Abstract
Background
The adipocytokine leptin may increase breast cancer risk, while adiponectin may be protective. We examined the association of the two circulating markers with mammographic density, a strong predictor of breast cancer risk.
Methods
For 183 premenopausal participants of a nutritional trial, mammograms performed at baseline, year 1, and year 2 were assessed for densities using a computer-assisted method. Serum samples obtained at the same time were analyzed for leptin and adiponectin by ELISA. We applied mixed models to incorporate the repeated measurements while adjusting for confounders including body mass index (BMI).
Results
At baseline, the mean age of the subjects was 42.6 ± 2.9 years; 40% were of Asian ancestry. Leptin was lower and adiponectin higher in normal weight than overweight women. Neither marker was related to absolute breast density. The significant inverse association of leptin with percent density disappeared when BMI was added to the model. After stratification by weight, percent density decreased with higher leptin levels in normal weight women, whereas it increased among overweight subjects. After adjustment for BMI, the positive association between percent density and adiponectin was greatly reduced and no longer significant.
Conclusions
These results do not support a strong association of leptin or adiponectin with breast cancer risk as assessed by mammographic density. On the other hand, the findings suggest the possibility that the inverse association of BMI with breast cancer risk in premenopausal women is mediated by adipocytokines.
Keywords: Mammographic density, breast cancer risk, adiposity, leptin, adiponectin, ethnicity
Introduction
Limited epidemiologic evidence suggests that the adipocytokines leptin and adiponectin are related to breast cancer development [1; 2]. Leptin, a protein produced mainly by adipose tissue, participates in pro-inflammatory responses, and may stimulate cancer growth [3; 4], thereby mediating the effect of obesity on cancer risk [4]. Despite many null findings, a few studies reported an increase in breast cancer risk with higher leptin levels [1; 5–9]. Interestingly, breast cancer risk in a study of young US women declined with higher leptin levels [10], a finding that agrees with the well established protective effect of body mass index (BMI) in premenopausal women [11]. Adiponectin is the most abundant protein in adipocytes [12; 13], decreases with higher levels of adipose tissue, and may protect against breast cancer due to its antiproliferative and anti-inflammatory effects [14]. Several epidemiologic studies suggest that lower circulating adiponectin confers an elevated breast cancer risk independent of BMI and leptin [14]. However, a prospective investigation described a non-significant 30% elevated risk among premenopausal women [15]. Both adipocytokines may affect the production of estrogens in adipose tissue, possibly through an effect on aromatase activity [16].
Mammographic density, the relative distribution of radiodense tissue to radiolucent fat in the breast, is a strong predictor of breast cancer risk [17]. A cross-sectional investigation detected a strong inverse association between leptin and mammographic density in postmenopausal women. However, given the relation between BMI and leptin, it disappeared after adjustment for BMI [18]. Similarly, in a study with premenopausal women that classified breast density into one of four parenchymal patterns, higher leptin levels were associated with less dense patterns, but the analysis was not adjusted for BMI [19]. As part of a nutritional intervention study, we assessed mammographic densities and measured serum levels of leptin and adiponectin in premenopausal women [20; 21]. Using these data, we examined the association of circulating leptin and adiponectin levels with mammographic density.
Methods
Study design
This investigation used data from a nutritional intervention trial [22]. As described elsewhere, 220 premenopausal participants with normal screening mammograms were randomized to a soy diet or to the control group and 189 women completed 2 years of intervention [22]. Women were excluded from this study due to use of oral contraceptives or other sex hormones, diagnosis of cancer, hysterectomy, no intact ovary or no regular menstrual periods, or high soy intake. As a result of drop-outs, missing mammograms, and the availability of serum, the current analysis includes 90 intervention and 93 control women. The Institutional Review Boards of the University of Hawaii approved the study protocol; participants signed informed consent and gave written permission to use frozen samples for future analyses.
Mammographic data collection
We scanned the mammograms performed at baseline and after 2 years for each woman, and, if available, also the image for the interim year using a Kodak LS85 Film Digitizer [20]. One of the authors (GM) performed computer-assisted density assessment [23; 24]. All mammograms for one woman were assessed during the same session, but the reader was blinded to the group status and the time sequence of the mammograms. The mammographic measures included the total breast area, the dense area, the nondense area computed as the difference between total and dense area, and percent density calculated as the ratio of the dense to the total area. We averaged the values for the right and the left breast. The intraclass correlation coefficients (ICC) for a sample of 219 duplicate readings were ICC = 0.93 (95% CI: 0.91–0.95) for the size of the dense areas and ICC = 0.998 (95% CI: 0.997–0.999) for the total breast area, resulting in an ICC of 0.95 for percent density (95% CI: 0.93–0.96) [20].
Analytical methods
This study made use of existing serum samples that were collected during the 2-year trial period 4–6 days after ovulation as determined by an ovulation kit [22]. Serum levels of leptin and adiponectin were assessed by double-antibody enzyme-linked-immunosorbent-assay (ELISA) (R&D Systems, Minneapolis, MN) according to the manufacturer's specifications [21]. The assay quality was assessed by 49 blinded controls from a pooled sample donated by 10 premenopausal center employees. The mean intra-batch coefficients of variation (CV) for leptin and adiponectin were 4.6% and 14.0%, whereas inter-batch CVs were 9.5% and 24.9%.
Statistical analysis
Statistical analyses were performed using the SAS statistical software package version 9.1 (SAS Institute, Inc., Cary, NC). Because the 90 women in the intervention group did not differ significantly from the 93 women in the control group and no intervention effect on leptin and adiponectin was observed [21], all subjects were analyzed together. Due to their non-normal distribution, BMI and leptin were log-transformed. Spearman correlation coefficients (rs) were computed to assess the relation between markers and breast density measures. After creating quartiles for the two markers, we computed means of breast density by category using Proc Mixed in SAS 9.1 [25; 26], a procedure that allows for an analysis of repeated measures with unbalanced times of measurement [27]. To assess significance of the association, we included the marker as a continuous variable while adjusting for age at mammogram. Ethnicity, parity, age at first live birth, and BMI were added for further adjustment because of their known association with breast density. The analyses were repeated after stratification by weight status, i.e., normal weight with a BMI <25 kg/m2 and overweight with a BMI of ≥25 kg/m2, while controlling for BMI as a continuous variable.
Results
The study population included 67 Caucasian, 23 Hawaiian, 49 Japanese, 14 Chinese, 11 Filipino, and 19 mixed/other women with substantial differences in BMI and mammographic measures (Table 1). Mean levels of leptin were significantly lower in Asian than in Caucasian women (p = 0.002), but the Native Hawaiian/Other group did not differ from Caucasians. On the other hand, adiponectin was significantly lower in Asian (p = 0.04) and in Hawaiian/Other women (p = 0.003) than in Caucasians. Mean BMI was significantly lower among Asians than in the two other groups (p = 0.002). The mean age of the study population was 42.6 ± 2.9 at the time of the baseline mammogram (Table 2). BMI and the size of the total breast increased over time, while the dense area and percent density decreased. Leptin and adiponectin were slightly higher after 2 years. Close to half of the women were overweight or obese and mean leptin levels were significantly higher in these women than in normal weight (20.8 and 40.4 vs. 10.7 ng/mL; p <0.0001). The relation for adiponectin was reversed; the respective means for normal weight, overweight and obese women were 9.5, 7.1, and 5.9 μg/mL (p <0.0001).
Table 1.
Study Population by Ethnicity at Baseline
| Variable | Caucasian | Asian | Hawaiian/Other |
|---|---|---|---|
| Number of women | 67 | 74 | 42 |
| Percent overweight (BMI 25–<30 kg/m2) | 22.4 | 25.7 | 33.3 |
| Percent obese (BMI >30 kg/m2) | 25.4 | 14.9 | 31.0 |
| Mean (SD) | |||
|---|---|---|---|
| Age at mammogram (y) | 42.3 (2.9) | 43.0 (2.7) | 42.2 (3.3) |
| Body mass index (kg/m2) | 27.0 (6.8) | 24.6 (4.4) | 27.4 (5.4) |
| Total breast area (cm2) | 130 (71) | 83 (42) | 112 (43) |
| Dense area (cm2) | 45 (29) | 36 (20) | 64 (43) |
| Nondense area (cm2) | 86 (72) | 47 (41) | 64 (43) |
| Percent density (%) | 41.1 (23.8) | 48.7 (24.9) | 48.1 (24.1) |
| Leptin (ng/mL) | 23.4 (18.5) | 14.8 (10.8) | 20.5 (15.0) |
| Adiponectin (μg/mL) | 9.4 (4.2) | 7.5 (3.7) | 6.7 (3.0) |
Table 2.
Characteristics of 183 Study Participants over 2 Years
| Variable | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| Number of women | 183 | 96 | 166 |
| Percent overweight (BMI 25–<30 kg/m2) | 26.2 | 29.2 | 30.7 |
| Percent obese (BMI >30 kg/m2) | 22.4 | 22.9 | 19.3 |
| Mean (SD) | |||
|---|---|---|---|
| Age at mammogram (y) | 42.6 (2.9) | 44.0 (2.8) | 45.0 (2.8) |
| Body mass index (kg/m2) | 26.1 (5.7) | 26.5 (6.2) | 26.5 (6.2) |
| Total breast area (cm2) | 106.9 (58.0) | 110.6 (64.8) | 110.8 (62.3) |
| Dense area (cm2) | 41.9 (25.1) | 41.4 (27.6) | 40.4 (25.9) |
| Nondense area (cm2) | 65.1 (57.2) | 69.2 (62.7) | 70.4 (60.9) |
| Percent density (%) | 45.8 (24.5) | 45.0 (26.6) | 42.5 (24.0) |
| Leptin (ng/mL) | 19.3 (15.4) | 20.7 (15.8) | 20.2 (16.6) |
| Adiponectin (μg/mL) | 8.0 (3.9) | 7.9 (3.9) | 8.2 (3.9) |
Leptin and adiponectin were strongly related to BMI (rs = 0.80 for leptin and rs = −0.41 for adiponectin; both p <0.0001). Percent density was significantly associated with leptin (rs = −0.48; p <0.0001) and adiponectin (rs = 0.24; p <0.0001), whereas the size of the dense are was not (rs = −0.04; p = 0.40 and rs = 0.07; p = 0.13). When adjusted means by quartiles of serum levels were computed (Table 3), both markers were also not related to absolute breast density. The 5.6% difference in dense area between the top and bottom quartiles of adiponectin (p = 0.14) was reduced after including other covariates. Leptin was significantly inversely associated with percent density (p <0.0001) when adjusted only for age, ethnicity, and reproductive factors with 10% lower densities in the highest vs. the lowest category. However, this association was attenuated to the null after adjustment for BMI. Percent density increased from 39.6 to 48.3% from the first to the fourth quartile of adiponectin (p = 0.001). When adding BMI to the model, this difference was reduced to 4.7% (p = 0.10). When we repeated these analyses for Asians and Caucasians separately, similar trends were found in both groups (data not shown).
Table 3.
Mean Mammographic Density by Quartiles of Adipocytokines
| Model | Quartiles of leptin (ng/mL)^ | P for trend | |||
|---|---|---|---|---|---|
| 0.9–8.4 | 8.5–15.6 | 15.7–26.2 | 26.3–88.0 | ||
| Dense area (cm2) | |||||
| Adjusted for age | 40.2 (2.4) | 40.5 (2.2) | 41.4 (2.2) | 42.3 (2.4) | 0.98 |
| Partially adjusted* | 41.7 (2.4) | 41.7 (2.2) | 42.1 (2.2) | 42.5 (2.4) | 0.53 |
| Fully adjusted# | 40.3 (2.5) | 41.1 (2.2) | 42.8 (2.2) | 44.2 (2.5) | 0.60 |
| Normal weight | 42.0 (2.1) | 41.7 (2.1) | 39.6 (2.6) | 39.6 (3.6) | 0.12 |
| Overweight | 36.4 (5.2) | 39.1 (4.0) | 41.0 (3.4) | 45.5 (3.4) | 0.22 |
| Percent density (%) | |||||
| Adjusted for age | 49.4 (2.2) | 46.5 (2.0) | 42.3 (2.0) | 38.7 (2.2) | <0.0001 |
| Partially adjusted* | 49.9 (2.2) | 46.8 (2.1) | 43.1 (2.1) | 39.5 (2.3) | <0.0001 |
| Fully adjusted# | 45.5 (2.1) | 44.7 (1.9) | 45.2 (1.9) | 45.1 (2.2) | 0.31 |
| Normal weight | 59.1 (2.5) | 56.0 (2.4) | 53.4 (3.2) | 51.7 (4.6) | 0.005 |
| Overweight | 26.7 (3.5) | 29.8 (2.7) | 32.6 (2.3) | 34.9 (2.3) | 0.03 |
| Quartiles of adiponectin (μg/mL) | |||||
|---|---|---|---|---|---|
| 0.7–4.8 | 4.9–7.5 | 7.6–10.2 | 10.3–24.5 | ||
| Dense area (cm2) | |||||
| Adjusted for age | 37.6 (2.4) | 40.0 (2.1) | 43.7 (2.1) | 43.2 (2.3) | 0.14 |
| Partially adjusted* | 38.6 (2.4) | 41.0 (2.1) | 44.7 (2.1) | 44.2 (2.3) | 0.14 |
| Fully adjusted# | 38.9 (2.4) | 41.2 (2.1) | 44.6 (2.2) | 43.9 (2.3) | 0.26 |
| Normal weight | 38.3 (2.8) | 40.4 (2.3) | 41.9 (2.1) | 43.0 (2.2) | 0.30 |
| Overweight | 38.9 (3.6) | 43.7 (3.3) | 46.0 (3.6) | 43.9 (4.0) | 0.28 |
| Percent density (%) | |||||
| Adjusted for age | 39.6 (2.3) | 42.7 (2.0) | 46.0 (2.0) | 48.3 (2.2) | 0.001 |
| Partially adjusted* | 40.0 (2.3) | 43.3 (2.0) | 46.7 (2.0) | 49.1 (2.2) | 0.0007 |
| Fully adjusted# | 42.5 (2.1) | 44.4 (1.8) | 46.5 (1.8) | 47.2 (2.0) | 0.10 |
| Normal weight | 51.9 (3.4) | 55.5 (2.7) | 56.4 (2.5) | 59.8 (2.6) | 0.14 |
| Overweight | 30.2 (2.4) | 33.4 (2.2) | 35.9 (2.4) | 33.4 (2.7) | 0.17 |
Adjusted for age, ethnicity, parity, and age at first live birth
Adjusted for age, ethnicity, parity, age at first live birth, and BMI
Means and standard errors are shown
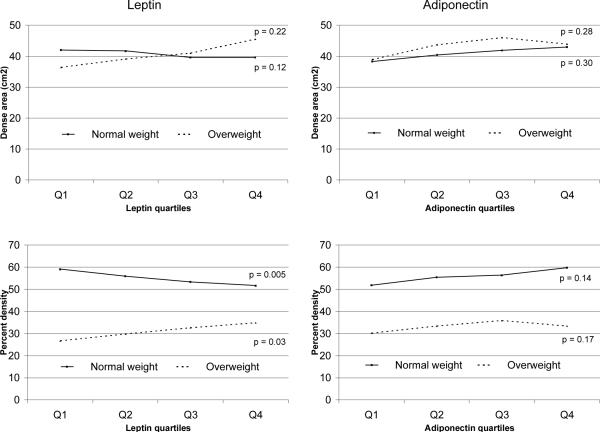
Stratification by weight status produced no significant differences when the dense area was modeled (Table 3 and Figure 1). For percent density, the well established difference in densities was observed; overweight/obese women had approximately 30% lower densities than normal weight women. The interaction between weight status, i.e., normal vs. overweight/obese, and leptin levels (p = 0.002) was significant. In the stratified analysis, normal weight women in the top quartile had 7.4% lower percent densities than those in the bottom quartile (p = 0.005), but this association was reversed among overweight women whose percent density was 8.2% higher in the top than in the bottom category (p = 0.03).
Figure 1.
Mean Breast Density for Quartiles of Markers Stratified by Weight Status*
*The cut-off for normal vs. overweight is 25 kg/m2; adjusted for age, ethnicity, reproductive variables, and BMI
Discussion
These findings among premenopausal women do not support an adverse effect of leptin or a protective effect of adiponectin on breast cancer risk as assessed by mammographic densities. In agreement with a previous report among postmenopausal women [18], adjustment for BMI eliminated the inverse association between leptin and breast density in this population of premenopausal women. The only previous study among premenopausal women used a qualitative method of breast density assessment and did not adjust for BMI [19]. Our stratified analysis suggests that leptin levels may be associated with elevated percent densities among overweight but not normal weight women. To our knowledge, no previous studies have looked at adiponectin and breast density. The suggestive positive association of percent density with adiponectin may be due to residual confounding by adiposity or it may indicate that adiponectin elevates risk in premenopausal women in contrast to the hypothesized protective effect in postmenopausal women, just as BMI [11] and possibly leptin [10] have opposite effects on breast cancer before and after menopause.
In agreement with the protective effect of overweight [11], three case-control studies among premenopausal women found that leptin was non-significantly associated with a lower breast cancer risk [6; 10; 28], whereas other studies described an increased risk with higher leptin levels in pre-and postmenopausal women [5; 7–9]. The adiponectin findings agree with a report from the Nurses Health Study that described a non-significantly elevated breast cancer risk with higher adiponectin levels in premenopausal women, while risk was lowered among postmenopausal women [15]. Given the current evidence, there is more support for a protective effect of adiponectin in post- than premenopausal women [14]. Our findings of lower adiponectin levels among Asian than Caucasian women despite lower BMI agree with a study among Japanese and Caucasian men; the former had lower adiponectin levels despite their lower BMI [29]. The authors suggest that the higher proportion of visceral adipose tissue as compared to subcutaneous adipose tissue in Japanese may explain this observation.
This investigation was limited by the strict selection criteria for the original trial that may limit the generalizability of our findings [22]. We used BMI to adjust for body fat although it is not a very representative measure across ethnic groups [30]; no other information on adiposity was available. Therefore, the few suggestive findings may be due to residual confounding by adiposity. Due to the limited power in subgroups, chance may be responsible for the opposite directions of the association between percent density and leptin after stratification by weight status. The small number of subjects in each ethnic group made it impossible to divide the population into more specific weight categories. On the other hand, the study had several strengths. We were able to use existing serum samples with excellent control of blood collection during luteal phase of the menstrual cycle under standardized conditions [22]. The long study period of 2 years and the availability of more than one observation for each subject were a great benefit because the study design addressed the intraindividual variability in markers. As shown by ICCs for leptin and adiponectin of 0.91 and 0.84, respectively, when all five measures of leptin and adiponectin were used, both markers were quite stable over time [21].
The question of what links adiposity to breast cancer risk has not been answered yet [31], but this study lends support to the contention that the protective effect of BMI on breast cancer risk in premenopausal women may be mediated by high leptin/low adiponectin because these markers were associated with breast density before but not after adjustment for BMI. However, given the strong correlation between BMI and leptin, it is not possible to disentangle their separate effects and draw conclusions in such a small study. Nevertheless, the effect of adipocytokines on breast cancer risk could be independent of breast density similar to those shown for circulating hormones [32] and body weight [33].
Acknowledgments
This research was supported by the National Cancer Institute grant R03 CA130061. The original study was supported by NCI grant R01 CA80843. We would like to thank the committed study participants ad the three dedicated summer students (Corey Kelsom, Brian Johnston, and William Cooney) who assisted with the lab assays.
References
- 1.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 3.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–40. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 4.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 5.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–82. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cust AE, Stocks T, Lukanova A, Lundin E, Hallmans G, Kaaks R, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat. 2009;113:567–76. doi: 10.1007/s10549-008-9958-8. [DOI] [PubMed] [Google Scholar]
- 7.Ozet A, Arpaci F, Yilmaz MI, Ayta H, Ozturk B, Komurcu S, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol. 2001;31:424–7. doi: 10.1093/jjco/hye097. [DOI] [PubMed] [Google Scholar]
- 8.Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu CL, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–6. [PubMed] [Google Scholar]
- 9.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–14. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Falk RT, Brinton LA, Madigan MP, Potischman N, Sturgeon SR, Malone KE, et al. Interrelationships between serum leptin, IGF-1, IGFBP3, C-peptide and prolactin and breast cancer risk in young women. Breast Cancer Res Treat. 2006;98:157–65. doi: 10.1007/s10549-005-9144-1. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tilg H, Wolf AM. Adiponectin: a key fat-derived molecule regulating inflammation. Expert Opin Ther Targets. 2005;9:245–51. doi: 10.1517/14728222.9.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 14.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 15.Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–6. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 16.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–9. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 18.Stuedal A, Ursin G, Veierod MB, Bremnes Y, Reseland JE, Drevon CA, et al. Plasma levels of leptin and mammographic density among postmenopausal women: a cross-sectional study. Breast Cancer Res. 2006;8:R55. doi: 10.1186/bcr1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol Biomarkers Prev. 2005;14:33–40. [PubMed] [Google Scholar]
- 20.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–94. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 21.Maskarinec G, Steude JS, Franke AA, Cooney RV. Inflammatory markers in a 2-year soy intervention among premenopausal women. J Inflamm (Lond) 2009;6:9. doi: 10.1186/1476-9255-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy SP, et al. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–44. [PubMed] [Google Scholar]
- 23.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 24.Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165–9. doi: 10.1097/00008469-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. SAS Institute Inc.; Cary, NC: 1996. [Google Scholar]
- 26.SAS Institute Inc. SAS OnlineDoc 9.1.2. SAS Institute Inc.; Cary, NC: 2004. [Google Scholar]
- 27.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; Oxford: 2003. [Google Scholar]
- 28.Mantzoros CS, Bolhke K, Moschos S, Cramer DW. Leptin in relation to carcinoma in situ of the breast: a study of pre- menopausal cases and controls. Int J Cancer. 1999;80:523–6. doi: 10.1002/(sici)1097-0215(19990209)80:4<523::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Kadowaki T, Sekikawa A, Okamura T, Takamiya T, Kashiwagi A, Zaky WR, et al. Higher levels of adiponectin in American than in Japanese men despite obesity. Metabolism. 2006;55:1561–3. doi: 10.1016/j.metabol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 31.Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesitycarcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–69. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178–87. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 33.Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–92. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]