Vitamin D is a 9,10-seco steroid, as shown by the numbering of its carbon skeleton. Vitamin D has 2 distinct forms: vitamin D2 and vitamin D3. Vitamin D2 is a 28-carbon molecule derived from the plant sterol ergosterol, whereas vitamin D3 is a 27-carbon derivative of cholesterol. Vitamin D2 differs from vitamin D3 in that it contains an extra methyl group and a double bond between carbons 22 and 23.
The most important aspects of vitamin D chemistry center on its cis-triene structure. This unique structure makes vitamin D and related metabolites susceptible to oxidation, ultraviolet (UV) light-induced conformational changes, heat-induced conformational changes, and attacks by free radicals. Most of these transformation products have less biological activity than does vitamin D. Research has now shown that vitamin D2 is much less bioactive than vitamin D3 in humans [1]. The parent compounds vitamin D2 and vitamin D3 are sometimes referred to as calciferol.
Hydroxylation reactions at both carbon 25 of the side chain and, subsequently, carbon 1 of the A ring result in the metabolic activation of vitamin D. Metabolic inactivation of vitamin D takes place primarily through a series of oxidative reactions at carbons 23, 24 and 26 of the molecule’s side chain. Metabolic activation and inactivation are well characterized and result in a plethora of vitamin D metabolites [2]. Of these metabolites, only 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D] provide any clinically relevant information. 25(OH)D2 and 25(OH)D3 are commonly known as calcifediol and the 1,25(OH)2D metabolites as calcitriol.
In this review I describe the current state of the science on the clinical assessment of circulating 25(OH) and 1,25(OH)2D.
Methods of 25(OH)D quantitation
The assessment of circulating 25(OH)D started its journey approximately four decades ago with the advent of the competitive protein-binding assay (CPBA) [3]. From that early time to the present we have progressed to radioimmunoassay (RIA), high-performance liquid chromatography (HPLC) and liquid chromatography coupled with mass spectrometry (LC/MS). I will provide a brief description of each technique.
Competitive protein-binding assay
A major factor responsible for the explosion of information on vitamin D metabolism and its relation to clinical disease was the introduction of a CPBA for 25(OH)D. Haddad and Chyu [3], introduced this CPBA almost four decades ago. The assay assessed circulating 25(OH)D concentrations using the vitamin D-binding protein (DBP) as a primary binding agent and 3H-25(OH)D3 as a reporter. Although this CPBA was valid, it was also relatively cumbersome. Technicians had to extract the sample with organic solvent, dry it under nitrogen, and purify it using column chromatography. This assay was suitable for the research laboratory but did not meet the requirements of a high-throughput clinical laboratory.
The major difficulty in measuring 25(OH)D is attributable to the molecule itself. 25(OH)D is probably the most hydrophobic compound measured by protein-binding assay (PBA), which constitutes either CPBA or radiommunoassay (RIA). The fact that the molecule exists in two forms, 25(OH)D2 and 25(OH)D3, compounds the difficulties with its quantitation by PBA. 25(OH)D’s lipophilic nature renders it especially vulnerable to the matrix effects of any PBA. Anything present in the sample assay vessel that is not present in the calibrator assay vessel can cause matrix effects. These matrix effect substances are usually lipid but in the newer direct assays, they could be anything contained in the serum or plasma sample. These matrix factors change the ability of the binding agent, antibody or binding protein to associate with 25(OH)D in the sample or standard in an equal fashion. When this occurs, it markedly diminishes the assay’s validity. Experience has demonstrated that the DBP is more susceptible to these matrix effects that antibodies [4]. The original Haddad procedure overcame the matrix problem by using chromatographic sample purification before CPBA [3].
Researchers had a strong desire to simplify this cumbersome CPBA for 25(OH)D, so Belsey et al. [5] developed a streamlined CPBA in 1974. The goal of this second-generation CPBA was to eliminate chromatographic sample purification as well as individual sample recovery using 3H-25(OH)D3. However, after several years of trying, researchers were unable to validate the Belsey assay due to matrix problems originating from ethanolic sample extraction [6].
The 25(OH)D CPBA’s did have the advantage of being cospecific for 25(OH)D2 and 25(OH)D3 and thus provided a ‘total’ 25(OH)D value if the assay was valid. The DBP’s binding cospecificity for 25(OH)D2 and 25(OH)D3 as well as its stability, made it an attractive candidate for incorporation into automated direct chemiluminescent assays. In fact, Nichols Institute Diagnostics used this approach when its researchers developed the Advantage 25(OH)D Assay. The US Food and Drug Administration (FDA) approved this assay for clinical use but Nichols ultimately withdrew it from the market place due to its propensity to overestimate total circulating 25(OH)D concentrations and its surprising inability to detect circulating 25(OH)D2 [7,8]. Although never described, these problems were probably linked to the DBP’s inability to resolve the matrix problems associated with direct sample assay. Currently, the CPBA for 25(OH)D is rarely used. Also, one cannot accurately compare most CPBA results for circulating 25(OH)D concentrations from the past with values from current methods because many of the matrix interferences were not linear in the old CPBA’s.
Radioimmunoassay
In the early 1980’s, my group decided that a nonchromatographic RIA for circulating 25(OH)D would be the best approach to measuring the substance. We therefore designed an antigen that would generate an antibody that was cospecific for 25(OH)D2 and 25(OH)D3 [9]. In addition, we designed a simple extraction method that allowed simple nonchromatographic quantification of circulating 25(OH)D [9]. In 1985 Immunonuclear Corp., now known as DiaSorin, introduced this 3H-based RIA as a kit on a commercial basis. This RIA was further modified in 1993 to incorporate a 125I-labeled reporter and calibrators (standards) in a serum matrix [10]. This modification finally made mass assessment of circulating 25(OH)D possible. In that same year this assay became the first FDA-approved device for the clinical diagnosis of nutritional vitamin D deficiency. Further, during these past 23 years, these DiaSorin tests have been utilized in the vast majority of large-clinical studies worldwide to define ‘normal’ circulating 25(OH)D levels in a variety of disease states. This test still remains today the only RIA-based assay that provides a ‘total’ 25(OH)D value.
Random-access automated instrumentation
DiaSorin Corporation, Roche Diagnostics, and the now defunct Nichols Institute Diagnostics all introduced methods for the direct (no extraction) quantitative determination of 25(OH)D in serum or plasma using completive protein assay chemiluminescence technology [11]. These assays appear quite similar on the surface but they are not.
In 2001, Nichols Diagnostics introduced the fully automated chemiluminescence Advantage 25(OH)D assay system. In this assay system, nonextracted serum or plasma was added directly into a mixture containing human DBP, acridinium-ester labeled anti-DBP, and 25(OH)D3-coated magnetic particles. Note that the primary binding agent was human DBP. Thus, this assay was a CPBA, much like the manual procedure introduced in 1974 by Belsey et al. [5]. The major difference between these procedures was that Belsey depotenized the sample with ethanol before assaying it. The calibrators for the Belsey assay were in ethanol. In the Advantage assay, the calibrators were in a serum-based matrix, and its developers assumed that this matrix would replicate the serum or plasma sample introduced directly into the assay system. In the end, the 1974 Belsey assay never worked and neither did the Advantage 25(OH)D Assay. The company removed the assay from the market in 2006.
In 2004, the DiaSorin Corporation introduced the fully automated chemiluminescence Liaison 25(OH)D Assay System [11]. This assay is very similar to the late Advantage assay, with one major difference – the Liaison assay uses an antibody as a primary-binding agent as opposed to the human DBP in the Advantage system. Thus, the Liaison is a true RIA method. Details on this procedure are available elsewhere [11]. The Liaison 25(OH)D assay is cospecific for 25(OH)D2 and 25(OH)D3, so it reports a ‘total’ 25(OH)D concentration. DiaSorin recently introduced a second-generation Liaison 25(OH)D assay. This new version has increased functional sensitivity and much improved assay precision. The Liaison 25(OH)D assay is the single most widely used 25(OH)D assay in the world for clinical diagnosis.
The most recent addition to the automated 25(OH)D assay platforms is from Roche Diagnostics. Their test is an RIA called vitamin D3(25-OH) and it can be performed on their Elecsys and Cobas systems. Roche only released this assay in 2007, so very little information on it is available. However, the assay can only detect 25(OH)D3, so it will not be a viable product in countries in which vitamin D2 is used clinically, including the USA [12].
Direct physical detection methods
Direct detection methodologies for determining circulating 25(OH)D include both HPLC and LC/MS procedures [13-17]. The HPLC methods separate and quantitate circulating 25(OH)D2 and 25(OH)D3 individually. HPLC followed by UV detection is highly repeatable and, in general, most people consider it the gold standard method. However, these methods are cumbersome and require a relatively large sample as well as an internal standard. Sample throughout is slow and is not suited to a high-demand clinical laboratory processing up to 10,000 25(OH)D assays per day.
Researchers have recently revitalized LC/MS as a viable method to assess circulating 25(OH)D [14-17]. As with HPLC, LC/MS quantitates 25(OH)D2 and 25(OH)D3 separately. When performed properly, LC/MS is a very accurate testing method. However, the equipment is very expensive and its overall sample throughput cannot, when performed properly, match that of the automated instrumentation format. As a methodology, LC/MS can compare favorably with RIA techniques [15,16]. One unique problem with LC/MS is its relative inability to discriminate between 25(OH)D3 and its inactive isomer 3-epi-25(OH)D3. This problem has been especially noticeable in the circulation of newborn infants [14]. Next to the DiaSorin assays, LC/MS is the next most utilized procedure for the clinical assessment of circulating 25(OH)D.
Determining analytical recovery of 25(OH)D2 and 25(OH)D3 in human serum or plasma
Questions constantly arise regarding the various 25(OH)D assay procedure’s ability to accurately measure total 25(OH)D [25(OH)D2 + 25 (OH)D3] levels in human samples [8]. A brief study recently has described the ability of the DiaSorin Liaison Total-D 25(OH)D Assay System to perform this task as compared to the “gold standard” hplc/UV quantitation of 25(OH)D2 and 25(OH)D3 [18]. Baseline scrum samples were obtained from nine volunteers which contained only 25(OH)D3. All subjects then consumed 50,000 IU/d vitamin D2 for a period of 14 days. Seven days following the final dose serum samples were again obtained. For exogenous in vitro recovery experiments 32 ng/ml of either 25(OH)D2 or 25(OH)D3 were added, in a small volume of ethanol, to each baseline serum sample. All samples were then subjected to direct hplc/UV quantitation to determine individual levels of 25(OH)D2 and 25(OH)D3 [9] or the DiaSorin Liaison Total-D Assay.
25(OH)D calibrators from The National Institute of Standards and Technology (NIST) were also tested. NIST describes the samples as Level 1; “normal” human serum; Level 2 “normal” human serum diluted 1/1 with horse serum; and Level 3 “normal” human serum “spiked” with 25(OH)D2 attempting to equal the amount of endogenous 25(OH)D3 contained in the sample. Horse serum from Sigma Chemical Company was also accessed.
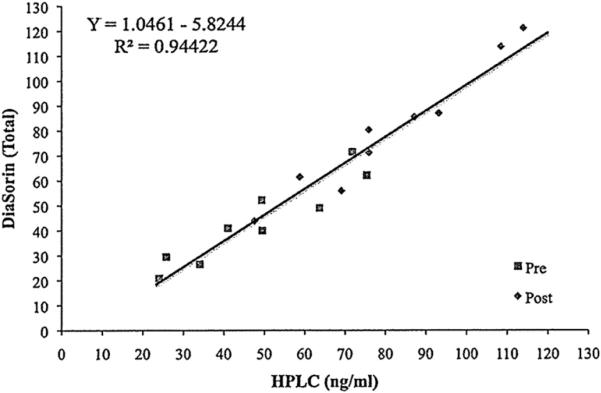
In the group of volunteers the baseline total 25(OH)D was 48.3 ± 19.0 and 43.7 ± 16.8 ng/ml () by hplc-UV and Liaison, respectively. In these baseline samples hplc-UV analysis demonstrated 99% of the circulating 25(OH)D to be of the D3 form, only 2 of 9 subjects had detectable (>1.0 ng/ml) 25(OH)D2. Following 14 days of oral vitamin D2 supplementation, total 25(OH)D levels were determined to be 81.1 ± 21.9 and 80.0 ± 25.5 ng/ml by hplc-UV and Liaison, respectively. By hplc analysis the elevations in 25(OH)D2 ranged from 25-88 ng/ml. In these post-supplementation samples, hplc-UV analysis also revealed 25(OH)D3 to be 43.5% of the total while the remaining 56.5 was 25(OH)D2. The regression relationship of pre and post samples between hplc-UV and Liaison was Liaison Total-D = 1.04 (hplc-UV) – 5.27, r2 = 0.95 (Figure 1). The recovery of exogenously added 25(OH)D2 or 25(OH)D3 to baseline samples was 98.3 ± 5.7 and 99.0 ± 6.7%, respectively by hplc-UV analysis, and 22.8 ± 19.7 and 62.7 ± 24.8%, respectively by Liaison analysis [18].
Fig. 1.
Elevations in plasma total 25(OH)D in volunteers following supplementation with vitamin D2 as measured by the DiaSorin Liaison Total method versus HPLC. Volunteers were given vitamin D2. Presupplementation concentrations are represented by the closed boxes and postsupplementation by the closed diamonds.
NIST Level 1 concentrations measured by the Liaison compared favorably with HPLC results. However, NIST Level 2 as higher (Liaison vs. HPLC) and Level 3 was lower Liaison vs. HPLC. The higher concentration in the NIST Level 2 can be attributed to the impact of the horse serum matrix and lower levels in NIST Level 3 can be attributed to the lack of recovery of exogenous material by the Liaison system.
The data reveals an important artifact that could lead to false conclusions about the ability of direct competitive antibody-based chemiluminescence assays to quantitatively detect 25(OH)D2 and/or 25(OH)D3 in patient samples. It has proven difficult to produce an antibody that is co-specific for the detection of 25(OH)D2 and 25(OH)D3 in human serum. In fact, only one such antibody has been reported and that is the antibody utilized in the DiaSorin 25(OH)D assays [9].
In the U.S. it is imperative that any 25(OH)D assay used for clinical diagnosis have the ability to detect total 25(OH)D, a sum of 25(OH)D2 and 25(OH)D3. With a single exception, all competitive protein binding assays introduced commercially have discriminated against 25(OH)D2 including the now defunct Nichols Advantage 25(OH)D assay system. It is also a fact that approximately 99% of the U.S. population has undetectable 25(OH)D2 in their circulation. This is because vitamin D2 is rarely used as a supplement anymore and patients only receive it when being treated for vitamin D deficiency by a physician. Since blood samples in the general population rarely contain significant amounts of 25(OH)D2, and because the compound is usually discriminated against by most antibody-based assays, it is the compound most often added exogenously to human serum to assess cross-reactivity and determine analytical recovery.
We have assumed since the early 1970’s that when one adds exogenous 25(OH)D to a blood sample it rapidly binds to its carrier protein, the vitamin D-binding protein (DBP) with little interaction to other blood components [19]. Up to this point in vitamin D assay technology, exogenous addition of 25(OH)D2 or 25(OH)D3 has served us well in our testing of quantitative analytical recoveries of these compounds [9]. Problems were never encountered because extraction procedures were based on organic solvents of one kind or another and they all destroyed the DBP and liberated the 25(OH)D into solution. The direct serum or plasma assays emerging today do not destroy the carrier proteins. Instead they rely on pH changes and/or blocking agents that liberate the 25(OH)D from its carrier protein but do not affect the ability of the steroid to bind to a specific antibody. This later disruption method is the method employed in the Liaison assay [11].
The results clearly demonstrate that exogenously added 25(OH)D2 or 25(OH)D3 do not distribute themselves on the DBP as occurs when assembled in vivo. The other possibility is that exogenously added 25(OH)D distribute to moieties other than the DBP. This is suggested by the clear linear relationship observed from in vivo human samples containing elevated amounts of 25(OH)D2 when assayed by the Liaison method versus HPLC-UV. On the other hand the failure of quantitative recovery is apparent from exogenously added 25(OH)D2 or 25(OH)D3 to the same samples and the assay methods compared (Table 1). This study describes an in vitro anomaly that really has no physiological relevance but could result in erroneous conclusions about 25(OH)D assay performance when comparing sample destruction methods such as HPLC-UV versus the newer sample disruption method such as the Liaison assay [18]. Extreme caution is warranted when preparing samples for such comparisons as is being done by vitamin D External Quality Assessment Scheme (DEQAS) and NIST.
Table 1.
Comparison of 25(OH)D concentrations measured by the DiaSorin Liaison and HPLC as a result of various exogenous and endogenous treatments.
| Sample ID | DiaSorin Liaison | HPLC |
|---|---|---|
| Total 25(OH)D (ng/ml) | ||
| Baseline | 43.7 ± 16.8 | 48.3 ± 19.0 |
| Vitamin D2 | 81.1 ± 21.9 | 80.0 ± 23.5 |
| 1Baseline + 25(OH)D2 | 51.0 ± 16.8 (22.8%) | 79.7 ± 19.0 (98.3%) |
| 1Baseline + 25(OH)D3 | 63.7 ± 20.4 (62.7%) | 80.0 ± 18.5 (99.0%) |
| Horse Serum | 12.7 ± 1.0 | 4.7 ± 0.2 |
| 2NIST Level 1 [22-24] | 24.4 ± 0.8 (106%) | 26.0 ± 1.1 (113%) |
| 2NIST Level 2 [12-14] | 19.8 ± 0.5 (152%) | 15.9 ± 0.7 (122%) |
| 2NIST Level 3 [42-46] | 27.2 ± 1.0 (61.8%) | 48.1 ± 3.0 (109%) |
32 ng/ml was added to each of 9 samples. Values in parentheses represent amount of 25(OH)D recovered as a % of mean values.
Values in brackets are expected values provided by NIST. Values in parentheses represent amount of 25(OH)D recovered as a % of mean values.
Determining and defining a ‘normal’ circulating 25(OH)D level
To define a ‘normal’ circulating level of a given substance or nutrient one usually obtains blood samples from a diverse population, measures the substance in question, plots the data by Gaussian distribution and determines normality. This method works well for nutrients such as folate or vitamin E and was precisely how normative circulating levels of 25(OH)D were defined in humans beginning about 40 years ago by Haddad and Chyu [3]. They sampled a population of ‘normal’ individuals whom were asymptomatic for disease, assessed circulating 25(OH)D and determined a mean value. In their study they also assessed 25(OH)D in a group of lifeguards and demonstrated their levels to be 2.5 times that of the ‘normals’. Countless similar studies performed over the ensuing decades reiterated the same conclusion. I, however, interpreted the original Haddad data differently; I suggested that the 25(OH)D levels in the lifeguards are normal and the ‘normals’ were actually vitamin D deficient [20]. This interpretation has largely been validated by the current research.
For all practical purposes, vitamin D does not naturally occur in foodstuffs that humans eat. There are exceptions such as oily fish and fish liver oil. The fact is, from an evolutionary standpoint, humans did not require vitamin D in their food supply because over millions of years humans evolved a photosynthetic mechanism in their skin to produce large amounts of vitamin D3. Thus, our skin is part of the vitamin D endocrine system, and vitamin D3 is really a preprohormone. The problem now is that humans avoid the sun, wear sunscreen and reside in latitudes that we are not programmed to live. To make matters worse, the dietary requirement for vitamin D in adults is 200 IU/d, as defined by the Adequate Intake (AI) by the Food and Nutrition Board and is essentially meaningless [21]. As a result of these factors, we now define a ‘normal’ circulating 25(OH)D range using various biomarkers of physiology or disease as opposed to a random population Gaussian distribution.
The first use of biomarkers to define ‘normal’ 25(OH)D levels, of course, started with parameters that affected skeletal integrity such as parathyroid hormone, bone mineral density and intestinal calcium absorption [20]. These parameters demonstrated that a minimum circulating level of 25(OH)D should be at least 32 ng/ml (80 nmol) [20,22]. Presently, the ‘normal’ circulating 25(OH)D level also relies on data based on the other diverse physiological function of 25(OH)D including cancer prevention [23-31], infectious disease [32-37], cardiovascular health [38-45], diabetes [45-48] and autoimmune control [49-51]. Because of the diverse interaction of vitamin D with our genome this list is certain to grow [52]. For the present it is generally agreed that a normal level of circulating 25(OH)D is 32-100 ng/ml (80-250 nmol). Please take note that 32 ng/ml is not an ‘optimum’ level but a minimum ‘normal’ level. What constitutes an ‘optimum’ level remains to be determined and may well be different for different physiological processes.
Clinical reporting of circulating 25(OH)D concentrations
As highlighted earlier, all DiaSorin 25(OH)D assays are approved by the FDA for clinical utility. Thus, the diagnostic 25(OH)D tests sold by DiaSorin and IDS Diagnostics (Fountain Hills, Arizona, USA) are under strict FDA control and monitoring for assay performance and reliability. In what we consider a distributing trend, many clinical reference laboratories are replacing these FDA-approved tests with ‘home-brew’ LC/MS methods that are diverse and not under FDA scrutiny. The reasons for this switch in utilization are the ‘perceived’ advantages of LC/MS technology being more accurate, precise, specific, cost effective and providing the separate determination of 25(OH)D2 and 25(OH)D3. First, with respect to accuracy and precision, the DiaSorin and IDS RIA methods perform at least as well as LC/MS methods according to the Vitamin D External Quality Assessment Scheme (DEQAS) operated out of London, UK. As far as specificity goes, the DiaSorin tests appear more specific than LC/MS methodology in that the DiaSorin assays do not detect the inactive 3-epimer of 25(OH)D3 [14]. Finally, LC/MS assays are marketed on their ability to separately measure 25(OH)D2 and 25(OH)D3 in a blood sample. Clinically, however, there is no advantage to this separate measurement claim. Not a single scientific publication exists that demonstrates separate 25(OH)D2 and 25(OH)D3 whose measurements are superior to a ‘total’ 25(OH)D value as supplied by the DiaSorin tests. In fact, this separate reporting has been shown to confuse the clinician [53]. The truth is, LC/MS laboratories report separate values because that is how LC/MS technology has to report the data [14-17] and is not a reason to ‘spin’ it into a clinical advantage. Also, the FDA has made their opinion on the separate reporting of 25(OH)D2 and 25(OH)D3 quite clear. In 2007, ESA Biosciences Inc. (Chelmsford, MA) submitted a 510K application to the FDA seeking approval of an hplc procedure to determine 25(OH)D2 and 25(OH)D3 separately in blood. The FDA responded “Assays intended for clinical use must have a clinical indication as well as an analytical claim. Please provide additional information to establish the clinical validity of separate 25(OH)D2 and 25(OH)D3 in diagnosis”. Of course, no such data exist so ESA’s test was only approved after they agreed to report only ‘total’ 25(OH)D. Thus, separate reporting of 25(OH)D2 and 25(OH)D3 in the eyes of the FDA is of no medical advantage or use. Some LC/MS laboratories have actually billed inappropriate CPT codes to enhance return for these separate reported values. We consider this practice to be abusive and fraudulent and feel it must end. Further, 99% of all patient samples assayed will not contain any 25(OH)D2.
Replacement of FDA-controlled devices such as the DiaSorin and IDS assays with ‘home-brew’ LC/MS assays from a clinical diagnostic standpoint is, again, disturbing. It is disturbing because the DiaSorin assays have and continue to be the standard of clinical 25(OH)D assessment. We can say this because the ‘normal’ range of circulating 25(OH)D is almost entirely based on clinical studies using the DiaSorin tests. In fact, Labcorp (Burlington, North Carolina, USA) uses a publication by Hollis [20] on which to base their clinical range of 25(OH)D levels. In turn, this publication is based on DiaSorin assay-based clinical studies so unless a given LC/MS method is calibrated against the DiaSorin methods, this reference range should not be reported against.
Many years and clinical studies have gone into establishing the DiaSorin reference range and as we stated earlier, this consists of thousands of scientific publications. To prove our point we have selected some large significant clinical studies on which the ‘normal’ circulating level of 25(OH)D is based, most of which utilized DiaSorin and some IDS assays as their method of analysis. I have not included any LC/MS clinical studies because basically none exist, which is my point exactly.
The DiaSorin RIA has been used to generate all of the 25(OH)D data from the third National Health and Nutrition Examination Survey (NHANES III). We have included selected references on this topic to validate our claim [38,48,53-56]. Many more studies from NHANES exist with respect to vitamin D and all use the DiaSorin RIA. Studies from the huge NIH sponsored Women’s Health Initiative (WHI) used the DiaSorin LIAISON assay for the first two major publications [31,57] with others to follow.
The Harvard-based studies, the Health Professionals’ Follow-up Study (HPFAS) and the Nurses’ Health Study (NHS) have been used to establish much of the information in the last decade with regard to the relationship of circulating 25(OH)D levels and various disease states such as cancer, autoimmune, cardiovascular and renal. All of these studies again utilized DiaSorin-based assays [24-30,34,35,45-49]. Of course, we cannot forget the relationship of vitamin D status, PTH and skeletal integrity. Hundreds of papers have been published on this topic; most using DiaSorin assays none using LC/MS testing.
What then should LC/MS laboratories do? If they are going to use the current DiaSorin-based reference range [20] they had better target their values to that of the DiaSorin test. In fact, this is basically how the FDA approves new devices for 25(OH)D assessment through the 510 K process since the DiaSorin RIA was the first device approved in 1993. The alternative is that each LC/MS site establish their own reference range which will take years of clinical study since a normal Gaussian distribution is useless in establishing a normative 25(OH)D range. In fact, this ‘normalization’ of values is quite common between other 25(OH)D assays and DiaSorin testing as recent articles demonstrate [58].
Finally, clinical reference laboratories should simply use a single reference range to report circulating 25(OH)D levels as does Labcorp, 32-100 ng/ml. Compare this to the Mayo Clinic which reports four different ‘classes’ of 25(OH)D status. This type of reporting is confusing and should be discontinued.
Methods of 1,25(OH)2D quantitation
Of all the steroid hormones, 1,25(OH)2D represented the most difficult challenge to the analytical biochemist with respect to quantitation. 1,25(OH)2D circulates at pmol concentrations (too low for direct UV or MS quantitation), is highly lipophilic and its precursor, 25(OH)D, circulates at nmol levels. The development of simple, rapid assay for this compound has proven to be a daunting task.
Radioreceptor assays
The first radioreceptor assay (RRA) for 1,25(OH)2D was introduced in 1974 [59]. Although this initial assay was extremely cumbersome, it did provide invaluable information with respect to vitamin D homeostasis. This initial RRA required a 20 ml serum sample, which was extracted using Bligh-Dyer organics. The extract had to be purified by three successive chromatographic systems, and chickens had to be sacrificed and the vitamin D receptor (VDR) harvested from their intestines. By 1976, the volume requirement for this RRA had been reduced to a 5 ml sample and sample pre-purification had been modified to include hplc [60]. However, the sample still had to be extracted using a modified Bligh-Dyer procedure and then pre-purified on Sephadex LH-20. Chicken intestinal VDR was still utilized as a binding agent.
A major advancement occurred in 1984 with the introduction of a radically new concept for the RRA determination of circulating 1,25(OH)2D [61]. This new RRA utilized solid-phase extraction of 1,25(OH)2D from serum along with silica cartridge purification of 1,25(OH)2D. As a result, the need for hplc sample pre-purification was eliminated. Also, this assay utilized VDR isolated from calf thymus, which proved to be quite stable and thus had to be prepared only periodically. Further, the volume requirement was reduced to 1 ml of serum or plasma. This assay opened the way for any laboratory to measure circulating 1,25(OH)2D. This procedure also resulted in the production of the first commercial kit for 1,25(OH)2D measurement. This RRA was further simplified in 1986 by decreasing the required chromatographic purification steps [62]. This method has become a citation classic [63].
As good as the calf thymus RRA for 1,25(OH)2D was, it still possessed two serious shortcomings. First, VDR had to be isolated from thymus glands. Second, because the VDR is so specific for its legend, only 3H-1,25(OH)2D3 could be used as a reporter, eliminating the use of I125 or chemiluminscent reporter. This was a major handicap, especially for the commercial laboratory.
Radioimmunoassay
In 1978, the first RIA for 1,25(OH)2D was introduced [64]. Although it was an advantage not to have to isolate the VDR as a binding agent, this RIA was relatively nonspecific, so the cumbersome sample preparative steps were still required. Over the next 18 years all RIA’s developed for 1,25(OH)2D suffered from the same shortcomings. In 1996, we developed the first significant advance in 1,25(OH)2D quantification in a decade [65]. This RIA incorporated and 125I-reporter, as well as standards in an equivalent serum matrix, so individual sample recoveries were no longer required. The sample purification procedure is the same one previously used for the rapid RRA procedure [62]. This assay has 100% cross-reactivity between 1,25(OH)2D2 and 1,25(OH)2D3 and is FDA-approved for clinical diagnosis in humans.
Another 125I-based RIA for 1,25(OH)2D is also commercially available from IDS Ltd. The basis of this kit is a selective immunoextraction of 1,25(OH)2D from serum or plasma with a specific monoclonal antibody bound to a solid support. This antibody is directed toward the H-hydroxylated A ring of 1,25(OH)2D [66]. This assay procedure has never been published in detail so critical evaluation is difficult. We concluded that this immunoextraction procedure was highly specific for the 1-hydroxylated forms of vitamin D. However, we also believe that this procedure overestimated circulating 1,25(OH)2D levels. Evidence of this overestimation is evident in a recent publication which shows a correlation of circulating 25(OH)D and 1,25(OH)2D at physiologic levels [67] indicating that 25(OH)D maybe interfering with the assay.
ELISA’s for circulating 1,25(OH)2D determinations do exist commercially from Immunodiagnostik and IDS. However, their performance has never been published in detail.
Direct physical detection methods
Direct detection methodologies for determining circulating 1,25(OH)2D is very problematic because of the low concentration (pmol) in the blood. Because of this fact direct UV detection is not possible. Recently two commercial laboratories Mayo Clinic and Quest Diagnostics have begun to offer LC/MS detection of circulating 1,25(OH)2D. However, details of these analyses are not in the public domain.
Determining and defining a ‘normal’ circulating 1,25(OH)2D level
Unlike 25(OH)D, a normal circulating level of 1,25(OH)2D can be determined from a Gaussian distribution of subjects. This has been accomplished over the last three decades and a normal adult level has been defined as 16-56 pg/ml with a mean of 37.6 [68]. Circulating 1,25(OH)2D is diagnostic for several clinical conditions, including vitamin D-dependent rickets types I and II, hyperclacemia associated with sarcoidosis, and other hypercolcemic disorders causing increased 1,25(OH)2D levels. These other disorders include tuberculosis, fungal infections, Hodgkin’s disease, lymphoma, and Wegener’s granulomatosis. In all other clinical conditions involving the vitamin D endocrine system, including hypoparathyroidism, hyperparathyroidism, and chronic renal failure, the assay of 1,25(OH)2D is a confirmatory test. It is also important to remember that circulating 1,25(OH)2D provides essentially no information with respect to the patient’s nutritional vitamin D status. Thus, circulating 1,25(OH)2D should not be used as an indicator for hypo- or hypervitaminosis D when nutritional factors are suspected.
A very interesting condition that has a profound effect on circulating 1,25(OH)D levels is pregnancy [69]. In pregnancy circulating 1,25(OH)2D rise dramatically. In my recently completed NIH-funded pregnancy and vitamin D trial circulating 1,25(OH)2D levels averaged 130 pg/ml with levels of 300-400 pg/ml observed in some patients. These levels are so dramatic they could almost be used as a pregnancy test. The physiologic purpose of this dramatic elevation remains to be determined.
Stability of 25(OH)D and 1,25(OH)2D in serum or plasma
Researchers have known for nearly 30 that endogenous 25(OH)D and 1,25(OH)2D are extremely stable in serum or plasma [70]. Lissner et al [70] showed that vitamin D metabolites in blood stored at 24°C for up to 72 h remain intact. Recent studies on the stability of 25(OH)D in plasma or serum that has undergone many freeze-thaw cycles have reported the same stability [71]. I have used the same pooled human 25(OH)D and 1,25(OH)2D internal controls stored at −20°C for > 10 y with no detectable degradation of either compound. The Vitamin D External Quality Assessment Scheme (DEQAS), a major vitamin D quality assessment organization, ships its serum samples used by laboratories for quality assessment by ground post worldwide without affecting 25(OH)D and 1,25(OH)2D values.
I have performed experiments to try to destroy endogenous 25(OH)D and 1,25(OH)2D in plasma to obtain a vitamin D-free human plasma to prepare various immunoassay procedure calibrators. When I placed crystalline 25(OH)D3 or 1,25(OH)2D3 in ethanol in an open glass petri dish and exposed the dish to intense UV light, the UV light destroyed the compounds within a few minutes. When IU conducted the same experiment by using serum or plasma, however, the 25(OH)D3 and 1,25(OH)2D3 levels did not change after 2 d of UV light exposure. I therefore stopped trying to use this procedure to produce vitamin D-free plasma.
Why are vitamin D and its metabolites so stable in serum or plasma when they are insulted with UV light, temperature shifts, or oxidation? One reason is that UV light penetrates aqueous media very poorly. However, the main reason is probably that in serum or plasma, vitamin D and its metabolites are essentially bound completely to the serum DBP and this complex resists potential insults to the vitamin D molecule very effectively. In conclusion, 25(OH)D and 1,25(OH)2D are very stable in serum or plasma, so they require only minimal attention to storage conditions.
Standardization of 25(OH)D and 1,25(OH)2D analysis
DEQAS (Internet: www.deqas.org) was founded in 1989 to compare the performance of then-available 25(OH)D tests. DEQAS has since become the largest vitamin D quality assessment program in the world, with ≈600 participating laboratories worldwide. The organization’s major aim today is to assess the analytic reliability of 25(OH)D and 1,25(OH)2D assays. The organization achieves this goal by:
Distributing serum pools at regular intervals;
Conducting statistical analyses of submitted results;
Appropriately manipulating pools to provide information on assay specificity and recovery;
Assigning GC-MS target values to selected 25(OH)D pools;
Helping participants and manufacturers evaluate methods by providing samples, technical support, and impartial advice;
Offering advice and support to participants having difficulty achieving an acceptable level of assay performance; and
Providing a forum for exchanging information on all aspects of vitamin D assay methodology.
My laboratory has participated in both the DEQAS 25(OH)D and DEQAS 1,25(OH)2D survey since 1997, and the survey has been invaluable in maintaining the integrity of our assay procedure. When DEQAS leaders question manufacturers about inconsistencies in their methods, most manufacturers attempt to address the issue identified.
One example of the value DEQAS offers occurred when DEQAS informed Nichols Institute Diagnostics that its Advantage 25(OH)D automated assay was overestimating total 25(OH)D concentrations and that, contrary to the manufacturer’s claims, the method could not detect circulating 25(OH)D2 concentrations [8]. Nichols Institute Diagnostics chose not to respond to the concerns that DEQAS identified. The company subsequently went out of business and its Advantage 25(OH)D assay is no longer on the market. As this example shows, DEQAS provides an invaluable service to the vitamin D assay community. In the future, I hope that DEQAS can incorporate the new National Institute of Standards and Technology 25(OH)D calibrators into its survey in some fashion.
The DEQAS survey has shown that most current 25(OH)D assay protocols perform in a comparable fashion with respect to absolute values, assay linearity, and assay precision. However, the survey results also show that the only assays that quantitatively detect total 25(OH)D are HPLC methods, LC/MS methods, and the DiaSorin assays.
Summary
The assessment of circulating 25(OH)D, and to a lesser degree 1,25(OH)2D, are rapidly becoming important clinical tools in the diagnosis and management of many diverse pathologies. At present, the reference range for circulating 25(OH)D and 1,25(OH)2D are 32-100 ng/ml and 16-56 ng/ml, respectively, and are largely based on clinical data derived from the FDA-cleared DiaSorin assay procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 2.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin endocrine system. Endocrine Rev. 1995;16:200–57. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 3.Haddad JG, Chyu KJ. Competitive protein-binding radioassay for 25-hyroxycholecalciferol. J Clin Endocrinol Metab. 1971;33:992–5. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon R, Van Herck E, Jans I, et al. Two direct nonchromatographic assays for 25-hydroxyvitamin D. Clin Chem. 1984;30:1731–6. [PubMed] [Google Scholar]
- 5.Belsey RE, DeLuca HF, Potts JT. A rapid assay for 25-OH-vitamin D3 without preparative chromatography. J Clin Endocrinol Metab. 1974;38:1046–51. doi: 10.1210/jcem-38-6-1046. [DOI] [PubMed] [Google Scholar]
- 6.Dorantes LM, Amaud SB, Arnaud CD, et al. Importance of the isolation of 25 hydroxyvitamin D before assay. J Lab Clin Med. 1978;91:791–6. [PubMed] [Google Scholar]
- 7.Leventis HC, Garrison L, Sibley M, et al. Underestimation of serum 25-hydroxyvitamin D by the Nichols Advantage Assay in patients receiving vitamin D2 replacement therapy. Clin Chem. 2005;51:1072–4. doi: 10.1373/clinchem.2005.048355. [DOI] [PubMed] [Google Scholar]
- 8.Carter GD, Jones JC, Berry JL. The anomalous behavior of exogenous 25-hydroxyvitamin D in competitive binding assays. J Steroid Biochem Mol Biol. 2007;103:480–2. doi: 10.1016/j.jsbmb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem. 1985;31:1815–19. [PubMed] [Google Scholar]
- 10.Hollis BW, Kamerud JO, Selvaag SR, et al. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 11.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical; validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Cavalier E, Wallac AM, Knox S, et al. Vitamin D measurement may not reflect what you give to our patients. J Bone Mineral Res. 2008;23:1864–5. doi: 10.1359/jbmr.080608. [DOI] [PubMed] [Google Scholar]
- 13.Lensmeyer GL, Wiege DA, Binkley N, et al. HPLC measurement for 25-hydroxyvitamin D measurement:: comparison with contemporary assays. Clin Chem. 2006;52:1120–26. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 14.Singh RJ, Taylor RL, Reddy GS, et al. C-3 epimers can account for a significant proportion of total circulating 25(OH)D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 15.Mansell Z, Wright DJ, Rainbow SJ. Routine Isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51:1683–90. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, McCoy LF, Schleicher RL, et al. Measurement of 25(OH)D2 and 25(OH)D3 in human serum using liquid chromatography-tandem mass spectrometry and it comparisons to a radioimmunoassay meth. Clin Chem Acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-Hydroxyvitamin D2 and D3 using HPLC-tandem mass spectrometry and examination if reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–20. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 18.Horst RL. In vivo versus in vitro recovery of 25(OH)D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin Liaison Total-D assay: Limitations of the NIST Controls. (In Press) [DOI] [PubMed]
- 19.Belsey R, Clark MB, Bernat M, et al. The physiologic significance of plasma transport of vitamin D and metabolites. Amer J Med. 1973;57:50–56. doi: 10.1016/0002-9343(74)90767-0. [DOI] [PubMed] [Google Scholar]
- 20.Hollis BW. Circulating 25-Hydroxyvitamin D Levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine 1997 . Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, fluoride. National Academy Press; Washington, DC: 1997. pp. 250–516. [PubMed] [Google Scholar]
- 22.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:515–16. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 23.Abbas S, Linseisen J, Slanger T, et al. Serum 25-hydroxyvitamin D and risk of postmenopausal breast cancer – results of a large case-control study. Carcinogenesis. 2008;29:93–9. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 24.Betone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 25.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–8. [PubMed] [Google Scholar]
- 26.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Heist RS, Liu G, et al. Circulating 25-Hydroxyvitamin D levels predict survival in early-state nonsmall-cell lung cancer patients. J Clin Oncol. 2007;25:474–85. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 28.Tworoger SS, Lee IM, Buring JE, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of incident ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:783–88. doi: 10.1158/1055-9965.EPI-06-0981. [DOI] [PubMed] [Google Scholar]
- 29.Mikhak B, Hunter DJ, Speigelman D, et al. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007;67:911–23. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 30.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–9. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 31.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 32.Liu PT, Sterdger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 33.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12:388–90. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich T, Nunn M, Dawson-Hughes B, et al. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–80. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich T, Joshipura KJ, Dawson-Hughes B, et al. Association between serum concentrations of 25-hydroxyvitamin D and periodontal disease in the US population. Am Clin Nutr. 2004;80:108–13. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 36.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutrition. 2009;139:1157–61. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol J. 2008;5:1–12. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scragg R, Sowers MF, Bell C. Serum 25-hydroxyvitamin D, ethnicity and blood pressure in the third national health and nutrition examination survey. Am J Hyperten. 2007;7:713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 40.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannucci E, Liu Y, Hollis BW, et al. A prospective study of 25-hydroxy-vitamin D and risk of myocardial infarction in men. Arch Inter Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilz JS, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–35. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 44.Pilz S, Dobnig H, Fischer JE, et al. Low vitamin D levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–13. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 45.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low 25(OH)D and 1,25(OH)2D levels will all-cause and cardiovascular mortality. Arch Internal Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 46.Chiu KC, Chu A, Go V, et al. Hypovitamninosis D is associated with insulin resistance and beta cell dysfunction. Amer J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 47.Zipitis CS, Abodeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 48.Chonchol M, Scragg R. 25-hydroxyvitamin D, insulin resistance and kidney function in the third national health and nutrition examination survey. Kidney Internat. 2007;71:134–9. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 49.Munger KL, Levin Ll, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;20:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 50.Ramagopalan SV, Maugeri NJ, Handunnetthi L, et al. Expression of multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5:e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–75. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavera-Mendoza LE, White JH. Cell defenses and sunshine vitamin. Sci Amer. 2007;297:62–72. doi: 10.1038/scientificamerican1107-62. [DOI] [PubMed] [Google Scholar]
- 53.Binkley N, Drezner MK, Hollis BW. Laboratory reporting of 25-hydroxyvitamin D results: potential for clinical misinterpretation. Clin Chem. 2006;52:2124–5. doi: 10.1373/clinchem.2006.074922. [DOI] [PubMed] [Google Scholar]
- 54.Nesby-O’Dell S, Scanion KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third national health and nutrition examination survey, 1988-1994. Amer J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 55.Looker JAC, Mussolino Me. Serum 25-hydroxyvitamin D and hip fracture risk in older white adults. J Bone Min Res. 2008;23:143–50. doi: 10.1359/jbmr.071003. [DOI] [PubMed] [Google Scholar]
- 56.Dawson-Hughes B. Calcium plus vitamin D and the risk of fractures. N Engl J Med. 2006;354:2285–7. [PubMed] [Google Scholar]
- 57.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 58.Rovner AJ, Stallings VA, Schall Jl, et al. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Amer J Clin Nutr. 2007;86:1694–9. doi: 10.1093/ajcn/86.5.1694. [DOI] [PubMed] [Google Scholar]
- 59.Brumbaugh PF, Haussler DH, Bressler R, et al. Radioreceptor assay for 1α,25-dihydroxyvitamin D3. Science. 1974;183:1089–91. doi: 10.1126/science.183.4129.1089. [DOI] [PubMed] [Google Scholar]
- 60.Eisman JA, Hamstra AJ, Kream BE, et al. A sensitive, precise, and convenient method for determination of 1,25dihydroxyvitamin D in human plasma. Arch Biochem Biophys. 1976;176(1):235–43. doi: 10.1016/0003-9861(76)90161-2. [DOI] [PubMed] [Google Scholar]
- 61.Reinhardt TA, Horst RL, Orf JW, Hollis BW. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: Application to clinical studies. Journal of Clinical Endocrinology and Metabolism. 1984;58:91–8. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- 62.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification. Clin Chem. 1986;32(11):2060–3. [PubMed] [Google Scholar]
- 63.Hollis BW. Phase switching SPE for faster 1,25(OH)2D analysis 1986. Clin Chem. 2008;54:446–7. doi: 10.1373/clinchem.2007.093914. [DOI] [PubMed] [Google Scholar]
- 64.Clemens TL, Hendy GN, Graham RF, et al. A radioimmunoassay for 1,25-dihydroxycholecalciferol. Clinical Science and Molecular Medicine. 1978;54:329–32. doi: 10.1042/cs0540329. [DOI] [PubMed] [Google Scholar]
- 65.Hollis BW, Kamerud JQ, Kurkowski A, et al. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42(4):586–92. [PubMed] [Google Scholar]
- 66.Fraser WD, Durham BH, Berry JL, et al. Measurement of plasma 1,25 dihydroxyvitamin D using a novel immunoextraction technique and immunoassay with iodine labeled vitamin D tracer. Ann Clin Biochem. 1997;34(Pt 6):632–7. doi: 10.1177/000456329703400606. [DOI] [PubMed] [Google Scholar]
- 67.El-Hajj Feleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91(2):405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 68.Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th edition Publ by The American Society for Bone and Mineral Research; 2006. p. 492. [Google Scholar]
- 69.Kumar R, Cohen WR, Silva P, et al. Elevated 1,25(OH)2D plasma levels in normal human pregnancy and lactation. J Clin Invest. 1979;63:342–4. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lissner D, Mason RS, Posen S. Stability of vitamin D metabolites in human blood serum and plasma. Clin Chem. 1981;27:773–4. [PubMed] [Google Scholar]
- 71.Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clin Chem. 2004;51:258–60. doi: 10.1373/clinchem.2004.041954. [DOI] [PubMed] [Google Scholar]