Abstract
Rationale
Activation of p38 Mitogen Activated Protein Kinase (MAPK) has a significant impact on cardiac gene expression, contractility, extracellular matrix remodeling and inflammatory response in heart. The p38 kinase pathway also has a controversial role in cardiac hypertrophy. MAPK Activated Protein Kinase-2 (MK2) is a well established p38 downstream kinase, yet its contribution to p38 mediated pathological response in heart has not been investigated.
Objective
We examined the specific contribution of MK2 to the pathological remodeling induced by p38.
Methods and Results
We used a cardiomyocyte specific and inducible transgenic approach to determine the functional and molecular impact of acute activation of the p38 pathway in heart in either a MK2 wildtype or a MK2 null background. p38 activation in wildtype mice led to a rapid onset of lethal cardiomyopathy associated with cardiomyocyte hypertrophy, interstitial fibrosis and contractile dysfunction. Inactivation of MK2 partially but significantly reduced cardiomyocyte hypertrophy, improved contractile performance, and prevented early lethality. MK2 inactivation had no effect on the mRNA levels of hypertrophic marker genes or the pro-inflammatory gene cyclooxygenase-2 (COX-2). However, MK2 had a major role in COX-2 protein synthesis without affecting the mRNA level or protein stability.
Conclusions
p38 activity in adult myocytes can contribute to pathological hypertrophy and remodeling in adult heart and that MK2 is an important downstream molecule responsible for specific features of p38 induced cardiac pathology.
Keywords: MK2, p38 MAPK, hypertrophy, COX-2, Heart Failure
Heart failure, a disease with high prevalence and mortality rates, can be induced by external stressors such as increased hemodynamic load, ischemia or reperfusion injury 1. A complex network of signaling molecules has been implicated in mediating the pathological effects of these extrinsic stressors, including p38 Mitogen Activated Protein Kinases (MAPK), a branch of the conserved MAP kinase family mostly implicated in stress signaling and regulation2.
In cultured cardiomyocytes, p38 activation induces myocyte hypertrophy and apoptosis 3, and promotes fetal gene expression and cytokine production 4, 5. However, p38 chronic activation in intact mouse hearts did not result in cardiac hypertrophy6, 7, but instead caused restrictive cardiomyopathy associated with extracellular matrix remodeling 6 and contractile dysfunction 8. In addition, elevated p38 activity suppresses hypertrophy via the negative regulation of NFAT activity in culture 9. Furthermore, cardiomyocyte specific genetic knockout of p38α results in a normal hypertrophic response subsequent to pressure overload, but is accompanied by accelerated heart failure, elevated myocyte death and interstitial fibrosis 10. Mice expressing a dominant negative cardiac specific p38α displayed normal hypertrophy but are resistant to fibrosis 11. Thus, the p38 pathway is generally considered not to be a major component of the hypertrophy pathway in heart, but is thought to be important for both adaptive and pathological remodeling. However, these in vivo studies employed chronic activation or inactivation approaches to characterize p38 function in heart. In vivo, p38 activity is mostly induced in a transient fashion under stressed conditions 2. Therefore, the impact of short-term activation of p38 activity in adult heart has not been investigated using genetic models.
MAPK Activated Protein Kinase-2 (MK2) is a major downstream p38 MAPK substrate that plays an important role in p38 mediated inflammatory regulation in immune cells. The MK2 deficient mouse develops normally, but has a significantly increased resistance to endotoxic shock with impaired cytokine induction 12. MK2 is an important regulator of mRNA stability for COX-213, IL-6, TNFα and MIP 14, 15, and its known substrates include serum response factor 16, 14-3-3 17, CREB and ATF-1 18. Shiroto et al. also demonstrated that MK2 deficiency protects the heart against ischemia/reperfusion injury19. However, the specific contribution of MK2 in p38 mediated pathological responses in heart has not been characterized.
In this report, we describe a new transgenic mouse model with conditional and cardiomyocyte-specific expression of a constitutively activated p38 specific upstream activator MKK3bE, and explore its biology in both wildtype and MK2 null mice. Unexpectedly, conditional activation of p38 in adult heart led to the rapid onset of cardiac hypertrophy accompanied by interstitial fibrosis and contractile dysfunction. MK2 loss significantly reduced MKK3bE induced hypertrophy, improved the contractile performance, and rescued lethality. However, MK2 inactivation did not affect the mRNA levels of hypertrophic marker genes or pro-inflammatory molecules. In contrast, MK2 inactivation had a major impact on COX-2 protein synthesis, without a significant effect on the COX-2 mRNA level or protein stability. These data are the first in vivo evidence demonstrating that acute p38 activation in adult heart is sufficient to induce lethal cardiac hypertrophy and other pathological heart remodeling. We also reveal a previously uncharacterized MK2 dependent regulation of COX-2 protein synthesis in heart and find that MK2 activity plays an important role in p38 mediated cardiac hypertrophy and heart failure.
METHODS
Transgenic animals
The conditional αMHC-flox-MKK3bE (MKK3) mouse line 6, the tamoxifen inducible Mer-Cre-Mer mouse line (Cre, a kind gift from Dr. Molkentin, Cincinnati) 20, 21, and the MK2 null mouse line (kindly provided by Dr. Huiping Jiang, Borehinger Ingelheim Pharmaceutical Inc.) 12 were already established as described. Details of crossbreeding and tamoxifen induction are described in Supplemental Information.
Statistical Analysis
Comparisons between more than two groups were accomplished using a factorial analysis of variance (ANOVA) with Fisher’s Protected Least Significant Difference post-hoc test. Comparisons between pairs of survival curves was accomplished by a Chi Square test. Analysis was carried out using the StatView program (Abacus Concepts, Berkeley, CA). In all cases a significant result was defined as p<0.05.
A detailed description of all methods used is available in the Online Data Supplement.
RESULTS
The Effect of MK2 Inactivation on p38 MAPK Activation in Adult Mouse Heart
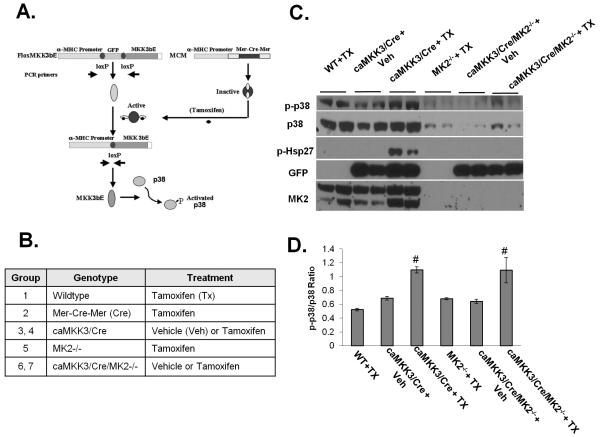
In an earlier study we demonstrated, using a tamoxifen inducible Cre combined with a floxed MKK3bE transgenic line, that we can induce MKK3bE expression and specific p38 activation in adult mouse heart 22. Using 4-6 month old adult mice with the five different genotypes of wildtype, Mer-Cre-Mer (Cre), MKK3/Cre, MK2−/− and MKK3/Cre/MK2−/− (Figure 1B and Methods), we observed that loss of MK2 significantly reduced the total p38 protein in heart, similar to the previous observations made in other tissues (Figure 1C) 23. However, phospho-p38/total p38 ratios remained elevated in the tamoxifen treated MKK3/Cre/MK2−/− hearts relative to other controls at a comparable level as observed in the tamoxifen treated MKK3/Cre hearts (Figure 1C). Therefore, loss of MK2 significantly reduced the total p38 protein but did not affect the upstream MKK3 dependent p38 phosphorylation/activation. As expected, phosphorylation of Hsp27, a well established downstream target of the p38/MK2 pathway, was readily detected in the tamoxifen treated MKK3/Cre hearts but was completely abolished in the tamoxifen treated MKK3/Cre/MK2−/− hearts, suggesting the loss of MK2 downstream signaling in MK2 null hearts.
Figure 1. Inducible p38 activation in adult mouse heart.
A) A diagram of the heart specific, inducible gene activation based on cre-loxP mediated DNA recombination. αMHC promoters provide heart specific expression, while a floxed GFP cassette and stop sequences prevent MKK3bE translation. B) A list of the genotypes and treatment groups used in this report. C) Transgenic mice and their littermate controls were injected with vehicle (+ Veh) or tamoxifen (+TX) as described in the Methods. After 8 days, the hearts were removed and total protein extracts were prepared and subjected to Western blot analysis using antibodies as indicated. D). The average ratio of phospho-p38 and total p38 signals as measured by densitometry. # p<0.05 vs. WT+TX as control.
The Impact of MK2 Inactivation on MKK3 Induced Cardiac Hypertrophy and Dysfunction
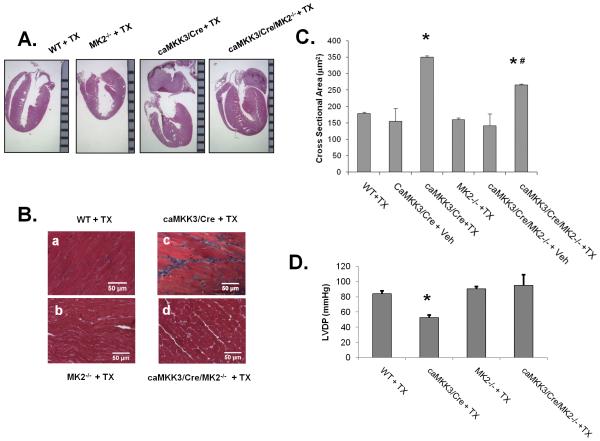
At eight days post induction, MKK3bE expression resulted in significant changes in the gross morphology of the heart, including ventricular hypertrophy and enlargement of the left atria with a prominent presence of thrombotic clot, indicating a state of hypertrophic cardiomyopathy (Figure 2). The status of ventricular hypertrophy was supported quantitatively by the average myocyte cross-sectional area (CSA) measured from histological sections (Figure 2B and 2C), end-systolic posterior wall thickness of the left ventricle measured from echocardiography (Figure 3), and left ventricular weight vs. body weight ratio in tamoxifen treated MKK3/Cre mice comparing to other control groups (Online Table II). In the MK2 null background, the extent of cardiac hypertrophy was partially but significantly ameliorated as determined from CSA (Figure 2C) and posterior wall thickness (Figure 3) and a strong trend of reduction in left ventricular weight (Online Table II).
Figure 2. Cardiac hypertrophy and dysfunction in p38 activated Heart.
A) Transgenic mice and their littermate controls were induced with tamoxifen as described in Figure 1, and the hearts removed and fixed on day 8. The hearts were embedded in paraffin and sectioned coronally (4 chamber view), then stained with hematoxylin and eosin. A ruler with mm marks was used as scale bar. B) Heart sections were prepared as in A, sectioned in a cross-section orientation at the mid-left ventricle, and the sections were stained by Masson’s Trichrome. C) The heart sections were quantified for myocyte cross sectional area (CSA) in μm2 from 10 random images of each heart and 10 random cells in each image. The resultant average of 100 cells per heart counted as a sample size of 1. D) Left ventricular developed pressure (LVDP, mmHg) measured from different genotype groups as indicated are presented as mean ± S.E. *, p<0.05 vs. WT+TX; #, p<0.05 vs. caMKK3/Cre + TX.,
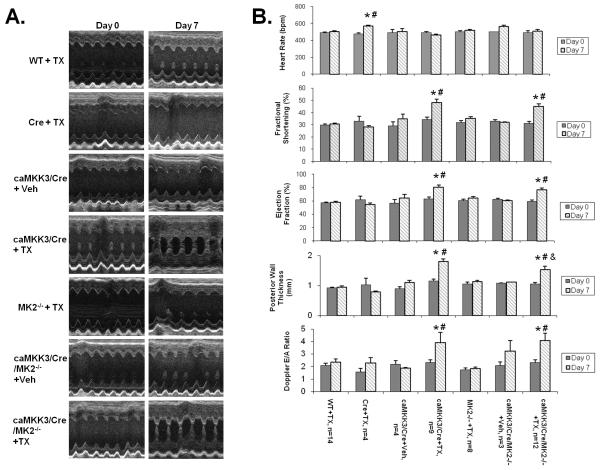
Figure 3. Cardiac function and morphology measured by echocardiography.
A) Representative M-mode images from the short-axis mid-left ventricle of different genotype mice as indicated are shown before (Day 0) and after (Day 7) tamoxifen induction. B) Functional and morphometric parameters from different genotype mice as indicated before (Day 0) and after (Day 7) tamoxifen induction. *, p<0.05 vs. respective WT or MK2−/− control. #, p<0.05 vs. Day 0 of the same group. &,.p<0.05 vs. MKK3/Cre+TX Day 7.
Also using echocardiography, we determined that MKK3 induction in adult hearts led to a significant increase in fractional shortening and ejection fraction, supporting again the status of restrictive and concentric hypertrophy (Figure 3). However, impaired cardiac function manifested in an increased mitral valve Doppler E/A ratio (Figure 3), suggesting elevated stiffness of the myocardium. Contractile dysfunction was further measured from a pressure catheter placed directly into the left ventricular chamber (Online Table I) which showed a significant reduction of left-ventricular developed pressure, an increase in end-diastolic pressure and tau following p38 activation (Online Table I). In the MK2-null background, MKK3 induced loss of LVDP was partially reversed (Figure 2D) while changes in E/A Doppler ratio and tau were not affected. Therefore, MK2 activity appears to contribute to specific aspects of contractile dysfunction in MKK3 induced hearts.
Cardiac fibrosis as determined by Masson’s Trichrome staining was induced in tamoxifen treated MKK3/Cre hearts in higher level in WT than in the MK2 null background (Figure 2B). Considering the potential deleterious impact of tamoxifen treatment on cardiac function as reported by Koitabashi et al. 24, we also examined the function of tamoxifen treated Mer-Cre-Mer transgenic mice (Cre + TX). Under our treatment regimen, the MCM (Cre) mice showed no cardiomyopathy as indicated by echocardiography (Figure 3), heart mass (Online Table II) or gene expression (Figure 5) in agreement with the earlier observations made by Sohal et al. 20, 21.
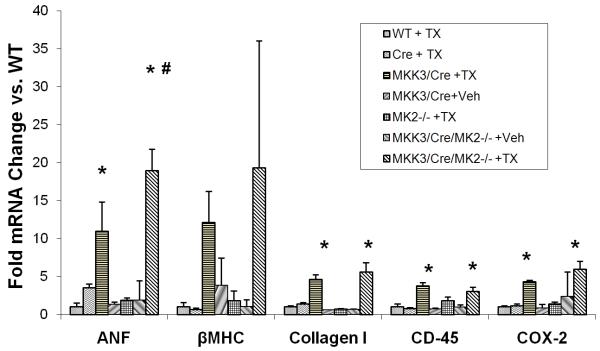
Figure 5. Stress and inflammatory gene activation in transgenic mice.
Mice with different genotypes as shown were induced with tamoxifen and sacrificed on day 8. mRNA levels of different genes (as indicated) were quantified by qPCR. *, p<0.05 vs. WT + TX; #, p<0.05 vs. caMKK3/Cre + TX.
The Role of MK2 in MKK3 Induced Lethal Heart Failure and Gene Expression
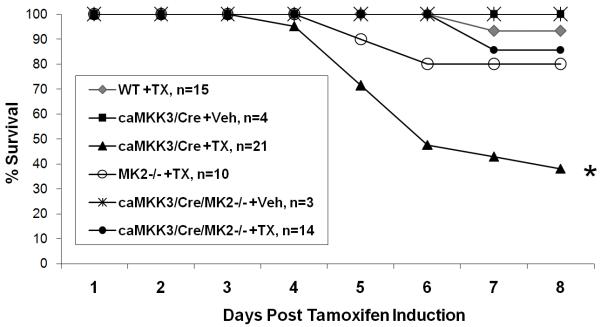
Acute tamoxifen induction of MKK3 activity in adult mice led to significant lethality with 38% survival at day 8 (Figure 4). By longitudinal telemetric monitoring, we observed that MKK3 induced mice developed progressive bradycardia accompanied by a gradual lowering of the body temperature as they approach death, a common observation made in end-stage heart failure (Online Figure I). Inactivation of MK2 significantly reduced the mortality observed in MKK3 induced animals. These data support the notion that MK2 inactivation in MKK3/Cre mice significantly reduced lethal heart failure. Therefore, MK2 contributes to MKK3 induced contractile dysfunction and the development of heart failure.
Figure 4. Mouse survival after transgene activation.
All six genotype/treatment groups were induced with tamoxifen or vehicle as indicated for three consecutive days (day 1-3) and allowed to proceed to day 8 before termination of the experiment. The survival of each group as a percentage is tracked here throughout the 8 day treatment period. *, p<0.05 vs. WT + TX .
Despite a significant impact on myocyte hypertrophy and cardiac function, MK2 inactivation did not reduce MKK3 induced expression of hypertrophic marker genes, including ANF and βMHC (Figure 5). In addition, Collagen I induction was also not affected. Lastly, MK2 inactivation did not affect COX-2 mRNA induction in the MKK3 expressing hearts. In previous report, MK2 null mice were found to have a normal or increased induction of TNFα mRNA but impaired TNFα protein biosynthesis 12, 25. This observation raised the question whether MK2 might also regulate COX-2 expression at a post-transcriptional level in p38 activated mouse heart.
MK2 Regulates COX-2 Protein Expression In Vitro
COX-2 induction is associated with hypertrophic cardiomyopathy (Streicher JMCC, in Press). To further characterize MK2 mediated COX-2 expression at the post-transcriptional level in cardiomyocytes, we infected neonatal rat ventricular myocytes (NRVM) with adenoviral constructs encoding constitutively active MK2 (caMK2), dominant negative MK2 (dnMK2), and constitutively active MKK3 (caMKK3), which specifically activates p38. Both caMK2 and caMKK3 robustly induce COX-2 expression over the control adenovirus (LacZ), while the dnMK2 was able to significantly antagonize COX-2 protein induction by caMKK3 (Figure 6A). Both caMK2 and caMKK3 also robustly induced Hsp27 phosphorylation, a downstream target of MK2, validating the functionality of these vectors. These data indicate that MK2 activation is able to induce COX-2 protein expression in cardiomyocytes and that MK2 activity is necessary for MKK3 mediated COX-2 induction.
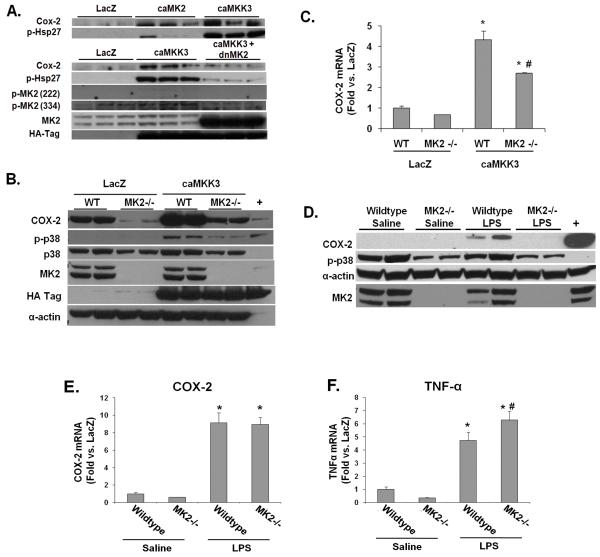
Figure 6. Characterization of COX-2 regulation by MK2.
A) NRVMs were transduced with the indicated adenoviral constructs. Forty-eight hours later cells were harvested. Immunoblots were performed on total protein extracts prepared from the treated cells, using antibodies for COX-2, phospho-Hsp27, phospho-MK2, total MK2, HA-tag (for HA-caMKK3), phospho-ERK and total ERK as indicated. B) Wildtype (WT) or MK2−/− MEF cells were cultured in DMEM + 2% FBS, transduced with the indicated adenovirus vectors, and analyzed after 48 hours. Immunoblots of total protein extracts were performed using the indicated antibodies. C) Wildtype (WT) or MK2−/− MEF cells were cultured and transduced with adenovirus as in panel A. COX-2 mRNA levels were determined by qPCR. * p<0.05 vs. LacZ control. # p<0.05 vs. WT caMKK3. D) MK2 WT and KO mice were injected with saline or LPS (5 mg/kg). Six hours post injection, the hearts were removed and protein extracts were prepared. Immunoblots are shown for COX-2 following immunoprecipitation and, for the other indicated proteins, from protein lysates. E) and F) COX-2 and TNFα mRNA levels were measured by qPCR from total RNA prepared from wildtype or MK2−/− hearts treated with saline or with LPS. * p<0.05 vs. saline control, # p<0.05 vs. wildtype treated with LPS.
The role of MK2 in COX-2 protein regulation was also observed in mouse embryonic fibroblasts (MEFs). In MK2−/− MEF cells, COX-2 protein was significantly reduced both at basal levels and following p38 activation (Figure 6B). In contrast, qPCR analysis revealed no difference in COX-2 mRNA levels between MK2+/+ and MK2−/− MEFs at the basal condition (Figure 6C), and p38 induced COX-2 mRNA was only partially reduced in MK2−/− MEFs. This finding supports the notion that MK2 has a role in regulating COX-2 protein in mammalian cells.
MK2 Promotes COX-2 Protein Induction in Mouse Heart
To determine whether MK2 has any functional role in COX-2 regulation in intact heart, we stimulated COX-2 induction in mouse heart by treating MK2+/+ and MK2−/− mice with LPS for 6 hours. LPS treatment led to a robust COX-2 protein induction in MK2+/+ hearts. In contrast, this induction was completely abolished in MK2−/− mice (Figure 6D). However, cardiac COX-2 mRNA levels were robustly induced in both MK2+/+ and MK2−/− hearts (Figure 6E). Thus, MK2 inactivation had no impact on the COX-2 mRNA induction in response to LPS but completely abolished COX-2 protein induction. We confirmed that the LPS treatment stimulates an inflammatory cardiac response in MK2−/− mice by showing a similarly robust stimulation of TNFα mRNA accumulation in both wildtype and MK2−/− hearts (Figure 6F). Therefore, both in vitro and in vivo observations strongly suggest that MK2 activity plays an essential role in the expression of COX-2 at the protein level in response to inflammatory stimulation.
MK2 Regulates COX-2 Protein Synthesis Without Affecting Its Degradation
Steady state protein levels can be regulated by either (or both) the rate of synthesis and the rate of degradation. To determine the mechanism of MK2 mediated COX-2 protein regulation, we metabolically labeled MK2+/+ and MK2−/− MEF cells with 35S-methionine (see Supplementary Methods). By continuously labeling cells with radioactive methionine over time, we were able to measure the accumulation rate of newly synthesized COX-2 protein (Figure 7A). Based on the linear curve of radioactive methionine incorporation in COX-2 immunoprecipitates between 2 hours and 4 hours post labeling, the newly synthesized COX-2 protein accumulation rate (K) in wildtype MEFs was nearly 2.4 fold faster than in MK2−/− MEFs (0.56 vs. 0.23 hour−1)(Figure 7A).
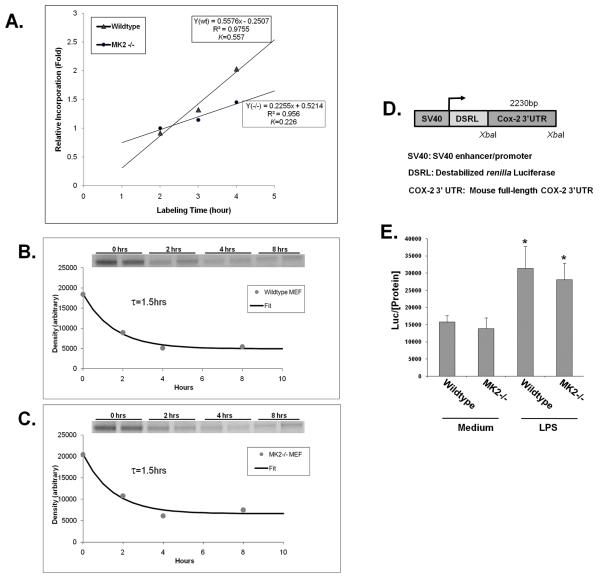
Figure 7. MK-2 mediated regulation of COX-2 protein synthesis.
A) MK2 WT and MK2−/− cells were labeled with 35S-methionine for the indicated time course. Total protein was harvested, and COX-2 was immunoprecipitated, separated by SDS-PAGE and autoradiographs were prepared. Densitometry data was collected from the films and fitted with a linear curve (See Methods for details). The COX-2 protein accumulation rates in wildtype and MK2−/− MEFs are derived from the linear equations provided. COX-2 protein turn-over rate: B) MK2 wildtype and C) MK2−/− cells were labeled with 35S-methionine for 0.5 hrs before being switched to non-radioactive methionine medium for the indicated time course. Radiolabeled COX-2 protein was immunoprecipitated and quantified as described above (see Inserts). The t1/2 of COX-2 degradation (i.e. the half-life) was measured from the fitted exponential curve. The data shown are representative of three independent experiments, each with the same results. D) Diagram of the COX-2 mRNA 3′UTR luciferase reporter construct. E) MK2 WT and KO cells were cultured and transfected with the reporter, and stimulated with medium or medium + LPS for 6 hours. Cells were lysed and luciferase levels in the extracts were measured with a luminometer and normalized to the protein concentration. * p<0.05 vs. medium treatment in wildtype MEFs.
Since the rate of protein accumulation is a combined result of protein synthesis and degradation, we also determined the COX-2 protein degradation rates in MK2+/+ and MK2−/− MEF cells, using a pulse-chase labeling method 26. As shown in Figure 7B/C, COX-2 protein turn-over rates (τ) were the same for MK2+/+ and MK2−/− cells (τcox2 =1.5 hours). The global synthesis and degradation protein profiles were also obtained in the same samples as described in the Supplementary Methods and were found to be the same for the two cell types (τtotal= 2.4 hours)(Online Figures II and III). This finding thus suggests that MK2 promotes COX-2 protein expression most likely by enhancing its translational rate rather than affecting COX-2 protein stability or turn-over.
One potential site for MK2 to regulate COX-2 translation is through the COX-2 mRNA 3′UTR, which has been shown to bind translational regulators like TIA-1 and tristetraprolin/tis-11 25, 27. To test this possibility, we transfected a reporter containing a constitutive promoter driving luciferase cDNA followed by the full-length COX-2 3′UTR (Figure 7D) into MK2+/+ and MK2−/− MEF cells. We then stimulated these cells with LPS, which is reported to stabilize the COX-2 mRNA through the 3′UTR 15. We did indeed observe an induction in the luciferase signal in response to LPS, as expected (Figure 7E). However, we detected no differences in luciferase activity between the MK2+/+ and MK2−/− MEFs, under either stimulated or basal conditions (Figure 7E). These data indicate that the loss of COX-2 protein seen in the MK2−/− cells is most likely via a mechanism independent of the COX-2 3′UTR.
DISCUSSION
In this study, we have established an inducible, heart specific model of MKK3 induction that permits the examination of acute p38 MAPK activation. By breeding the inducible transgene alleles into the MK2−/− background, we examined the role of MK2 in acute p38 activation in adult heart. Induction of MKK3 led to cardiomyocyte hypertrophy, cardiac remodeling, contractile dysfunction, pathological gene activation, and lethal bradycardic heart failure. Removal of the MK2 gene rescued some aspects of this pathologic response, including partially ameliorated hypertrophy and contractile dysfunction, and prevention of early lethality. Thus, MK2 has an important role in mediating some aspects of p38 induced heart failure. Its contribution to p38 signaling can be mediated by either affecting the total p38 protein level and signaling intensity (Figure 1) or by specifically blocking the MK2 dependent downstream signaling (as demonstrated by Hsp27 phosphorylation in Figure 1). From both in vitro and in vivo analysis, we also established a previously uncharacterized role for MK2 in COX-2 protein synthesis regulation. Therefore, our study not only demonstrates a specific contribution of MK2 in p38 induced cardiomyopathy but also reveals a new mechanistic link between p38 and inflammatory gene expression in heart.
The finding that p38 activation was sufficient to induce ventricular myocyte hypertrophy is supported by wall thickness measured in vivo by echocardiography, normalized ventricular mass and myocyte CSA from histological sections. The finding that p38 induces hypertrophy in adult heart is in accordance with in vitro myocyte experiments 3. However, most in vivo models of p38 activation have not shown myocyte hypertrophy 6, 7. One potential explanation for this discrepancy lies in the timing and duration of p38 activation. Earlier in vivo studies used constitutively activated promoters to achieve p38 activation in an unregulated manner at earlier developmental stages 6, 7. In contrast, our current study used a tamoxifen dependent Cre gene to activate the p38 pathway in adult heart (see Methods). This may eliminate the potential confounding factors involving p38 mediated regulation of cardiomyocyte proliferation and differentiation as implicated in other reports 28, or secondary compensatory changes. However, we cannot exclude other complicating factors such as contribution of tamoxifen treatment 24and GFP expression 29 although we have different control groups to support the specificity of the observed phenotype in MKK3bE expressing hearts.
Considering the fact that p38 is usually activated in a transient fashion in response to acute stress stimuli, our model may be more relevant to pathological induction of the p38 pathway in heart. Our data also shows that MK2 had a major role in p38 mediated cardiac hypertrophy. MK2 inactivation partially reduced LV weight, cross-sectional area and LV wall thickness induced by p38 activation. In short, our data suggests that activation of the p38 pathway in adult heart may still have a significant contribution to cardiomyocyte hypertrophy in addition to other aspects of pathological remodeling. Furthermore, MK2 is a significant downstream signaling component in p38 induced hypertrophy.
In addition to hypertrophy, we also showed that p38 MAPK activation is linked to cardiac remodeling and contractile defects as supported by histology, gene expression and functional data, in good agreement with previous findings 6, 8. MK2 inactivation significantly improved cardiac contractility in MKK3 transgenic hearts based on hemodynamic measurements. However, MK2 did not rescue the observed myocardial stiffness or fibrotic remodeling. One possibility is that MK2 acts directly downstream of p38 MAPK to mediate negative inotropic signaling on the contractile apparatus 8. However, the underlying molecular mechanism needs to be further investigated.
In this study, we demonstrate that MK2 has a previously uncharacterized role in regulating COX-2 protein expression. Our data suggests that MK2 modulates the rate of COX-2 protein synthesis without affecting its degradation. We also suggest that the MK2 mediated modulation of COX-2 protein synthesis does not involve the COX-2 mRNA 3′UTR. This observation differs from previous reports that implicates p38 and MK2 in COX-2 induction through mRNA stabilization 14, 15, 30. MK2 mediated regulation of COX-2 protein synthesis is an important addition to the regulatory mechanisms linking stress stimuli to inflammatory responses in heart cells.
COX-2 protein induction has been found in human patients with myocardial infarction or heart failure 31, and prostaglandins such as PGF2α are induced in heart failure patients as well 32. COX-2 inhibition protects the heart from endotoxin and lipoteichoic acid treatment 33, 34. COX-2 inhibition also protects the heart from ischemia related damage in coronary artery ligated rat and mouse heart 35, 36. However, a number of other st udies have reported no effect, or the opposite effect of COX-2 inhibition in myocarditis and ischemia (reviewed in 37). Evidence from our lab suggests that COX-2 expression in heart is sufficient to induce cardiac hypertrophy and fetal gene expression 38. Clearly, more investigation will be needed to establish the specific function of COX-2 in MK2 mediated regulation of cardiac pathology and to uncover the underlying molecular basis by which MK2 regulates COX-2 protein synthesis.
The MK2 KO mouse appears to tolerate the loss of MK2 protein well without adverse effects 12. Therefore, MK2 inhibition may serve as an alternative means of preventing cardiac hypertrophy, heart failure and inhibiting inflammatory response. Indeed, specific MK2 inhibitors are already being evaluated for rheumatoid arthritis 39 and their efficacy in treating heart failure remains to be examined. Nevertheless, the in vivo evidence and molecular data presented in this study suggest that MK2 is a potential target of intervention for cardiomyopathy and inflammation.
NOVELTY AND SIGNIFICANCE.
What is known?
Chronic activation of a stress inducible p38 mitogen activated protein kinase (MAPK) pathway can lead to pathological changes in the heart, including gene expression, contractile dysfunction, extracellular remodeling, and myocyte death. However, its specific contribution to cardiac hypertrophy remains controversial.
MAPK activated protein kinase-2 (MK2) is a major downstream protein kinase of p38 MAPK; however, its role in p38 induced cardiac pathology is unknown.
Cyclooxygenase-2 (COX2) is a known downstream target of p38 signaling and has a role in the development of hypertrophy. However, the significance and mechanism of MK2 mediated pathway in COX2 expression in heart is unknown.
What new information does this article contribute?
Inducible activation of p38 pathway in adult heart led to hypertrophy and pathological remodeling, a phenotype different from the one that resulted from chronic activation of the p38 pathway.
MK2 inactivation has a significant but selective impact on the pathological effect of p38 activation in the intact heart.
MK2 is a critical signal molecule for stress-induced COX2 expression in heart and other cells. The underlying mechanism involves protein synthesis instead of transcriptional regulation, mRNA stability, or protein degradation.
Summary
Chronic activation of p38 MAPK can lead to pathological changes in gene expression, contractility, extracellular matrix remodeling, and inflammatory response in the heart. However, the specific role of the p38 kinase pathway in cardiac hypertrophy is controversial. MK2 is a well established p38 downstream kinase, yet its contribution to p38 mediated pathological response in heart has not been investigated. In this report, we developed new transgenic mouse models with cardiomyocyte specific and inducible activation of the p38 pathway in the heart in either an MK2 wildtype or an MK2 null background. Through phenotypic and molecular characterizations, we have established that acute p38 activation in the adult mouse heart causes rapid onset of lethal cardiomyopathy associated with cardiomyocyte hypertrophy, different from the phenotype resulting from chronic p38 activation. Our study also demonstrates for the first time the selective contribution of MK2 to p38 induced pathological changes, including a major role in the post-transcriptional regulation of the pro-inflammatory protein COX-2. This study provides a new understanding of the functional significance of a major stress-response signaling pathway in the heart and of the downstream signaling mechanisms.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Jing Gao, Na Du and Haiying Pu for assistance in mouse breeding and handling. The authors also thank Riccardo Olcese (UCLA) for assistance in data analysis.
Sources of Funding
The work described here was supported by grants from National Institutes of Health HL70079, HL080111, HL088640 (YW) and CA084572 (HH). YW is an Established Investigator of American Heart Association.
ABBREVIEATIONS
- MAP Kinase
Mitogen activated protein kinases
- MK-2
MAP kinase activated protein kinase-2
- COX-2
cyclooxygenase-2
- NFAT
Nuclear factor of activated T-cells
- Il-6
Interleukin-6
- TNFα
Tumor necrotic factor alpha
- MIP
macrophage inflammatory protein
- CREB
cAMP response element binding protein
- ATF-1
activating transcription factor 1
- MKK3b
MAP kinase kinase 3b
- αMHC
alpha myosin heavy chain
- CSA
cross-section area
- LVDP
Left ventricle developed pressure
- ANF
atrial natriuretic factor
- βMHC
beta-myosin heavy chain
- NRVM
neonatal rat ventricular myocytes
- MEF
mouse embryonic fibroblasts
- LPS
lipopolysaccharides
- 3′UTR
3′ untranslated region
- GFP
green fluorescent protein
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Ono K, Han J. The p38 signal transduction pathway: Activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 4.Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. P38 map kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 6.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 map kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martindale JJ, Wall JA, Martinez-Longoria DM, Aryal P, Rockman HA, Guo Y, Bolli R, Glembotski CC. Overexpression of mitogen-activated protein kinase kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. J Biol Chem. 2005;280:669–676. doi: 10.1074/jbc.M406690200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao P, Wang SQ, Wang S, Zheng M, Zhang SJ, Cheng H, Wang Y, Xiao RP. P38 mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 mapk promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. P38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the grb2-p38 mapk signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. Mapkap kinase 2 is essential for lps-induced tnf-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 13.Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR. Regulation of cyclooxygenase 2 mrna stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. Mk2 targets au-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem. 2002;277:3065–3068. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau S, Morrice N, Peggie M, Campbell DG, Gaestel M, Cohen P. Inhibition of sapk2a/p38 prevents hnrnp a0 phosphorylation by mapkap-k2 and its interaction with cytokine mrnas. Embo J. 2002;21:6505–6514. doi: 10.1093/emboj/cdf639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. Mapkap kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 17.Powell DW, Rane MJ, Joughin BA, Kalmukova R, Hong JH, Tidor B, Dean WL, Pierce WM, Klein JB, Yaffe MB, McLeish KR. Proteomic identification of 14-3-3zeta as a mitogen-activated protein kinase-activated protein kinase 2 substrate: Role in dimer formation and ligand binding. Mol Cell Biol. 2003;23:5376–5387. doi: 10.1128/MCB.23.15.5376-5387.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. Fgf and stress regulate creb and atf-1 via a pathway involving p38 map kinase and mapkap kinase-2. Embo J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- 19.Shiroto K, Otani H, Yamamoto F, Huang CK, Maulik N, Das DK. Mk2-/-gene knockout mouse hearts carry anti-apoptotic signal and are resistant to ischemia reperfusion injury. J Mol Cell Cardiol. 2005;38:93–97. doi: 10.1016/j.yjmcc.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 21.Petrich BG, Molkentin JD, Wang Y. Temporal activation of c-jun n-terminal kinase in adult transgenic heart via cre-loxp-mediated DNA recombination. Faseb J. 2003;17:749–751. doi: 10.1096/fj.02-0438fje. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell S, Ota A, Foster W, Zhang B, Fang Z, Patel S, Nelson SF, Horvath S, Wang Y. Distinct gene expression profiles in adult mouse heart following targeted map kinase activation. Physiol Genomics. 2006;25:50–59. doi: 10.1152/physiolgenomics.00224.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, Lin LL, Gaestel M. Distinct cellular functions of mk2. Mol Cell Biol. 2002;22:4827–4835. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced mercremer gene deletion models. Circ Res. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mrna stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273(Pt 1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon DA, Balch GC, Kedersha N, Anderson P, Zimmerman GA, Beauchamp RD, Prescott SM. Regulation of cyclooxygenase-2 expression by the translational silencer tia-1. J Exp Med. 2003;198:475–481. doi: 10.1084/jem.20030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel FB. Cardiomyocyte proliferation: A platform for mammalian cardiac repair. Cell Cycle. 2005;4:1360–1363. doi: 10.4161/cc.4.10.2081. [DOI] [PubMed] [Google Scholar]
- 29.Huang WY, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med. 2000;6:482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- 30.Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxgenase-2 mrna stability by taxanes: Evidence for involvement of p38, mapkapk-2, and hur. J Biol Chem. 2003;278:37637–37647. doi: 10.1074/jbc.M301481200. [DOI] [PubMed] [Google Scholar]
- 31.Abbate A, Santini D, Biondi-Zoccai GG, Scarpa S, Vasaturo F, Liuzzo G, Bussani R, Silvestri F, Baldi F, Crea F, Biasucci LM, Baldi A. Cyclo-oxygenase-2 (cox-2) expression at the site of recent myocardial infarction: Friend or foe? Heart. 2004;90:440–443. doi: 10.1136/hrt.2003.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF, Jr., Sawyer DB, Cupples LA, Colucci WS, Sam F. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J. Card. Fail. 2006;12:122–127. doi: 10.1016/j.cardfail.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: Evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–2764. doi: 10.1161/01.cir.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 34.Grandel U, Hopf M, Buerke M, Hattar K, Heep M, Fink L, Bohle RM, Morath S, Hartung T, Pullamsetti S, Schermuly RT, Seeger W, Grimminger F, Sibelius U. Mechanisms of cardiac depression caused by lipoteichoic acids from staphylococcus aureus in isolated rat hearts. Circulation. 2005;112:691–698. doi: 10.1161/CIRCULATIONAHA.104.503938. [DOI] [PubMed] [Google Scholar]
- 35.Saito T, Rodger IW, Hu F, Robinson R, Huynh T, Giaid A. Inhibition of cox pathway in experimental myocardial infarction. J. Mol. Cell. Cardiol. 2004;37:71–77. doi: 10.1016/j.yjmcc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 36.LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am. J. Physiol. Heart. Circ. Physiol. 2004;286:H1416–1424. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]
- 37.Streicher JM, Wang Y. The role of cox-2 in heart pathology. Cardiovasc Hematol Agents Med Chem. 2008;6:69–79. doi: 10.2174/187152508783329948. [DOI] [PubMed] [Google Scholar]
- 38.Streicher JM, Kamei K, Ishikawa TO, Herschman H, Wang Y. Compensatory hypertrophy induced by ventricular cardiomyocyte-specific cox-2 expression in mice. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.01.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson DR, Meyers MJ, Vernier WF, Mahoney MW, Kurumbail RG, Caspers N, Poda GI, Schindler JF, Reitz DB, Mourey RJ. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (mk-2) J Med Chem. 2007;50:2647–2654. doi: 10.1021/jm0611004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.