cell 98, 281–284 [DOI] [PubMed] [Google Scholar]
- 26.Angrave F. E., Avery S. V. (2001) Antimicrobe Agents Chemother. 45, 2939–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeom J., Jeon C. O., Madsen E. L., Park W. (2009) J. Biochem. 145, 481–491 [DOI] [PubMed] [Google Scholar]
- 28.Singh N. P., Stephens R. E., Singh H., Lai H. (1999) Mutat. Res. 429, 159–168 [DOI] [PubMed] [Google Scholar]
- 29.Albesa I., Becerra M. C., Battán P. C., Páez P. L. (2004) Biochem. Biophys. Res. Commun. 317, 605–609 [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Zhao X. (2009) Antimicrob. Agents Chemother 53, 1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez M. E., Newman D. K. (2001) Cell Mol. Life Sci. 58, 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavrodi D. V., Bonsall R. F., Delaney S. M., Soule M. J., Phillips G., Thomashow L. S. (2001) J. Bacteriol. 183, 6454–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckert B., Beck C. F. (1989) J. Bacteriol. 171, 3557–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies B. W., Kohanski M. A., Simmons L. A., Winkler J. A., Collins J. J., Walker G. C. (2009) Mol. Cell 36, 845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassett D. J., Imlay J. A. (2007) ACS Chem. Biol. 2, 708–710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.