Abstract
Aged mice treated peripherally with lipopolysaccharide (LPS) show an exaggerated neuroinflammatory response and cognitive deficits compared to adults. Considerable evidence suggests resveratrol, a polyphenol found in red grapes, has potent antiinflammatory effects in the periphery, but its effects on the central inflammatory response and cognitive behavior are unknown. Therefore, the current study investigated if resveratrol dietary supplementation would inhibit neuroinflammation as well as behavioral and cognitive deficits in aged mice given LPS to mimic a peripheral infection. In initial studies, adult (3–6 months) and aged (22–24 months) mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected intraperitoneally (i.p.) with saline or LPS, and locomotor activity and spatial working memory were assessed. As anticipated, deficits in locomotor activity and spatial working memory indicated aged mice are more sensitive to LPS compared to adults. More importantly, the LPS-induced deficits in aged animals were mitigated by dietary supplementation of resveratrol. In addition, resveratrol consumption reduced LPS-induced interleukin-1β (IL-1β) in plasma and the IL-1β mRNA in the hippocampus of aged mice. Finally, pretreatment of BV-2 microglial cells with resveratrol potently inhibited LPS-induced IL-1β production. These data show that aged mice are more sensitive than adult mice to both the inflammatory and cognitive effects of peripheral immune stimulation and suggest that resveratrol may be useful for attenuating acute cognitive disorders in elderly individuals with an infection.
Introduction
Interleukin-1β (IL-1β), a proinflammatory cytokine produced in the brain predominantly by microglia, plays a dual role in hippocampal-dependent learning and memory processes. A basal level of IL-1β and IL-1 receptor type 1 signaling is essential for hippocampal learning and memory.1 However, if IL-1β activity exceeds a normal physiological range, hippocampal neurogenesis is reduced and the consolidation of hippocampal-dependent memories is inhibited.2,3. The potential role of IL-1β in cognitive aging is of growing interest because the gene expression profile of cognitively impaired aged animals is indicative of increased brain inflammation.4–6 In addition, the constitutive expression of IL-1β in the brain of old, but otherwise healthy, animals is often higher than young cohorts.7,8 Evidence further suggests the aging process sensitizes microglia to signals from the peripheral immune system.9–11 For example, after isolating microglia from whole brain and staining for CD11b and IL-1β, Henry et al.11 found a higher percentage of IL-1β–positive microglia in aged mice compared to adults following peripheral injection of lipopolysaccharide (LPS). Moreover, after peripheral injection of LPS, old mice expressed higher levels of IL-1β in the hippocampus and had greater deficits in spatial working memory compared to young adults.8 Recently, the exaggerated sickness behavior in aged mice caused by peripheral injection of LPS was attenuated by intracerebroventricular injection of IL-1 receptor antagonist (IL-1RA).12 Therefore, inhibiting IL-1β production by microglial cells may be useful for slowing cognitive aging or preventing infection-related cognitive disorders.
Resveratrol, (3,5,4′-trihydroxy-trans-stilbene), is a polyphenol found mainly in grapes and red wine and has diverse biological activities that confer protection against oxidative stress, inflammation, cardiovascular disease, and cancer.13–19 Resveratrol is of particular interest for modulating diseases with an inflammatory component because several studies found it to inhibit production of reactive oxygen species (ROS) by neutrophils, monocytes, and macrophages20–24 as well as activation of several transcription factors including nuclear factor-κB (NF-κB) and activator protein-1 (AP-1).25,26 Recently, a number of animal studies have focused on the neuroprotective effects of resveratrol, showing it to slow the neuropathology associated with Alzheimer27 and Parkinson disease28 and to protect against injury from brain trauma29 and cerebral ischemia.30 It was further shown to modulate cholinergic neurotransmission and improve cognition in diabetic rats.31
That all of the aforementioned conditions are associated with increased expression of IL-1β and other inflammatory cytokines in the brain presents the distinct possibility that the beneficial effects of resveratrol are conferred through its antiinflammatory properties. Despite the number of studies that show resveratrol inhibits the production of inflammatory molecules by stimulated microglial cells in vitro,32–35 there have been no studies in aged animals investigating the potential for dietary resveratrol to reduce IL-1β in the brain during infection and protect against deficits in cognition.
Therefore, the purpose of the present study was to determine if resveratrol dietary supplementation would inhibit IL-1β expression in the hippocampus as well as behavioral and cognitive deficits in aged mice given LPS to mimic a peripheral infection. The results suggest infection-related neuroinflammation and cognitive deficits in elderly subjects can be minimized by resveratrol supplementation.
Materials and Methods
Animals and diet
Adult (3- to 6-month-old) and aged (22- to 24-month-old) male BALB/c mice from our specific pathogen-free colony were maintained as described previously.12 To eliminate the effect of phytoestrogens present in standard rodent chow diet, we used an isoflavone-free diet (AIN93G) formulated with casein rather than soy. Before the start of each experiment, mice were provided a standard AIN-93G mouse diet (Research Diets, Inc, New Brunswick, NJ) for a 1-week acclimation period. Some animals were provided AIN-93G throughout the study, whereas others were provided AIN-93G plus 0.4% resveratrol. Resveratrol (cat. no. 70675; lot no. 125673; Cayman Chemicals, Ann Arbor, MI) was homogeneously blended into the AIN-93G control diet, pelleted, and preserved in a manner to ensure the stability of resveratrol. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Behavioral tests
Locomotor Activity
Home cage activity was measured using the EthoVision animal tracking system (Noldus Information Technologies, The Netherlands) and total distance moved was determined during 5-min tests. Tests were conducted during the dark phase (between 0800 and 1700) of the photoperiod under infrared lighting to aid video recording.
Spatial Working Memory
A version of the Morris water maze was used to determine hippocampal-dependent leaning and memory. The testing apparatus used has been described previously.36 Animal training took place during a 5-day acquisition phase with three massed trials administered each day. To begin each trial, mice were placed on the platform for 30 sec preceding the start of each session and then pseudo-randomly placed in the water in one of three predetermined locations 2 cm from the edge of the tank facing the wall. Mice were allowed to swim freely for a maximum of 60 sec or until the platform was located. After the mouse reached the platform, it was required to remain there for 30 sec. If the platform was not located during the 60 sec, mice were guided to the platform and allowed to remain there for 30 sec. After completion of three successive trials, mice were returned to their home cage. Performance parameters that were determined using the EthoVision animal tracking system included swim speed, latency to the platform, and distance swam.
To evaluate working memory performance, mice were tested in the same matching-to-place paradigm described above, except that the platform was relocated to the opposite quadrant of the pool; all distal visual cues remained constant. Animals were placed on the platform for 30 sec preceding the start of the reversal test and given three trials to locate the platform in the new target quadrant. All performance parameters remained the same as described above.
Experimental design
Escherichia coli LPS (1.0 μg/mouse; serotype 0127:B8, Sigma, St. Louis, MO) was dissolved in sterile saline immediately prior to an experiment. Adult (n = 40) and aged (n = 48) mice were provided an AIN-93G standard diet or AIN-93G plus 0.4% resveratrol diet for 4 weeks. Food intake and body weight were measured on a weekly basis for the duration of the study.
After 4 weeks of diet supplementation, locomotor activity was evaluated at the onset of the dark phase. Immediately after the behavioral test, mice received a 100-μL intraperitoneal (i.p.) injection of sterile saline or LPS (1.0 μg). After the injections, mice were returned to their home cage and locomotor activity was determined again 2, 4, 8, and 24 h later. At 24 h after injection, mice were killed by CO2 asphyxiation, and blood and hippocampal tissue were collected for determination of IL-1β protein and mRNA levels, respectively.
To determine the effects of resveratrol on changes in hippocampal-dependent learning, a separate study was conducted wherein a reversal learning version of the Morris water maze was used 4 and 24 h following peripheral immune stimulation. During the fourth week of diet supplementation, animals were trained in a 5-day acquisition phase with three massed trials administered each day. The platform remained in a constant location during the acquisition phase. Animals were allowed a 2-day rest period and on day 8 mice were administered LPS and then 4 and 24 h later were subjected to a reversal test in which the platform was moved to the opposite quadrant of the pool but all distal visual cues remained constant.
Determination of IL-1β protein and mRNA expression
Plasma samples were assayed for IL-1β protein using a bead-based immunoassay kit combined with a Cytokine Reagent kit as described by manufacturer (Bio-Rad, Hercules, CA). The multiplex assay was sensitive to <3 pg/mL for IL-1β. The interassay and intraassay coefficients of variation were <8%.
Total RNA was isolated from hippocampus using the Tri Reagent protocol (Sigma, St. Louis, MO). A Quanti Tect reverse transcription kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination as described previously.37 Quantitative real-time PCR was performed using the Assay-on-Demand gene expression protocol (Applied Biosystems, Foster City, CA) as described.12 Briefly, cDNA was amplified by PCR, where a target cDNA (Mm00434228_ml for IL-1β) and a reference cDNA (Mn99999915_gl for glucose-3 phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′-fluorescent reporter dye (6-carboxyfluorescein) and a 3′-nonfluorescent quencher dye. Fluorescence was determined on an ABI PRISM 7900HT sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results were expressed as fold difference.
BV-2 microglial cell culture
The immortalized murine microglia cell line, BV-2 (a gift from Linda Van Eldik, Northwestern University, Evanston, IL), was maintained in 150-cm2 tissue culture flasks (BD Falcon) in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 units/mL penicillin/streptomycin at 37°C in a humidified incubator under 5% CO2. In all experiments, cells were resuspended in DMEM supplemented with 10% FBS and seeded in six-well plates (BD Falcon) before being subjected to treatments. Cell viability was measured by the tetrazolium compound MTS cell proliferation assay according to the manufacturer's instructions (Promega, Madison, WI).
BV-2 cells were pretreated with vehicle (0.05% ethanol vol/vol) or resveratrol (0–50 μM) for 1 h and incubated with 100 ng/mL of LPS for 4 h to determine supernatant IL-1β concentration. The doses for resveratrol were chosen based on findings that resveratrol reduced prostaglandin E2 (PGE2) production and free radical formation in LPS-activated primary rat microglia.35 IL-1β protein was determined using a Quantikine mouse IL-1β immunoassay kit as described by the manufacturer (R&D Systems, Minneapolis, MN). The assay was sensitive to <3 pg/ml for IL-1β. The inter- and intraassay coefficients of variation were <8%.
Statistical analysis
Data were analyzed using the Mixed Procedure of the Statistical Analysis System (SAS Inst., Cary, NC). All data were subjected to a univariate analysis to ensure normality. Behavioral results were subjected to a three-way analysis of variance (ANOVA) using repeated measures in which test hour (0, 2, 4, 8, and 24 h) was a within-subjects measure, and age (adult or aged), diet (standard or resveratrol diet), and LPS (saline or 1.0 μg/mouse) were between-subjects measures. IL-1β mRNA and protein levels were analyzed using a three-way ANOVA in which age (adult or aged), diet (standard or 4 g of resveratrol/kg diet), and LPS (saline or 1.0 μg/mouse) were between-subjects measures. IL-1β secretion from BV-2 cells was analyzed using a two-way ANOVA in which pretreatment (0 μM ethanol, 25 μM or 50 μM resveratrol) and LPS (media or 100 ng/mL) were between measures. A post hoc Student t-test of least square means with a Bonferroni adjustment was employed to determine if treatment means were significantly different from one another (p < 0.05). All data are presented as means ± standard error of mean (SEM).
Results
Resveratrol protects aged but not adult mice from LPS-induced behavioral deficits
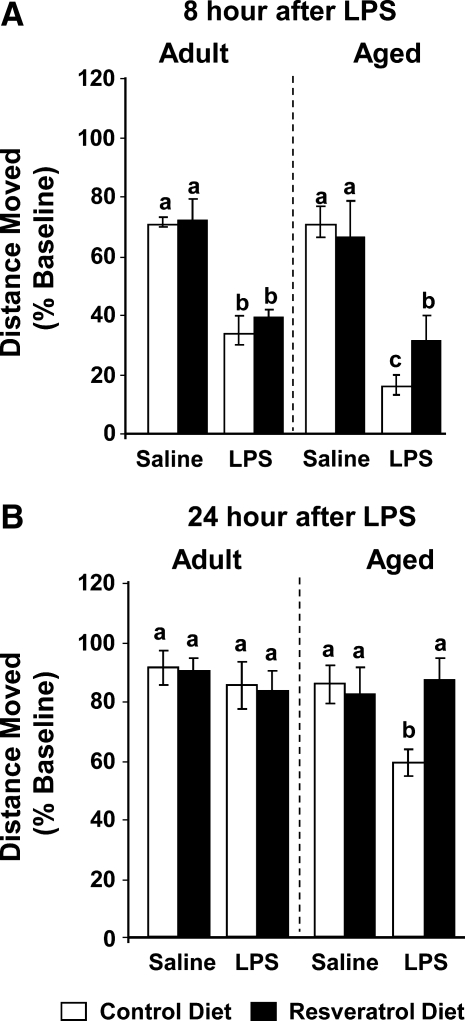
To assess the role of resveratrol in mediating behavioral deficits associated with peripheral immune activation in the aged, mice were provided standard or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or LPS. On the basis of daily food intake, both adult and aged mice consumed an average of 16.33 mg of resveratrol/day, and resveratrol supplementation did not alter food intake nor body weight of adult or aged mice (data not shown). Three-way ANOVA of locomotor activity (Fig. 1A,B) revealed significant main effects of age (F[1, 35] = 39.03, p < 0.0001) and LPS (F[1, 35] = 258.24, p < 0.0001) as well as a tendency for diet (F[1, 35] = 3.14, p = 0.08). In addition, there were significant age × LPS (F (1, 35) = 4.52, p = 0.04) and age × diet × LPS (F[1, 35] = 16.79, p = 0.0002) interactions. Post hoc comparisons showed LPS reduced locomotor activity similarly in both adult and aged mice 2–4 h postinjection and that there was no difference within diet supplementation groups (data not shown). Diet supplementation did not affect LPS-induced sickness behavior in adults at any time postinjection. However, resveratrol supplementation ameliorated LPS-induced locomotor deficits in aged mice beginning at 8 h (t [35] = −2.84, p = 0.007) and completely restored the longer lasting depression of locomotor activity seen in aged mice 24 h post LPS (t[36] = −3.42, p = 0.04). These data show that resveratrol inhibits LPS-induced sickness behavior in aged, but not adult mice.
FIG. 1.
Resveratrol protected aged mice but not adult mice from lipopolysaccharide (LPS)-induced deficits in locomotor behavior. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or LPS. Locomotor activity was measured for both adult and aged mice at 8 h (A) and 24 h (B) after LPS injection. Bars represent the mean ± standard error of the mean (SEM) (n = 10–11). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Effects of resveratrol on spatial working memory
We next tested mice in a version of the Morris water maze to determine if resveratrol supplementation inhibited LPS-induced disruption in hippocampal-dependent learning and memory. Adult and aged mice were trained in the water maze during the fourth week of diet supplementation (days 1–5) and on day8 administered LPS and tested 4 and 24 h later in a working memory version of the water maze. During the acquisition phase (Fig. 2A,B), repeated-measures ANOVA revealed main effects of age for distance (F[1, 353] = 58.35, p < 0.0001) and latency (F[1, 337] = 52.70, p < 0.0001) in which aged animals swam further and took longer to reach the platform than did adult animals. There were also main effects of day for these two parameters in which the performance of both age groups improved, as evidenced by decreases in distance swam (F[4, 353] = 93.47, p < 0.0001) and time it took to reach the platform (F[4, 337] = 50.11, p < 0.0001) over the 5 days of acquisition. These data demonstrate that, although the performance of both adult and aged mice improved over time, aged mice swam further and longer to locate the platform during the acquisition phase. The reduced performance of aged mice did not appear to be due to lack of general motor ability or motivation because swim speed did not significantly differ by age across test sessions of the acquisition phase (Fig. 2C). Prior to peripheral immune stimulation, there was no difference between diet supplementation groups, suggesting that resveratrol had no effect on the animal's ability to learn the task.
FIG. 2.
Performance of adult and aged mice during a 5-day acquisition phase in the Morris water maze. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks, and during the fourth week of diet supplementation animals were trained in a 5-day acquisition phase. Distance swam (A), latency to platform (B), and swim speed (C) were measured for both adult and aged mice. Data points represent the mean ± standard error of the mean (SEM) (n = 11–13). Means marked with and asterisk (*) or number sign (#) are significantly different (p < 0.05) from aged treatment-matched baseline controls, respectively.
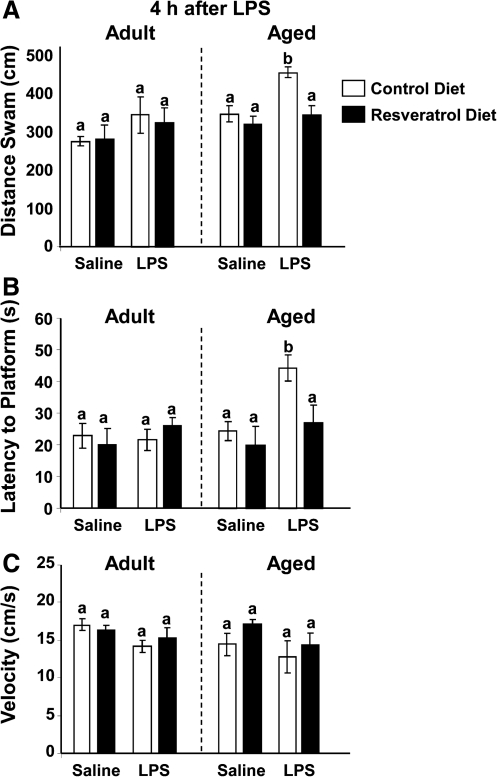
A separate three-way ANOVA examined the effects of resveratrol on working memory performance during reversal testing 4 h (Fig. 3A–C) after LPS. Analysis of distance swam 4 h post LPS injection revealed significant main effects of diet (F[1, 61] = 5.01, p = 0.02) and LPS (F[1, 61] = 12.70, p = 0.007). Analysis of latency to the platform 4 h post LPS revealed significant main effects of age (F[1, 61] = 4.52, p = 0.03) and LPS (F[1, 61] = 6.70, p = 0.01) and a trend toward significance of diet (F[1, 61] = 2.82, p = 0.09), as well as a trend toward significance of age × diet (F[1, 61] = 3.67, p = 0.06) and age × LPS (F[1, 61] = 3.40, p = 0.06) interactions. Post hoc comparisons showed LPS impaired learning and memory as assessed by distance swam (t[61] = 3.86, p = 0.006) and latency to the platform (t[61] = 3.87, p = 0.007) 4 h after LPS in only aged mice. Remarkably, this LPS-induced inhibition of working memory in the aged was completely blocked by resveratrol dietary supplementation as assessed by distance swam (t[61] = 3.57, p = 0.01) and latency to the platform (t[61] = 3.72, p = 0.01).
FIG. 3.
Resveratrol improves impaired spatial working memory in aged mice 4 h post LPS injection. After 5 days of acquisition training, mice were evaluated in a reversal test 4 h after lipopolysaccharide (LPS) injection. Distance swam to platform (A), latency to find the platform (B), and swim speed (C) were evaluated for both adult and aged mice. Bars represent the mean ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
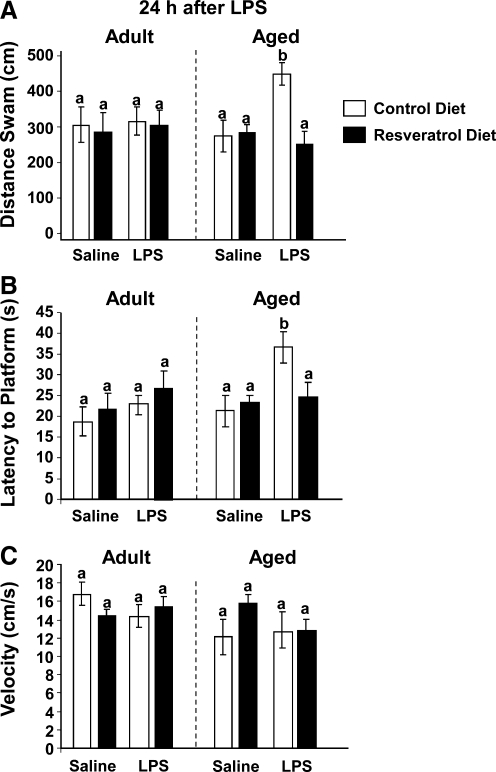
A similar effect on reversal learning was observed at 24 h (Fig. 4A–C). Analysis of distance swam revealed a significant main effect of diet (F[1, 65] = 3.17, p = 0.05) as well as a trend toward significance of diet × LPS (F[1, 65] = 3.18, p = 0.06) interaction. Analysis of latency to the platform 24 h post LPS revealed significant main effects of age (F[1, 63] = 3.94, p = 0.05) and LPS (F[1, 63] = 4.84, p = 0.03), as well as a trend toward significance of diet × LPS (F[1, 63] = 3.15, p = 0.08) interaction for latency to platform. Post hoc comparisons showed LPS-induced impaired learning persisted in only aged mice as assessed by distance swam (t[65] = 3.43, p = 0.04) and latency to the platform (t[63] = 3.39, p = 0.03). As with the 4-h time point in aged animals given LPS, working memory was completely restored by resveratrol dietary supplementation as evaluated by distance swam (t[65] = 3.03, p = 0.06) as well as latency to the platform (t[63] = 2.23, p = 0.04). These data indicate that the ability to integrate the new platform position with existing memories of the spatial cues is disrupted by peripheral immune stimulation and compounded with age. They further showed that a resveratrol-supplemented diet provided significant protection against these decrements in performance.
FIG. 4.
Resveratrol improves impaired spatial working memory in aged mice 24 h post LPS injection. After 5 days of acquisition training, mice were evaluated in a reversal test 24 h after lipopolysaccharide (LPS) injection. Distance swam to platform (A), latency to find the platform (B), and swim speed (C) were evaluated for both adult and aged mice. Bars represent the mean ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
Resveratrol inhibits the LPS-induced increase in IL-1β in both periphery and hippocampus of aged mice
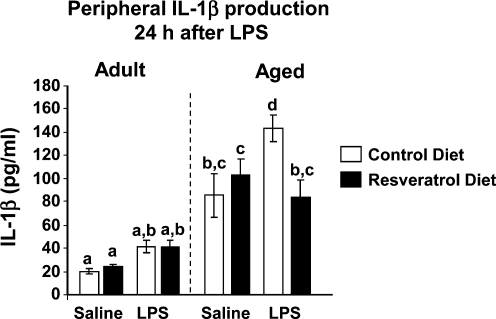
We next investigated whether resveratrol would attenuate IL-1β production in the periphery of mice injected with LPS (Fig. 5). Three-way ANOVA of plasma IL-1β protein revealed main effects of age (F[1, 35] = 78.10, p < 0.0001), LPS (F[1, 35] = 6.33, p = 0.01) as well as significant diet × LPS (F[1, 35] = 7.08, p = 0.02) and age × diet × LPS (F[1, 35] = 5.71, p = 0.02) interactions. In addition, there was a trend for an age × diet interaction (F[1, 35] = 3.17, p = 0.08). While IL-1β was not increased by LPS in adults, probably owing to the fact that blood samples were not collected until 24 h after injection, levels of IL-1β in aged animals were significantly elevated after LPS (t[35] = 4.20, p = 0.0048) compared to aged saline control. More importantly, the LPS-induced increase in IL-1β production in aged mice was reduced by resveratrol supplementation (t[35] = 4.38, p = 0.0028).
FIG. 5.
Dietary supplementation with resveratrol inhibited the LPS-induced increase in interleukin-1β (IL-1β) in periphery of aged mice. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or lipopolysaccharide (LPS). After the final behavioral test or cognitive test (24 h after injection), blood was collected and IL-1β production was measured by an IL-1β Quantikine assay. Bars represent means ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
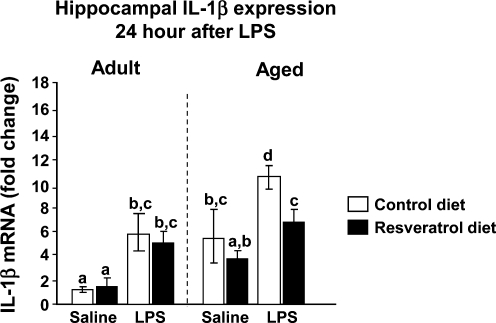
Because increased expression of IL-1β in the hippocampus has been associated with disruption of cognitive processing after peripheral injection of LPS,8,38 we next measured hippocampal IL-1β mRNA levels 24 h after LPS in both adult and aged mice (Fig. 6). Three-way ANOVA of hippocampal IL-1β mRNA levels revealed main effects of age (F[1, 41] = 34.13, p < 0.0001), diet (F[1, 41] = 5.85, p = 0.02) and LPS (F[1, 41] = 45.41, p < 0.0001) as well as significant age × diet (F[1, 41] = 8.38, p = 0.006), age × LPS (F[1, 41] = 5.82, p = 0.02) interactions. In addition, there were trends for diet × LPS (F[1, 41] = 3.09, p = 0.08) and age × diet × LPS (F[1, 41] = 3.35, p = 0.07) interactions. Notably, hippocampal levels of IL-1β mRNA in aged animals were significantly elevated after LPS (t[41] = 6.43, p < 0.0001). Consistent with the behavioral data, the LPS-induced increase in IL-1β mRNA in aged mice was reduced by resveratrol supplementation (t[41] = 4.58, p < 0.0001).
FIG. 6.
Dietary supplementation with resveratrol inhibited the lipopolysaccharide (LPS)-induced increase in interleukin-1β (IL-1β) mRNA in aged mice. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or LPS. After the final behavioral test or cognitive test (24 h after injection), hippocampal tissue was collected and IL-1β mRNA was measured by quantitative real-time PCR. Bars represent means ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
Resveratrol suppresses LPS-induced IL-1β secretion in BV-2 microglial cells
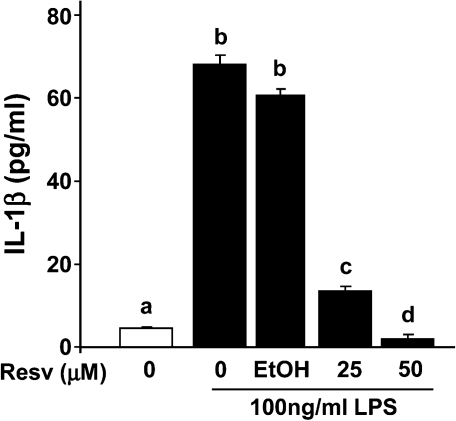
To determine if resveratrol can directly mediate IL-1β production by microglia, BV-2 microglial cells were pretreated with resveratrol (0, 25, and 50 μM) and stimulated with 100 ng/mL LPS for a 4-h incubation period (Fig. 7). IL-1β concentration in supernatant of BV-2 cells stimulated with LPS increased to 69.76 pg/mL (t[55] = 37.44, p < 0.0001). Pretreatment of BV-2 cells with 25 and 50 μM of resveratrol reduced LPS-stimulated IL-1β by 81% (t[55] = −22.85, p < 0.0001) and 91% (t[55] = −42.78, p < 0.0001), respectively. The highest concentration of resveratrol (50 μM) blocked IL-1β secretion completely. Neither LPS nor resveratrol affected cell survival or proliferation as determined by MTS assay (data not shown). Taken together, these data indicate that resveratrol is a potent inhibitor of LPS-induced IL-1β production in BV-2 microglial cells and may provide protection by reducing peripheral responses to LPS or by directly acting on brain microglia.
FIG. 7.
Resveratrol inhibits lipopolysaccharide (LPS)-induced interleukin-1β (IL-1β) secretion in BV-2 cells. BV-2 cells were pretreated with resveratrol (0–50 μM) for 1 h and stimulated with LPS (100 ng/mL) for a 4-h incubation period. IL-1β secretion was measured by an IL-1β Quantikine immunoassay. Concentration of IL-1β in supernatants from BV-2 cells stimulated with LPS was ∼ 70.0 pg/mL. Bars represent the mean ± standard error of the mean (SEM) from three independent experiments. Means with different letters (a, b, c, or d) are significantly different (p < 0.05) from each other. Resv, Resveratrol; EtOH, ethanol.
Discussion
Recent evidence indicates aging sensitizes microglial cells to signals from the peripheral immune system, resulting in an exaggerated neuroinflammatory response and behavioral pathology during peripheral infection.5,8,11 Thus, to promote healthy aging and facilitate recovery after peripheral infection, it is vital that new strategies be envisioned to mitigate the dysregulated communication between the immune system and brain in elderly subjects. Therefore, the goal of the present study was to determine if consuming a diet supplemented with the polyphenol resveratrol afforded aged mice protection from the excessive production of IL-1β in the brain and severe behavioral and cognitive deficits that occur during infection. The significant results showed that dietary supplementation of resveratrol inhibited the production of IL-1β in the periphery and brain as well as the deficits in spatial working memory when LPS was administered to aged mice to mimic a peripheral infection. Resveratrol was further shown to inhibit IL-1β production by LPS-stimulated microglia in vitro. Thus, the present findings in aged mice suggest dietary resveratrol can constrain the central response to signals from the peripheral immune system and promote recovery after peripheral infection.
Markers of inflammation have been reported to increase in both the peripheral blood39,40 and brain41 with advancing age. In the periphery, this is due in part to the increased capacity of mononuclear cells in elderly subjects to produce proinflammatory cytokines.42 This is also the case in the brain as microglia, which are derived from mononuclear myeloid progenitors, from aged mice produced higher levels of proinflammatory cytokines both in the absence and presence of immune stimuli.41,43 Peripheral injection of LPS has been found to cause an exaggerated inflammatory cytokine response in the aged brain.5,8 Moreover, anorexia, depression-like behavior, and deficits in hippocampal-dependent learning and memory are more evident in old mice than in young adults after peripheral LPS administration.5,8,44 Importantly, this was confirmed in the present study, because aged mice compared to young adults had: (1) higher circulating levels of IL-1β in the absence and presence of LPS stimulation; (2) higher levels of IL-1β mRNA in the hippocampus in the absence and presence of LPS stimulation; (3) LPS-induced sickness behavior of a greater magnitude and duration; and (4) LPS-induced deficits in spatial working memory. Thus, the model appeared to be optimal for assessing the ability of resveratrol to mitigate the interaction between the peripheral immune system and brain in aged subjects.
In this study we adopted a pragmatic approach by delivering resveratrol as a dietary supplement. Similar to other polyphenols, the oral bioavailability of resveratrol is low due to rapid excretion and extensive metabolism into various glucuronide and sulfate conjugates.45,46 Nonetheless, moderate consumption of red wine, where resveratrol is highly concentrated, is associated with reduced risk of cardiovascular disease and cancer.47,48 Moreover, several reports have confirmed orally administered resveratrol to be absorbed and cross the blood–brain barrier and incorporate into the brain.49–52 The antiinflammatory effects of resveratrol in aged mice could be linked to its ability to inhibit factors involved in gene transcription such as mitogen-activated protein kinase (MAPK), AP-1, and NF-κB.25 Resveratrol affects NF-κB by inhibiting Iκ-B kinase, thereby preventing translocation of NF-κB into the nucleus.25,53 How this occurs is not clear; however, it may be that resveratrol activates SIRT1, an enzyme of the sirtuin class of nicotinamide adenine dinucleotide (NAD)+-dependent histone deacetylases, which deacetylates NF-κB, thereby inactivating the transcription factor.54,55
Recently, Adler et al.56 demonstrated that inhibition of NF-κB signaling in old mice reverted the tissue characteristics and global gene expression to those of young mice. This kind of “rejuvenation” suggests that the continuous activation of NF-κB signaling could promote the aging process. Several studies have indicated that SIRT1 is a potent inhibitor of NF-κB transcription.32,54 The signaling link between SIRT1 and NF-κB is especially interesting with respect to aging because according to a number of studies SIRT1 acts to extend lifespan by inhibiting NF-κB signaling, and this is sufficient to reverse gene expression changes associated with age in mice.54,56,57 It is important to note that resveratrol did not inhibit the effects of LPS in young adults. This is important because immunological and behavioral responses to infection are intended to help the host contend against infective agents. However, in LPS-treated old mice, resveratrol reduced IL-1β in the periphery and brain and improved locomotor behavior and hippocampal-dependent spatial working memory. Accordingly, resveratrol may prove to be neuroprotective against age-related neuroinflammation by downregulating NF-κB signaling and restoring the response to LPS to that of their younger cohorts.
Regardless of the mechanism, the current findings suggest that dietary supplementation with resveratrol may play an important role in reversing the deleterious effects of infection on behavior and cognition in elderly subjects. These findings may also support the role of natural compounds as a possible preventative and/or complementary therapy for several neurodegenerative diseases caused by neuroinflammation.
Acknowledgments
This research was supported by National Institutes of Health (NIH) grants AG16710 and MH069148 (to R.W.J.). J.A. is supported by a NIH Ruth L. Kirschstein Institutional National Research Service Award 5T32 DK59802 from the The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to the Division of Nutritional Sciences at the University of Illinois.
Author Disclosure Statement
There are no actual or potential competing interests.
References
- 1.Avital A. Goshen I. Kamsler A. Segal M. Iverfeldt K. Richter-Levin G. Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 2.Pugh RC. Fleshner M. Watkins LR. Maier SF. Rudy JW. The immune system and memory consolidation: a role for the cytokine IL- 1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 3.Ben Menachem-Zidon O. Goshen I. Kreisel T. Ben Menahem Y. Reinhartz E. Ben Hur T. Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- 4.Blalock EM. Chen KC. Sharrow K. Herman JP. Porter NM. Foster TC. Landfield PW. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godbout JP. Chen J. Abraham J. Richwine AF. Berg BM. Kelley KW. Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 6.Swanson KS. Vester BM. Apanavicius CJ. Kirby NA. Schook LB. Implications of age and diet on canine cerebral cortex transcription. Neurobiol Aging. 2009;30:1314–1326. doi: 10.1016/j.neurobiolaging.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Griffin R. Nally R. Nolan Y. McCartney Y. Linden J. Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen J. Buchanan JB. Sparkman NL. Godbout JP. Freund GG. Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilger RN. Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparkman NL. Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry CJ. Huang Y. Wynne AM. Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham J. Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G. Lewis K. Pistell PJ. Poosala S. Becker KG. Boss O. Gwinn D. Wang M. Ramaswamy S. Fishbein KW. Spencer RG. Lakatta EG. Le Couteur D. Shaw RJ. Navas P. Puigserver P. Ingram DK. de Cabo R. Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kris-Etherton PM. Hecker KD. Bonanome A. Coval SM. Binkoski AE. Hilpert KF. Griel AE. Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 15.Athar M. Back JH. Tang X. Kim KH. Kopelovich L. Bickers DR. Kim AL. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar S. Singh G. Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 17.King RE. Kent KD. Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem-Biol Interact. 2005;151:143–149. doi: 10.1016/j.cbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim IJ. Beck HN. Lein PJ. Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J Neurosci. 2002;22:4530–4539. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S. Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 20.Rotondo S. Rajtar G. Manarini S. Celardo A. Rotillo D. de Gaetano G. Evangelista V. Cerletti C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang DS. Kang BS. Ryu SY. Chang IM. Min KR. Kim Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705–712. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- 22.Surh YJ. Hurh YJ. Kang JY. Lee E. Kong G. Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinez J. Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865–870. doi: 10.1016/s0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- 24.Moreno JJ. Resveratrol modulates arachidonic acid release, prostaglandin synthesis, and 3T6 fibroblast growth. J Pharmacol Exp Therapeut. 2000;294:333–338. [PubMed] [Google Scholar]
- 25.Manna SK. Mukhopadhyay A. Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 26.Estrov Z. Shishodia S. Faderl S. Harris D. Van Q. Kantarjian HM. Talpaz M. Aggarwal BB. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 27.Wang J. Ho L. Zhao Z. Seror I. Humala N. Dickstein DL. Thiyagarajan M. Percival SS. Talcott ST. Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 28.Jin F. Wu Q. Lu YF. Gong QH. Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur J Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Sonmez U. Sonmez A. Erbil G. Tekmen I. Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 30.Della-Morte D. Dave KR. DeFazio RA. Bao YC. Raval AP. Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmatz R. Mazzanti CM. Spanevello R. Stefanello N. Gutierres J. Correa M. da Rosa MM. Rubin MA. Chitolina Schetinger MR. Morsch VM. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;610:42–48. doi: 10.1016/j.ejphar.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Chen J. Zhou Y. Mueller-Steiner S. Chen LF. Kwon H. Yi S. Mucke L. Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 33.Meng XL. Yang JY. Chen GL. Wang LH. Zhang LJ. Wang S. Li J. Wu CF. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem-Biol Interact. 2008;174:51–59. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Bureau G. Longpre F. Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 35.Candelario-Jalil E. de Oliveira AC. Graf S. Bhatia HS. Hull M. Munoz E. Fiebich BL. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflamm. 2007;4:25. doi: 10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosczyk HA. Sparkman NL. Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krzyszton CP. Sparkman NL. Grant RW. Buchanan JB. Broussard SR. Woods J. Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1109–R1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparkman NL. Buchanan JB. Heyen JR. Chen J. Beverly JL. Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daynes RA. Araneo BA. Ershler WB. Maloney C. Li GZ. Ryu SY. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol. 1993;150:5219–5230. [PubMed] [Google Scholar]
- 40.Ershler WB. Sun WH. Binkley N. Gravenstein S. Volk MJ. Kamoske G. Klopp RG. Roecker EB. Daynes RA. Weindruch R. Interleukin-6 and aging: Blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 41.Ye SM. Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 42.Fagiolo U. Cossarizza A. Scala E. Fanales-Belasio E. Ortolani C. Cozzi E. Monti D. Franceschi C. Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 43.Ye SM. Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 44.Godbout JP. Moreau M. Lestage J. Chen J. Sparkman NL. OC J. Castanon N. Kelley KW. Dantzer R. Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wenzel E. Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutrit Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 46.Lambert JD. Sang S. Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharmaceut. 2007;4:819–825. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 47.Lefevre J. Michaud SE. Haddad P. Dussault S. Menard C. Groleau J. Turgeon J. Rivard A. Moderate consumption of red wine (cabernet sauvignon) improves ischemia-induced neovascularization in ApoE-deficient mice: Effect on endothelial progenitor cells and nitric oxide. FASEB J. 2007;21:3845–3852. doi: 10.1096/fj.06-7491com. [DOI] [PubMed] [Google Scholar]
- 48.Brown L. Kroon PA. Das DK. Das S. Tosaki A. Chan V. Singer MV. Feick P. The biological responses to resveratrol and other polyphenols from alcoholic beverages. Alcohol Clin Exp Res. 2009;33:1513–1523. doi: 10.1111/j.1530-0277.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asensi M. Medina I. Ortega A. Carretero J. Bano MC. Obrador E. Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q. Xu J. Rottinghaus GE. Simonyi A. Lubahn D. Sun GY. Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 51.Abd El-Mohsen M. Bayele H. Kuhnle G. Gibson G. Debnam E. Kaila Srai S. Rice-Evans C. Spencer JP. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- 52.Juan ME. Maijo M. Planas JM. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J Pharmaceut Biomed Anal. 2010;51:391–398. doi: 10.1016/j.jpba.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Holmes-McNary M. Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 54.Yeung F. Hoberg JE. Ramsey CS. Keller MD. Jones DR. Frye RA. Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howitz KT. Bitterman KJ. Cohen HY. Lamming DW. Lavu S. Wood JG. Zipkin RE. Chung P. Kisielewski A. Zhang LL. Scherer B. Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 56.Adler AS. Sinha S. Kawahara TL. Zhang JY. Segal E. Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adler AS. Kawahara TL. Segal E. Chang HY. Reversal of aging by NFkappaB blockade. Cell Cycle (Georgetown, TX) 2008;7:556–559. doi: 10.4161/cc.7.5.5490. [DOI] [PubMed] [Google Scholar]