Abstract
An organocatalytic asymmetric Nazarov cyclization of diketoesters has been developed that proceeds by means of a dual activation mechanism. Screening of a number of catalysts led to a new thiourea that incorporates a primary amino group. The method gives rise to cyclic products with two adjacent quaternary asymmetric carbon atoms, one of which is an all carbon atom stereocenter, with complete or nearly complete diastereoselectivity, and in high or very high enantiomeric excess. A brief and very convenient synthesis of the acyclic diketoester starting materials through nucleophilic addition to a ketene has been described.
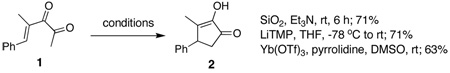
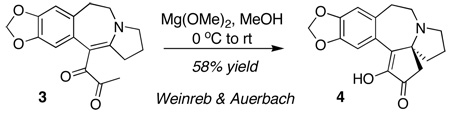
In earlier work we have described cyclizations of α-ketoenones under a variety of mild reaction conditions. For example, ketoenone 1 can be converted to α-hydroxycyclopentenone 2 in 71% yield by exposure to silica gel and triethylamine in the absence of solvent at room temperature (eq 1).1 Alternatively, treatment of 1 with lithium tetramethylpiperidide or with Yb(OTf)3 and pyrrolidine leads to 2 in 71% and 63% yield, respectively.2 There are earlier examples of diketone cyclizations that lead to α-hydroxycyclopentenones that may may proceed through a similar mechanism. For example, in 1975 Weinreb and Auerbach, inspired by an observation published in 1965 by Muxfeldt and coworkers,3 described the cyclization of diketone 3 to 4 under the influence of Mg(OMe)2 during their synthesis of cephalotaxine (eq 2).4,5 Both Muxfeldt and
 |
[1] |
 |
[2] |
Weinreb described the cyclization as an intramolecular Michael reaction of a chelated magnesium enolate. Moreover, both groups noted that the cyclization did not proceed in the absence of Lewis acidic metal species. Although we cannot rule out the intramolecular Michael addition, we have described our reactions as Nazarov cyclizations6 for two reasons. First, the intramolecular Michael addition is a forbidden 5-endo-trig process7 and second, many of our cyclizations are favored by enolate substitution, whereas steric encumbrance of the nucleophile would be expected to inhibit a Michael reaction.
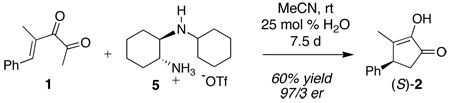
A longstanding goal in our group has been to develop a useful asymmetric organocatalytic Nazarov cyclization of α-ketoenones.8,9 Many Nazarov cyclizations require strongly acidic conditions, but the mild conditions for the cyclizations of 1 gave us reason to believe that an organocatalytic process could be developed. Our first iminium ion-mediated Nazarov cyclization of α-ketoenones proceeded via exposure of 1 to stoichiometric diamine triflate 5 to give (S)-2 in 60% yield and 97/3 er (eq 3).10 The reaction was slow (7.5 d) and a catalytic cycle was not established, presumably due to the exceptional stability of a covalent intermediate.
 |
[3] |
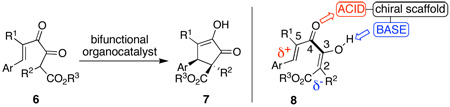
Our strategy toward overcoming this problem was the use of weaker non-covalent catalysts in combination with diketoesters, shown in eq 4. During the course of our studies we accumulated evidence that more highly enolic diketones underwent cyclization with greater facility. Moreover, enolic diketoesters are attractive substrates because either the E or
 |
[4] |
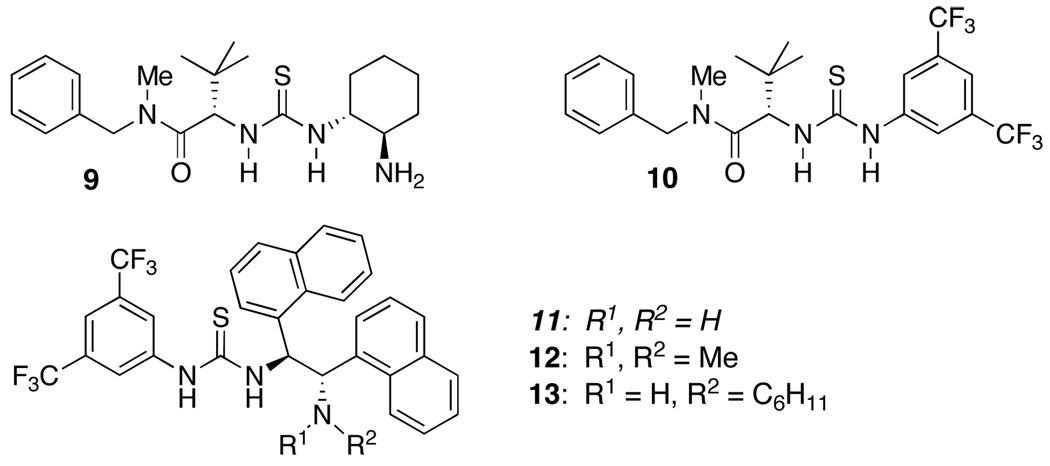
the Z enol isomer can be formed selectively,11 sparing us the labor of controlling the geometry of a tetrasubstituted alkene. These substrates also have the potential to generate two adjacent stereogenic carbon atoms diastereoselectively, one of which is an all-carbon stereocenter. Our catalytic system was designed to induce complementary polarization at the two terminal carbon atoms12 as indicated in 8 (eq 4), consequently a bifunctional organocatalyst combining Bronsted acidic and Lewis basic groups was developed (see Figure 1).
Figure 1.
Organocatalysts.
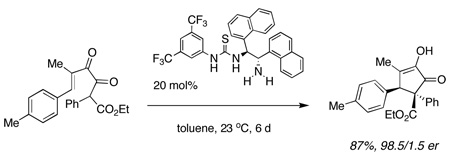
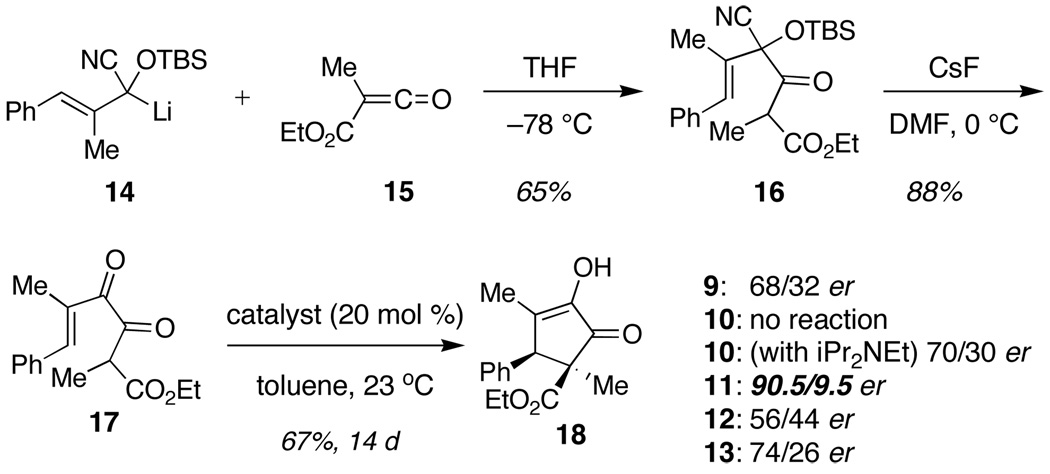
It remained for us to develop the general and convenient diketoester synthesis that is summarized in Scheme 1. Lithiated cyanohydrin silyl ether13 14 was added to ketene 15 leading to ketoester 16 in 65% yield. Exposure of 16 to CsF led to 17 in 88% yield. The ketene was formed conventionally, by treating the malonate mono-acid chloride with Hünig’s base in ether at −78° C.14 The commercial availability of several chiral thiourea catalysts allowed us to prove principle quickly.15,16 Exposure of 17 to 20 mol% of thiourea 9 led to the desired product 18 in 68/32 er. However, catalyst 10 that lacks a basic amino group was completely ineffective and did not lead to cyclization of 17. Addition of 0.2 equiv Hünig’s base to the reaction mixture of 17 and 10 induced catalytic activity and led to 18 in 70/30 er. In all cases the relative stereochemistry indicated a conrotatory process that had taken place from the E enol of 17. The absolute stereochemistry was assigned on the basis of X-ray crystallographic analysis. These data provide strong support for the dual activation mechanism that is implicit in 8 (eq 4) and are consistent with the observations of both Muxfeldt and Weinreb referred to above that also suggest dual activation.
Scheme 1.
Synthesis of diketoesters and cyclization.
A fairly extensive screening of bifunctional catalysts led to our choice of 11. A few trends revealed themselves during this work. For example, catalyst 12 that bears a tertiary amino group led to 18 in only 56/44 er, whereas 13 bearing a secondary amine led to product in 74/26 er. The optimal catalyst 11 led to 18 in 90.5/9.5 er (67%, 14 d). Since the cyclization of 17 to 18 could be induced by base alone, these results may reflect a competitive background reaction with more hindered amines.
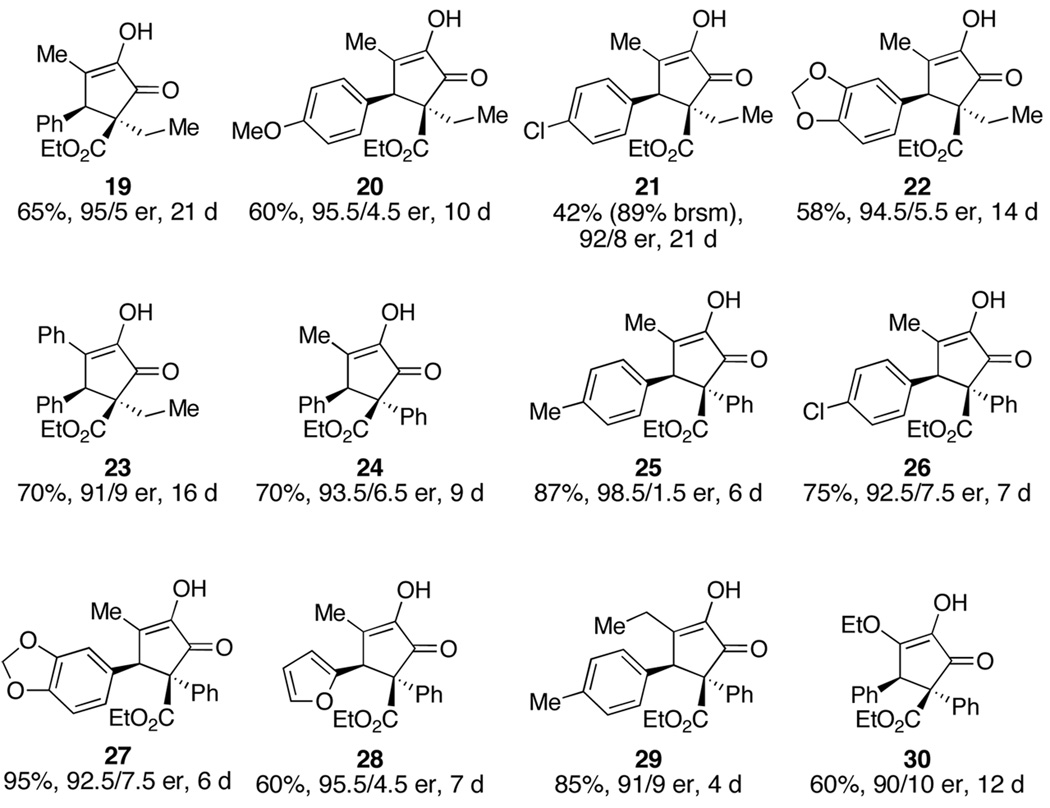
A number of examples of the cyclization of diketoesters under optimized conditions (20 mol% 11, 0.1 M in toluene, 23°C) are summarized in Figure 2. Reaction yields were generally good (58% to 95%) and er’s were good to excellent (90/10 to 98.5/1.5). The reactions were slow, requiring between 4 and 21 d for completion. This may reflect product inhibition, since the product is likely to engage the catalyst in a similar way to the enol form of the acyclic starting material. Support for this hypothesis was provided by 7 (Ar=R1=R2=Ph, R3=Et; 87%, 75/25 er, 2d) that precipitated from the reaction mixture and which was formed in the fastest reaction of the ones examined. In only four examples (7, 21, 25, 29) were we able to detect ca. 5% of the diastereomeric cyclopentenone product derived from the Z enol. In the absence of a C6 aryl group cyclization does not take place. The cyclization requires an aryl group at C6 but tolerates alkyl or phenyl groups at C2.17
Figure 2.
Examples of the organocatalytic cyclization.
If the mechanistic hypothesis implicit in 8 is valid, it raises the interesting question of how stereochemical information is transmitted to the developing C-C bond that is remote from the stereogenic carbon atoms of the catalyst. Since asymmetric induction in 18 requires imposing helicity in 17, it is plausible that coordination with the catalyst results in torsion of the C3–C4 bond. The other elements of novelty in this work are the synthesis of the starting materials and the discovery of an unusual organocatalytic process that generates two adjacent stereogenic carbon atoms, one of which is an all-carbon stereocenter.18 The acyclic substrates will likely prove to be versatile starting materials for several other variants of the Nazarov cyclization. We will explore these and will also address the problem of product inhibition.
Supplementary Material
Acknowledgments
Acknowledgment is made to the National Institutes of Health for generous support (GM57873).
Footnotes
Supporting Information Available: Detailed experimental and spectroscopic data and reproductions of 1H and 13C NMR data for 18–30, and of the intermediates leading to 18–30. X-ray structure of the (−)-camphanic acid derivative of 19. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Uhrich EA, Batson WA, Tius MA. Synthesis. 2006:2139. [Google Scholar]
- 2.Batson WA, Sethumadhavan D, Tius MA. Org. Lett. 2005;7:2771. doi: 10.1021/ol050970x. [DOI] [PubMed] [Google Scholar]
- 3.Muxfeldt H, Weigele M, Van Rheenen V. J. Org. Chem. 1965;30:3573. [Google Scholar]
- 4.Weinreb SM, Auerbach J. J. Am. Chem. Soc. 1975;97:2503. [PubMed] [Google Scholar]
- 5.MAT thanks Prof. Weinreb for alerting him to this work.
- 6. For reviews of the Nazarov reaction see: Habermas KL, Denmark S, Jones TK. In: Organic Reactions. Paquette LA, editor. Vol. 45. New York: John Wiley & Sons, Inc.; 1994. pp. 1–158. Harmata M. Chemtracts. 2004;17:416. Pellissier H. Tetrahedron. 2005;61:6479. Tius MA, M. A Eur. J. Org. Chem. 2005:2193. Frontier AJ, Collison C. Tetrahedron. 2005;61:7577. Nakanishi W, West FG. Curr. Opin. Drug Disc. Devel. 2009;12:732.
- 7.Baldwin JE, Cutting J, Dupont W, Kruse L, Silberman L, Thomas RC. J. Chem. Soc., Chem. Commun. 1976:736. [Google Scholar]
- 8. Catalytic asymmetric Nazarov cyclizations: Liang G, Trauner D. J. Am. Chem. Soc. 2004;126:9544. doi: 10.1021/ja0476664. Rueping M, Ieawsuwan W, Antonchick AP, Nachtsheim BJ. Angew. Chem. Int. Ed. 2007;46:2097. doi: 10.1002/anie.200604809. Walz I, Togni A. Chem. Comm. 2008:4315. doi: 10.1039/b806870d. Rueping M, Ieawsuwan W. Adv. Synth. Catal. 2009;351:78. Yaji K, Shindo M. Synlett. 2009:2524.
- 9. Recent examples of the Nazarov cyclization: He W, Huang J, Sun X, Frontier AJ. J. Am. Chem. Soc. 2008;130:300. doi: 10.1021/ja0761986. Rieder CJ, Fradette RJ, West FG. Chem. Comm. 2008:1572. doi: 10.1039/b800800k. Banaag AR, Tius MA. J. Org. Chem. 2008;73:8133. doi: 10.1021/jo801503c. Bitar AY, Frontier AJ. Org. Lett. 2009;11:49. doi: 10.1021/ol802329y. Grant TN, Rieder CJ, West FG. Chem. Comm. 2009:5676. doi: 10.1039/b908515g. Marx VM, Burnell DJ. J. Am. Chem. Soc. 2010;132:1685. doi: 10.1021/ja909073r. Wender PA, Stemmler RT, Sirois LE. J. Am. Chem. Soc. 2010;132:2532. doi: 10.1021/ja910696x. Wang M, Han F, Yuan H, Liu Q. Chem. Comm. 2010:2247. doi: 10.1039/b917703e.
- 10.Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA. Org. Lett. 2010;12:440. doi: 10.1021/ol9025765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babinski D, Soltani O, Frantz DE. Org. Lett. 2008;10:2901. doi: 10.1021/ol8010002. [DOI] [PubMed] [Google Scholar]
- 12.(a) Tius MA, Kwok C-K, Gu X-q, Zhao C. Synthetic Commun. 1994;24:871. [Google Scholar]; (b) He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. J. Am. Chem. Soc. 2008;130:1003. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- 13.Rawal VH, Appa Rao J, Cava MP. Tetrahedron Lett. 1985;26:4275. [Google Scholar]
- 14.(a) Newman MS, Zuech EA. J. Org. Chem. 1962;27:1436. [Google Scholar]; (b) Calter MA, Orr RK, Song W. Org. Lett. 2003;5:4745. doi: 10.1021/ol0359517. [DOI] [PubMed] [Google Scholar]
- 15. Catalyst 9: Huang H, Jacobsen EN. J. Am. Chem. Soc. 2006;128:7170. doi: 10.1021/ja0620890. Lalonde MP, Chen Y, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:6366. doi: 10.1002/anie.200602221. Catalyst 10: Zuend SJ, Coughlin MP, Lalonde MP, Jacobsen EN. Nature. 2009;461:968. doi: 10.1038/nature08484.
- 16.(a) Taylor MS, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:1520. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; (b) Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- 17.The C2 phenyl group increases the reaction rate (compare 21 and 26, 22 and 27) whereas aliphatic groups larger than ethyl (isopropyl, n-propyl) sharply decrease the reaction rate.
- 18. Reviews: Douglas CJ, Overman LE. PNAS. 2004;101:5363. doi: 10.1073/pnas.0307113101. Denissova I, Barriault L. Tetrahedron. 2003;59:10105. Christoffers J, Mann A. Angew. Chem. Int. Ed. 2001;40:4591. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.