Abstract
This study examined the relation of soy intake with hormonal and proliferation markers in benign and malignant breast tissue using tissue microarrays (TMAs). TMAs with up to 4 malignant and 4 benign tissue samples for 268 breast cancer cases were constructed. Soy intake in early life and in adulthood was assessed by questionnaire. The TMAs were stained for ERα, ERβ, PR, HER2/neu, PCNA, and Ki-67 using standard immunohistochemical methods. Logistic regression was applied for statistical analysis. A higher percentage of women showed positive marker expression in malignant than in benign tissue. With one exception, HER2/neu, no significant associations between soy intake and pathologic markers were observed. Early life soy intake was associated with lower HER2/neu and PCNA staining of malignant tissue. In benign tissue, early life soy intake showed higher ER and PR expression, but no difference in proliferation markers. The results of this investigation provide some assurance that soy intake does not adversely affect markers of proliferation. TMAs were shown to be a useful tool for epidemiologic research.
Keywords: Soy foods, Breast cancer, Immunohistochemistry, Tissue microarrays, Proliferation, Ethnicity
Evidence from epidemiological studies supports the hypothesis that soy intake protects against breast cancer, possibly due to an anti-estrogenic or anti-proliferative effect of isoflavones (1,2). It is well known that breast cancer development is strongly determined by endocrine conditions (3,4). On the histopathologic level, it has been shown that the most differentiated structures in breast tissue, the mature ducts and lobules, show less epithelial proliferation and fewer estrogen and progesterone receptors and are less susceptible to the development of cancer than the more undifferentiated structures such as terminal end buds and developing terminal ductal lobular units (4). Because estrogen exposure early in life appears to induce protection against cancer development (5), the weak estrogenic effects of isoflavones in soy beans, if consumed early in life, may accelerate differentiation of breast tissue and prevent tumor development later in life. In contrast, the effects of soy consumption during adulthood in the presence of endogenous estrogens may be more anti-estrogenic (6,7).
Estrogen receptor alpha (ERα) is the classical steroid hormone receptor acting as a transcriptional factor mediating the actions of endogenous and exogenous estrogens on breast tissue. Progesterone receptor (PR) expression is a sensitive indicator of estrogenic effects (8). The function of ERβ is not fully understood, but isoflavones are more likely to bind to ERβ than ERα (9). Proliferation of tumor cells is an important prognostic indicator. Ki-67 provides a snapshot of cell proliferation; its staining peaks in the S phase of the cell cycle with a half-life of 1 hour. Proliferating cell nuclear antigen (PCNA) assesses cumulative proliferative activity and is more abundant in cells than Ki67 because it may persist for hours to days after the cell has left the cell cycle (10,11). The proto-oncogene human epidermal growth factor receptor 2 (HER-2/neu) is a member of the epidermal growth factor receptor family and plays a key role in proliferation and survival of tumor cells in breast tissue (12). The aims of this study were to examine the relation of soy intake at different periods within the lifespan with markers of hormonal receptivity and function (ERα, ERβ, and PR), proliferation markers (PCNA and Ki-67) and the prognostic marker HER2/neu in breast cancer patients with Caucasian, Japanese, and other ethnic backgrounds and to explore the use of tissue microarrays (TMA) as a source for malignant and benign breast tissue in an epidemiologic study. Our hypothesis for this project was that women with high soy intake throughout life would show lower proliferation in normal breast tissue than women with low soy intake due to the protective effects from soy.
Methods
Study population and data collection
The study was conducted with approval from the Institutional Review Board at the University of Hawaii and Wake Forest University School of Medicine. For this investigation, 607 breast cancer cases who had participated in the Multiethnic Cohort (MEC) (13) and in a nested case-control study (NCC) of mammographic densities were recruited (14). All subjects had completed a 26-page self-administered questionnaire that included a dietary history, as well as information about demographics, body weight and height, and medication history (13). Through a linkage to the data base of the Tissue Repository (TR) within the Hawaii Tumor Registry (HTR), part of the Surveillance, Epidemiology, and End Results (SEER) program, we identified women with available pathologic blocks (15). Recruitment letters and lifetime soy questionnaires (LTSQ) were mailed to the remaining 430 subjects; 177 women were not contacted because their tissue was located at non-participating hospitals or because they were deceased. We received 323 consent forms and 311 LTSQ (75%). Another 12 women could be included because they were deceased and had tissue blocks available in the TR. Among these 335 women with available tissue blocks, 56 subjects did not have sufficient tissue to create TMAs leaving 279 women for tissue sampling.
The LTSQ asked for the frequency of intake for usual serving sizes of four soy foods (tofu, soy beans and sprouts, soy milk and drinks, other soy products) during childhood, adolescence, early adulthood, and late adulthood (16). In a previous investigation (16), we had compared results for 356 women who had completed the LTSQ twice; the κ values indicated substantial agreement during childhood and adolescence (0.61 and 0.62) and during early and late adulthood (0.55 and 0.46). Based on 8 frequency categories and 3 serving sizes, mean intake of soy foods during early life (up to age 20) and adulthood (20+ years) was estimated.
Tumor Microarrays
We created TMAs because this method allows high throughput and yields maximal use of limited tissue resources (17). Several archived tissue blocks from a given tumor (generally up to ten) were first grossly inspected by a surgical pathologist (JK) to identify 1–2 blocks with sufficient tissue. A single hematoxylin and eosin (H&E) slide was prepared from each of these blocks. On this slide, the same pathologist separately marked representative areas of malignant and benign tissue. The H&E slide was aligned with the corresponding “donor” block and a 0.6 mm cylindrical tissue specimen was taken from the selected area and transferred to a “recipient” (empty) paraffin block using a tissue-arraying instrument (Beecher Instruments, Sun Prairie, WI). Each tissue sample was mapped to a TMA grid with a unique ID number for reference (18). Up to 4 malignant cores and 4 benign cores per patient were placed in one of six blocks. Out of the 2,232 tissue samples to be placed, 12% of malignant and 29% of benign specimens could not be added because not enough tissue was available. Therefore, 1,773 tissue samples (79.4%) were arrayed along with samples from liver, spleen, and normal breast as quality controls. Sections of the TMA blocks (5 μm) were prepared and mailed vacuum-sealed to Wake Forest University for immunohistochemistry.
Immunohistochemistry
The TMAs were stained for the following markers: ERα, ERβ, PR, and PCNA (Clones 6F11, EMR02, 1A6, and PC10, respectively; all from Novocastra Labs, Newcastle-upon-Tyne, UK), Ki-67 (Clone SP6, Labvision NeoMarkers, Fremont, CA), and HER2/neu 1 (rabbit polyclonal, DAKO Corporation, Carpinteria, CA). The basic staining procedure used a avidin-biotin-alkaline phosphatase method, modified for antigen retrieval from paraffin-embedded tissue using the procedure of Shi et al (19). Following overnight incubation with the primary antibodies at 4°C, tissue sections were sequentially incubated with a biotinylated secondary antibody and a streptavidin-alkaline phosphatase conjugate at 37°C for 20 minutes respectively (Biogenex, San Ramon, CA, USA) and then visualized using the chromogen/substrate Vector Red (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared through p-Xylene and coverslipped. Appropriate positive and negative controls were included for each antibody.
Pathologic Evaluation
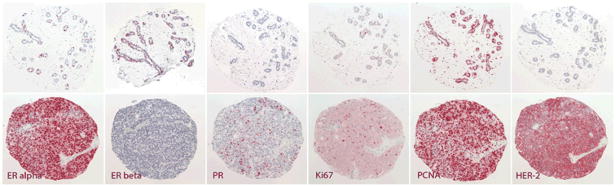
Once stained, a trained pathologist (JMC) evaluated all tissue specimens for the presence of epithelial tissue, malignancy, and tumor grade (Figure 1). About 7–9% of the samples had fallen off the slides during the staining process. The number was similar across epithelial markers, but twice as many benign as malignant samples were missing. During the pathologic evaluation, 118 breast tissue samples were re-categorized as benign or malignant and 550–600 tissue cores could not be evaluated for epithelial staining because the quality of dye was poor or because the core contained only connective or fat tissue, i.e., the tissue area of interest had been missed. As a result, approximately 80% of malignant and 40% of benign tissue samples were included in the analysis corresponding to 780 malignant and 270 benign cores per marker. On average, 3.1 malignant tissue samples and 1.7 benign specimens per woman were available. For 268 women at least one tissue sample was available (253 women with at least one malignant and 159 women with at least one benign specimen). Quantification of immunostaining was done on individual TMA core sections at a magnification of 20x, using a Nikon Labophot 2 microscope, a 3 megapixel digital camera (Infinity 2–3, Lumenera Inc., Ottawa, Ontario), and color imaging software (Image Pro Plus, Media Cybernetics, Bethesda, MD). The area of all nuclei in the core section was measured by a color selection corresponding to hematoxylin (A). The area of positively immunostained tissue was measured by a color selection corresponding to the Vector Red chromogen (B) after manually excluding artifacts by tracing them out of the image mask. For the nuclear stains, the percentage of positive staining was expressed as B/A × 100. In the event that a TMA core intended to include benign tissue actually represented malignant tissue or vice versa, classification of the core was amended to reflect the tissue actually present in the section.
Figure 1.
Representative photomicrographs of benign tissue (top row) and malignant tissue (bottom row).
Statistical Analysis
All statistical analyses were performed using the SAS software 9.1 (SAS Institute Inc., Cary, NC). We computed means of percent staining for each subject separately for benign and for malignant tissue before classifying ERα, ERβ, PCNA, and HER2/neu into two categories: no stain or less than 10% staining vs. positive (≥10% staining). Due to their strong left skew, PR and Ki-67 were classified into no vs. any stain. Because of the small sample size, early life and adult soy intake were categorized into <1 serving/week and ≥1 serving/week. We computed intraclass correlation coefficients (ICC) to evaluate the agreement of repeat core samples within-person and the kappa (κ) statistic to compare receptor results as reported by HTR and as assessed in our study. The relation of soy intake with marker expression was examined using logistic regression; odds ratios (OR) and 95% confidence intervals (95% CI) are presented. The models were adjusted for ethnicity, age, BMI, and hormone replacement therapy (HRT) use because of its well-known effect on epithelial markers. Log-likelihood tests indicated that energy intake and reproductive factors did not contribute to the models. Due to the exploratory nature of this investigation, we did not correct for multiple comparisons.
Results
The 268 women with at least one tissue sample available had comparable characteristics to participants of the NCC study of mammographic densities (Table 1). The major discrepancy was that women in our study were slightly younger than women of the original study and, therefore, also more likely to be premenopausal. The majority of women in this study had early-stage disease consisting of either ductal carcinoma in situ (21%) or invasive ductal carcinoma, stage 1 or stage 2 (66%). Most malignancies (58%) were ER and PR positive, 16% were negative for at least one receptor, and the remaining 26% had no results recorded in the HTR. The ICCs across the malignant specimens varied between 0.45 and 0.74 depending on the marker, whereas those for benign tissue were lower (0.20–0.42). The associations between ERα and PR as reported by the HTR and as assessed in the TMAs were κ = 0.39 (95% CI: 0.17–0.62) and κ = 0.34 (95% CI: 0.16–0.52), respectively, for subjects with three or more malignant cores. For 66% of subjects, at least three malignant cores were available, whereas only 10% of women had three or more benign cores. The median soy intakes during early life and adulthood were 0.3 (inter quartile range (IQR) = 1.3) and 1.2 (IQR = 2.0) servings per week, respectively. For 118 women, soy consumption was less than one serving per week during early life and during adulthood, whereas it was one serving or more during both periods for 69 women. Only 8 women reported high intake early in life and low intake during adulthood, while 73 were low during early life and high during adulthood.
Table 1.
Characteristics of women recruited for the TMA study and the original study*
| Variable | Original Study | TMA study |
|---|---|---|
| Sample size (N) | 607 | 268 |
| Ethnicity (%) | ||
| Caucasian | 185 (30.5) | 92 (34.3) |
| Hawaiian | 80 (13.2) | 35 (13.1) |
| Japanese | 287 (47.3) | 116 (43.3) |
| Other | 55 (9.1) | 25 (9.3) |
| Age at study entry | 59.4 ± 8.4 | 57.6 ± 8.4 |
| Body mass index (kg/m2) | 25.1 ± 5.1 | 24.8 ± 4.4 |
| Soy intake | ||
| Early life soy intake (svgs/wk) | 1.7 ± 3.4 | 1.4 ± 3.1 |
| Adult soy intake (svgs/wk) | 2.8 ± 4.3 | 2.4 ± 3.7 |
| Family history of breast cancer (%) | 104 (17.1) | 40 (14.9) |
| Age at menarche (%) | ||
| < 13 years | 334 (55.0) | 153 (55.4) |
| 13 –14 years | 218 (35.9) | 97 (35.1) |
| > 14 years | 55 (9.1) | 26 (9.4) |
| Number of children (%) | ||
| 0 – 1 | 171 (28.2) | 80 (29.9) |
| 2 – 3 | 309 (50.9) | 137 (51.1) |
| > 3 | 127 (20.9) | 51 (19.0) |
| Age at first live birth (%) | ||
| < 21 years | 80 (13.2) | 38 (14.2) |
| 21 – 30 years | 377 (62.1) | 163 (60.8) |
| > 30 years | 56 (9.2) | 20 (7.5) |
| N/A | 94 (15.5) | 47 (17.5) |
| HRT use at study entry (%) | ||
| Never | 218 (35.9) | 105 (39.2) |
| Current | 309 (50.9) | 124 (46.3) |
| Past | 80 (13.2) | 39 (14.5) |
| Menopausal Status (%) | ||
| Premenopausal | 99 (16.3) | 91 (34.0) |
| Postmenopausal | 508 (83.7) | 177 (66.0) |
Means ± standard deviation unless stated otherwise
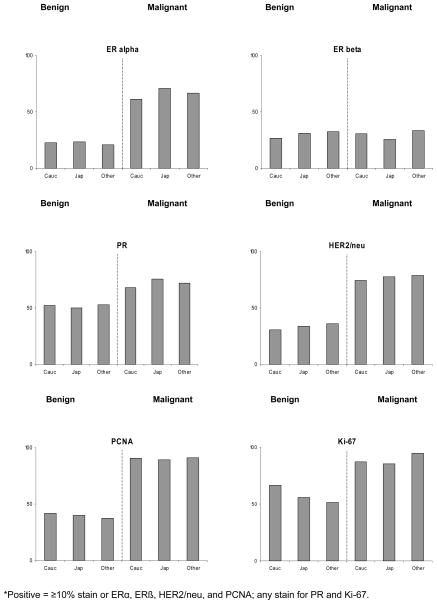
Epithelial marker expressions differed between malignant and benign breast tissue, except for ERβ, for which the respective proportions of positive staining in malignant and benign tissue were 28% and 29% (Figure 2). For ERα, PR, HER2/neu, PCNA, and Ki-67, malignant samples were considerably more likely to be in the higher category than benign samples. The respective percentages were 23% and 67% for ERα, 51% and 72% for PR, 33% and 76% for HER2/neu, 41% and 90% for PCNA, and 60% and 86% for Ki-67. ERα staining in malignant tissue was approximate three times as high as for ERβ (67% vs. 23%). The proportions stained did not differ significantly between Caucasian and Japanese subjects for any marker. The largest differences were for ERα in malignant tissue with 61% in Caucasians and 71% in Japanese (p = 0.15), for PR in malignant tissue with 68% and 76% (p = 0.25), and Ki-67 in benign tissue with 67% vs. 56% (p = 0.23).
Figure 2.
Percent of women by ethnicity and positive marker expression in malignant and benign tissue*
*Positive = ≥10% stain or ERα, ERβ, HER2/neu, and PCNA; any stain for PR and Ki-67
With one exception, we observed no statistically significant association between soy intake and marker expression (Tables 2 and 3). Therefore, emphasis was placed on general consistency and direction of results. Looking at malignant breast tissue (Table 2), women with a soy intake of ≥1 serving/week during early life were slightly more likely to have elevated expression of PR and Ki-67 than women with low soy intake. In contrast, women with high soy consumption during early life were less likely to have HER2/neu and PCNA staining. Women with high soy intake in adulthood were less likely to show HER2/neu staining (OR = 0.46; p = 0.03) and more likely to show ERβ staining (OR = 1.24; p = 0.49) in their tumors. Otherwise, there was little association of adult soy consumption with any of the other markers in malignant tissue. Women were slightly less likely to have tumors with a higher grade for higher soy intake in childhood (OR = 0.72; p = 0.32) or adulthood (OR = 0.79; p = 0.44). When we repeated the analyses for Caucasian and Japanese women separately, we observed divergent results for the association of adult soy intake with PR, Ki-67, and tumor grade. The respective ORs for Caucasians were 2.49 (95%CI: 0.71–8.77), 2.24 (95%CI: 0.46–10.9), and 0.52 (95%CI: 0.17–1.54), whereas they were 0.64 (95%CI: 0.23–1.77), 0.59 (95%CI: 0.15–2.33), and 1.43 (95%CI: 0.61–3.36) for Japanese women. However, the numbers were very small, all of the 95% CIs overlapped, and the interaction terms were not statistically significant.
Table 2.
Soy intake in early life and adulthood and expression of epithelial markers in malignant tissue*
| Soy intake | Early life (<20 years) |
Adulthood (20+ years) |
||||
|---|---|---|---|---|---|---|
| N in category 1 | N in category 2 | OR (95% CI) | N in category 1 | N in category 2 | OR (95% CI) | |
| ERα1 | ERα1 | |||||
| < 1 svg/wk | 63 | 115 | 1.00 | 41 | 76 | 1.00 |
| ≥ 1 svg/wk | 21 | 53 | 1.15 (0.59–2.25) | 43 | 92 | 1.03 (0.57–1.87) |
| p-value | 0.68 | 0.92 | ||||
| ERβ1 | ERβ1 | |||||
| < 1 svg/wk | 125 | 53 | 1.00 | 85 | 32 | 1.00 |
| ≥ 1 svg/wk | 53 | 20 | 1.01 (0.51–2.01) | 93 | 41 | 1.24 (0.67–2.29) |
| p-value | 0.97 | 0.49 | ||||
| PR2 | PR2 | |||||
| < 1 svg/wk | 53 | 126 | 1.00 | 34 | 83 | 1.00 |
| ≥ 1 svg/wk | 17 | 57 | 1.28 (0.63–2.62) | 36 | 100 | 1.06 (0.57–1.97) |
| p-value | 0.49 | 0.86 | ||||
| HER2/neu1 | HER2/neu1 | |||||
| < 1 svg/wk | 41 | 137 | 1.00 | 23 | 94 | 1.00 |
| ≥ 1 svg/wk | 18 | 56 | 0.67 (0.33–1.45) | 36 | 99 | 0.46 (0.23–0.92) |
| p-value | 0.33 | 0.03 | ||||
| PCNA1 | PCNA1 | |||||
| < 1 svg/wk | 16 | 163 | 1.00 | 11 | 106 | 1.00 |
| ≥ 1 svg/wk | 9 | 65 | 0.54 (0.19–1.50) | 14 | 122 | 1.05 (0.42–2.62) |
| p-value | 0.24 | 0.92 | ||||
| Ki-672 | Ki-672 | |||||
| < 1 svg/wk | 22 | 156 | 1.00 | 13 | 102 | 1.00 |
| ≥ 1 svg/wk | 8 | 65 | 1.20 (0.45–3.19) | 17 | 119 | 0.85 (0.36–2.00) |
| p-value | 0.72 | 0.70 | ||||
| Tumor grade3 | Tumor grade3 | |||||
| < 1 svg/wk | 58 | 120 | 1.00 | 36 | 80 | 1.00 |
| ≥ 1 svg/wk | 29 | 45 | 0.72 (0.38–1.37) | 51 | 85 | 0.79 (0.44–1.43) |
| p-value | 0.32 | 0.44 | ||||
| Tumor stage4 | ||||||
| < 1 svg/wk | 155 | 26 | 1.00 | 103 | 14 | 1.00 |
| ≥ 1 svg/wk | 67 | 10 | 0.87 (0.36–2.06) | 119 | 22 | 1.43 (0.65–3.15) |
| p-value | 0.74 | 0.38 | ||||
Categories for ERα, ERβ, PCNA, and HER2/neu: Category 1 = no stain or <10% stain and Category 2 = ≥10% stain.
Categories for PR and Ki-67: Category 1 = no stain and Category 2 = any stain.
Category 1 = Grade 1&2; Category 2 = Grade 3
Category 1 = in-situ & localized; Category 2 = regional
Odds ratios (OR) were obtained by logistic regression and adjusted for ethnicity, age, BMI, and HRT use; totals may differ due to variations in the number of readable cores
Table 3.
Soy intake in early life and adulthood and expression of epithelial markers in benign tissue*
| Soy intake | Early life (<20 years) |
Adulthood (20+ years) |
||||
|---|---|---|---|---|---|---|
| N in category 1 | N in category 2 | OR (95% CI) | N in category 1 | N in category 2 | OR (95% CI) | |
| ERα1 | ERα1 | |||||
| < 1 svg/wk | 86 | 23 | 1.00 | 52 | 15 | 1.00 |
| ≥ 1 svg/wk | 36 | 12 | 1.33 (0.53–3.35) | 70 | 20 | 0.86 (0.37–2.02) |
| p-value | 0.55 | 0.73 | ||||
| ERβ1 | ERβ1 | |||||
| < 1 svg/wk | 78 | 31 | 1.00 | 48 | 18 | 1.00 |
| ≥ 1 svg/wk | 32 | 16 | 1.52 (0.65–3.54) | 62 | 29 | 1.23 (0.56–2.70) |
| p-value | 0.33 | 0.61 | ||||
| PR2 | PR2 | |||||
| < 1 svg/wk | 54 | 54 | 1.00 | 34 | 33 | 1.00 |
| ≥ 1 svg/wk | 23 | 27 | 1.46 (0.68–3.16) | 43 | 48 | 1.13 (0.56–2.30) |
| p-value | 0.33 | 0.73 | ||||
| HER2/neu1 | HER2/neu1 | |||||
| < 1 svg/wk | 75 | 34 | 1.00 | 45 | 22 | 1.00 |
| ≥ 1 svg/wk | 29 | 18 | 1.34 (0.60–3.04) | 59 | 30 | 0.88 (0.42–1.85) |
| p-value | 0.48 | 0.73 | ||||
| PCNA1 | PCNA1 | |||||
| < 1 svg/wk | 65 | 43 | 1.00 | 42 | 25 | 1.00 |
| ≥ 1 svg/wk | 30 | 20 | 1.06 (0.49–2.30) | 53 | 38 | 1.21 (0.59–2.48) |
| p-value | 0.88 | 0.61 | ||||
| Ki-672 | Ki-672 | |||||
| < 1 svg/wk | 43 | 63 | 1.00 | 24 | 42 | 1.00 |
| ≥ 1 svg/wk | 22 | 27 | 1.00 (0.46–2.16) | 41 | 48 | 0.68 (0.33–1.41) |
| p-value | 1.00 | 0.30 | ||||
Categories for ERα, ERβ, PCNA, and HER2/neu: Category 1 = no stain or <10% stain and Category 2 = ≥10% stain.
Categories for PR and Ki-67: Category 1 = no stain and Category 2 = any stain.
Odds ratios (OR) were obtained by logistic regression and adjusted for ethnicity, age, BMI, and HRT use; totals may differ due to variations in the number of readable cores
None of the associations in benign breast tissue was significant (Table 3). In terms of trends, women with high soy intake early in life were 30–50% more likely to be in the high expression category for ERα, ERβ, PR, and HER2/neu, but the ORs for PCNA and Ki-67 were very close to one. High soy intake during adulthood was not associated much with any marker. After stratification by ethnicity, Caucasian and Japanese women had divergent ORs for ERβ and Ki-67. They were 2.61 (95%CI: 0.57–11.95) and 1.87 (95%CI: 0.46–7.56) for Caucasians and 0.69 (95%CI: 0.20–2.36) and 0.32 (95%CI: 0.10–1.11) for Japanese.
When we examined the proliferation markers in combined soy intake categories, we found no elevated expression of markers for women with low intake in early life and high intake during adulthood. In benign tissue, the ORs for PCNA and Ki-67 were 0.85 and 0.73 as compared to low intake throughout life. The respective values for malignant tissue were 1.04 and 0.91. The ORs for PCNA and Ki-67 in women with high vs. low intake throughout life were also not significant (1.24 and 0.76 in benign; 0.64 and 1.01 in malignant tissue).
Discussion
Using TMAs from a population-based TR, we were able to obtain results for 268 of 442 (61%) subjects that had been contacted. Contrary to our hypothesis, we observed no significant associations between soy consumption and epithelial markers in breast tissue samples with the exception of adult soy intake who were less likely to have HER2/neu expressed in malignant tissue, probably a chance finding. Overall, a higher percentage of breast cancer patients showed positive marker expression in malignant tissue than in benign tissue except for ERβ; this finding is consistent with the putative role of ERβ in maintaining the differentiated phenotype of the breast (20). As to non-significant trends, women with early life soy intake had lower HER2/neu and PCNA staining in malignant tissue and women with high soy intake early or late in life had lower grade tumors. In benign tissue, early life soy intake suggested higher ERα, ERβ, PR, and HER2/neu expression. As indicated by ICCs, the agreement between tissue specimens was considerably higher for malignant than for benign tissue.
Our findings agree with previous reports showing that 10–20% of epithelial cells in benign breast tissue express ERα (21). However, the results do not support the concern raised by several small interventions in women that had noted an increase in some measure of cell proliferation after short-term soy intake (22–25). They also disagree with an observation of higher ERα expression for monkeys exposed to soy that may have been a chance finding (26). The relatively high percentage of positives Ki-67 staining is due to the use of “any staining” as a cut-off, but this cut-off for Ki67 is reasonably close to the 2.7% cutoff used by others in the development of prognostic models for breast cancer outcomes (27).
This project had a number of limitations. Despite the population-based TR maintained by the HTR (15), we were not able to include all breast cancer cases from our previous study because not all hospitals participate in the TR to the same degree (28). Furthermore, close to 10% of specimens fell off during staining and another 30% could not be evaluated because they did not contain epithelial tissue or the staining was inadequate. These percentages are not unusual; similar losses have been reported by others (28–30). The small size of cores (0.6 mm) and the selection of benign tissue aggravated the problem in our study (Dr. Christopher A. Moskaluk, University of Virginia, personal communication). Larger size needles may have alleviated some of the loss because the specimens would have been more likely to include epithelial tissue and because they may have adhered better to the slides. Unfortunately, the small size of tissue introduces the problem that the tumor may not be represented well; this explains the κ values of 0.26–0.37 for PR and ERα, but variations in assays conducted in different hospitals may also contribute to the weak agreement. The morphologic classification and interpretation of stains are based on findings within one small highly defined tissue area in TMAs (29). Nevertheless, good agreement between immunohistochemical results for single specimen slides and TMAs was shown repeatedly (17,31). Although our pathologic evaluation technique did not account for the intensity of stains, we believe that the use of staining controls, training of observers, and repeated measurements for assessment of interobserver and intraobserver variability constitute an improvement over the completely subjective assessments currently used in clinical practice. A serious issue is the fact that the so-called benign tissue in our study originates from surgery specimens. Therefore, the tissue may not be truly benign, or may be affected by paracrine signals from the nearby malignant tissue. On the other hand, the TMA approach saved tissue and allowed high level standardization for immunohistochemical staining because all specimens were stained under the same conditions (32).
The validity of the LTSQ is difficult to ascertain. Recall of diet for childhood adds another layer to the well known issues in dietary assessment (33), but a study in adults who recalled their adolescent diet after a 4-year period found acceptable agreement (34). Our own questionnaire indicated moderate reproducibility after 5 years (16). Other reports that have presented associations between adolescent diet and breast cancer risk also support the idea that adolescent diet can be assessed with some level of validity (35). Although soy intake prior and after adolescence would be a more valid comparison, we consider our classification an appropriate indicator for the presence of soy in the early life diet.
This investigation did not detect evidence for our hypothesized association between soy consumption and proliferative markers in breast tissue, but provides some assurance that soy intake does not adversely affect markers of proliferation. Given the widespread publicity about the health effects of soy foods, it is important to understand if the weak estrogenic effects of isoflavones have the ability to stimulate cell proliferation under certain conditions (25,36). Because isoflavones have estrogenic and anti-estrogenic effects, the final effect on carcinogenesis may depend on the dose and on the time of life when isoflavones were administered (37). To answer questions about the potential proliferative effect of soy isoflavones (25), it would be optimal to examine breast tissue from women without breast cancer. However, given the discomfort of needle biopsies and the ethical issues of biopsying unaffected women, large numbers of samples for epidemiologic studies cannot be obtained. Our approach of utilizing pathologic blocks from the HTR enabled us to examine a sizeable number of specimens for a variety of markers in breast tissue.
Acknowledgments
The breast pathology study and the nested case-control study were funded by grants from the National Cancer Institute (R21 CA1080250 and R01 CA85265). The Multiethnic Cohort Study has been supported by USPHS (National Cancer Insitute) Grant R37 CA 54281 (PI: Dr. L.N. Kolonel). We are grateful to the study participants and to the staff of the Hawaii Tumor Registry for their support. We thank Hugh Luk for the preparation of the TMAs, Hermina Borgerink for the staining of the TMAs, and Joseph Finley for the assessment of stains.
References
- 1.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 2.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson BE, Pike MC, Bernstein L, Ross RK. In: Breast cancer. Schottenfeld D, editor. Oxford University Press; New York: 1996. pp. 1022–1039. [Google Scholar]
- 4.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 5.Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, et al. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci U S A. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, et al. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 7.Lamartiniere CA. Timing of exposure and mammary cancer risk. J Mammary Gland Biol Neoplasia. 2002;7:67–76. doi: 10.1023/a:1015722507237. [DOI] [PubMed] [Google Scholar]
- 8.Nardulli AM, Greene GL, O’Malley BW, Katzenellenbogen BS. Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen’s effect on progesterone receptor synthesis and degradation. Endocrinology. 1988;122:935–944. doi: 10.1210/endo-122-3-935. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 10.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, et al. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol. 1992;45:416–419. doi: 10.1136/jcp.45.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 13.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Mammographic Density and Breast Cancer Risk: The Multiethnic Cohort. Am J Epidemiol. 2005;162:743–752. doi: 10.1093/aje/kwi270. [DOI] [PubMed] [Google Scholar]
- 15.Goodman MT, Hernandez BY, Hewitt S, Lynch CF, Cote TR, et al. Tissues from population-based cancer registries: a novel approach to increasing research potential. Hum Pathol. 2005;36:812–820. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, Aylward AG, Erber E, Takata Y, Kolonel LN. Soy intake is related to a lower body mass index in adult women. Eur J Nutr. 2008 doi: 10.1007/s00394-008-0707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XR, Charette LA, Garcia-Closas M, Lissowska J, Paal E, et al. Construction and validation of tissue microarrays of ductal carcinoma in situ and terminal duct lobular units associated with invasive breast carcinoma. Diagn Mol Pathol. 2006;15:157–161. doi: 10.1097/01.pdm.0000213453.45398.e0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson WF, Luo S, Chatterjee N, Rosenberg PS, Matsuno RK, et al. Human epidermal growth factor receptor-2 and estrogen receptor expression, a demonstration project using the residual tissue respository of the Surveillance, Epidemiology, and End Results (SEER) program. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 20.Speirs V, Walker RA. New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. J Pathol. 2007;211:499–506. doi: 10.1002/path.2130. [DOI] [PubMed] [Google Scholar]
- 21.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 22.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, et al. Stimulatory influence of soy protein isolate on breast secretion in pre-and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–794. [PubMed] [Google Scholar]
- 23.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 24.McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431S–1435S. doi: 10.1093/ajcn/68.6.1431S. [DOI] [PubMed] [Google Scholar]
- 25.Messina M, Caskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 26.Wood CE, Kaplan JR, Stute P, Cline JM. Effects of soy on the mammary glands of premenopausal female monkeys. Fertil Steril. 2006;85 (Suppl 1):1179–1186. doi: 10.1016/j.fertnstert.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 27.Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez BY, Frierson HF, Moskaluk CA, Li YJ, Clegg L, et al. CK20 and CK7 protein expression in colorectal cancer: demonstration of the utility of a population-based tissue microarray. Hum Pathol. 2005;36:275–281. doi: 10.1016/j.humpath.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, et al. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: a comparison between whole sections and tissue microarrays. J Clin Pathol. 2007;60:397–404. doi: 10.1136/jcp.2005.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni S, Patil DB, Diaz LK, Wiley EL, Morrow M, et al. COX-2 and PPARgamma expression are potential markers of recurrence risk in mammary duct carcinoma in-situ. BMC Cancer. 2008;8:36. doi: 10.1186/1471-2407-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillett CE, Springall RJ, Barnes DM, Hanby AM. Multiple tissue core arrays in histopathology research: a validation study. J Pathol. 2000;192:549–553. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH721>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Quraishi I, Rishi M, Feldman M, Wargovich MJ, Weber B. Clinical validation of breast cancer biomarkers using tissue microarray technology. Appl Immunohistochem Mol Morphol. 2007;15:45–49. doi: 10.1097/01.pai.0000213129.86288.34. [DOI] [PubMed] [Google Scholar]
- 33.Kipnis V, Midthune D, Freedman LS, Bingham S, Schatzkin A, et al. Empirical Evidence of Correlated Biases in Dietary Assessment Instruments and Its Implications. Am J Epidemiol. 2001;153:394. doi: 10.1093/aje/153.4.394. [DOI] [PubMed] [Google Scholar]
- 34.Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, et al. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol. 2005;161:89–97. doi: 10.1093/aje/kwi019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu XO, Jin F, Dai Q, Wen W, Potter JD, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- 36.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 37.Bouker KB, Hilakivi-Clarke L. Genistein: does it prevent or promote breast cancer? Environ Health Perspect. 2000;108:701–708. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]