Abstract
Objectives
Intravenous immunoglobulin (IVIG) has demonstrated improvement in chronic inflammatory demyelinating polyneuropathy (CIDP) patients in placebo controlled trials. However, IVIG is also much more expensive than alternative treatments such as corticosteroids. The objective of the paper is to evaluate, from a Canadian perspective, the cost-effectiveness of IVIG compared to corticosteroid treatment of CIDP.
Methods
A markov model was used to evaluate the costs and QALYs for IVIG and corticosteroids over 5 years of treatment for CIDP. Patients initially responding to IVIG could remain a responder or relapse every 12 week model cycle. Non-responding IVIG patients were assumed to be switched to corticosteroids. Patients on corticosteroids were at risk of a number of adverse events (fracture, diabetes, glaucoma, cataract, serious infection) in each cycle.
Results
Over the 5 year time horizon, the model estimated the incremental costs and QALYs of IVIG treatment compared to corticosteroid treatment to be $124,065 and 0.177 respectively. The incremental cost per QALY gained of IVIG was estimated to be $687,287. The cost per QALY of IVIG was sensitive to the assumptions regarding frequency and dosing of maintenance IVIG.
Conclusions
Based on common willingness to pay thresholds, IVIG would not be perceived as a cost effective treatment for CIDP.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired immune-mediated inflammatory disorder that targets the myelin sheaths that wrap the nerves of the peripheral nervous system. The motor weakness symptoms of CIDP resemble those of Guillain-Barre syndrome (GBS), and CIDP is sometimes considered to be a chronic counterpart of GBS[1]. The course of CIDP may be chronic progressive, stepwise, or monophasic. CIDP can occur at all ages and in both sexes, but is more common in older individuals and males. It is believed that the older age group is more likely to have a chronic progressive course of CIDP, and in younger patients, a relapse remitting course[2]. The prevalence rate of CIDP has been reported to be between 1.0 to 1.9 per 100,000 population[3,4].
CIDP has both motor and sensory symptoms, with motor being the predominant feature. There is symmetrical involvement of both arms and legs, including both proximal and distal muscles, resulting in global muscle weakness and a general reduction or absence of deep tendon reflexes[2]. Occasionally, muscle weakness becomes profound, and patients are unable to walk[5]. A prevalence study conducted by Lunn and colleagues[3] reported that 54% of patients had been severely disabled at some point in the past, and 13% were still severely disabled at the time of the prevalence assessment.
Patients with CIDP show improvement after treatment with corticosteroids and Plasma Exchange (PE),[6,7] but both treatments have disadvantages. Due to the chronic nature of the disease, long-term use of corticosteroids is usually required, and this carries the risk of numerous Adverse Events (AEs) and serious adverse events (SAEs)[8]. The benefit from PE is usually transient, therefore it is usually employed concomitantly with other therapy[7]. Also, PE must be carried out in specialized centres, and the repeated procedures require good vascular access[9].
In September 2008, the Food and Drug Adminsitration (FDA) granted Talecris Biotherapeutics supplemental licenses for their IVIG products to include CIDP as an indication[10]. The Health Products and Food Branch of Health Canada granted their approval for this indication in October 2008[11]. IVIG has demonstrated improvement in CIDP patients in placebo-controlled trials[9,12-14]. However, IVIG is also expensive. A recent report estimated the annual IVIG maintenance costs to be over $70,000 in Canada[15].
Canada has one of the highest per capita rates of consumption of IVIG in the world, and the consumption rate has been increasing annually over the past decade[16,17]. Escalating cost, increasing demand for an expanding number of indications, and a recent IVIG shortage has prompted Canada to adopt new approaches to manage and prioritize IVIG use. Assessing the impact of IVIG in patients with CIDP has been identified as a priority. This is because of its relatively high utilization rates in Canada, the potential availability of alternative treatments, and the uncertainty of a therapeutic advantage over alternative therapy.
The objective of this study is to evaluate the cost-utility of IVIG compared to corticosteroids for the treatment of CIDP in Canada.
Methods
Overview
A cost-utility analysis was conducted using a Markov model to compare IVIG to corticosteroids for the treatment of CIDP. The population entering the model are assumed to be 54 years of age and weighing 75 kg. These assumptions are based on the average age and weight of patients in the trial that compared IVIG and corticosteroid treatment in patients with CIDP[18]. The analysis is taken from the perspective of a Canadian publicly funded health care system. Although IVIG forms part of the budget for Canadian Blood Services (CBS), its costs are borne by Canadian public health care payers as part of their payments to CBS[19]. The effectiveness measure is quality adjusted life years (QALY). In the basecase analysis, the time horizon of the model is set to five years. Alternate time horizons are assumed in sensitivity analyses. Both costs and effects were discounted at a rate of 5% annually.
Model structure
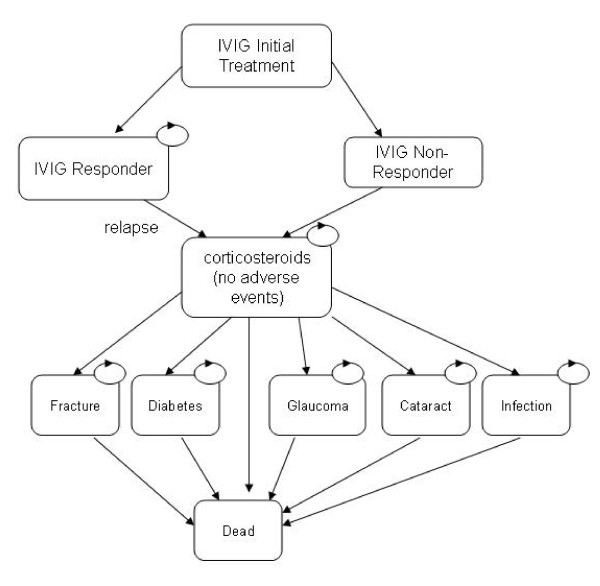
The structure of the model, including the transitions between health states, is presented in Figures 1 and 2. Figure 1 presents the model structure for the IVIG treatment strategy. Each box represents different health states in the model. Transitions between one health state to another are indicated by straight arrows in the figures. Circled arrows indicate patients can remain in a health state from one model cycle to the next. As shown, all patients enter the model in the IVIG initial treatment health state. Each model cycle represents 12 weeks of time. After this initial twelve week cycle, a proportion of patients are either IVIG responders or IVIG non-responders. Patients who respond to treatment are assumed to receive maintenance IVIG each twelve week cycle until they relapse, and therefore no longer respond to treatment. Once patients relapse, they are assumed to switch to corticosteroid treatment. Patients not responding to initial IVIG treatment are also assumed to switch to corticosteroid treatment.
Figure 1.
Structure of IVIG treatment arm of the model.
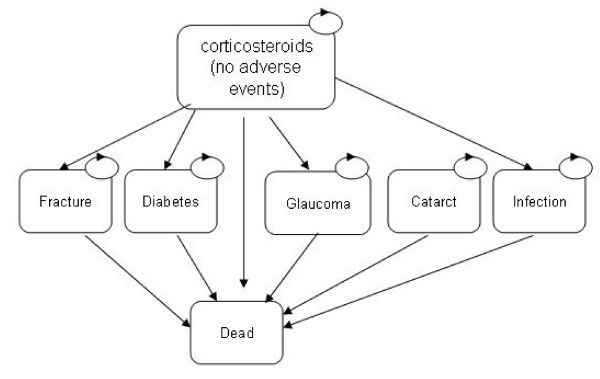
Figure 2.
Structure of corticosteroid treatment arm of the model.
Once patients start corticosteroid treatment, they are at risk of a number of AE's in each twelve week cycle. The AEs used in the model included fracture, diabetes, glaucoma, cataract and serious infection. Though this is not an exhaustive list of side-effects associated with steroid use, we evaluated these as they were incorporated into an economic evaluation of corticosteroids for the treatment of rheumatoid arthritis[20]. This study was used as the source for a number of AE related model inputs. Once patients have an AE, it is assumed that patients discontinue steroid treatment. It is assumed that once treatment is stopped, HbA1C (diabetes), and elevated intraocular pressure (glaucoma) return to normal. Furthermore, it is assumed that these conditions lasted for 1 year duration before discovery and steroid discontinuation. For each adverse event, patients are assigned an increased risk of mortality, increased costs, and a reduction in quality of life.
Figure 2, represents the model structure for the corticosteroid treatment strategy. As shown, it is similar to the structure of the IVIG arm, except no distinction is made between steroid responders and steroid non-responders. There are a number of reasons why no distinction is made. First, the one clinical trial comparing IVIG with corticosteroids in CIDP patients[18] did not report treatment response or relapse as an outcome. Second, because IVIG treatment is so much more expensive than corticosteroids, it is more important to distinguish the proportion of patients that respond and therefore incur maintenance treatment costs, compared to corticosteroids. Finally, the only study that compared utility values in IVIG and corticosteroid treated CIDP patients[21] did not report utility values by responder status.
Model Inputs
A number of different model input parameters were used to populate the model. These include: initial IVIG response rate; IVIG relapse rates; corticosteroid AE rates; mortality rates; IVIG treatment costs; corticosteroid treatment costs; AE related costs and finally, utility values associated with treatments and AEs. These are discussed below.
IVIG response and relapse rates
A literature review was conducted to identify randomized controlled trials that evaluated IVIG for CIDP patients. Six trials were identified that evaluated IVIG and reported response rates[9,12,14,22-25]. Response rates from the IVIG treatment arms of these studies were pooled using a random effects meta-analysis[26]. Table 1 presents details of the meta-analysis. As shown, the pooled IVIG response rate was estimated to be 0.473 (95% CI 0.361, 0.585). The IVIG relapse rate was based upon data from the ICE study[14]. This was the only study that reported relapse rates over a six month period. The 25 week relapse rate for IVIG in this study was estimated to be 13%. This is equivalent to a 12 week relapse rate of 6.5%. The cumulative relapse rate from the 25 week ICE study was extrapolated in the model by applying a constant relapse rate of 6.5% to patients in the IVIG responder health state in each cycle throughout the model time horizon.
Table 1.
IVIG response rate
Corticosteroid adverse event probabilities
The probabilities of corticosteroid related adverse events were taken from a published cost-effectiveness study comparing corticosteroids with Cox-2 inhibitors for the treatment of rheumatoid arthritis[20]. Bae et al.[20] used studies by McDougall et al.[27] as their source for fracture and cataract probabilities. Saag et al.[28] was used as their source for the probabilities of diabetes, glaucoma and serious infection. Table 2 presents the annual corticosteroid AE probabilities used in the model.
Table 2.
Corticosteroid adverse events
| Adverse event | Annual probability |
|---|---|
| Fracture | 0.0098 |
| Diabetes | 0.0043 |
| Cataract | 0.0114 |
| Glaucoma | 0.0008 |
| Serious Infection | 0.0035 |
Utilities
Background utility values for the model were based upon utilities from a U.K. general population[29]. Utility values by age and gender are presented in Table 3. Utility gains from IVIG treatment were added to the background utility values, while utility losses from corticosteroid related adverse events were subtracted from background utility values.
Table 3.
General population utility values
| Utility Values | ||
|---|---|---|
| Age | Females | Males |
| 35-44 | 0.91 | 0.91 |
| 45-54 | 0.85 | 0.84 |
| 55-64 | 0.81 | 0.78 |
| 65-74 | 0.78 | 0.78 |
| 75+ | 0.71 | 0.75 |
The incremental gain in utility from IVIG treatment compared to corticosteroid treatment was assumed to be 0.12. This was based on findings from McCrone et al.[21] who measured utility at baseline and at 6 weeks in CIDP patients treated with either IVIG or corticosteroids. This utility gain was added to the baseline utility values for all IVIG treated patients for the full duration of the first 12 week model cycle. This utility gain was also applied to patients for the full duration of each subsequent cycle where they remain IVIG responders.
The disutility due to fracture was estimated using an unpublished Canadian model evaluating treatments for corticosteroid induced osteoporosis. This unpublished model is a modification of an osteoporosis model published by Goeree et al.[30] The disutility associated with diabetes was estimated using the Ontario Diabetes Economic Model (ODEM)[31]. The ODEM was run for 30 years under different scenarios. First it was run assuming an elevated HbA1C for the first year. Second it was run assuming no elevated HbA1C for the first year. Disutilities were calculated as the difference in utilities predicted by ODEM under these 2 scenarios. Table 4 presents the disutilities associated with fracture and diabetes by first and subsequent years.
Table 4.
Disutility, incremental mortality, and costs for the first year and subsequent years after fracture and diabetes
| Fracture | Diabetes | |||
|---|---|---|---|---|
| Age | 1st year | Subsequent years | 1st year | Subsequent years |
| Disutility by age group | ||||
| 40-44 | -0.0833 | -0.0293 | -0.000179 | -0.000333 |
| 45-49 | -0.0971 | -0.0324 | -0.000173 | -0.000247 |
| 50-54 | -0.1047 | -0.0349 | -0.000160 | -0.000263 |
| 55-59 | -0.1068 | -0.0371 | -0.000074 | -0.000681 |
| 60-64 | -0.1094 | -0.0391 | -0.000040 | -0.000727 |
| 65-69 | -0.1113 | -0.0412 | -0.000003 | -0.000618 |
| 70+ | -0.1212 | -0.0425 | -0.000128 | -0.000754 |
| Incremental mortality by age group | ||||
| 40-44 | 0.0092 | 0.0001 | 0.000390 | 0.000320 |
| 45-49 | 0.0115 | 0.0001 | 0.000715 | 0.000285 |
| 50-54 | 0.0127 | 0.0001 | 0.000875 | 0.000685 |
| 55-59 | 0.0142 | 0.0002 | 0.001160 | 0.000320 |
| 60-64 | 0.0187 | 0.0003 | 0.001525 | 0.000755 |
| 65-69 | 0.0260 | 0.0006 | 0.002025 | 0.001035 |
| 70+ | 0.0541 | 0.0018 | 0.002645 | 0.000850 |
| Costs by age group | ||||
| 40-44 | $3,926 | $63 | $12 | $24 |
| 45-49 | $4,643 | $68 | $13 | $32 |
| 50-54 | $5,041 | $73 | $16 | $55 |
| 55-59 | $5,159 | $78 | $21 | $93 |
| 60-64 | $5,302 | $83 | $24 | $151 |
| 65-69 | $7,901 | $87 | $25 | $260 |
| 70+ | $10,880 | $744 | $27 | $341 |
The disutility associated with the development of cataracts in the model was assumed to be 0.38 while waiting for surgery and 0.10 after surgery[32]. These values were based on a cost-effectiveness study on reducing waiting times for cataract surgery in Ontario[32]. It was assumed that patients would have a 109 day wait for cataract surgery[32]. The disutility for glaucoma was assumed to be 0.061[33]. For serious infection a disutility of 1.0 for two weeks duration was assumed. This assumption was used in Bae et al.[20]
Mortality
Background mortality rates by age were based on the most recent Canadian life table data[34]. The average of male and female mortality rates were used in the model. The increased risk of death after fracture was derived from the same model which provided the utilities[30]. The increased risk of death from diabetes was estimated using the ODEM[31]. The increased risk of death after fracture and diabetes is presented in Table 4. The acute risk of death from serious infection was based upon data from a Canadian study on in-hospital mortality from community acquired pneumonia[35]. This study reported mortality rates of 0.018 and 0.111 for patients aged between 25-65 and those over 65 respectively. No increase in the probability of death was assumed for the other corticosteroid related adverse events.
IVIG costs
The initial and maintenance IVIG treatment cost estimates were based on the dose and frequency of IVIG administration and the cost per each IVIG administration. The dose and frequency of IVIG treatment assumed in the model was based upon the monograph of the product approved for CIDP treatment in Canada[36]. This includes an initial loading dose of 2 grams of IVIG per kg of body weight over two to four days along with maintenance dosing of 1 g/kg over one to two days every three weeks. This is the same dosing regimen used in the study used to estimate IVIG relapse rates[14]. For the purpose of the model, it is assumed that the initial treatment is given as two 1 g/kg doses, and that maintenance IVIG treatment is given as a single 1 g/kg dose every 3 weeks.
The cost per gram of IVIG ($59.19) was provided by Canadian Blood Services (personal communication). The cost per hour for a nurse ($32) was based on the Canadian Salary Survey[37]. Based on a 1 g/kg dose, a 75 kg patient and 3.5 hours of nurse supervision time, the total cost per IVIG administration is calculated as $4551.25. In the initial 12 week cycle patients are assumed to be given two 1 g/kg loading dose treatments of IVIG. They are also assumed to receive 1 g/kg maintenance doses at weeks 3, 6, 9 and 12, resulting in a total cost of $27,307.50 for the initial model cycle. In subsequent twelve week cycles, patients are assumed to have four 1 g/kg IVIG maintenance treatments, resulting in IVIG costs of $18,205. This cost is applied to patients who remain IVIG responders.
Corticosteroid Costs
The costs of corticosteroid treatment were based upon the reimbursement rate for a 50 mg pill ($0.0913) and a 5 mg pill ($0.022) of prednisone from the Ontario Drug Benefit formulary[38]. Patients on corticosteroids were assumed to take a bisphosphonate to help protect them from fracture. The cost of etidrocal ($19.99 per 400 mg/500 mg 90 tablet kit) was derived from the Ontario Drug Benefit formulary[38]. Based upon expert opinion, it was assumed that patients would be prescribed 60 mg per day of prednisone for the first 4 weeks of treatment. The dose would then be reduced by 10 mg per day in each of the next 20 weeks. After 24 weeks, the dose was assumed to be tapered down to 5 mg per day. While taking 60 mg of prednisone per day, patients were assumed to take one 50 mg pill and two 5 mg pills per day. While taking 50 mg of prednisone patients were assumed to take a single 50 mg pill. While taking less than 50 mg of prednisone per day, patients were assumed to take multiple 5 mg pills.
An 8%[39] pharmacy markup and a $7.00[39] dispensing fee were incorporated into the corticosteroid costs. Based upon the unit drug costs, pharmacy markup, pharmacy dispensing fee and the assumed treatment regimen, the cost for the first, 2nd and subsequent model cycles are $51.19, $43.57, and $39.87 respectively.
Cost of corticosteroid related adverse events
The cost of fracture was estimated using the same model that provided the utility and risk of death after fracture values[30]. The cost of diabetes was estimated using the ODEM[31]. The diabetes and fracture related costs used in the model for the first and subsequent years are provided in Table 4. The cost related to development of cataracts ($6,218) was taken from Hopkins et al.[32] and was primarily comprised of surgery costs. The costs related to development of glaucoma ($152) and serious infection ($24,334) were based on the estimates used by Bae et al.,[20] inflated to 2008 Canadian dollars. This conversion from U.S. to Canadian dollars was done using the December 2008 currency exchange rate[40]. Inflation from 1999 Canadian dollars to 2008 Canadian dollars was done using the health care component of the consumer price of the consumer price index[41].
Uncertainty
The variability of cost-effectiveness results according to patient characteristics was assessed using one-way sensitivity analysis. The model was run assuming different patient weights and starting ages. Because patient weight affects IVIG dosing, it also affects the costs. The structural uncertainty of the model was evaluated using one-way sensitivity analyses varying the discount rates and the model duration. The model was also evaluated using different assumptions about the utility gain from IVIG, the extrapolation of IVIG relapse rates, treatment switching for corticosteroid patients after suffering an AE, and on the dosing and frequency of maintenance IVIG treatment.
Parameter uncertainty was evaluated using probabilistic sensitivity analysis and expressed as cost-effectiveness acceptability curves (CEACs) based upon 1000 2nd order Monte Carlo simulations. Beta distributions were used for parameters whose values are constrained between zero and one. These include probability parameters, baseline utility variables and utility weight parameters. Gamma distributions were used for corticosteroid adverse event cost parameters as the values of the cost parameters need to be non-negative. No distributions were applied to the unit costs of IVIG or corticosteroids. Normal distributions were applied to the incremtal utility from IVIG response, along with disutility from corticosteroid AEs, an incremental mortality from adverse events. Selected distributions and parameters values that were used in the probabilistic sensitivity analysis appear in Table 5.
Table 5.
Probabilsitic parameters
| Variable | Mean Value | Distribution (parameters) | 95% C.I. based on parameters |
|---|---|---|---|
| IVIG Response Rate | 0.473 | beta (α = 35.52,β = 39.61) | (0.361, 0.585) |
| IVIG relapse rate (25 weeks) | 0.13 | beta (α = 4.03, β = 26.97) | (0.039, 0.266) |
| IVIG incremental utility | 0.12 | normal (μ = 0.12,β = 0.08) | (-0.05, 0.29) |
| Corticosteroid adverse events annual probailites | |||
| Fracture | 0.0098 | beta (α = 1.20,β = 120.8) | (0.000,0.033) |
| Diabetes mellitus | 0.0043 | beta (α = 0.48,β = 111.52) | (0.000,0.022) |
| Cataract | 0.0114 | beta (α = 1.39,β = 120.61) | (0.001,0.036) |
| Glaucoma | 0.0008 | beta (α = 0.09,β = 111.91) | (0.000,0.008) |
| Serious Infection | 0.0035 | beta (α = 0.39,β = 120.8) | (0.000,0.020) |
| Glaucoma utility weight | 0.96 | beta (α = 214.32, β = 13.68) | (0.906,0.967) |
| Cataract utility weight-before surgery | 0.62 | beta (α = 62, β = 38) | (0.586,0.767) |
| Cataract utility weight post-surgery | 0.90 | beta (α = 90, β = 10) | (0.835,0.951) |
| Glaucoma utility weight | 0.96 | beta (α = 214.32, β = 13.68) | (0.906,0.967) |
Results
Basecase
Table 6 presents the basecase cost-effectiveness results. As shown, the total cost for the IVIG treatment arm over the 5 year duration of the model is estimated to be $124,065. This compares with $2,196 for the corticosteroid treatment arm, resulting in an incremental cost of IVIG compared to corticosteroids of $121,869. Over 5 years, IVIG was estimated to have 3.962 QALYs compared to 3.785 for corticosteroids. The resulting incremental cost-utility ratio of IVIG compared to corticosteroids is $687,287 per QALY gained. Based on these results if society is willing to pay $687,287 or more for a QALY, IVIG would be considered the cost-effective treatment. If societal willingness to pay for a QALY is less than $687,287, corticosteroids would be considered the cost-effective strategy.
Table 6.
Basecase Results
| Treatment | Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR |
|---|---|---|---|---|---|
| corticosteroids | $2,196 | 3.785 | ref | ref | |
| IVIG | $124,065 | 3.962 | $121,869 | 0.177 | $687,287 |
Uncertainty
One way sensitivity analyses were conducted on a number of patient characteristics (age and weight) and various model assumptions. The results of sensitivity analysis are presented in Table 7. As shown, the cost per QALY of IVIG compared to corticosteroids for patients weighing 35 kg is $327,665, while the cost per QALY of IVIG for patients weighing 95 kg is $867,090. The incremental cost per QALY for patients with a starting age of 35 years is $686,130, while the cost per QALY for patients with a starting age of 75 years is $683,219 per QALY. Using different discount rates or model time horizons had little impact on the cost-utility estimates. Assuming a larger incremental utility impact of IVIG does impact the results. If a 0.25 utility gain is assumed, the cost per QALY becomes $335,038. If patients in the corticosteroid arm are assumed to switch to IVIG after an adverse event the cost per QALY of IVIG becomes $682,309. If no extrapolation of the IVIG relapse beyond 25 weeks is applied to the model, the cost per QALY of IVIG becomes $672,616. In the basecase analysis, it was assumed that maintenance IVIG was given in 1 mg/kg doses, once every 3 weeks. If it is assumed that maintenance IVIG is 1 mg/kg once every 8 weeks, the cost per QALY of IVIG becomes $288,535. If it is assumed that maintenance IVIG is 0.4 mg/kg once every 8 weeks, the cost per QALY of IVIG becomes $148,518.
Table 7.
Sensitivity analysis
| Parameter varied | Incremental Costs (IVIG-corticosteroids) | Incremental QALYs (IVIG-corticosteroids | $/QALY IVIG vs. corticosteroids |
|---|---|---|---|
| Patient Weight | |||
| 35 kg | $58,102 | 0.177 | $327,665 |
| 45 kg | $74,043 | 0.177 | $417,569 |
| 55 kg | $89,985 | 0.177 | $507,474 |
| 65 kg | $105,927 | 0.177 | $597,378 |
| 75 kg | $121,869 | 0.177 | $687,282 |
| 85 kg | $137,810 | 0.177 | $777,186 |
| 95 kg | $153,752 | 0.177 | $867,090 |
| Starting Age | |||
| 35 years old | $122,293 | 0.178 | $686,130 |
| 45 years old | $122,077 | 0.178 | $686,759 |
| 55 years old | $121,504 | 0.177 | $687,371 |
| 65 years old | $119,999 | 0.176 | $683,643 |
| 75 years old | $116,182 | 0.170 | $683,219 |
| Discount rate | |||
| 0% | $126,340 | 0.185 | $682,390 |
| 3% | $123,542 | 0.180 | $685,346 |
| Model time horizon | |||
| 1 year | $59,546 | 0.078 | $764,917 |
| 3 years | $102,457 | 0.146 | $702,569 |
| 5 years | $121,869 | 0.177 | $687,282 |
| 10 years | $139,986 | 0.209 | $670,396 |
| 20 years | $143,690 | 0.218 | $658,267 |
| Incremental IVIG utility gain = 0.25 | $121,869 | 0.364 | $335,038 |
| Assume corticosteroid patients switch to IVIG after adverse event | $113,444 | 0.166 | $682,309 |
| Relapse rate for IVIG not extrapolated beyond 25 weeks | $173,189 | 0.257 | $672,616 |
| Maintenance IVIG dose and frequency | |||
| 1.0 mg/kg every 3 weeks | $121,869 | 0.177 | $687,282 |
| 1.0 mg/kg every 6 weeks | $65,304 | 0.177 | $368,284 |
| 1.0 mg/kg every 8 weeks | $51,163 | 0.177 | $288,535 |
| 0.4 mg/kg every 3 weeks | $55,661 | 0.177 | $313,905 |
| 0.4 mg/kg every 6 weeks | $32,200 | 0.177 | $181,595 |
| 0.4 mg/kg every 8 weeks | $26,335 | 0.177 | $148,518 |
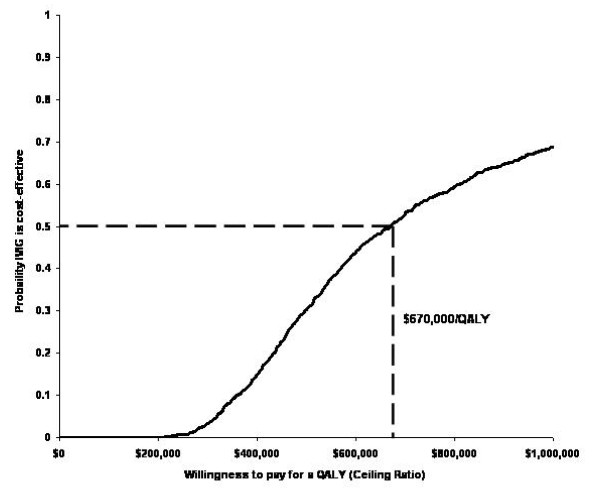
Figure 3 presents the cost-effectiveness acceptability curve for the IVIG treatment arm using the basecase model assumptions. As shown, at a willingness to pay for QALY threshold of $670,000, the probability that IVIG is cost effective is 50%. At the commonly quoted willingness to pay threshold of $50,000 per QALY, the probability that IVIG is cost-effective is less than 1%.
Figure 3.
Cost-effectiveness acceptability curve.
Discussion
In this cost-utility analysis in patients with CIDP, the incremental cost of IVIG treatment compared to corticosteroid treatment was estimated to incur $124,065 more costs and result in 0.177 more QALYs compared to the corticosteroid treatment arm over 5 years. The resulting incremental cost-utility ratio of IVIG compared to corticosteroids is $687,287 per QALY gained. The ICUR varied from $327,665 to $867,090 when patient weight was decreased to 35 kg and increased to 95 kg, respectively. Assuming that maintenance treatment with IVIG consists of 0.4 mg/kg doses every 8 weeks instead of 1.0 mg/kg doses every three weeks resulted in a cost per QALY estimate $148,518. Probabilistic sensitivity analysis found that at a willingness to pay for a QALY threshold of $670,000, the probability that IVIG is cost-effective is 50%. Our results are consistent with those from the only other economic evaluation we identified that compared IVIG with corticosteroids for CIDP treatment[21]. In this 6 week trial based economic evaluation, the authors reported that at a willingness to pay threshold of £250,000, there was a 50% chance that IVIG was cost-effective compared to corticosteroids.
The economic analysis has a number of limitations. As is the case for all models, our analysis had to make a number of assumptions. This includes the extrapolation of the non-statistically significant 0.12 (p = 0.07) utility gain from IVIG found by McCrone et al.[21] over the five year time horizon of the model. Another limitation is the reliance on this single source of utility gain from IVIG treatment[21]. Another limitation is that the reliance on a single source[20] to define the corticosteroid related adverse events used in the model. Because a public health care payer perspective was taken, indirect costs were not included. If a societal perspective was taken and indirect costs taken into consideration, the cost-utility of IVIG compared to corticosteroids may have been more favourable.
Despite the high costs, IVIG remains a popular treatment in Canada. This is likely due to its potential for better patient outcomes compared to other treatments. Another possible reason is that IVIG funding comes directly from jurisdictional health budgets and do not comprise part of individual hospital budgets.
Conclusions
IVIG is much more expensive compared to corticosteroids for the treatment of CIDP. Our model estimates the incremental cost per QALY of IVIG compared to corticosteroids to be $687,287. Based on commonly quoted willingness to pay thresholds, IVIG treatment for CIDP is unlikely to be considered a cost-effective use of health care resources. Results varied according to the frequency and dose of IVIG administration.
Competing interests
CC received funding from Talecris Biotherapeutics Ltd. and is the primary investigator in a multi-centre study funded by Baxter Canada. No payments were received by him or by patients who were enrolled in the study at the time.
Authors' contributions
GB conceptualized the economic analysis, and was primarily responsible for the data analysis and write up of the study. KG was responsible for conducting the review of the clinical literature review that was used to estimate efficacy variables for the economic analysis. FX helped develop the economic model and assisted with the writing of the manuscript. KC was the information specialist for the manuscript. NA assisted with the overall design of the study and preparation of the manuscript. JET assisted with the preparation of the manuscript. DOR assisted with the preparation of the manuscript. CC provided clinical expertise in the development of the economic model, and assisted with the preparation of the manuscript. ML provided clinical expertise in the development of the economic model and helped write the manuscript. RG assisted with the overall design of the study and preparation of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Gord Blackhouse, Email: blackhou@mcmaster.ca.
Kathryn Gaebel, Email: gaebk@mcmaster.ca.
Feng Xie, Email: fengxie@mcmaster.ca.
Kaitryn Campbell, Email: kcampbe@mcmaster.ca.
Nazila Assasi, Email: assasin@mcmaster.ca.
Jean-Eric Tarride, Email: tarride@mcmaster.ca.
Daria O'Reilly, Email: oreilld@mcmaster.ca.
Colin Chalk, Email: colin.chalk@mcgill.ca.
Mitchell Levine, Email: levinem@mcmaster.ca.
Ron Goeree, Email: goereer@mcmaster.ca.
Acknowledgements
Funding for this project was provided by the Canadian Agency for Drugs and Technologies in Health.
References
- van Schaik I, Van den Bergh P, de Haan R, Vermeulen M. Intravenous immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD004429.pub2. [DOI] [PubMed] [Google Scholar]
- Lewis RA. Chronic inflammatory demyelinating polyneuropathy. Neurol Clin. 2007;25:71–87. doi: 10.1016/j.ncl.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Lunn MP, Manji H, Choudhary PP, Hughes RA, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. J Neurol Neurosurg Psychiatry. 1999;66:677–680. doi: 10.1136/jnnp.66.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol. 1999;46:910–913. doi: 10.1002/1531-8249(199912)46:6<910::AID-ANA14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Guillain-Barré Syndrome Support Group. GBS CIDP guide. Sleaford (UK): The Group; 2005. [Google Scholar]
- Dyck PJ, O'Brien PC, Oviatt KF, Dinapoli RP, Daube JR, Bartleson JD. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol. 1982;11:136–141. doi: 10.1002/ana.410110205. [DOI] [PubMed] [Google Scholar]
- Merkies I, Bril V, Dalakas M, Deng C, Donofrio P, Hanna K. 133rd Annual Meeting of the American Neurological Association. Salt Lake City; 2008. Quality-of-Life (QoL) Improvements with Immune Globulin Intravenous, 10% Caprylate/Chromatography Purified (IGIV-C) in Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) [abstract M-3] [Google Scholar]
- Dyck PJ. Intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy and in neuropathy associated with IgM monoclonal gammopathy of unknown significance. Neurology. 1990;40:327–328. doi: 10.1212/wnl.40.2.327. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain. 1996;119:1067–1077. doi: 10.1093/brain/119.4.1067. [DOI] [PubMed] [Google Scholar]
- Gamunex [FDA product approval information] Rockville (MD): Center for Biologics Evaluation and Research, U.S. Food and Drug Administration; 2008. [Google Scholar]
- Notices of compliance. Ottawa: Health Canada; 2008. Gamunex. [Google Scholar]
- Thompson N, Choudhary P, Hughes RA, Quinlivan RM. A novel trial design to study the effect of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 1996;243:280–285. doi: 10.1007/BF00868527. [DOI] [PubMed] [Google Scholar]
- van Doorn PA, Brand A, Strengers PF, Meulstee J, Vermeulen M. High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study. Neurology. 1990;40:209–212. doi: 10.1212/wnl.40.2.209. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- Gaebel K, Blackhouse G, Campbell K, Robertson D, Xie F, Assasi N. Intravenous immunoglobulin for chronic inflammatory demyelinating polyneuropathy: clinical and cost-effectivelness analyses. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- IVIG usage in Canada. Ottawa: Canadian Blood Services; 2005. [Google Scholar]
- IVIG usage in Canada. Ottawa: Canadian Blood Services; 2004. [Google Scholar]
- Hughes R, Bensa S, Willison H, Van den BP, Comi G, Illa I. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50:195–201. doi: 10.1002/ana.1088. [DOI] [PubMed] [Google Scholar]
- Anderson D, Ali K, Blanchette V, Brouwers M, Couban S, Radmoor P. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21:S9–56. doi: 10.1016/j.tmrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Bae SC, Corzillius M, Kuntz KM, Liang MH. Cost-effectiveness of low dose corticosteroids versus non-steroidal anti-inflammatory drugs and COX-2 specific inhibitors in the long-term treatment of rheumatoid arthritis. Rheumatology (Oxford) 2003;42:46–53. doi: 10.1093/rheumatology/keg029. [DOI] [PubMed] [Google Scholar]
- McCrone P, Chisholm D, Knapp M, Hughes R, Comi G, Dalakas MC. Cost-utility analysis of intravenous immunoglobulin and prednisolone for chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2003;10:687–694. doi: 10.1046/j.1351-5101.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- Zinman LH, Sutton D, Ng E, Nwe P, Ngo M, Bril V. A pilot study to compare the use of the Excorim staphylococcal protein immunoadsorption system and IVIG in chronic inflammatory demyelinating polyneuropathy. Transfus Apheresis Sci. 2005;33:317–324. doi: 10.1016/j.transci.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Barohn RJ, Freimer ML, Kissel JT, King W, Nagaraja HN. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56:445–449. doi: 10.1212/wnl.56.4.445. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Litchy WJ, Kratz KM, Suarez GA, Low PA, Pineda AA. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 1994;36:838–845. doi: 10.1002/ana.410360607. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, van Doorn PA, Brand A, Strengers PF, Jennekens FG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: a double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 1993;56:36–39. doi: 10.1136/jnnp.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- McDougall R, Sibley J, Haga M, Russell A. Outcome in patients with rheumatoid arthritis receiving prednisone compared to matched controls. J Rheumatol. 1994;21:1207–1213. [PubMed] [Google Scholar]
- Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96:115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Center for Health Economics, University of York; 1999. [Google Scholar]
- Goeree R, Blackhouse G, Adachi J. Cost-effectiveness of alternative treatments for women with osteoporosis in Canada. Curr Med Res Opin. 2006;22:1425–1436. doi: 10.1185/030079906X115568. [DOI] [PubMed] [Google Scholar]
- O'Reilly D, Hopkins RB, Blackhouse G, Clarke P, Hux J, Guan J. Development of an Ontario diabetes economic model (ODEM) and application to a multidisciplinary primary care diabetes management program. Hamilton (ON): Program for Assessment of Technology in Health, McMaster University/St. Joseph's Healthcare Hamilton; 2006. [Google Scholar]
- Hopkins RB, Tarride JE, Bowen J, Blackhouse G, O'Reilly D, Campbell K. Cost-effectiveness of reducing wait times for cataract surgery in Ontario. Can J Ophthalmol. 2008;43:213–217. doi: 10.3129/I08-002. [DOI] [PubMed] [Google Scholar]
- Jampel HD. Glaucoma patients' assessment of their visual function and quality of life. Trans Am Ophthalmol Soc. 2001;99:301–317. [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. Life tables, Canada, provinces and territories, 2000-2002. Ottawa: Statistics Canada; 2006. [Google Scholar]
- Talecris Biotherapeutics. FDA Grants Priority Review of Gamunex® as a Treatment for Neurological Disorder CIDP. 2008.
- Talecris Biotherapeutics. Gamunex: Immune Globulin Intravenous (Human),10%. Product Monograph. Mississauga (ON): Talecris; 2008. [Google Scholar]
- Service Canada. Labour Market Information: Wages and Salaries. Ottawa: Public Works and Government Services Canada; 2008. [Google Scholar]
- Ontario Ministry of Health and Long-Term Care. e-Formulary. Ontario drug benefit formulary/comparative drug index: electronic version. 1.4. Toronto: Queen's Printer for Ontario; 2007. [Google Scholar]
- Ontario Ministry of Health and Long-Term Care. Notice to pharmacies: regulation changes made to the Ontario drug benefit act (ODBA) and the drug interchangeability and dispensing fee act (DIDFA) Toronto: Queen's Printer for Ontario; 2009. [Google Scholar]
- Bank of Canada. Rates and Statistics. Ottawa: Bank of Canada; 2008. [Google Scholar]
- Statistics Canada. Table 326-0020 - Consumer price index (CPI), 2005 basket, monthly (2002 = 100) Ottawa: Statistics Canada; 2008. [Google Scholar]