Abstract
Background
Cholera toxin (CT) and toxin-co-regulated pili (TCP) are the major virulence factors of Vibrio cholerae O1 and O139 strains that contribute to the pathogenesis of disease during devastating cholera pandemics. However, CT and TCP negative V. cholerae strains are still able to cause severe diarrheal disease in humans through mechanisms that are not well understood.
Methodology/Principal Findings
To determine the role of other virulence factors in V. cholerae pathogenesis, we used a CT and TCP independent infection model in the nematode Caenorhabditis elegans and identified the hemolysin A (hlyA) gene as a factor responsible for animal death and developmental delay. We demonstrated a correlation between the severity of infection in the nematode and the level of hemolytic activity in the V. cholerae biotypes. At the cellular level, V. cholerae infection induces formation of vacuoles in the intestinal cells in a hlyA dependent manner, consistent with the previous in vitro observations.
Conclusions/Significance
Our data strongly suggest that HlyA is a virulence factor in C. elegans infection leading to lethality and developmental delay presumably through intestinal cytopathic changes.
Introduction
Understanding the nature of the biological determinants that underlie severe illness has been a longstanding goal of V. cholerae research. Cholera toxin (CT) and toxin-coregulated pili (TCP) are the major virulence determinants of V. cholerae O1 and O139 strains. However, other V. cholerae sero groups and vaccine strains that lack CT and TCP are also capable of causing diarrheal illness [1], [2], [3], [4]. In addition to these two virulence factors, other toxins such as hemolysin/cytolysin (VCC), zonula occludens toxin (Zot), and accessory cholera enterotoxin (Ace) have also been identified. Although cytopathic effects of these toxins have been well characterized in vitro, their role regarding the molecular mechanisms underlying the disease pathogenesis is not clear, and requires further research in animal models [5].
Pore-forming toxins (PFTs) are the most common class of toxins that are implicated in bacterial virulence [6]. VCC is an 80 kDa PFT that is expressed in most V. cholerae strains including O1 biotype El Tor, O139, and non-O1/non-O139 isolates. VCC peptides, encoded by the hlyA gene, assemble into heptameric channels following proteolytic activation by exogenous proteases [7], [8], [9]. The effects of VCC on eukaryotic hosts have been documented at both the cellular and organism level. In vitro, VCC is associated with cellular degenerative events such as autophagy, vacuolization, lysis, apoptosis, and necrosis [10], [11], [12], [13], [14], [15]. In infant mouse and rabbit ileal loop models, VCC was found to be responsible for the residual toxicity and diarrhea observed after the administration of vaccine strains into the gastrointestinal system [10].
Additionally, VCC seemed to be the major contributor to the lethality of streptomycin-fed adult mice after gastrointestinal exposure to high doses of El Tor strains [16].
The nematode Caenorhabditis elegans has been used as an invertebrate host to identify and assess virulence factors of several human pathogens including Pseudomonas aeruginosa, Salmonella Typhimurium and Yersinia pseudotuberculosis [17], [18], [19], [20], [21], [22], [23]. V. cholerae causes lethal infection in C. elegans via a CT and TCP independent process that provides an excellent model to investigate the roles of other V. cholerae virulence factors [23]. In this model, the nematode killing by V. cholerae has been associated with LuxO regulated genes in the quorum sensing (QS) pathway such as the transcriptional regulator hapR and hapR-regulated metalloprotease PrtV [23].
We used the CT negative vaccine strains CVD109 and CVD110 to examine the roles of additional V. cholerae virulence factors. CVD110 is derived from its parental strain CVD109 with an additional mutation in the hlyA locus [24]. In the C. elegans infection model, we observed a decrease in nematode killing after feeding CVD110, in comparison to CVD109, pointing to the deleterious effects of hlyA. This finding led to the identification of V. cholerae hemolysin/cytolysin as a virulence factor that contributes to the pathogenesis of C. elegans infection. Using the high throughput Complex Object Parametric Analyzer and Sorter (COPAS) assay and microscopy, we also found that the hlyA gene causes growth retardation in C. elegans. We determined the severity of lethal infection after feeding nematodes with wild type V. cholerae biotypes that have differences in hlyA gene structure and expression, and found a correlation between worm lethality and varying levels of bacterial hemolytic activity. Furthermore, we showed that the hlyA gene-encoded hemolysin/cytolysin is responsible for the formation of intestinal vacuoles in C. elegans during V. cholerae infection. Altogether, our findings provide an in vivo model for further research on the virulence mechanisms of V. cholerae hemolysin/VCC using C. elegans as a host organism.
Materials And Methods
Bacterial strains, plasmids, media and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. V. cholerae strains were cultured in tryptic soy broth (TSB, Becton Dickinson Microbiology Systems, BBL, Cockeysville, MD) media supplemented with 1% NaCl at 30°C. Escherichia coli OP50 was grown in LB culture medium.
Table 1. C. elegans and bacterial strains, and plasmids used in this study.
| Relevant genotype and/or phenotype | Source or reference | |
| C. elegans strains | ||
| N2 | Wild type Bristol isolate | Caenorhabditis Genetics Center |
| SS104 | glp-4 (bn2) | Caenorhabditis Genetics Center |
| Bacterial strains | ||
| OP50 | E. coli | Caenorhabditis Genetics Center |
| A1552 | V. cholerae Wild-type O1 El Tor, Ogawa | Fitnat Yildiz, UCSC |
| N16961 | V. cholerae Wild-type O1 El Tor, Ogawa | DVA* Strain collection |
| 569B | V. cholerae Wild-type O1 classical | DVA strain collection |
| VC395 | V. cholerae Wild-type O1 classical | DVA strain collection |
| E7946 | V. cholerae Wild-type O1 El Tor, Ogawa | DVA strain collection |
| CVD110 | Δ(ctxAB zot ace) hlyA::(ctxB mer) Hgr Parental strain: E7946 | James B. Kaper, University of Maryland, School of Medicine |
| CVD109 | Δ(ctxAB zot ace) Parental strain: E7946 | James B. Kaper, University of Maryland, School of Medicine |
| HNC44 | CVD110/pHNC44 | This study |
| HNC45 | E7946 ΔhlyA | This study |
| LS38 | S. aureus | DVA Strain collection |
| Plasmids | ||
| pHNC44 | pMMB66EH: hlyA | This study |
| pCWΔhlyA | ΔhlyA | K. Satchel, University of Illinois |
*Division of Virulence Assessment, CFSAN, FDA.
Cloning and complementation of V. cholerae hlyA gene
For complementation of the hlyA mutation in CVD110, a plasmid containing the entire hlyA gene was introduced into CVD110. For this procedure, a 2497 bp DNA segment containing the entire hlyA gene was amplified from E7946 by PCR using primers VchlySalIF 5′CAGTGTCGACTGACGAGGGTAACCCATGA and VchlyPstIR 5′CAGTCTGCAGTTTCAGGGCATGCTTCCA which were designed to contain SalI and PstI sites (underlined) for subsequent cloning. PCR was performed in 20 µl solutions containing 1 µl of bacterial cell lysate, primers (400 nM) and 17 µl (0.019 U/ml) Platinum Blue PCR Supermix (Invitrogen). PCR conditions for amplification were the following: 95°C for 5 minutes followed by 35 cycles of denaturation at 94°C for 30 seconds; annealing at 56°C for 30 seconds; and extension at 72°C for 60 seconds; with a final extension of 72°C for 7 minutes. The amplified product was cloned into a SalI, PstI digested pMMB66EH [25]. For complementation experiments the resulting plasmid pHNC44 was introduced into a spontaneous streptomycin resistant mutant of CVD110 (resulting in strain HNC44) using a conjugation method described by Datta et al. [26].
Construction of ΔhlyA of V. cholerae E7946
HNC45, the strain containing a deletion in hlyA locus, was generated via introduction of pCWΔhlyA [16] into a spontaneous streptomycin resistant mutant of E7946 using conjugation. Resulting mutants were analyzed by PCR to ensure the loss of the hlyA gene.
C. elegans strains and maintenance
C. elegans wild type strain N2 Bristol and SS104 glp-4(bn4) strain were obtained from the Caenorhabditis Genetic Center (CGC, Minneapolis, MN), and were maintained at 20°C and 16°C, respectively in C. elegans habitation media (CeHM) [27] in tissue culture flasks on a platform shaker.
Caenorhabditis elegans Lethality Assay
100 µl of overnight cultures of V. cholerae strains were seeded onto the center of the surface of a five cm NGM agar plates and incubated at room temperature (∼22°C) overnight prior to addition of about 50 L4 stage glp-4(bn2) worms onto the plates for each treatment (20 to 30 worms per plate). glp-4(bn2) temperature sensitive sterile mutants were used to prevent the worms from having progeny during the assay. For lethality assays L4 stage worms were shifted from 16°C to 25°C as soon as they were placed onto the plates. At least three replicates were made for each experimental condition. E. coli OP50 (C. elegans food strain) was used as a baseline control in each experiment. Plates were incubated at 25°C during the experiment and scored for live worms every 24–48 hours. We censored the missing worms from the analysis at the time of the event. 100 µg/ml ampicillin was added to NGM agar plates used for complementation experiments to maintain the plasmid during the survival assay. The Prism version 4.0 (GraphPad, San Diego, CA) was used to analyze and to plot the data according to a Kaplan-Meier method and survival curves were compared using the logrank test. Statistical significance was set at p-value <0.05.
Microscopy
Live nematodes were mounted on an agar pad on a slide and covered with a cover glass. Sodium azide was used to anesthetize the worms [28]. L1 stage worms were exposed to test bacteria on NGM agar plates for 48 hours at 20°C before examination. The different developmental stages of C. elegans are defined as L1, L2, L3, L4 and adult, and are described as follows [29]. L1 stage: Gonad consists of 4 to 12 cells. L2 stage: More than 12 cells present in the gonad, and vulva development has not started yet. L3 stage: Vulva development is in progress, gonad arms grow towards anterior and posterior ends of the worm. L4 stage: The vulva cells move together and create a channel like opening between uterine cavity and outside environment, gonad arms reflects to grow back towards the middle of the animal where vulva is located, and the somatic gonad is differentiated into the uterus, spermatica and oviduct. Adult stage: Gonad development is complete, oocytes and fertilized eggs are present. Intestinal tracts, somatic gonad and vulva were examined under Nomarski optics using a Zeiss AxioImager D1 microscope (Carl Zeiss MicroImaging, Inc, Thornwood, NY).
COPAS analysis
A COPAS biosorter [30] (Union Biometrica, MA) was used to assess worm growth. L1 stage animals were synchronized by treating gravid adults with hypochlorite and incubating released eggs in M9 buffer overnight [28]. Experiments were started with synchronized L1 stage animals. After exposure to test bacteria on the NGM agar plates for 72 hours, worms were washed out of the plates into 15 ml Falcon tubes with M9 buffer, washed twice in M9 buffer and sorted following calibration and sample analysis methods [31]. For each experimental condition, worm growth was assessed by measuring the parameter “Extinction” (EXT; represents the optical density of the worms, which measures the decrease in laser light intensity when an object passes through the laser beam). To evaluate the life stage composition of a worm culture, EXT values were plotted against the frequency of events by binning the readings in increments of 50. Sums of EXT values of 1000 worms for each condition were calculated as an index of population growth, and Student's t test was used to compare growth.
Hemolysin assay
CAMP test was performed using Staphylococcus aureus strain LS38 on five percent sheep RBC plates according to the procedure described by Christie et al [32]. Staphylococcus aureus strain LS38 was streaked in the middle of the agar plate in a straight line. The V. cholerae strain to be tested was streaked perpendicularly to the LS38 streak without allowing the two streaks to touch each other. The presence of enhanced hemolytic reaction was evaluated where the tested bacterial strains are in close proximity with Staphylococcus aureus strain LS38, which appears like a clear arrowhead.
Results
hlyA is required for lethality during V. cholerae infection in C. elegans
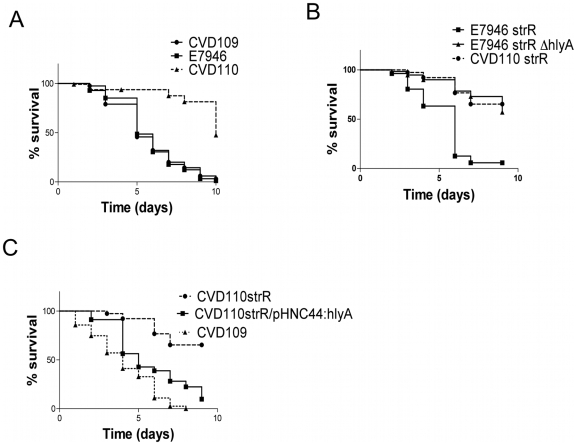
Worms fed with a wild type V. cholerae strain die faster than the ones fed with E. coli OP50, the standard nematode food, or UV-killed V. cholerae, and this lethality seems to be the result of a lethal infection that is independent of the major virulence factors, CT and TCP ([23] and our unpublished results). To determine the role of other virulence factors in nematode killing, we fed worms with V. cholerae vaccine strains that are deficient in several known virulence factors, and assayed lethality under these conditions. The vaccine strain CVD110 lacks the virulence genes zot, ace, ctxA, and hlyA [24], Table 1. The ctxB gene locus was deleted in CVD110 genome, but it was reinserted into the hlyA locus to inactivate hlyA gene and keep the immunogenicity elicited by CtxB protein. For full toxicity, CtxB requires the presence of CtxA, and since CVD110 does not have ctxA gene, the virulence mediated by cholera toxin is lacking in this strain [24]. We observed an attenuated killing response in CVD110 fed worms in comparison to the worms fed with the isogenic wild type V. cholerae strain E7946 (Fig. 1A), suggesting that one or more of these deficient factors might be responsible for increased lethality in C. elegans. When we fed worms with CVD110's immediate parental strain CVD109, which has an intact hlyA, we found that the presence of hlyA gene was sufficient to kill the worms at a rate comparable to that of observed for V. cholerae E7946 (Fig. 1A). To further evaluate the role of hlyA gene, we created the E7946-derived strain HNC45 that has a single locus deletion of hlyA gene. HNC45 fed worms showed decreased lethality similar to that of observed for CVD110 (Fig. 1B). When we reintroduced a functional copy of hlyA into CVD110 via conjugation of a plasmid expressing hlyA, the resulting strain showed lethality when fed to C. elegans (Fig. 1C) similar to that of the wild type strain E7946. Together, these findings indicated that V. cholerae hlyA is responsible for lethality during infection in C. elegans.
Figure 1. hlyA is required for killing during V. cholerae infection in C. elegans.
Lethality analysis was performed in glp-4(bn2) worms that were fed with indicated bacterial strains (Table 1). Agar plates were kept at 25°C and scored for survivors at 24–48 hour intervals. Data were plotted according to a Kaplan-Meier method and survival curves were compared using the logrank test. p<0.005. CVD110 and CVD109 represent the hlyA deficient and hlyA containing vaccine strains respectively, and they are isogenic with E7946. strR, streptomycin resistance; ΔhlyA, hlyA deletion; CVD110 strR/pHNC44:hlyA is the complementation strain. A) Comparison of lethality caused by vaccine strains CVD110 (hlyA-), CVD109 (hlyA+) and V. cholerae WT strain E7946. CVD110 exposed worms: median survival–10 days, CVD109 exposed worms: median survival–5 days, E7946 exposed worms: median survival-5 days. p<0.0001 for CVD110 versus CVD109 and for CVD110 versus E7946. p = 0.3455 for E7946 versus CVD109. B) Comparison of lethality caused by hlyA deletion mutant, WT strain E7946 and CVD110. p<0.0001 for E7946 strR versus E7946 strR ΔhlyA and for E7946 strR versus CVD110 strR. p = 0.1383 for E7946 strR ΔhlyA versus CVD110 strR. C) Comparison of lethality caused by CVD110, CVD110 with hlyA expressing plasmid and CVD109. p<0.0001 for each curve comparison in this graph.
V. cholerae infection causes developmental delay in C. elegans via hlyA
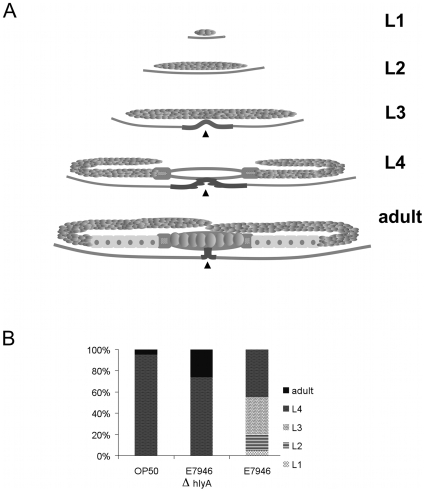
Because the worm lethality assay measures the longevity of non-reproducing worm populations and allows observations only for adult animals, we wanted to evaluate other possible outcomes in relation to C. elegans infection that could be attributed to hlyA. To characterize how the exposure to V. cholerae affects the life cycle of C. elegans, we fed strain E7946 to wild type growing worms and examined their development using two approaches. In the first assay, synchronized L1 stage worms were initiated on bacterial feeding, and were examined under Nomarski optics at 48 hours for subsequent attainment of larval stages. To identify developmental stages, we used the size and shape of the somatic gonad and vulva as developmental landmarks (Methods, and Fig. 2A). While all the worms that were fed with the control bacterium OP50 reached L4 stage and beyond at 48 hours, only 45% of the E7946 fed worms attained L4 stage during this time (Fig. 2B), suggesting that a developmental delay was induced by V. cholerae exposure. Using this assay, we evaluated the effects of hlyA on larval development and found that all L1 worms that were fed on the hlyA-deficient V. cholerae strain HNC45 reached L4 stage and beyond at 48 hours (Fig. 2B).
Figure 2. V. cholerae causes developmental delay in C. elegans via hlyA gene.
A) Illustrative pictures of gonad and vulva in each developmental stage. Black arrowhead indicates vulva. B) Synchronized L1 stage N2 worms (n = 50 per condition) were fed the indicated bacterial strains on agar plates for 48 hours at 20°C. The developmental stage of growing worms was determined under Nomarski optics using gonad development as milestones.
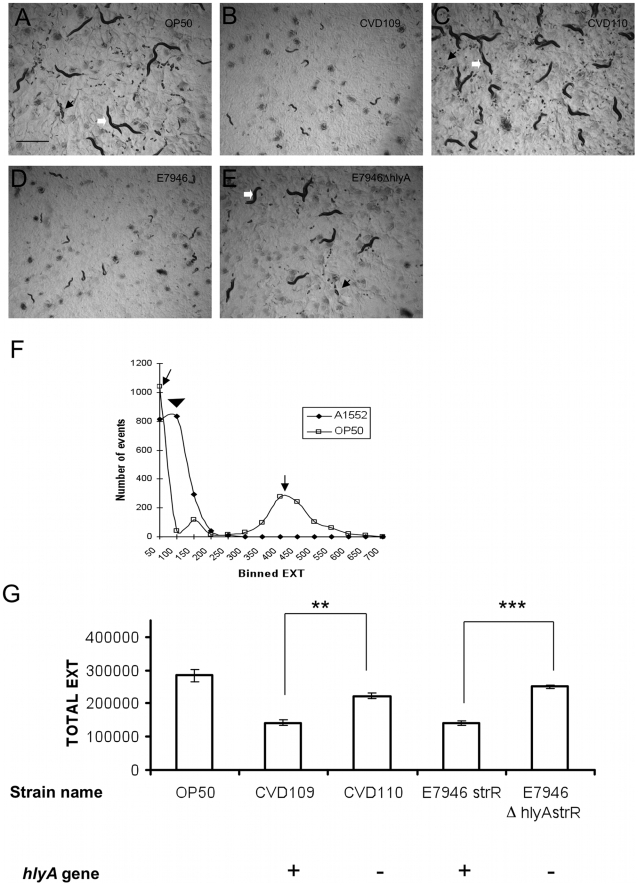
In an independent approach, the COPAS biosorter [30] was used to quantify the optical density distributions of growing worm populations to evaluate the composition of life stages in a given feeding condition. For this assay, synchronized L1 stage worms were fed on hlyA intact (E7946 and CVD109) and hlyA deficient (CVD110 and HNC45) strains of V. cholerae for 72 hours, and the optical density of the worms were evaluated by EXT measurements (Methods). A qualitative assessment of images taken on samples of worm cultures prior to sorting showed that the worms fed with hlyA deficient strains and with E. coli OP50 contained mostly adults and eggs. In contrast, worms fed with the hlyA intact strains contained smaller animals and no eggs (Fig. 3A–E). We observed distinct density distribution curves for E. coli OP50 and V. cholerae A1552 fed worms, which is consistent with the life stage composition of their respective worm cultures (Figure 3F). We compared the total optical density values that reflects the population growth (methods), and found that the worms exposed to hlyA(+) strains showed developmental delay while the worms exposed to hlyA(-) strains grew similar to the worms fed with the OP50 bacteria (Fig. 3G). Altogether, these results confirmed our findings in lethality assays, and further implicated a role for hlyA as a virulence factor that impairs nematode development.
Figure 3. V. cholerae causes growth retardation of worm cultures via hlyA gene.
Synchronized L1 stage N2 worms were fed on indicated bacterial strains on agar plates for 72 hours at 22°C. Worms washed into M9 buffer were sorted using COPAS (n = 1000). Images in A to E indicate the composition of worm cultures prior to sorting. Black arrows indicate eggs, white arrows indicate adult nematodes. (F) Curve representing the optical density (EXT) distribution of sorted worms was plotted. E. coli OP50 fed worm populations showed two separable peak domains (indicated with arrows), V. cholerae A1552 fed worms showed a single peak (indicated with an arrowhead) falling between E. coli OP50 induced peaks that is consistent with a population of worms mostly larger than eggs but fail to reach adult sizes EXT, extinction; strR, streptomycin resistance. (G) Population growth of C. elegans fed with hlyA deleted, hlyA intact V. cholerae strains and OP50. Student's t test was used to compare growth. ** denotes a statistical significance of P<0.001 according to Student's t test; *** P<0.0001. Total EXT represents the sum of EXT values for sorted worms per condition.
Varying levels of hemolytic activity in V. cholerae strains correlate with severity of the nematode infection
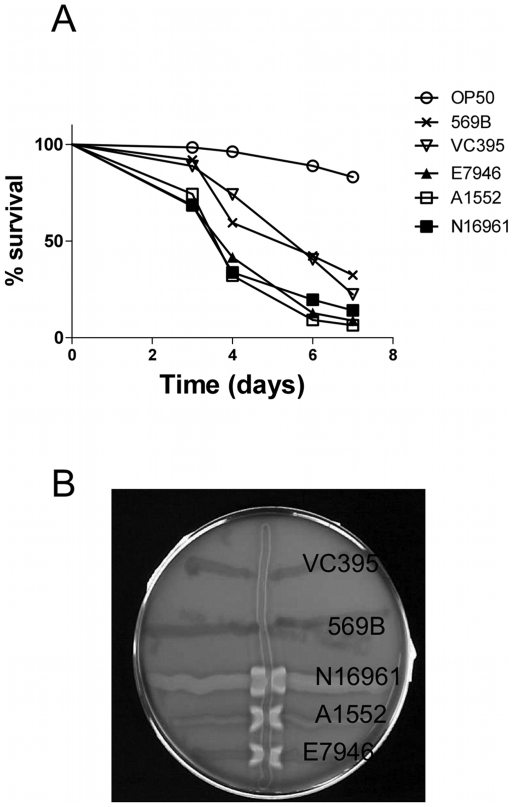
One of the major differences between the two biotypes of V. cholerae is that the Classical strains exhibit lower hemolytic activity than the El Tor strains [33]. A variation in hlyA expression presumably explains the decrease in hemolytic activity, because the levels of hlyA expression were found to be lower in Classical biotype than in El Tor biotype [34]. The classical strain 569B also contains a 11 bp deletion in the open reading frame of hlyA gene that results in a stop codon and a predicted 244 aa long truncated gene product [35]. Truncated HlyA peptide still has cytotoxic effects, but the molecular lesion lessens the severity of its cytotoxicity [10]. To compare the lethality caused by two biotypes of V. cholerae, Classical strains 569B and 395, and El Tor strains N16961, E7946, A1552 were fed to the worms. All the strains tested showed increased lethality in comparison to E. coli OP50 baseline, but the Classical strains caused a lower level of lethality than the El Tor strains (Fig. 4A). The results of the CAMP hemolytic assay revealed that while all the El Tor strains had identifiable hemolytic activity, the Classical strains showed no hemolytic activity under assay conditions (Methods; Fig. 4B). Assessing the modulation of the severity of infection by varying levels of hemolytic activity in wild type isolates of V. cholerae provided a further clue on how the activity of hlyA gene might be related to virulence mechanisms in the nematode infection. Altogether, our results derived from the interactions of different V. cholerae isolates with the nematode supported the notion that hlyA is a virulence factor in C. elegans infection.
Figure 4. Correlation between level of hemolytic activity and severity of lethality.
A) Lethality analysis as described in the legend of Fig. 1 was performed in glp-4(bn2) worms that were fed indicated bacterial strains (Table 1). O1 El Tor strains A1552, E7946 and N16961 induce higher lethality than O1 Classical strains 569B and VC395 with a statistical significance of p<0.01 according to logrank test. Curve comparisons within the classical (p = 0.5389) and El tor (p = 0.7224) strains were not statistically significant. B) Hemolytic activity of indicated bacterial strains was determined by the CAMP test (Methods).
hlyA expression contributes to formation of vacuoles in the intestine of C. elegans
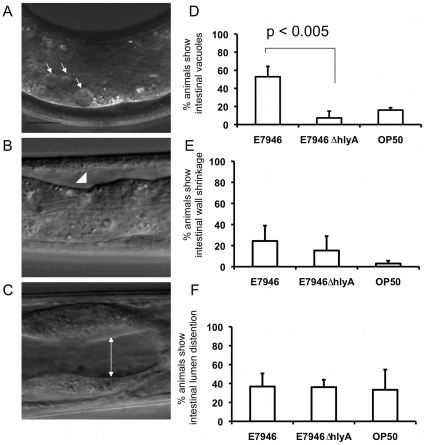
V. cholerae O1 El Tor strains cause lethality in C. elegans through intestinal colonization [23]. In addition, we observed tissue damage in the form of vacuole formation and intestinal wall shrinkage along the gut in worms feeding on wild type V. cholerae (Fig. 5A and 5B). Since it has been shown that VCC causes cellular vacuolation in cultured cells [12], [13], [36], we investigated the contribution of hlyA to intestinal lesions in C. elegans. L1 stage worms were fed with bacterial strains for 48 hours, and examined under Nomarski optics for the presence or absence of anatomical changes that indicate intestinal pathology including appearance of vacuoles, wall shrinkage, and lumen distention. We found that animals fed with the hlyA deficient strain HNC45 showed a lower degree of intestinal vacuolization in comparison to the hlyA intact E7946-fed worms (Fig. 5A and D), suggesting that the hlyA expression may contribute to the formation of intestinal vacuoles during V. cholerae infection. There were no statistically significant differences in other anatomical features such as intestinal wall shrinkage, and luminal distention between E7946 and HNC45 fed animals (Fig. 5B, C, E, and F). Together, these results indicate that hlyA has a specific role in eliciting intestinal vacuolation during V. cholerae infection in C. elegans that may represent a crucial step in pathogenesis leading to developmental delay and lethality.
Figure 5. hlyA expression is required for formation of vacuoles in the intestine.
glp-4(bn2) worms were fed wild type strain E7946 and hlyA deficient HNC45 for 48 hours at 20°C and examined under Nomarski optics. A) Intestinal vacuoles (as indicated by arrows) appear in the gut of V. chlorea fed nematodes. B) Arrowhead marks a region of intestinal wall shrinkage, and C) the extent of a distended lumen is indicated by the white line. Quantifications of these anatomical changes as represented by the percent of animals carrying these changes are shown in D) for intestinal vacuoles, in E) for intestinal wall shrinkage, and in F) for distended lumen. Statistical significance is derived according to Student's t test. n = 20.
Discussion
CT is a powerful toxin, and the severity of symptoms induced by it in human gastroenteritis limits our understanding of the role of accessory toxins in the disease process. Although several vaccine studies have uncovered possible roles for accessory toxins as virulence determinants in human diarrheal disease [4], [37], [38], [39], the contribution of these toxins to the pathogenesis of gastroenteritis is not well understood. Since the interaction of V. cholerae with various host organisms mimics only partial aspects of the clinical picture in humans, the development of additional host models to examine the mechanisms of virulence is essential. The exposition of the accessory toxin VCC as a virulence determinant via vaccine studies [37] was confirmed by findings in animal and cell culture models [7], [10], [16] and further elucidation of its role in the pathogenesis of V. cholerae infection requires the advance of novel animal models. Here, using a CT independent infection model in the nematode C. elegans, we demonstrated the hlyA gene as a virulence factor contributing to the pathogenesis of infection and lethality in this system. Our findings open the way to further research not only on the interaction of VCC with other proteins on the pathogen side, but also on its interactions and effects regarding host's innate immune system, developmental pathways, and target cells where main events leading to pathogenesis take place.
Previous studies have shown that hemolysins and other members of PFTs produced by several bacterial species induce lethality in C. elegans [18], [40], [41], [42]. The α-hemolysin of S. aureus and the hemolysin ShlA of S. marcescens are required for C. elegans killing [18], [40], [42]. In addition, B. thuringiensis Crystal (Cry) PFTs are toxic to C. elegans, producing vacuole-like structures, pitting, and constrictions in the intestinal tissue of the exposed worms [41]. Similarly, we observed tissue damage in the form of vacuoles and constrictions along the intestines of the nematodes that were feeding on wild type V. cholerae, and found that this vacuolization was modulated by hlyA expression. Our findings are consistent with the results of previous reports stating that VCC cause vacuolization in cultured cells [12], [13], [36], and extend those observations to an in vivo experimental setting.
Although the lethality assay is a widely used method in analyzing the host response of C. elegans to microorganisms, it measures the longevity of worm populations only in the adult stage. We extended our observations on the V. cholerae infection of adult worms to the larval stages of C. elegans to assess development. Using microscopy and COPAS to assess population characteristics of growing worms, we showed that V. cholerae infection impairs nematode development via hlyA gene. Further research is required to investigate whether developmental pathways are altered via VCC during V. cholerae infection in C. elegans.
What other genes in V. cholerae might be interacting with hlyA gene during pathogenesis of C. elegans infection? A candidate interactor would be PrtV, a protease regulated by the LuxO–HapR pathway in V. cholera that has been reported to be involved in C. elegans lethality [23]. PrtV and VCC may interact by an activating relationship because they both induce lethality in C. elegans. A prtV mutation in O1 El tor strain O17 does not seem to affect the hemolytic activity of this strain [43], but Ou et al. recently suggested that an 80 kDa protoxin form of VCC be a potential substrate for PrtV using a biochemical assay [44]. Further research is needed to address the interaction of VCC and PrtV in vivo.
What happens to cells during PFT exposure? Early effect of pore formation is increased permeabilization of the plasma membrane to ions, in particular calcium, possibly leading to osmotic stress [45]. However, recent studies revealed that cells might be actively responding to the attack of PFTs via major signaling pathways to alleviate cellular destruction [46], [47]. p38 mitogen activated protein kinase (p38 MAPK) pathway has been thought to be a major player in innate immunity, and it was found to be upregulated transcriptionally in response to B. thuringiensis Cry5B toxin in C. elegans [47]. The same pathway was also shown to be involved in protection of mammalian cells against PFT aerolysin [47]. Cells might deal with PFTs using pathways that regulate cell death. Caspase-1, a major player in apoptosis, appeared to promote cell survival by activating sterol regulatory element binding proteins (SREBPs) upon PFT exposure [46]. Our recent work revealed that hypoxic response pathway protects C. elegans against PFTs, including VCC [48]. Cultured cells were found to have an increased autophagic response to VCC, and it was thought that this mechanism was required to override cytotoxicity and prevent cell death [14], [49]. Besides enabling high throughput approaches and powerful genetic and genomic methods, further investigations in C. elegans -V. cholerae host pathogen model will be important not only in identifying host immune responses against VCC attack, but also in characterizing the changes underlying pathogenesis at subcellular resolution in a readily accessible way.
Acknowledgments
The authors would like to thank Jane Michalski and James B. Kaper of the Center for Vaccine Development, School of Medicine, University of Maryland for V. cholerae vaccine strains CVD110 and CVD109. The authors are also indebted to Karla J. Fullner Satchel for hlyA deletion clone, and Hulusi Cinar for critical reading of the manuscript. C. elegans strains used in this paper were provided by the Caenorhabditis Genetics Center. We are grateful to Nick Olejnick, Thomas Black, Oluwakemi Odusami, Mitzi Aguilar for technical help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by Food and Drug Administration intramural funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, et al. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci U S A. 2004;101:2123–2128. doi: 10.1073/pnas.0308485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MM, Kaper JB, Herrington D, Ketley J, Losonsky G, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 3.Ottaviani D, Leoni F, Rocchegiani E, Santarelli S, Masini L, et al. Prevalence and virulence properties of non-O1 non-O139 Vibrio cholerae strains from seafood and clinical samples collected in Italy. Int J Food Microbiol. 2009;132:47–53. doi: 10.1016/j.ijfoodmicro.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Tacket CO, Losonsky G, Nataro JP, Cryz SJ, Edelman R, et al. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a delta ctxA delta zot delta ace derivative of El Tor Ogawa Vibrio cholerae. J Infect Dis. 1993;168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 5.Fullner Satchell KJ. Toxins of Vibrio cholerae: Consensus and Controversy. In: Hecht IG, editor. Microbial Pathogenesis and the Intestinal Epithelial Cell. Washington, DC: ASM press; 2003. pp. 481–502. [Google Scholar]
- 6.Alouf J, Popoff M, editors. London: Academic Press; 2005. The Comprehensive Source Book of Bacterial Protein Toxins. [Google Scholar]
- 7.Ichinose Y, Yamamoto K, Nakasone N, Tanabe MJ, Takeda T, et al. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55:1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikigai H, Akatsuka A, Tsujiyama H, Nakae T, Shimamura T. Mechanism of membrane damage by El Tor hemolysin of Vibrio cholerae O1. Infect Immun. 1996;64:2968–2973. doi: 10.1128/iai.64.8.2968-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson R, Gouaux E. Crystal structure of the Vibrio cholerae cytolysin (VCC) pro-toxin and its assembly into a heptameric transmembrane pore. J Mol Biol. 2005;350:997–1016. doi: 10.1016/j.jmb.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Alm RA, Guerry P, Power ME, Lior H, Trust TJ. Analysis of the role of flagella in the heat-labile Lior serotyping scheme of thermophilic Campylobacters by mutant allele exchange. J Clin Microbiol. 1991;29:2438–2445. doi: 10.1128/jcm.29.11.2438-2445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho A, Andrade JR, Vicente AC, Dirita VJ. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect Immun. 2000;68:1700–1705. doi: 10.1128/iai.68.3.1700-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa-Arredondo P, Heuser JE, Akopyants NS, Morisaki JH, Giono-Cerezo S, et al. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect Immun. 2001;69:1613–1624. doi: 10.1128/IAI.69.3.1613-1624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra R, Figueroa P, Mukhopadhyay AK, Shimada T, Takeda Y, et al. Cell vacuolation, a manifestation of the El tor hemolysin of Vibrio cholerae. Infect Immun. 2000;68:1928–1933. doi: 10.1128/iai.68.4.1928-1933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saka HA, Gutierrez MG, Bocco JL, Colombo MI. The autophagic pathway: a cell survival strategy against the bacterial pore-forming toxin Vibrio cholerae cytolysin. Autophagy. 2007;3:363–365. doi: 10.4161/auto.4159. [DOI] [PubMed] [Google Scholar]
- 15.Zitzer A, Palmer M, Weller U, Wassenaar T, Biermann C, et al. Mode of primary binding to target membranes and pore formation induced by Vibrio cholerae cytolysin (hemolysin). Eur J Biochem. 1997;247:209–216. doi: 10.1111/j.1432-1033.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- 16.Olivier V, Haines GK, 3rd, Tan Y, Satchell KJ. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect Immun. 2007;75:5035–5042. doi: 10.1128/IAI.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aballay A, Ausubel FM. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr Opin Microbiol. 2002;5:97–101. doi: 10.1016/s1369-5274(02)00293-x. [DOI] [PubMed] [Google Scholar]
- 18.Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, et al. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 2003;22:1451–1460. doi: 10.1093/emboj/cdg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurz CL, Ewbank JJ. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 2000;8:142–144. doi: 10.1016/s0966-842x(99)01691-1. [DOI] [PubMed] [Google Scholar]
- 20.Kurz CL, Ewbank JJ. Infection in a dish: high-throughput analyses of bacterial pathogenesis. Curr Opin Microbiol. 2007;10:10–16. doi: 10.1016/j.mib.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurz CL, Ewbank JJ. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet. 2003;4:380–390. doi: 10.1038/nrg1067. [DOI] [PubMed] [Google Scholar]
- 23.Vaitkevicius K, Lindmark B, Ou G, Song T, Toma C, et al. A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc Natl Acad Sci U S A. 2006;103:9280–9285. doi: 10.1073/pnas.0601754103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalski J, Galen JE, Fasano A, Kaper JB. CVD110, an attenuated Vibrio cholerae O1 El Tor live oral vaccine strain. Infect Immun. 1993;61:4462–4468. doi: 10.1128/iai.61.10.4462-4468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, et al. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 26.Datta AR, Kaper JB, MacQuillan AM. Shuttle cloning vectors for the marine bacterium Vibrio parahaemolyticus. J Bacteriol. 1984;160:808–811. doi: 10.1128/jb.160.2.808-811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nass R, Hamza I. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and growth culture conditions and compound exposure parameters. Current Protocols in Toxicology. 2007. pp. 1–17. [DOI] [PubMed]
- 28.Sulston J, Hodgkin J. Wood WB, editor. Methods. 1988. The Nematode Caenorhabditis elegans New York: Cold Spring Harbor Laboratory Press.
- 29.Wood WB, editor. New York: Cold Spring Harbor Laboratory Press; 1988. The Nematode Caenorhabditis elegans. [Google Scholar]
- 30.Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol Biol. 2006;351:275–286. doi: 10.1385/1-59745-151-7:275. [DOI] [PubMed] [Google Scholar]
- 31.Sprando RL, Olejnik N, Cinar HN, Ferguson M. A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a Complex Object Parametric Analyzer and Sorter, and axenic liquid media. Food Chem Toxicol. 2009;47:722–728. doi: 10.1016/j.fct.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Christie R, Atkins NE, Munch-Peterson E. A note on a lytic phenomenon shown by group B streptococi. Aust J Exp Biol Med Sci. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- 33.Richardson K, Michalski J, Kaper JB. Hemolysin production and cloning of two hemolysin determinants from classical Vibrio cholerae. Infect Immun. 1986;54:415–420. doi: 10.1128/iai.54.2.415-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beyhan S, Tischler AD, Camilli A, Yildiz FH. Differences in gene expression between the classical and El Tor biotypes of Vibrio cholerae O1. Infect Immun. 2006;74:3633–3642. doi: 10.1128/IAI.01750-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rader AE, Murphy JR. Nucleotide sequences and comparison of the hemolysin determinants of Vibrio cholerae El Tor RV79(Hly+) and RV79(Hly-) and classical 569B(Hly-). Infect Immun. 1988;56:1414–1419. doi: 10.1128/iai.56.6.1414-1419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschioni M, Tombola F, de Bernard M, Coelho A, Zitzer A, et al. The Vibrio cholerae haemolysin anion channel is required for cell vacuolation and death. Cell Microbiol. 2002;4:397–409. doi: 10.1046/j.1462-5822.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- 37.Levine MM, Kaper JB, Herrington D, Losonsky G, Morris JG, et al. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket CO, Kotloff KL, Losonsky G, Nataro JP, Michalski J, et al. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am J Trop Med Hyg. 1997;56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 39.Tacket CO, Losonsky G, Nataro JP, Comstock L, Michalski J, et al. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 40.Bae T, Banger AK, Wallace A, Glass EM, Aslund F, et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marroquin LD, Elyassnia D, Griffitts JS, Feitelson JS, Aroian RV. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics. 2000;155:1693–1699. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sifri CD, Begun J, Ausubel FM, Calderwood SB. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect Immun. 2003;71:2208–2217. doi: 10.1128/IAI.71.4.2208-2217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogierman MA, Fallarino A, Riess T, Williams SG, Attridge SR, et al. Characterization of the Vibrio cholerae El Tor lipase operon lipAB and a protease gene downstream of the hly region. J Bacteriol. 1997;179:7072–7080. doi: 10.1128/jb.179.22.7072-7080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ou G, Rompikuntal PK, Bitar A, Lindmark B, Vaitkevicius K, et al. Vibrio cholerae cytolysin causes an inflammatory response in human intestinal epithelial cells that is modulated by the PrtV protease. PLoS One. 2009;4:e7806. doi: 10.1371/journal.pone.0007806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aroian R, van der Goot FG. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr Opin Microbiol. 2007;10:57–61. doi: 10.1016/j.mib.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 47.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, et al. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci U S A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellier A, Chen CS, Kao CY, Cinar HN, Aroian RV. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLoS Pathog. 2009;5:e1000689. doi: 10.1371/journal.ppat.1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutierrez MG, Saka HA, Chinen I, Zoppino FC, Yoshimori T, et al. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc Natl Acad Sci U S A. 2007;104:1829–1834. doi: 10.1073/pnas.0601437104. [DOI] [PMC free article] [PubMed] [Google Scholar]