Abstract
Objective
Vezf1 is an early zinc finger transcription factor that is essential for normal vascular development and functions in a dose-dependent manner. Here, we investigated the role of Vezf1 during processes of endothelial cell differentiation and maturation by studying mutant Vezf1 ES cells using the in vitro embryoid body differentiation model and the in vivo teratocarcinoma model.
Methods and Results
Vezf1−/− ES cell-derived embryoid bodies failed to form a well-organized vascular network and showed dramatic vascular sprouting defects. Our results indicate that the retinol pathway is an important mediator of Vezf1 function, and that loss of Vezf1 results in reduced retinol/Vitamin A signaling and aberrant extracellular matrix formation. Unexpectedly, we also uncovered defects during in vitro differentiation of Vezf1−/− ES cells along hematopoietic cell lineages. Vezf1−/− ES cell-derived teratocarcinomas were able to spontaneously differentiate into cell types of all three germ layers. However, histological and immunohistochemical examination of these tumors showed decreased cell proliferation, delayed differentiation, and large foci of cells with extensive deposition of extracellular matrix. Embryoid bodies and teratocarcinomas derived from heterozygous ES cells displayed an intermediate phenotype.
Conclusion
Together, these results suggest that Vezf1 is involved in early differentiation processes of the vasculature by regulating cell differentiation, proliferation, and ECM distribution and deposition.
Keywords: Vezf1, zinc finger gene, vascular development, gene knock-out, ES cells, embryoid body differentiation, teratocarcinoma
Introduction
Development of the vascular system involves two distinct processes: coalescence of mesoderm-derived angioblasts to form a primitive vascular plexus (vasculogenesis); and subsequently sprouting, branching, remodeling, and maturation of the primary plexus to give rise to the mature blood vasculature (angiogenesis) 1-3. These complex developmental processes require the precise temporal and spatial expression of a large number of genes. Much progress has been made over the past 15 years to identify relevant genes, and to understand their participation in signaling pathways and their role in vascular development and disease 1, 4, 5. In contrast, much less is understood about how the precise expression of vascular-specific genes is mediated by the coordinated action of transcription factors. Promoter analysis of endothelial-specific genes in vitro identified crucial transcriptional regulatory factors. Their roles in vascular development and in the adult endothelium have been elucidated through transgenic and gene targeting studies (reviewed in 6-8). The emerging picture from these studies suggests that most if not all transcription factors that are important for blood vessel formation are not endothelial-specific either in their expression or in their function. In fact, several of these factors are involved in both hematopoiesis and vascular development. Furthermore, expression of endothelial genes during blood vessel formation and homeostasis often requires unique combinations of multiple transcription factors, possibly in combination with tissue-specific co-factors or bridging proteins 6, 7.
In a gene trap screen for early cardiovascular genes, we have identified a novel zinc finger transcription factor, Vezf1 9-11. The C2H2 (Krüppel-like) zinc finger protein VEZF1 is highly conserved among vertebrates, and its 518 amino acid sequence shows only six amino acid differences between mouse and human 12, suggesting that Vezf1 plays a conserved role in mammals. Vezf1 is unique amongst endothelial transcription factors: although it is expressed in the endothelium as well as in neuronal and mesodermal tissues 10, 13, its function appears to be restricted to the vascular system. In vitro studies indicate that Vezf1 regulates the proliferation, migration and network formation of endothelial cells 14. In addition, its human ortholog DB1/ZNF161 functionally interacts with Rho GTPases and associated regulatory proteins and regulates expression of the human endothelin-1 gene 15-17. Targeted inactivation of Vezf1 in mice results in embryonic lethality at midgestation, and Vezf1 was found to control development of the blood vascular and lymphatic system in a dose-dependent fashion 18. Homozygous mutant embryos showed defects in vascular remodeling and loss of vascular integrity resulting in localized hemorrhaging, concomitant with defects in endothelial cell adhesion and junction formation in the mutant vessels. Haploinsufficiency was observed in 20% of heterozygous embryos, and those displayed lymphatic hypervascularization associated with hemorrhaging and edema.
Here, we address the specific role(s) of Vezf1 in endothelial cell proliferation, sprouting, and remodeling by using an in vitro differentiation assay of mutant ES cells into embryoid bodies (EB). Vasculogenesis and angiogenesis occur in successive steps during EB formation, and these can be easily followed by using immunohistochemical methods 19-22, making this in vitro model suitable for studies of targeted mutations in ES cells 20, 23, 24. In addition, pluripotent ES cells, when transplanted into an ectopic location in syngeneic or athymic host mice, can develop tumors of different tissue types called teratocarcinoma 25. We have employed this in vivo differentiation model to examine Vezf1 gene function during tissue differentiation and tumor development. Our results show that Vezf1 is involved in multiple processes of vascular formation by regulating cell differentiation, proliferation, as well as ECM distribution and deposition.
Methods
ES cell culture, EB differentiation, and angiogenic sprouting assay
Wild-type R1 ES cells (wt; Vezf1+/+), Vezf1+/− (clone V2) and Vezf1−/− ES cells (clone F3) 18 were maintained on γ-irradiated primary mouse embryo fibroblast (MEF) feeder cells in high-glucose DMEM (Gibco BRL) supplemented with 20% fetal bovine serum (FBS; Gemini), 0.1 mM β-mercaptoethanol, 1 mM L-glutamine, 0.1 mM non-essential amino acids, 20mM HEPES and 1,000 U/ml of LIF (ESGRO; Chemicon, USA). In vitro differentiation of ES cells into embryoid bodies (EBs) was initiated using the hanging drop culture protocol 20. Briefly, drops containing ES cells (2,500 cells in 30 μl) were spotted onto the lid of 150-mm dishes and incubated as “hanging drops” in DMEM supplemented with 10% FBS (Atlas Biologicals), β-mercaptoethanol, L-glutamine, non-essential amino acids and HEPES as described above, in the absence of LIF. EBs were collected after 2 days and grown in suspension culture between 1 to 12 days before harvesting. Alternatively, EBs grown for 2 days in hanging drops were cultured for 1 day in suspension and then transferred to 2D attachment cultures on gelatin-coated tissue culture plates for spontaneous differentiation. Lymphatic endothelial cell (LEC) differentiation was induced in EBs at day-5 or day-14 in suspension culture by adding 20ng/ml recombinant human VEGF-A165 (R&D Systems) and 200ng/ml recombinant human VEGF-C (R&D Systems) 26, 27, and cultured for up to 21 days post induction. At indicated time points, EBs were collected, fixed in 4% paraformaldehyde, and embedded in OCT. Angiogenic sprouting assays were performed in semi-solid 3-D collagen gels 21. Briefly, EBs from day-12 suspension culture were harvested, embedded in type I collagen gel containing 20 ng/ml VEGF and cultured in small droplets for 2 to 5 days. EBs were fixed in situ, transferred to glass slides, air-dried, and stored at −20°C for immunostaining.
Immunofluorescence and immunohistochemistry
Protocols for immunofluorescence and immunohistochemistry were essentially as described 18. Primary antibodies used were rat anti-mouse PECAM-1/CD31 (1:200; Pharmingen), rat anti-mouse MECA-32 (1:15; Pharmingen), ICAM-1 (1:100; Seikagaku Corp.), goat anti-mouse VE-cadherin (1:20; R&D Systems), rabbit anti-mouse collagen IV (1:200; Chemicon), rabbit anti-human Caspase-3 (1:200; Cell Signaling Technology, Inc.), rabbit Annexin V (1:200; Abcam), and rabbit anti-mouse LYVE1 (1:200; AngioBio). For each antibody, an IgG isotype antibody was used as a control. Secondary antibodies included TRITC-conjugated goat anti-rabbit IgG, F(ab’)2, FITC-conjugated goat anti-rat IgG (1:100), Cy3-conjugated donkey anti-rat IgG (1:300), Cy3-conjugated goat anti-rabbit IgG (1:300), Cy5-conjugated donkey anti-goat IgG (1:500), HRP-conjugated donkey anti-rabbit IgG (1:200), and HRP-conjugated donkey anti-rabbit IgG(H+L) (1:200). Nuclei were counterstained with DAPI using Vectashield mounting medium (Vector Labs). Images were taken under a Zeiss Axioplan 2 IE MOT microscope using digital ORCA-ER-1394 camera.
Quantitative RT-PCR
Total RNA was extracted from ES cells following MEF depletion and from day-5 EBs using TRIzol Reagent (Invitrogen). cDNA was prepared from 1 μg RNA using Superscript III Reverse Transcriptase and random hexamers (Invitrogen) in a 40 μl reaction. A 1:20 aliquot of the cDNA reaction was used for Real Time PCR in a 25-μl reaction using SYBR Green technology (Applied Biosystems). Gene expression was measured on a BioRad IQ5 Multicolor PCR Cycler (BioRad) using primers listed below, and was normalized using primers against mouse β-actin. To confirm specificity of the reaction, melting point analysis was incorporated at the end of the PCR.
| Gene | Forward Primer | Reverse primer | Product size in bp |
|---|---|---|---|
| RBP4 | gacaaggctcgtttctctgg | aaaggaggctacaccccagt | 243 |
| TTR | gacaggatggcttcccttcg | ccaggactttgaccatcagagg | 117 |
| RAR-β | gcctgcagaagtgctttgaagt | gctttccggatcttctcagtga | 150 |
| Aldh2 | gagcaggggcctcaggtgga | tcattggcccgccccacaac | 248 |
| Col-IV(a)1 | ttcagattccgcagtgcccta | ttctcatgcacacttggcagc | 310 |
| Col-IV(a)2 | tggctgaggaggaaatcaagc | aatggcgttgcacggaagt | 232 |
| β-actin | gtgggccgctctaggcaccaa | ctctttgatgtcacgcacgatttc | 539 |
Protein isolation and western blots
Conditioned medium was collected, filtered, and snap frozen from wt and Vezf1−/− ES cells cultured for 24 hours, and from EBs maintained in medium supplemented with 50ng/mL VEGF-A and 100ng/mL bFGF (R&D Systems). Protein concentration was determined by using the BCA™ Protein Assay (Pierce, IL). 50μg of protein from conditioned media was separated on 4-12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Whatman GmbH, Dassel) using the NUPAGE® Electrophoresis and Transfer System (Invitrogen, CA). Pre-stained low range molecular weight standard (Invitrogen, CA) and purified RBP4 (kind gift from Dr. William Blaner, Columbia University) were also included. Membranes were blocked in 2% skim milk in Tris-buffered saline (0.05% Tween 20; 1 hour at room temperature) and incubated overnight with rabbit polyclonal anti-RBP4 antibody (1:500; provided by Dr. William Blaner, Columbia University). Secondary antibody used was horseradish peroxidase-conjugated donkey anti rabbit (1:5000, 1 hour at room temperature). The ECL Plus Western Blotting Reagent (Amersham Biosciences) was used to visualize RBP4 protein.
Flow Cytometry
At indicated time points, EBs and undifferentiated ES cells were dissociated to single-cell suspension using 0.2% collagenase A (Sigma). Dissociated ES cells were MEF-depleted by incubation on gelatin-coated dishes (0.7%; Sigma). ES cells and EB-derived cells were fixed in 2% paraformaldehyde and washed in staining buffer (0.01% NaN3 in PBS). For oct3/4 expression, cells were incubated with 1% saponin (Sigma) for 15 minutes prior to washing. Cells were incubated for Fc receptor blockage with rat anti-mouse CD16/CD32 (1μg/106 cells) and stained either alone with Alexa647-conjugated rat anti-mouse Oct3/4 (1μg/106 cells; eBiosciences) or in combination with PE-CY7-conjugated rat anti-mouse PECAM-1/CD31 (0.5μg/106 cells; e-Biosciences), Alexa 488-conjugated rat anti-mouse VE-Cadherin (1μg/106 cells; eBiosciences) and PE-conjugated rat anti-mouse CD41 (1μg/106 cells; Becton-Dickinson Biosciences). Cells were washed, re-suspended in PBS then filtrated using cell-strainer caps. Unstained cells were used to set the cut-off for background fluorescence. Data acquisition was done using FACs Canto (Becton-Dickinson Bioscience) and analysis was performed using FlowJo Software.
Generation of ES cell clones with LacZ knock-in into the Vezf1 locus
The original Vezf1 targeting construct contained a deletion of the transcription start site, resulting in a non-functional LacZ gene 18. A new construct termed Vezf1lacZ was generated, in which the transcriptional start site was restored and the IRES-lacZgt1.2neo cassette was replaced by an SDK-lacZ-PGKneo cassette. XhoI-linearized DNA was introduced into R1 ES cells by electroporation, and six correctly targeted clones were identified by Southern blot analysis as described 18. Expression of LacZ in EB derived from targeted ES cell clones was detected by using a b-galactosidase staining kit (Activemotif, CA).
Teratocarcinoma formation
A total of 1×107 cells of clone F3 (Vezf1−/−) or clone V2 (Vezf1+/−) suspended in 150μl PBS was injected subcutaneously into the left flank of 6- to 8-week old male nude mice. The right flank was injected with wt ES cells as a control. Two weeks after injection, animals were sacrificed, and the teratocarcinomas were dissected from the subcutaneous fascial planes, weighed and fixed overnight in 4% paraformaldehyde. Half of each teratocarcinoma was embedded in OCT and the other half in paraffin, sectioned at 6 μm and used for immunostaining or H&E staining, respectively. The weight of each individual control teratocarcinoma was used as 100%, and the weight of the experimental teratocarcinoma from the same animal was expressed as percentage of the control. A total of six mice were used for each ES cell clone.
Results
Delayed attachment and reduced growth of Vezf1−/− EBs
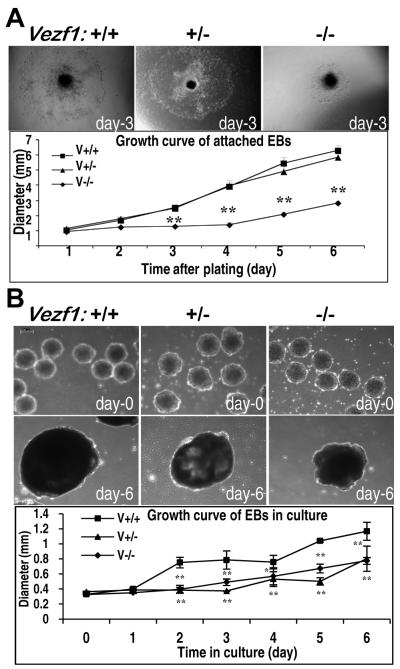
To investigate in detail the role of Vezf1 during vascular development, we examined the formation of vascular structures in EBs derived from ES cells that are homozygous (Vezf1−/−), heterozygous (Vezf1+/−), or wild type (wt) for a targeted Vezf1 null allele 18. We have shown previously that Vezf1 mRNA is absent in Vezf1−/− ES cells and EBs, and reduced to about half in Vezf1+/− EBs 18. The in vitro differentiation of ES cells was initiated in hanging drop cultures, followed by growth either in suspension culture or in 2D attachment culture. We first assessed the ability of EB to attach to substrate and for outgrowth of differentiating cells. After overnight culture on gelatin-coated dishes, almost all EBs derived from Vezf1+/− and wt ES cells were attached and had formed a rim of spreading, differentiating cells, whereas about one-third of Vezf1−/− EBs did not firmly attach overnight, or even within 6 days of culture (not shown). Furthermore, cellular outgrowth from those Vezf1−/− EBs that attached to the culture dishes was significantly delayed and slower, with diameters of attached Vezf1−/− EBs about 50% reduced compared to Vezf1+/− and wt EBs (Fig. 1A). Interestingly, when grown in suspension culture, mutant and wt EBs formed cystic structures; however, both Vezf1+/− and Vezf1−/− EBs grew significantly slower than wt EBs, and by day-6 they were about 1/3 smaller compared to wt controls (Fig. 1B).
Figure 1. Growth characteristics of wild-type and Vezf1 mutant embryoid bodies.
(A) Growth in 2D attachment culture. EBs were grown for 3 days in suspension culture and switched to 2D attachment culture. EBs attached over night to gelatin-coated plates, and differentiating cells grew out from the center to the periphery. Upper panels: Morphology of representative EBs derived from wt (Vezf1+/+), Vezf1+/−, and Vezf1−/− ES cells, respectively, at day-3 after attachment. Images were taken under an inverted Olympus IX70 microscope. The graph shows growth of EB between day-1 and day-6 of 2D attachment culture. Diameters of EBs (n=14 for each genotype) were measured in 24-hour intervals using Image-Pro Plus software. The experiment was repeated three times, and the graph depicts the results from one representative experiment. **, P<0.01 comparison between wt vs. Vezf1+/− and wt vs. Vezf1+/+ EBs were performed by the student’s t-test. (B) Growth in suspension culture. Upper panels: Representative images of wt, Vezf1+/−, and Vezf1−/− EBs, respectively, at day-0 and day-6 in suspension culture. Images were taken under a Leica DMIL inverted microscope. Bottom panel: Diameters of EBs in suspension culture were measured from day-0 to day-6 using Leica Application Suite (n=5 for each genotype). Comparison between groups were performed by Two-way ANOVA with Bonferroni post tests. *P<0.01, **P<0.001 vs. Vezf1+/− and Vezf1−/− EBs. Data shown as mean ±SEM.
Vascular structure defects in mutant Vezf1 EBs
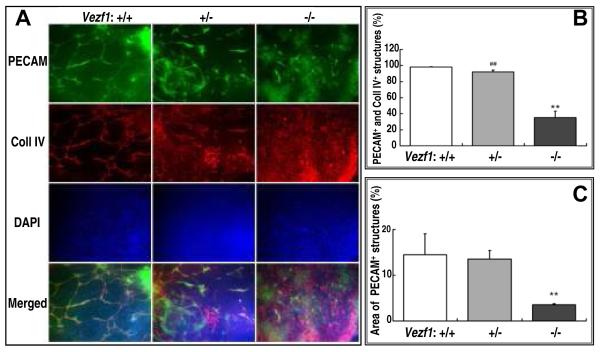
We next examined the differentiation of endothelial cells and formation of vascular structures in EBs. For this, EBs were grown in 2D attachment culture and double-stained at day-3, -6, -9, and -12 with antibodies specific for the mouse pan-endothelial marker PECAM-1/CD31 and the ECM protein collagen type IV. Differences between mutant and wt EBs became apparent at day-6 when endothelial cell cords were beginning to develop, and were most obvious at day 9 (shown in Fig. 2A). Thus, wt (Vezf1+/+) EBs displayed cords with PECAM-1/collagen IV positive endothelial cells that were organized into extensive vascular networks (Fig. 2A, left panel). In contrast, Vezf1−/− EBs formed only short PECAM-1 positive cell cords that did not develop into a proper vascular network. Overall, vascular coverage, as measured by the area covered by PECAM-1 positive structures, was reduced from 14.5% in wt to 3.6% in Vezf1−/− EBs (Fig. 2B). In addition, most of the PECAM-1 staining in Vezf1 null EBs was localized in patches of cells with undifferentiated morphology (Fig. 2A, top right panel). High levels of collagen IV expression were visible on large sheets of undifferentiated cells (Fig. 2A, bottom right panel), and generally did not co-localize with PECAM-1 expression (35% co-localization of PECAM-1 and collagen IV in Vezf1−/− EBs vs. 98% in wt EBs; Fig. 2C). No changes in vascular structure formation were detected when 2D cultures were extended to 12 days, indicating that the observed defects were not due to delayed vascular differentiation. Interestingly, Vezf1+/− EBs displayed an intermediate phenotype with shortened and thickened cell sheets that contained PECAM-1/collagen IV positive cells; however these had failed to form an extensive vascular network (Fig. 2A, middle panel).
Figure 2. Aberrant vascular structure formation and collagen IV deposition in mutant EBs.
(A) EBs in 2D culture Shown are representative images of day-9 EBs derived from wt (Vezf1+/+), Vezf1+/− and Vezf1−/− ES after fixation and immunostaining for PECAM-1 and type IV collagen. Cell nuclei were counter-stained with DAPI. Bottom row depicts merged images from PECAM-1, collagen IV and DAPI staining. Note the extensive vascular network in wt EBs. In contrast, vascular structures did not form properly in Vezf1−/− EBs. Vezf1+/− EBs displayed an intermediate phenotype. Images were taken under a Zeiss Axioplan 2 IE MOT using a 20x objective.
(B) Quantitative analysis of vascular coverage. The vasculature as shown by PECAM-1+ staining was analyzed for area density and expressed as percentage of the PECAM-1+ area. At least three independent measurements were performed in each group. Comparison of Vezf1−/− vs. Vezf1+/− and Vezf1+/+ groups were performed by student’s t-test; **, P<0.01. (C) Quantitative analysis of co-expression of PECAM-1 and collagen IV. Shown is the percentage of collagen IV+ areas that was adjacent to or overlapped with PECAM-1+ areas. Comparisons between two groups were done using student’s t-test. **, P<0.01 Vezf1−/− vs. Vezf1+/+; ##, P<0.01 Vezf1−/− vs. Vezf1+/−.
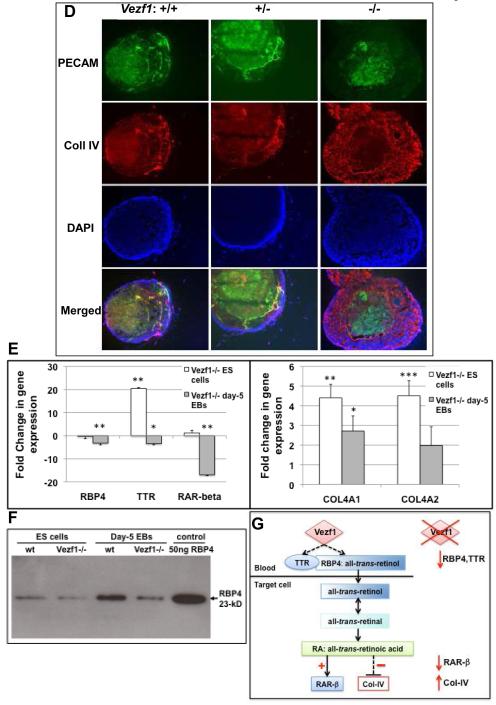
(D) Suspension culture EBs. Sections of EBs grown for 9 days in suspension culture were immunostained for PECAM-1 and collagen IV. Cell nuclei were counter-stained with DAPI. Bottom row depicts merged images from PECAM-1, collagen IV and DAPI staining. Well-developed PECAM-1 and collagen IV positive vascular structures were visible in wt and, to a lesser degree, in Vezf1+/− EBs. In Vezf1−/− EBs, PECAM-1 positive sheets of cells were visible in the center, but these failed to form cell cords and they did not co-stain with collagen IV.
(E) Expression of retinol pathway genes Rbp4, Ttr, and RAR-β, and collagen 4a1 and 4a2 in ES cells and embryoid bodies. Gene expression levels were determined by quantitative RT-PCR and normalized to β-actin. Shown are changes in expression in Vezf1−/− vs. wt ES cells and day-5 EBs. Comparisons between wt and Vezf1−/− ES cells and EBs were performed by student’s t-test (n=3 for each genotype).
(F) Detection of secreted RBP4 protein by western blot analysis. Representative western blot using conditioned media (50μg protein) from wt and Vezf1−/− ES cells and day-5 EBs. 50 ng of purified 23-kD RBP4 protein was loaded as a control. RBP4 levels are reduced in Vezf1−/− EBs and, to a lesser extent, in Vezf1−/− ES cells.
(G) Model proposing how Vezf1 regulates the retinol/Vitamin A pathway. Left: Vezf1 activates expression of the all-trans retinol carrier Rbp4 and transthyretin, directly or indirectly. TTR binds to RBP4:all-trans retinol, preventing its renal clearance and resulting in increased cellular uptake. Retinol is converted to retinal and retinoic acid, which directly transactivates expression of its receptor RAR-β and indirectly represses that of Collagen IV. Right: Loss of Vezf1 function in EBs results in deregulation of the retinol/Vitamin A pathways with reduced expression of Rbp4, Ttr and RAR-β, and accumulation of collagen IV.
We also examined vascular differentiation in EBs grown for 3, 6, and 9 days in suspension culture (representative sections of day-9 cystic EBs are shown in Fig. 2D). PECAM-1 and collagen IV in wt and Vezf1+/− EBs co-localized to endothelial cell cords that were mostly restricted to a region underneath an outer layer of tightly packed visceral endodermal cells (Fig. 2D, left and middle panel). These vascular structures were well developed. In contrast, Vezf1−/− EBs contained a broad, presumably mesodermal layer that was filled with collagen IV-positive cells but devoid of PECAM-1-positive cells. PECAM-1 positive cells were restricted to the center of the EBs, but these failed to coalesce into cords (Fig. 2D, right panel).
The expression patterns in wt and mutant EBs of endothelial ICAM-1 in 2D attachment culture (Suppl. Fig. 1), and of endothelial VE-cadherin (Suppl. Fig. 2) and MECA-32 (not shown) in suspension culture, respectively, was similar to that observed for PECAM-1. Thus, we observed patches of endothelial cells that lack to properly form vascular cords. VE-cadherin staining partially overlapped with PECAM-1 in Vezf1 null EBs (Suppl. Fig. 2, bottom row). Together, these results confirm that endothelial lineage determination and differentiation is unaffected but that vascular structure formation is defective in EBs derived from Vezf1−/− ES cells. To test for the possibility that apoptosis could be the cause of the observed vascular defects, EBs were grown for 9 days in 2D attachment culture and co-stained for PECAM-1 and Caspase 3. However, no increase in the numbers of Caspase 3-positive cells was detected within the PECAM-1 positive cell population, or overall in the EBs (Suppl. Fig. 3). Similarly, when sections of day-9 suspension culture EBs were stained for Annexin V, we observed no increase in apoptosis in PECAM-1 positive or negative cells in mutant Vezf1 EBs (Suppl. Fig. 4).
Finally, we investigated the formation of lymphatic endothelial structures in EBs derived from wt and mutant Vezf1 ES cells. LYVE-1+/MECA-32− or LYVE-1+/PECAM-1+ cells became apparent in about 80% of wt EBs at day-7 in suspension culture and were found up to day-26 in cord-like structures. Fewer LYVE-1 positive EBs were obtained from Vezf1+/− and Vezf1−/− ES cells and at later time points of suspension culture. No obvious differences in the morphology of the putative lymphatic structures were found (data not shown).
Suppression of retinol metabolism in Vezf1−/− EBs
A recent microarray screen for Vezf1 target genes in E9.5 embryos identified several candidate genes involved in retinol metabolism 28. Specifically, expression of retinol binding protein 4 (Rbp4), a retinol/Vitamin A carrier in the plasma, and transthyretin (Ttr), which binds to RBP4 and prevents it from renal clearance 29, 30, was significantly decreased in Vezf1−/− embryos. Intriguingly, several studies reported that all-trans-retinoic acid inhibits collagen type IV expression in vitro in human and rat cells 31-33. To investigate if expression of key retinol pathways genes and collagen IV are similarly affected during in vitro differentiation in mutant EBs, we performed quantitative RT-PCR on ES cells and day-5 EB. Expression of Rbp4, Ttr and the retinoic acid target gene RAR-β34, 35 was significantly reduced in Vezf1−/− day-5 EBs but not in undifferentiated ES cells (Fig. 2E, left panel). The levels of secreted RBP4 protein were also decreased in supernatant medium harvested from Vezf1−/− EBs, and to a lesser extent in ES cells, when compared to wt controls (Fig. 2F). Consistent with the observed strong and aberrant immunostaining (Fig. 2A and D), increased expression of α1 and α2 chains of collagen type IV was detected in Vezf1−/− EBs (Fig. 2E, right panel). Together, these results support our model (Fig. 2G) that Vezf1 activates the retinol/vitamin A pathway, either directly by transactivation of Rbp4 and Ttr, or indirectly. Deregulation of retinol/vitamin A signaling in Vezf1 null EBs results in elevated collagen IV expression and accumulation (Fig. 2G).
Sprouting angiogenesis is impaired in Vezf1−/− EBs
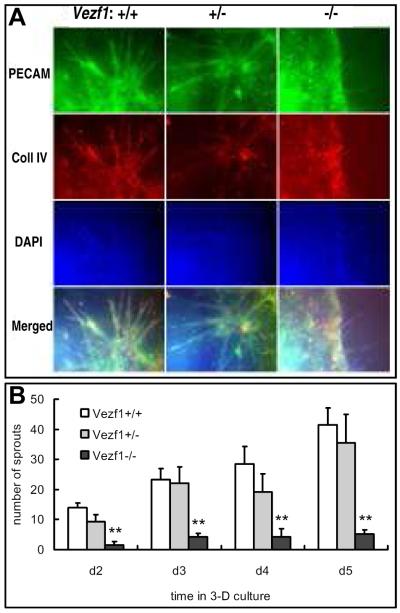
When ES-derived EBs are embedded in a three-dimensional matrix of type I collagen in the presence of angiogenic factors, sprouting outgrowth of endothelial cells from the initial cord-like vascular structures in EBs can be observed that closely mimics sprouting angiogenesis in the developing embryo 36, 37. The three-dimensional system is a powerful tool to study this angiogenesis process on a cellular and molecular level. To examine the ability of endothelial cells derived from Vezf1 null ES cells to undergo sprouting angiogenesis, EBs were grown in suspension culture for 12 days and subsequently embedded in collagen type I gels in the presence of the pro-angiogenic growth factor VEGF. Generally, initial spouting was detected after 24 hours in wt and Vezf1+/− EBs in the collagen matrix. The sprouts continued to grow in number and length, and after 3-4 days the primary sprouts began to interconnect and formed secondary sprouts. Co-immunostaining for PECAM-1 and collagen IV of day-5 cultures indicated that the sprouts contained endothelial cells (Fig. 3A, left and middle column, and Fig. 3B). In contrast, no or few sprouts were visible after 24 hours in collagen-embedded Vezf1−/− EBs. At day-5 in collagen matrix, Vezf1−/− EBs had significantly fewer primary sprouts and rarely formed secondary sprouts (Fig. 3A, right column, and Fig. 3B). In conclusion, sprouting angiogenesis of mutant ES cell-derived endothelial cells was severely impaired.
Figure 3. Vascular sprout formation in EBs cultured in collagen matrix.
(A) EBs were cultured for 12 days in suspension culture, followed by 5-day culture in collagen type I gel drops. Endothelial cell sprouts were visualized by whole-mount staining of embedded EBs for PECAM-1 and type IV collagen. Cell nuclei were counter-stained with DAPI. Bottom row depicts merged images from PECAM-1, collagen IV and DAPI staining. Sprout formation was significantly impaired in Vezf1−/− EBs when compared to wt and Vezf1+/− EBs. (B) Quantitative analysis of vascular sprout formation. Individual vascular sprouts from EBs, as indicated by PECAM-1+ staining in corresponding images, were counted at day 2, 3, 4 and 5 in collagen drops (n=10 from each genotype). Comparison of Vezf1−/− vs. Vezf1+/− and Vezf1+/+ groups were performed by student’s t-test; **, P<0.01.
Quantitative analysis of undifferentiated, endothelial, hematopoietic cell populations
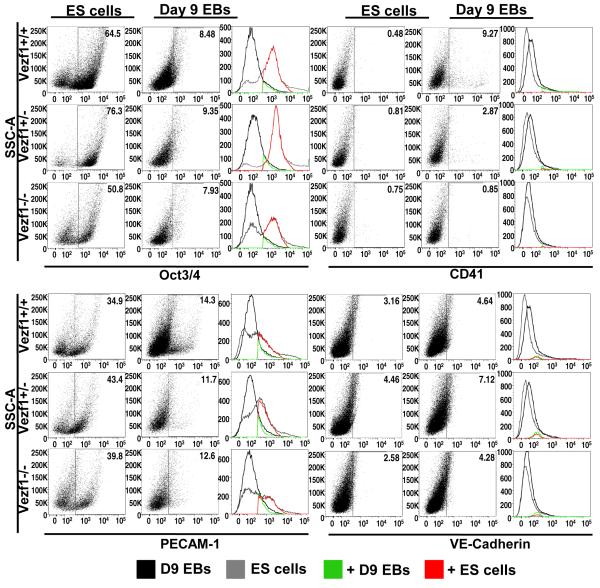
We further examined the differentiation potential of wt and Vezf1 mutant ES cells along the endothelial and hematopoietic lineages by using flow cytometry. Cell suspensions of wt, Vezf1+/− and Vezf1−/− ES cells and of day-9 EBs were analyzed for the presence of undifferentiated cells (Oct3/4-positive fraction), endothelial cell lineage (PECAM-1 positive and VE-cadherin positive fractions), and hematopoietic cell lineage (CD41 fraction) (Fig. 4). Wt and Vezf1 mutant ES cells were able to differentiate at comparable levels, as judged by a similar decrease in Oct-3/4-positive cells (6- to 8-fold decrease during 9 days of in vitro differentiation; upper left panels in Fig. 4). Importantly, we did not detect a significant difference in endothelial marker gene expression between wt and mutant cells, as shown by the percentage of PECAM-1 positive and VE-cadherin positive cells before and after differentiation (Fig. 4, bottom panels). Thus, loss-of-function of Vezf1 does not appear to block endothelial lineage determination and differentiation. We noted a 1.6-fold increase in VE-cadherin-positive cells with all three genotypes. We also observed an overall 3- to 4-fold decrease in PECAM-1 positive cells upon differentiation; the large fraction of PECAM-1 positive ES cells is consistent with its previously reported expression in the inner cell mass of blastocysts 38. Unexpectedly, significantly lower numbers of CD41-positive hematopoietic cells were detected upon differentiation in Vezf1 mutant EBs as compared to wt EBs. Thus, whereas the fraction of CD41-positive cells increased during wt EB differentiation by 19-fold, and during Vezf1+/− EB differentiation by 4-fold, no significant change was found during differentiation of Vezf1−/− EBs (0.75% vs. 0.85% CD41+ cells; Fig. 4, upper right panel). To determine which specific hematopoietic lineages are affected, hematopoietic progenitor assays 39, 40 were performed (Suppl. Fig. 5). Day-6 EBs derived from wt and Vezf1+/− ES cells gave rise to all types of hematopoietic progenitors with similar efficiencies, including primitive erythroid, definitive erythroid, macrophage, bipotential definitive erythroid/macrophage, and multipotential precursor colonies. In contrast, greatly reduced numbers of primitive erythroid and bipotential colonies, and no definitive erythroid, macrophage, and multipotential progenitor colonies were obtained from Vezf1−/− day-6 EBs. Together, these results indicate that Vezf1 null ES cells differentiate efficiently in vitro into PECAM-1 positive and VE-cadherin positive endothelial cells, but that differentiation into cells of the hematopoietic lineages is compromised.
Figure 4. Analysis of Oct3/4, CD41, PECAM-1, and VE-Cadherin gene expression in undifferentiated ES cells and differentiated cells from day-9 EBs.
Wild-type, Vezf1+/−, and Vezf1−/− ES cells (106 cells each) and dissociated cells from day-9 suspension culture EBs (550, 880, and 3,300 EBs from wt, Vezf1+/−, and Vezf1−/− EBs, respectively) were analyzed by flow cytometry for the expression of Oct3/4, CD41, PECAM-1 and VE-Cadherin. The gates correspond to Oct3/4+, CD41+, PECAM-1+ or VE-Cadherin+ populations. The histograms depict the number of day-9 EB-derived cell population (black), ES cell population (grey), positive cells from day 9 EB cell population (green), and positive cells from the ES cell population (red).
Aberrant cell differentiation in teratocarcinomas derived from Vezf1-null ES cells
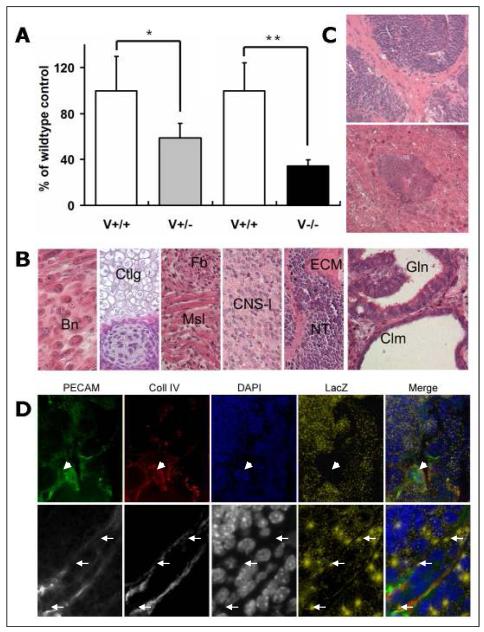
To study the differentiation of mutant Vezf1 ES cells in vivo, Vezf1−/− or Vezf1+/− ES cells were transplanted subcutaneously into one flank of athymic nude mice, and wt ES cells were injected as control into the opposite flank. Two weeks later, teratocarcinomas were dissected, weighted and fixed. The growth of Vezf1−/− tumors and, to a lesser extend, of Vezf1+/− tumors was significantly reduced compared to wt controls (35% and 58% of wt control weight, respectively) (Fig. 5A). H&E staining of tumor sections revealed that both mutant and wt-derived teratocarcinomas contained a spectrum of somatic tissues derived from ecto-, endo- and mesoderm; these included epithelium, ciliatum, cartilage, bone, muscle cells, adipose tissue, neural and connective tissues (Fig. 5B). Overall, neural tissue was prominent in the tumors and consisted of neural rosettes and tubes, and central nervous system (CNS)-like tissue. In addition, an abundance of columnar epithelial cells lining cystic spaces formed glands and solid nests (Fig. 5B). Although Vezf1−/− ES cells were able to differentiate into every cell type found in the control teratocarcinomas, we detected notable differences. First, neural tube structures in Vezf1−/− teratocarcinomas were typically less differentiated (Fig. 5C), whereas wt tumors contained neural tissue of the more differentiated CNS-like phenotype. A second pathological feature was the large number of ECM-rich foci in connective tissue present in Vezf1−/− tumors, some of which formed bone-like structures (Fig. 5C). In contrast, wt tumors contained overall more gland-like structures with well-defined spaces that were surrounded by columnar epithelium, similar to those shown in the far right panel of Fig. 5B.
Figure 5. Teratocarcinoma formation in nude mice.
(A) Comparison of weight of teratocarcinomas derived from Vezf1+/− and Vezf1−/− ES cells with their wt controls 2 weeks after subcutaneous injection. The weight of each individual teratocarcinoma was expressed as a percentage of the control (100%) co-injected into the same animal. Six mice each were used for Vezf1+/− and Vezf1−/− ES cells. Vezf1+/− and Vezf1−/− teratocarcinomas were significantly smaller than the wt controls. Values are shown as mean±SE. Comparisons between two groups were performed using student’s t-test. * P<0.05; wt vs. Vezf1+/−, ** P<0.01 wt vs. Vezf1−/−. (B) Histology of representative H&E stained sections of Vezf1−/− teratocarcinomas. From left to right: Bn, bone; Ctlg, cartilage; Fb, fibroblasts; Msl, muscle; CNS-l, central nervous system-like tissue; ECM, extracellular matrix; NT, neural tube; Gln, gland; Clm, cilium. (C) Vezf1−/− teratocarcinomas contained an abundance of areas with less differentiated tissues: multi-cell layered neural tube-like structures with more increased cellularity and decreased lumen formation (top), and extensive deposition of extracellular matrix surrounding neural tube structures (top), or within bone-like structures (bottom). (D) Cryosections of Vezf1lacZ/+ ES cell-derived teratocarcinomas were stained for PECAM-1 and collagen IV, and β-galactosidase. Cell nuclei were counterstained with DAPI. To visualize lacZ in the merged pictures, β-galactosidase staining was shown in a pseudocolor (yellow dots). Most of the PECAM-1, collagen IV positive vessels were negative for lacZ (arrow head). A small fraction of blood vessels contained cells that showed β-galactosidase expression (arrow). Top: 20x objective; bottom: 63x objective.
Reduced blood vessel formation in teratocarcinomas
We generated ES cell clones with a LacZ knock-in into the endogenous Vezf1 locus (Vezf1lacZ/+) (Suppl. Fig. 6). This approach allowed us to visualize Vezf1 gene expression during in vivo and in vitro differentiation. β-galactosidase staining of Vezf1lacZ/+ EBs grown in 2D attachment culture revealed that Vezf1 expression was widespread but not ubiquitous. Prior to day-7, LacZ expression was highest located in the center of attached EBs in presumably mesodermal cells. Beginning at day-8 in culture, localized and elevated expression became apparent in cell clusters and tubes similar in appearance and shape to PECAM-1 positive structures, suggesting that these correspond to areas of early endothelial differentiation (Suppl. Fig. 6, bottom).
Teratocarcinomas derived from Vezf1−/− ES cells were smaller, and they contained fewer and less organized vascular structures that co-stained with PECAM-1 and Collagen IV when compared to wt or Vezf1+/− tumors (Fig. 6, and not shown). In addition, vascular structures in mutant teratocarcinomas rarely displayed ZO-1 positive cells (not shown). This prompted us to investigate if blood vessels in teratocarcinomas are derived from the host, from differentiating ES cells, or both. For this, we injected Vezf1lacZ/+ ES cells into nude mice, allowing us to visualize ES cell-derived cells in the blood vessels. Sections from the resulting tumors were examined for co-localization of PECAM-1 and collagen IV immunostaining with β-galactosidase staining. The majority of PECAM-1 positive small vessels and the surrounding tissue were negative for LacZ (Fig. 5D, upper panel) and thus were presumably host-derived. However, a small portion of PECAM-1 positive cells within the vascular structures showed β-galactosidase activity, and these cells were interspersed with apparently host-derived endothelial cells within the same vessel (Fig. 5D, arrows in lower panel). Thus, our results suggest that Vezf1 null ES cells are capable to differentiate into endothelial cells and to contribute to the formation of vessels within the teratocarcinomas.
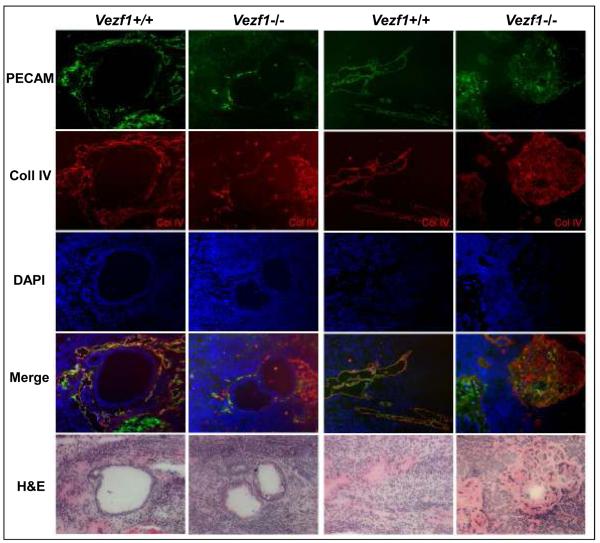
Figure 6. Vascular structure defects in Vezf1−/− teratocarcinomas.
Representative sections from 2 wt and 2 contra-lateral Vezf1−/− teratocarcinomas are shown. Sections were immunostained for PECAM-1 and collagen IV, and cell nuclei were counterstained with DAPI. Merged images are from single PECAM-1, collagen IV and DAPI images. Bottom row: close-by H&E stained section. Note the absence of well-formed vascular structures around glandular epithelial structures in Vezf1−/−, but not wt teratocarcinomas (left 2 columns). Mesenchyme-rich areas in wt teratocarcinomas contained PECAM-1, collagen IV positive vessels. In contrast, Vezf1−/− teratocarcinomas contained large mesenchymal foci with high collagen IV deposition and much lower PECAM-1 expression. These areas did not contain recognizable vascular structures (right 2 columns).
Strikingly, distinct areas in Vezf1−/− ES cell-derived teratocarcinomas displayed low cellularity and high ECM content, as evidenced by H&E staining (Fig. 5C; Fig. 6, far right Vezf1−/−panel). In consecutive sections, these regions showed high levels of collagen IV deposition but few PECAM-1 positive cells, indicating rudiment and disorganized vascular structures (Fig. 6). In contrast, wild-type teratocarcinomas contained well-organized vascular structures with PECAM-1 and collagen IV co-localization in ECM-rich regions (Fig. 6, Vezf1+/+ panels). These areas were similar in their morphology to mesenchymal or connective tissue where the majority of blood vessels are normally localized. The area density of blood vessels in wt and Vezf1−/− ES derived teratocarcinomas was 21.59±2.54% and 6.67±4.50%, respectively (P<0.01). Since the majority of the vessels are host-derived, indirect, non-endothelial effects may contribute to the reduced vascularity. The results suggest that the cellular microenvironment inside the teratocarcinomas may play an additional role in the formation of the tumor vasculature.
Discussion
Blood vessel development during mammalian embryogenesis is a finely tuned process that is controlled by a wide variety of genes. We have recently reported on the crucial and dosage-dependant role of the zinc finger transcription factor gene Vezf1 during vascular system development 18. Although Vezf1 is expressed in the endothelium of the developing vasculature and in mesodermal and neuronal cells, the primary defect in Vezf1 null embryos appears to have its origin in the endothelium. Thus, ultrastructural and immunohistochemical studies demonstrated loss of vascular wall integrity concomitant with defects in endothelial cell junctions and the ECM as the underlying cause of hemorrhaging and embryonic death 18. In this study, we investigated in greater detail the involvement of Vezf1 in various processes of angiogenesis. We used ES cells that are heterozygous or homozygous for a targeted Vezf1 allele, and induced their differentiation in vitro into embryoid bodies (EBs) and in vivo during teratocarcinoma formation. EB formation recapitulates the key developmental processes during peri- and early post-implantation development 41; these include vascular lineage differentiation and the formation of a network of endothelial cell cords and tubes 22, 42, 43. The EB system is particularly useful to study loss-of-function mutations when the phenotype is embryonic lethal, as is the case with Vezf1 null embryos.
Our results add new insights into the role of Vezf1 during vascular development. We observed three major phenotypes in EBs derived from mutant ES cells: delay in growth and attachment, defects in the formation of vascular structures, and extensive and abnormal collagen IV deposition. Importantly, these phenotypes were recapitulated when mutant ES cells were induced to form teratocarcinomas. In contrast to wt EBs that formed a well-organized vascular network surrounded by a defined collagen IV positive ECM, Vezf1−/− EBs contained mostly dispersed endothelial cells that failed to form a recognizable network. We also observed reduced angiogenic sprouting and branching in mutant EBs. It is unlikely that these defect are solely a consequence of the slower growth rate of the mutant EBs since overall differentiation was not affected whereas vascular cord formation did not catch up upon extended periods of culture. Rather, it is likely that the defects are the result of differences in endothelial cell polarization, migration and/or alignment. Of interest, intermediate phenotypes were observed in Vezf1+/− EBs. Since Vezf1 mRNA levels are reduced to half in heterozygous EBs, these results provide further support for the notion that Vezf1 acts in a strictly dosage-dependant fashion.
Loss-of-Vezf1 gene function resulted in the abnormal distribution and deposition of collagen IV. Loss of collagen IV co-localization with PECAM-1 positive endothelial cells is consistent with the reduced levels of collagen deposition found around capillaries of Vezf1 null embryos 18. Collagen IV is a main component of the vascular basement membrane, a specialized ECM on the basal side of endothelial cells, which is important for endothelial differentiation and proliferation and for providing mechanical support 44, 45. Mice with a targeted inactivation of the Col4α1/2 locus die between E10.5-11.5 due to structural failure of the basement membrane 46. We propose that loss-of-Vezf1 causes aberrant accumulation on non-endothelial cells and incomplete deposition of collagen IV on endothelial cells, resulting in the destabilization of the endothelial basement membrane and the emerging vascular structures, vascular remodeling defects, and the loss of vascular integrity. Importantly, our studies have uncovered retinol/Vitamin A metabolism as a potential molecular pathway linking Vezf1 function to collagen IV expression and its distribution in the ECM (model in Fig. 2G). Consistent with this model, Vitamin A deficiency in rats has been shown to increase the expression of collagen type IV 47. We propose that Vezf1 regulates the retinol/Vitamin A pathway by activating expression of Rbp4 and Ttr. Regulation of Rbp4 and Ttr by Vezf1 could be achieved either through direct transcriptional transactivation, or indirectly via the transcription factor HNF4α 48.
Previous studies using Vezf1 antisense RNA resulted in reduced numbers of endothelial cells in G2/M and an increase in apoptosis 14. In contrast, we did not observe increased apoptosis in Vezf1−/− EBs, and therefore apoptosis can be excluded as a cause of the observed angiogenesis defects. Certain metabolic products of collagen IV, including tumstatin 49, 50, and canstatin 51, inhibit angiogenesis by inducing apoptosis. The accumulation of collagen IV in Vezf1−/− EBs and teratocarcinomas possibly may be explained by increased local expression and/or low metabolic turnover, which in turn may explain the low number of apoptotic cells.
Unexpectedly, we uncovered striking defects during in vitro differentiation of Vezf1-null ES cells along the definitive hematopoietic lineages; in addition, differentiation into primitive erythrocytes was significantly reduced. It should be noted that in Vezf1 null embryos formation of primitive erythrocytes and megakaryocytes appeared to be normal 18. However, lethality of homozygous mutant embryos before the onset of definitive hematopoiesis precluded the analysis of potential differentiation defects in vivo.
Histological analyses of teratocarcinomas revealed that loss-of-Vezf1 does not result in a general block of cell lineage differentiation in these tumors; however, it does affect the tumorigenic ability of ES cells in vivo. Thus, Vezf1−/− derived tumors were significantly smaller and displayed an overall less differentiated phenotype, including an increase in neural tubes-like structures, and reduced formation of epithelia with well-defined lumen. It is important to note that significant fewer PECAM-1-positive vascular structures were detected in Vezf1−/− teratocarcinomas around and within differentiated tissue structures when compared to wt controls. Lower vascularity may lead to reduced supply of the tumors with nutrients and oxygen, likely resulting in reduced tumor growth. Interestingly, we found that the majority of vascular structures within the tumors were host-derived, whereas ES cell-derived endothelial cells contributed in a mosaic fashion to the vascular cords. Thus, the lower vascular content cannot be attributed solely to reduced differentiation or proliferation of mutant ES cell-derived endothelial structures; rather, differences in angiogenic factors that are present in the tumor microenvironments may play a role as well. It is possible that loss-of-function of Vezf1 in non-endothelial cells contributes to the observed phenotypes observed in embryoid bodies and teratocarcinomas. However, experimental evidence from the Vezf1 null mice suggested that endothelial cells are the origin of the primary defects. Results presented here substantiate this notion further: our findings indicate that overall differentiation of ES cells, as judged by cavitation and appearance of cells of the endothelial lineage, is very similar in wt and mutant embryoid bodies.
In conclusion, our studies using the EB and teratocarcinoma model provide new insights into the role of Vezf1 in the vascular system. Our data further support our hypothesis that Vezf1 is a transcription factor that controls the formation of a functional three-dimensional vascular network in a strictly dosage-dependant manner. Importantly, they suggest a direct involvement of Vezf1 in regulating endothelial differentiation, proliferation, and ECM distribution and deposition, with Collagen IV being an important mediator. A complete understanding of how Vezf1 exerts its role on a molecular level is still lacking. Several potential downstream target genes have been identified in vitro, these include endothelin-1 16, 17 and stathmin/OP18 14. We have recently performed a microarray screen using RNA from Vezf1−/− embryos and identified a small number of potential Vezf1 target genes; these include members of the retinoic acid metabolism pathway, members of the Apoa/b family of lipid transport proteins, fibrinogens, and members of the serpin family of proteases 28. In addition, increasing evidence suggests that Vezf1 function is modulated by protein-protein interactions with components of the Rho GTPase machinery, including RhoB and p68RacGAP 15, 17. Finally, an intriguing new role for Vezf1 in epigenetic regulation of gene expression has recently been uncovered 52. Vezf1 loss-of-function in ES cells results in down-regulation of the de novo methyltransferase Dnmt3b and affects widespread loss of DNA methylation of genomic DNA. Results from the present study suggest that the embryoid body system will be a powerful in vitro model that will allow studying genetic and epigenetic regulation of vascular system development in a quantitative fashion.
Supplementary Material
Acknowledgements
We thank Derek Lemons (UCSD) for generating the Vezf1lacZ/+ targeting vector, W. Blaner (Columbia University) for providing RBP4 antibody and purified RBP4 protein, G. Keller and M. Kennedy (McEwen Centre for Regenerative Medicine) with advice on the hematopoietic progenitor assay, Wendy LeVine for expert technical assistance with histology, and James Mtui for assistance with differentiating ES cells into lymphatic endothelial cells.
Sources of funding. This work was supported by NIH grants R01 HL65738 and R01 HL68648 to H.S., and a post-doctoral fellowship from the Swedish Research Council to P.A.O.
Footnotes
Disclosure. The authors declare that they have no relationships that could be perceived as real or apparent conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 6.Oettgen P. Transcriptional regulation of vascular development. Circ Res. 2001;89:380–388. doi: 10.1161/hh1701.095958. [DOI] [PubMed] [Google Scholar]
- 7.Minami T, Aird WC. Endothelial cell gene regulation. Trends Cardiovasc Med. 2005;15:174–184. doi: 10.1016/j.tcm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Xiong J-W, Battaglino R, Leahy A, Stuhlmann H. Large-scale screening for developmental genes in ES cells and embryoid bodies using retroviral entrapment vectors. Dev. Dyn. 1998;212:181–197. doi: 10.1002/(SICI)1097-0177(199806)212:2<181::AID-AJA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Xiong J-W, Leahy A, H-H Lee, Stuhlmann H. Vezf1: a Zn finger transcription factor restricted to endothelial cells and their precursors. Dev. Biol. 1999;206:123–141. doi: 10.1006/dbio.1998.9144. [DOI] [PubMed] [Google Scholar]
- 11.Kuhnert F, Stuhlmann H. Identifying early vascular genes through gene trapping in mouse embryonic stem cells. Curr. Top. Dev. Biol. 2004;62:261–281. doi: 10.1016/S0070-2153(04)62009-6. [DOI] [PubMed] [Google Scholar]
- 12.Kuhnert F, Stuhlmann H. Vezf1: a transcriptional regulator of the endothelium. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- 13.Lemons D, Sun X, Zou Z, Campagnolo L, Xiong J-W, Kuhnert F, English M, Licht J, Stuhlmann H. VEZF1 is an endothelial transcriptional transactivator that dispays sequence-specific DNA binding to the IL-3, flk-1 and flt-1 promoter. 2009. Manuscript under revision.
- 14.Miyashita H, Kanemura M, Yamazaki T, Abe M, Sato Y. Vascular endothelial zinc finger 1 is involved in the regulation of angiogenesis: possible contribution of stathmin/OP18 as a downstream target gene. Arterioscler. Thromb. Vasc. Biol. 2004;24:878–884. doi: 10.1161/01.ATV.0000126373.52450.32. [DOI] [PubMed] [Google Scholar]
- 15.Lebowitz PF, Prendergast GC. Functional interaction between RhoB and the transcription factor DB1. Cell Adhes. Commun. 1998;6:277–287. doi: 10.3109/15419069809010787. [DOI] [PubMed] [Google Scholar]
- 16.Aitsebaomo J, Kingsley-Kallesen ML, Wu Y, Quertermous T, Patterson C. Vezf1/DB1 is an endothelial cell-specific transcription factor that regulates expression of the endothelin-1 promoter. J. Biol. Chem. 2001;276:39197–39205. doi: 10.1074/jbc.M105166200. [DOI] [PubMed] [Google Scholar]
- 17.Aitsebaomo J, Wennerberg K, Der CJ, Zhang C, Kedar V, Moser M, Kingsley-Kallesen ML, Zeng GQ, Patterson C. p68RacGAP is a novel GTPase-activating protein that interacts with vascular endothelial zinc finger-1 and modulates endothelial cell capillary formation. J. Biol. Chem. 2004;279:17963–17972. doi: 10.1074/jbc.M311721200. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnert F, Campagnolo L, Xiong JW, Lemons D, Fitch MJ, Zou Z, Kiosses WB, Gardner H, Stuhlmann H. Dosage-dependent requirement for mouse Vezf1 in vascular system development. Dev. Biol. 2005;283:140–156. doi: 10.1016/j.ydbio.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wartenberg M, Gunther J, Hescheler J, Sauer H. The embryoid body as a novel in vitro assay system for antiangiogenic agents. Lab. Invest. 1998;78:1301–1314. [PubMed] [Google Scholar]
- 20.Kearney JB, Bautch VL. In vitro differentiation of mouse ES cells: hematopoietic and vascular development. Methods Enzymol. 2003;365:83–98. doi: 10.1016/s0076-6879(03)65006-8. [DOI] [PubMed] [Google Scholar]
- 21.Feraud O, Prandini MH, Vittet D. Vasculogenesis and angiogenesis from in vitro differentiation of mouse embryonic stem cells. Methods Enzymol. 2003;365:214–228. doi: 10.1016/s0076-6879(03)65015-9. [DOI] [PubMed] [Google Scholar]
- 22.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3421. [PubMed] [Google Scholar]
- 23.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- 24.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 25.Robertson EJ. Embryo-derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and embryonic stem cells: a practical approach. IRL Press; Oxford, England: 1987. [Google Scholar]
- 26.Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood. 2006;107:1214–1216. doi: 10.1182/blood-2005-08-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreuger J, Nilsson I, Kerjaschki D, Petrova T, Alitalo K, Claesson-Welsh L. Early lymph vessel development from embryonic stem cells. Arterioscler Thromb Vasc Biol. 2006;26:1073–1078. doi: 10.1161/01.ATV.0000217610.58032.b7. [DOI] [PubMed] [Google Scholar]
- 28.Chitnis S, Nichol D, Ocaya PA, Stuhlmann H. Gene expression profiling of mouse Vezf1 null embryos. 2010. Submitted.
- 29.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 30.von Eynatten M, Humpert PM. Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert Rev Mol Diagn. 2008;8:289–299. doi: 10.1586/14737159.8.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Chang YC, Kao YH, Hu DN, Tsai LY, Wu WC. All-trans retinoic acid remodels extracellular matrix and suppresses laminin-enhanced contractility of cultured human retinal pigment epithelial cells. Exp Eye Res. 2009;88:900–909. doi: 10.1016/j.exer.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 32.Hellemans K, Grinko I, Rombouts K, Schuppan D, Geerts A. All-trans and 9-cis retinoic acid alter rat hepatic stellate cell phenotype differentially. Gut. 1999;45:134–142. doi: 10.1136/gut.45.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin YH, Lei FY, Hu P, Pei J, Feng ZB, Pang YS. Effect of all-trans retinoic acid on renal expressions of matrix metalloproteinase-2, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rats with glomerulosclerosis. Pediatr Nephrol. 2009;24:1477–1486. doi: 10.1007/s00467-009-1166-1. [DOI] [PubMed] [Google Scholar]
- 34.de The H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 35.Sucov HM, Murakami KK, Evans RM. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feraud O, Cao Y, Vittet D. Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab. Invest. 2001;81:1669–1681. doi: 10.1038/labinvest.3780380. [DOI] [PubMed] [Google Scholar]
- 37.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc. Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 38.Robson P, Stein P, Zhou B, Schultz RM, Baldwin HS. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev Biol. 2001;234:317–329. doi: 10.1006/dbio.2001.0274. [DOI] [PubMed] [Google Scholar]
- 39.Keller GM. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 41.Leahy A, Xiong J-W, Kuhnert F, Stuhlmann H. Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J. Exp. Zool. 1999;284 doi: 10.1002/(sici)1097-010x(19990615)284:1<67::aid-jez10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 42.Choi K, Chung YS, Zhang WJ. Hematopoietic and endothelial development of mouse embryonic stem cells in culture. Methods Mol. Med. 2005;105:359–368. doi: 10.1385/1-59259-826-9:359. [DOI] [PubMed] [Google Scholar]
- 43.Gao F, Shi HY, Daughty C, Cella N, Zhang M. Maspin plays an essential role in early embryonic development. Development. 2004;131:1479–1489. doi: 10.1242/dev.01048. [DOI] [PubMed] [Google Scholar]
- 44.Madri JA. Extracellular matrix modulation of vascular cell behaviour. Transpl Immunol. 1997;5:179–183. doi: 10.1016/s0966-3274(97)80035-4. [DOI] [PubMed] [Google Scholar]
- 45.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 46.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 47.Marin MP, Esteban-Pretel G, Alonso R, Sado Y, Barber T, Renau-Piqueras J, Timoneda J. Vitamin A deficiency alters the structure and collagen IV composition of rat renal basement membranes. J Nutr. 2005;135:695–701. doi: 10.1093/jn/135.4.695. [DOI] [PubMed] [Google Scholar]
- 48.Duncan SA, Nagy A, Chan W. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of Hnf-4(−/−) embryos. Development. 1997;124:279–287. doi: 10.1242/dev.124.2.279. [DOI] [PubMed] [Google Scholar]
- 49.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 50.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 51.He GA, Luo JX, Zhang TY, Hu ZS, Wang FY. The C-terminal domain of canstatin suppresses in vivo tumor growth associated with proliferation of endothelial cells. Biochem Biophys Res Commun. 2004;318:354–360. doi: 10.1016/j.bbrc.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 52.Gowher H, Stuhlmann H, Felsenfeld G. Vezf1 regulates genomic DNA methylation through its effects on expression of DNA methyltransferase Dnmt3b. Genes Dev. 2008;22:2075–2084. doi: 10.1101/gad.1658408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.