Abstract
Health-related quality of life (HRQOL) is an important measure of the effects of chronic liver disease in affected patients that helps guide interventions to improve well-being. However, the relationship between HRQOL and survival in liver transplant candidates remains unclear. We examined whether the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores from the Short Form 36 (SF-36) Health Survey were associated with survival in liver transplant candidates. We administered the SF-36 questionnaire (version 2.0) to patients in the Pulmonary Vascular Complications of Liver Disease study, a multicenter prospective cohort of patients evaluated for liver transplantation in 7 academic centers in the United States between 2003 and 2006. Cox proportional hazards models were used with death as the primary outcome and adjustment for liver transplantation as a time-varying covariate. The mean age of the 252 participants was 54 ± 10 years, 64% were male, and 94% were white. During the 422 person years of follow-up, 147 patients (58%) were listed, 75 patients (30%) underwent transplantation, 49 patients (19%) died, and 3 patients were lost to follow-up. Lower baseline PCS scores were associated with an increased mortality rate despite adjustments for age, gender, Model for End-Stage Liver Disease score, and liver transplantation (P for the trend = 0.0001). The MCS score was not associated with mortality (P for the trend = 0.53). In conclusion, PCS significantly predicts survival in liver transplant candidates, and interventions directed toward improving the physical status may be helpful in improving outcomes in liver transplant candidates.
Cirrhosis and its complications create a substantial healthcare burden in the United States and have a negative impact on health-related quality of life (HRQOL) in affected patients.1,2 Liver transplantation is a life-saving treatment for cirrhosis that improves HRQOL.3,4 The current United Network of Organ Sharing policy for liver allocation is based on the Model for End-Stage Liver Disease (MELD) score,5 which has been found to be a robust predictor of survival in this patient population.6 However, even with the implementation of this policy, the waitlist for liver transplantation is long, and the HRQOL of patients on the waitlist is severely impaired.
Several generic and liver disease–specific HRQOL instruments have been developed and validated to assess HRQOL in patients awaiting liver transplantation.7 The HRQOL instruments assess physical, mental, functional, psychosocial, and certain liver disease–specific domains of quality of life. However, the association between these domains of HRQOL and mortality in liver transplant candidates remains uncertain. In a recent study, the liver-specific scales but not the generic Short Form 36 (SF-36) scales of the Liver Disease Quality of Life instrument8 were associated with mortality in patients with advanced liver disease.9 The interpretation of these findings is complicated by the small sample size, the availability of data from a single liver transplant center, and insufficient adjustment for liver transplantation. The SF-36 is a widely used instrument that captures the physical and emotional domains of HRQOL in decompensated cirrhosis.10,11 The Physical Component Summary (PCS) and Mental Component Summary (MCS) scales are independent predictors of mortality in other chronic diseases.12,13 Furthermore, the PCS scale has a moderate correlation with the MELD score.14,15
Hence, we sought to determine if the physical and emotional domains of HRQOL, as measured by the SF-36, were associated with survival in a large multi-center cohort of well-characterized patients with cirrhosis evaluated for liver transplantation.
PATIENTS AND METHODS
Study Design and Study Sample
The Pulmonary Vascular Complications of Liver Disease study enrolled a cohort of 536 consecutive out-patients evaluated for liver transplantation at 7 centers in the United States between 2003 and 2006. The only inclusion criterion was the presence of portal hypertension with or without intrinsic liver disease. We excluded patients with active infections, patients with recent (<2 weeks) gastrointestinal bleeding, and patients who had undergone previous liver or lung transplantation. For this study, we also excluded patients who were not initially presenting for liver transplant evaluation and those who did not complete the SF-36 questionnaire and who did not have complete data for analyses. The study was approved by the institutional review board of each center, and patients provided informed consent.
Data Collection and Variables
Patients underwent a physical examination and laboratory assessment. The MELD score was calculated.16 The etiology of the underlying liver disease, complications of liver disease (ascites, encephalopathy, variceal bleeding, and hepatocellular carcinoma), past medical history, comorbidities (diabetes mellitus, hypertension, left ventricular hypertrophy, congestive heart failure, and chronic obstructive pulmonary disease), current medications, social history, and New York Heart Association (NYHA) functional class were recorded. We administered the Medical Outcomes Study SF-36 (version 2.0) in the Liver Disease Quality of Life 1.0 questionnaire to the study participants.1 The SF-36 survey includes 8 multi-item scales (physical functioning, role limitations due to physical problems, bodily pain, general health, emotional well-being, role limitations due to emotional problems, energy/fatigue, and social functioning) that evaluate physical quality of life and emotional quality of life, which are represented broadly by the PCS and MCS scales. Both the PCS and MCS scores are norm-based scores that range from 8 to 73 and from 10 to 74, respectively.17,18 Higher scores indicate better HRQOL.
Survival, liver transplant status, and dates were obtained from the medical records, the subjects’ physicians, the subjects themselves, and the Social Security Death Index as of December 31, 2006. Patients who were alive were censored at this date.
Statistical Analyses
Continuous data were summarized with means and standard deviations, and categorical variables were summarized by frequency and percentage. Correlations between PCS or MCS and MELD were estimated with Spearman’s rho. The probability of liver transplantation with PCS and MCS was estimated separately with logistic regression.
Survival curves were estimated with the Kaplan-Meier method. Cox proportional hazards models were used to estimate the rate ratios [hazard ratios (HRs)] of death across quintiles of PCS and MCS. To determine whether certain baseline demographic and clinical characteristics might explain the association between PCS and survival, we constructed models with PCS and potential confounding variables. Rate ratios were adjusted for age, gender, MELD score, and liver transplantation, which was used as a time-varying covariate. A P value less than 0.05 was considered statistically significant for all analyses. All analyses were performed with available data with SAS version 9.1.3 (SAS Institute Cary, NC).
RESULTS
Patient Characteristics
Five hundred thirty-six patients were enrolled in the Pulmonary Vascular Complications of Liver Disease cohort. Four hundred seventy-three of these patients (88%) were newly evaluated; of these, 261 (55%) returned the questionnaire, and 252 had complete data and constituted the final study sample. The mean age of the study participants was 54 ± 10 years, 64% were male, and 94% were white. The most common etiology of liver disease was alcohol (43%), which was followed by hepatitis C virus (HCV) infection (38%). The mean MELD score was 13 ± 5. The mean PCS and MCS scores were 35 ± 11 and 42 ± 13, respectively. We compared the final study sample to those newly evaluated subjects with incomplete data or without questionnaires (n = 221). There were no apparent differences in terms of age, gender, race, ethnicity, etiology of liver disease, socioeconomic status, or complications of liver disease. The MELD score of excluded subjects was minimally higher than that of included subjects (14 ± 6 versus 13 ± 5), and this indicated that they had more severe liver disease. Further comparison of the 2 groups showed that 55 patients (25%) in the excluded group underwent liver transplantation, whereas 75 patients (30%) in the included group underwent liver transplantation (P = 0.23), and 58 (26%) in the excluded group died, whereas 49 (19%) in the included group died (P = 0.08).
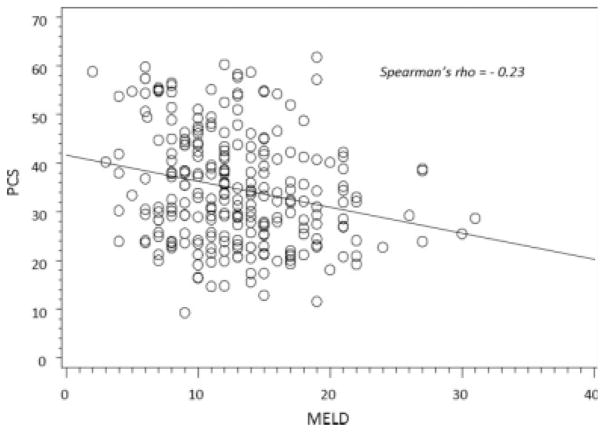
Table 1 shows demographic and clinical characteristics of the study sample grouped according to quintiles of PCS. Because these comparisons were not part of our primary analysis, we did not perform formal hypothesis testing. Age, sex, race, ethnicity, educational status, smoking status, etiology of liver disease, and medical comorbidities were similar across quintiles of PCS. Patients with a lower PCS score (indicating worse quality of life) were more likely to have less household income and more likely to have chronic obstructive pulmonary disease than those who had a higher PCS score. Patients with a lower PCS score had worse NYHA functional class and were more likely to have complications of liver disease such as ascites and encephalopathy in comparison with patients with a higher PCS score. PCS was associated with the MELD score (r = −0.23, P = 0.0002; Fig. 1).
TABLE 1.
Patient Characteristics by Quintiles of the Physical Component Summary
| n | Physical Component Summary |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| PCS score (mean) | 20 | 27 | 33 | 41 | 52 | |
| Number | 252 | 50 | 51 | 50 | 51 | 50 |
| Age, years | 252 | 54 ± 9 | 54 ± 7 | 55 ± 10 | 54 ± 11 | 52 ± 10 |
| Male, % | 252 | 56 | 65 | 52 | 72 | 74 |
| Race, % | 252 | |||||
| White | 92 | 94 | 96 | 90 | 96 | |
| Nonwhite | 8 | 6 | 4 | 10 | 4 | |
| Ethnicity, % | 252 | |||||
| Non-Hispanic | 94 | 96 | 90 | 92 | 92 | |
| Hispanic | 6 | 4 | 10 | 8 | 8 | |
| Education level, % | 248 | |||||
| High school/GED or less | 39 | 38 | 49 | 41 | 28 | |
| Vocational school/some college | 23 | 38 | 27 | 24 | 24 | |
| College degree | 19 | 16 | 20 | 27 | 24 | |
| Professional/graduate degree | 19 | 8 | 4 | 8 | 24 | |
| Household income, % | 223 | |||||
| <$25,000 | 34 | 36 | 40 | 20 | 13 | |
| $25,000–$50,000 | 34 | 32 | 18 | 24 | 15 | |
| $50,000–$75,000 | 18 | 9 | 22 | 15 | 24 | |
| >$75,000 | 14 | 23 | 20 | 41 | 48 | |
| Health insurance, % | 206 | |||||
| Public | 42 | 38 | 45 | 41 | 30 | |
| Private | 58 | 62 | 55 | 59 | 70 | |
| Etiology of liver disease, % | 252 | |||||
| Alcohol | 40 | 43 | 48 | 37 | 46 | |
| Hepatitis C infection | 46 | 37 | 42 | 41 | 26 | |
| Other | 14 | 20 | 10 | 22 | 18 | |
| MELD score | 252 | 14 ± 5 | 14 ± 6 | 13 ± 5 | 12 ± 5 | 11 ± 4 |
| Alanine aminotransferase, U/L | 251 | 55 ± 41 | 52 ± 38 | 51 ± 49 | 63 ± 57 | 71 ± 66 |
| Aspartate aminotransferase, U/L | 251 | 73 ± 45 | 81 ± 61 | 66 ± 43 | 87 ± 93 | 75 ± 50 |
| Albumin, g/dL | 246 | 3 ± 0.5 | 3 ± 0.6 | 3 ± 0.6 | 3 ± 0.6 | 3.6 ± 0.6 |
| Sodium, mEq/L | 252 | 136 ± 5 | 136 ± 4 | 136 ± 4 | 137 ± 4 | 138 ± 4 |
| Complications of cirrhosis, % | ||||||
| Ascites | 247 | 56 | 40 | 40 | 28 | 27 |
| Encephalopathy | 251 | 53 | 49 | 58 | 35 | 20 |
| Variceal bleeding | 252 | 12 | 29 | 28 | 31 | 24 |
| Hepatocellular carcinoma | 252 | 8 | 6 | 6 | 22 | 10 |
| Smoking status, % | 241 | 18 | 21 | 12 | 13 | 12 |
| Comorbidities, % | ||||||
| Diabetes mellitus | 252 | 38 | 20 | 34 | 27 | 24 |
| Hypertension | 252 | 24 | 31 | 26 | 33 | 18 |
| Left ventricular hypertrophy | 222 | 41 | 31 | 30 | 29 | 27 |
| Congestive heart failure | 252 | 4 | 2 | 6 | 2 | 0 |
| COPD | 252 | 10 | 14 | 6 | 2 | 2 |
| Medications, % | 252 | |||||
| Diuretic use | 68 | 57 | 58 | 49 | 38 | |
| Diabetes mellitus medications | 26 | 18 | 18 | 20 | 12 | |
| Beta blockers | 42 | 53 | 36 | 49 | 36 | |
| Liver transplant status, % | 252 | 40 | 25 | 36 | 27 | 20 |
| NYHA functional status, % | 252 | |||||
| I | 14 | 25 | 20 | 33 | 42 | |
| II | 34 | 39 | 52 | 47 | 54 | |
| III | 42 | 29 | 28 | 18 | 4 | |
| IV | 10 | 6 | 0 | 2 | 0 | |
NOTE: Data are means and standard deviations or percentages.
Figure 1.
Correlation between the Physical Component Summary (PCS) and the Model for End-Stage Liver Disease (MELD).
Patients with a lower MCS score were less educated and were more likely to have HCV infection than those with a higher MCS score (Table 2). There were more active smokers and diabetics among patients with a lower MCS score in comparison with patients with a higher MCS score. Unlike PCS, MCS was not associated with the MELD score.
TABLE 2.
Patient Characteristics by Quintiles of the Mental Component Summary
| n | Mental Component Summary |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| MCS score (mean) | 23 | 36 | 42 | 51 | 59 | |
| Number | 252 | 50 | 51 | 50 | 51 | 50 |
| Age, years | 252 | 54 ± 8 | 55 ± 9 | 54 ± 10 | 54 ± 10 | 54 ± 11 |
| Male, % | 252 | 64 | 63 | 56 | 67 | 70 |
| Race, % | 252 | |||||
| White | 94 | 96 | 98 | 96 | 84 | |
| Nonwhite | 6 | 4 | 2 | 4 | 16 | |
| Ethnicity, % | 252 | |||||
| Non-Hispanic | 92 | 88 | 94 | 96 | 94 | |
| Hispanic | 8 | 12 | 6 | 4 | 6 | |
| Education level, % | 248 | |||||
| High school/GED or less | 58 | 37 | 39 | 29 | 33 | |
| Vocational school/some college | 23 | 29 | 22 | 33 | 26 | |
| College degree | 17 | 20 | 24 | 22 | 24 | |
| Professional/graduate degree | 2 | 14 | 14 | 16 | 16 | |
| Household income, % | 223 | |||||
| <$25,000 | 37 | 37 | 26 | 25 | 20 | |
| $25,000–$50,000 | 27 | 22 | 28 | 17 | 31 | |
| $50,000–$75,000 | 15 | 15 | 16 | 12 | 29 | |
| >$75,000 | 22 | 26 | 30 | 46 | 20 | |
| Health insurance, % | 206 | |||||
| Public | 50 | 40 | 40 | 29 | 39 | |
| Private | 50 | 60 | 60 | 71 | 61 | |
| Etiology of liver disease, % | 252 | |||||
| Alcohol | 44 | 47 | 42 | 39 | 42 | |
| Hepatitis C infection | 46 | 47 | 36 | 31 | 32 | |
| Other | 10 | 6 | 22 | 30 | 26 | |
| MELD score | 252 | 13 ± 4 | 13 ± 5 | 13 ± 5 | 13 ± 5 | 13 ± 6 |
| Alanine aminotransferase, U/L | 251 | 67 ± 59 | 67 ± 62 | 49 ± 40 | 46 ± 40 | 63 ± 50 |
| Aspartate aminotransferase, U/L | 251 | 84 ± 56 | 92 ± 94 | 69 ± 51 | 61 ± 40 | 76 ± 48 |
| Albumin, g/dL | 246 | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.2 ± 0.6 | 3.3 ± 0.6 | 3.2 ± 0.6 |
| Sodium, mEq/L | 252 | 137 ± 4 | 136 ± 5 | 136 ± 4 | 136 ± 5 | 137 ± 4 |
| Complications of cirrhosis, % | ||||||
| Ascites | 247 | 38 | 40 | 37 | 36 | 40 |
| Encephalopathy | 251 | 46 | 54 | 46 | 33 | 36 |
| Variceal bleeding | 252 | 26 | 8 | 28 | 31 | 32 |
| Hepatocellular carcinoma | 252 | 8 | 12 | 14 | 4 | 14 |
| Smoking status, % | 241 | 22 | 22 | 13 | 10 | 8 |
| Comorbidities, % | ||||||
| Diabetes mellitus | 252 | 40 | 29 | 26 | 25 | 22 |
| Hypertension | 252 | 38 | 20 | 22 | 33 | 20 |
| Left ventricular hypertrophy | 222 | 38 | 30 | 33 | 36 | 31 |
| Congestive heart failure | 252 | 6 | 4 | 2 | 2 | 0 |
| COPD | 252 | 4 | 12 | 4 | 12 | 2 |
| Medications, % | 252 | |||||
| Diuretic use | 52 | 65 | 48 | 57 | 48 | |
| Diabetes mellitus medications | 30 | 12 | 20 | 18 | 14 | |
| Beta blockers | 46 | 29 | 54 | 39 | 48 | |
| Liver transplant status, % | 252 | 20 | 31 | 34 | 29 | 34 |
| NYHA functional status, % | 252 | |||||
| I | 28 | 25 | 20 | 37 | 24 | |
| II | 36 | 43 | 58 | 35 | 54 | |
| III | 34 | 25 | 14 | 25 | 22 | |
| IV | 2 | 6 | 8 | 2 | 0 | |
NOTE: Data are means and standard deviations or percentages.
Survival Analyses
There were 422 person years of follow-up. One hundred forty-seven patients (58%) were listed, 75 patients (30%) underwent liver transplantation, 49 patients (19%) died, and 3 patients were lost to follow-up. PCS (P = 0.15) and MCS (P = 0.22) were not associated with the probability of transplantation.
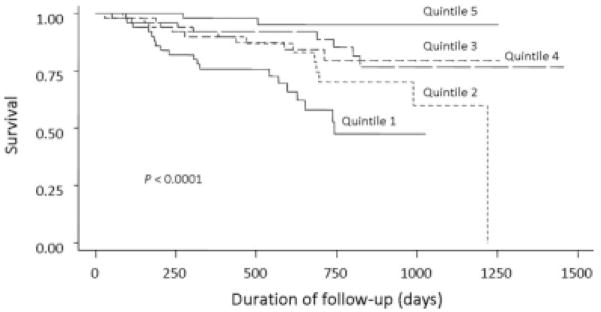
A lower PCS score was associated with worse survival (P < 0.001; Fig. 2). Patients in the lower quintiles of PCS had a higher risk of death in comparison with patients in the highest quintile of PCS (Table 3; P value for the trend < 0.0001). This monotonic association between PCS and mortality persisted even after adjustments for age, gender, and MELD score. We also adjusted for liver transplant status as a time-varying covariate, but this did not change the association between PCS and mortality.
Figure 2.
Kaplan-Meier estimates of survival across quintiles of the Physical Component Summary.
TABLE 3.
Mortality Rate Ratios by Quintiles of the Physical Component Summary
| Physical Component Summary |
P Value for the Trend† | |||||
|---|---|---|---|---|---|---|
| Quintile 1 (9–23.9) | Quintile 2 (24–29.7) | Quintile 3 (29.9–36.8) | Quintile 4 (37–44.8) | Quintile 5 (45–62) | ||
| Rate ratio (95% CI) | 12 (3–50) | 6 (1.3–27) | 4 (0.9–19) | 3 (0.7–15) | 1.0 | <0.0001 |
| Adjusted rate ratio (95% CI)* | 9 (2–39) | 3.5 (0.7–16) | 2.8 (0.6–13) | 2.3 (0.5–11) | 1.0 | 0.0001 |
Adjusted for age, gender, Model for End-Stage Liver Disease score, and liver transplantation.
The linear trend in the hazard ratio was tested by the introduction of the value of the quintile as a continuous variable in Cox’s model.
To investigate whether the traditional demographic and clinical risk factors could explain the association between PCS and mortality, we performed additional adjusted analyses. The multivariate mortality rate ratios for patients in the first quintile of PCS remained significantly higher than those for patients in the fifth quintile of PCS after adjustments for white race [HR = 9.0, 95% confidence interval (CI) = 2–40], Hispanic origin (HR = 8.3, 95% CI = 2–37), household income (HR = 9.5, 95% CI = 2–41), health insurance (HR = 9.0, 95% CI = 2–40), HCV-related liver disease (HR = 9.0, 95% CI = 2–38), and alcohol-related liver disease (HR = 9.0, 95% CI = 2–39). This association remained persistent even after adjustments for complications of liver disease such as ascites (HR = 18, 95% CI = 2–136), encephalopathy (HR = 9.3, 95% CI = 2–41), hepatocellular carcinoma (HR = 9.4, 95% CI = 2–41), and variceal bleeding (HR = 9.0, 95% CI = 2–38). Furthermore, adjustments for smoking status (HR = 7.7, 95% CI = 2–34), NYHA functional class (HR = 8.0, 95% CI = 2–37), or medical comorbidities such as diabetes mellitus (HR = 7.0, 95% CI = 2–32), congestive heart failure (HR = 8.0, 95% CI = 2–36), left ventricular hypertrophy (HR = 8.0, 95% CI = 2–36), and chronic obstructive pulmonary disease (HR = 8.0, 95% CI = 2–35) did not influence the finding that patients in the first quintile of PCS had a higher mortality rate than those in the fifth quintile of PCS. Controlling for the study site also did not have any influence on the association between PCS and survival (HR = 9.0, 95% CI = 2–41).
MCS was not associated with mortality (Table 4; P = 0.22). Mortality rate ratios were similar across all the quintiles of MCS, and there was no change in this result even after adjustments for age, gender, MELD score, or liver transplantation (Table 4; P value for the linear trend = 0.16).
TABLE 4.
Mortality Rate Ratios by Quintiles of the Mental Component Summary
| Mental Component Summary |
P Value for the Trend† | |||||
|---|---|---|---|---|---|---|
| Quintile 1 (11–30.9) | Quintile 2 (31–39) | Quintile 3 (39.4–46) | Quintile 4 (47–54.2) | Quintile 5 (54.4–70) | ||
| Rate ratio (95% CI) | 1.6 (0.6–4) | 1.2 (0.4–3) | 1.8 (0.7–5) | 0.9 (0.3–2) | 1.0 | 0.22 |
| Adjusted rate ratio (95% CI)* | 1.7 (0.7–4) | 1.1 (0.4–3) | 1.1 (0.4–3) | 0.8 (0.3–2) | 1.0 | 0.16 |
Adjusted for age, gender, Model for End-Stage Liver Disease score, and liver transplantation.
The linear trend in the hazard ratio was tested by the introduction of the value of the quintile as a continuous variable in Cox’s model.
DISCUSSION
In this large multicenter study, we found that PCS, the summary measure of physical domains of HRQOL, was significantly associated with survival, whereas MCS, the summary measure of mental and emotional domains of HRQOL, was not. Patients with lower PCS scores had a markedly increased risk of mortality. Importantly, this association between PCS and survival was not explained by differences in demographics, etiology or severity of liver disease, complications of cirrhosis, important clinical variables, medical comorbidities, or the use of liver transplantation. Our findings suggest that perceived physical components of HRQOL are an important determinant in the survival of patients awaiting liver transplantation in addition to liver disease severity.
The finding that the patients’ perception of their physical health was strongly associated with survival is consistent with previous studies; Yeo et al.19 showed that baseline European Organization for Research and Treatment of Cancer Core 30 Quality of Life Questionnaire scores related to physical health were significantly associated with survival in patients with unresectable hepatocellular carcinoma, the majority of whom had chronic liver disease due to hepatitis B virus. A study by Jones et al.20 showed that fatigue, a symptom that directly influences the physical domain of HRQOL, was associated with survival in patients with primary biliary cirrhosis. Ascites, encephalopathy, diuretic use, and severity of liver disease11 were associated with poor physical status as in our study, but the association between PCS and survival was independent of these variables. MCS, a summary measure of mental HRQOL derived from the SF-36, was not predictive of survival; therefore, patients’ perception of their mental HRQOL at the time of LT evaluation may not be an important indicator of their survival chances.
A recent study by Kanwal et al.9 showed that PCS and MCS were not predictive of survival in patients with advanced chronic liver disease. In contrast to their study, we found that PCS was an independent predictor of mortality. It is unclear why our findings were different, but in comparison with the study by Kanwal et al., we had a larger and more diverse population derived from a cohort of well-characterized patients with cirrhosis from 7 different liver transplant centers. Moreover, to determine whether liver transplantation may have influenced the association between PCS and survival, we adjusted for liver transplantation as a time-dependent covariate in our analyses. Also, we found that there was a moderate negative correlation between PCS and MELD scores that did not influence the association between PCS and mortality, and this finding suggests that PCS may be complementary to the MELD score in predicting the survival of transplant candidates.
From an epidemiological perspective, the major finding in our study is that self-reported HRQOL of liver transplant candidates predicted the subsequent risk of all-cause mortality after adjustments for age, gender, MELD score, and liver transplant status. This finding is strengthened by the fact that this is a large, multicenter study, and the study population was selected from a cohort of well-characterized patients with cirrhosis. Among the various prognostic indicators studied in cirrhosis,21 the MELD score by far has been the most accurate predictor of mortality risk6 and has therefore been widely used for the allocation of livers.22 Consistent with previous literature, we found in our study that the MELD score was a strong independent predictor of mortality in all the multivariate models. This study reinforces the idea that in addition to the MELD score, patients’ perception of their physical HRQOL plays an important role in predicting outcomes of liver transplant candidates.
There are several limitations of this study. First, there could be selection bias introduced by the inclusion of only patients who returned the questionnaire and had complete data for the analyses. Excluded patients did have modestly more severe liver disease as reflected in the MELD score. Although there were no statistically significant differences between the included and excluded patients in terms of the proportion who underwent liver transplantation and those who died, we could not exclude that these 2 groups may have been distinct. However, we found no other apparent differences between these excluded patients and the study sample. Second, because the HRQOL measures are self-reports, some patients might have overestimated or underestimated their activities or may have misinterpreted the questions. Third, 94% of the patients included in the study were white, and a majority of the patients were recruited from transplant centers in the Northeast and Southeast regions of the United States. Moreover, certain cultural and ethnic issues may not have been measured by the HRQOL instrument that we used. All these factors may limit the generalizability of our findings to the overall transplant population in the nation. Nevertheless, the association between HRQOL and survival could have been explained by inadequately measured or unmeasured confounders.
From a clinical perspective, the finding that the baseline self-reported physical quality of life of liver transplant candidates is an important prognostic indicator of survival presents a potential opportunity for intervention. Previous studies have shown that regular physical activity is associated with significantly better PCS scores in liver transplant recipients.23 Furthermore, increasing physical activity improves both objective and self-reported physical functioning in such patients.24 Hence, identifying liver transplant candidates with poor self-reported physical quality of life and targeting treatment interventions toward improving their physical activity may prolong their survival. Determining if exercise interventions are feasible and improve PCS scores in liver transplant candidates is an important area for study.
Acknowledgments
This study was financially supported by the National Institutes of Health (DK064103, DK065958, RR00645, RR00585, RR00046, and RR00032).
The Pulmonary Vascular Complications of Liver Disease study group also includes the following members: Jeffrey Okun, B.A., Daniel Rabinowitz, Ph.D., Evelyn M. Horn, M.D., Lori Rosenthal, N.P., Debbie Rybak, M.A., and Sonja Olsen, M.D. (Columbia University); Vijay Shah, M.D., Russell Wiesner, M.D., and Linda Stadheim, R.N. (Mayo Clinic); Dottie Faulk, J. Stevenson Bynon, M.D., Devin Eckhoff, M.D., Harpreet Singh, Raymond L. Benza, M.D., and Keith Wille, M.D. (University of Alabama); Lisa Forman, M.D., David Badesch, M.D., and Ted Perry (University of Colorado); Steven Zacks, M.D., Roshan Shrestha, M.D., and Carrie Nielsen, R.N. (University of North Carolina); Darren B. Taichman, M.D., Ph.D., Vivek Ahya, M.D., Harold Palevsky, M.D., Michael Harhay, Sandra Kaplan, R.N., and Rajender Reddy, M.D. (University of Pennsylvania); and Neil Kaplowitz, M.D., and James Knowles, M.D., Ph.D. (University of Southern California).
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- GED
graduate education diploma
- HCV
hepatitis C virus
- HR
hazard ratio
- HRQOL
health-related quality of life
- MCS
Mental Component Summary
- MELD
Model for End-Stage Liver Disease
- NYHA
New York Heart Association
- PCS
Physical Component Summary
- SF-36
Short Form 36
Footnotes
This article was written for the Pulmonary Vascular Complications of Liver Disease study group.
References
- 1.Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, Artinian L, et al. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease—the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotardo DR, Strauss E, Teixeira MC, Machado MC. Liver transplantation and quality of life: relevance of a specific liver disease questionnaire. Liver Int. 2008;28:99–106. doi: 10.1111/j.1478-3231.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, McCormick M, Price LL, Boparai N, Farquhar L, Henderson JM, Guyatt G. Impact of liver transplantation on health-related quality of life. Liver Transpl. 2000;6:779–783. doi: 10.1053/jlts.2000.18499. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RB, Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. The new liver allocation system: moving towards evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 6.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Gutteling JJ, de Man RA, Busschbach JJ, Darlington AS. Overview of research on health-related quality of life in patients with chronic liver disease. Neth J Med. 2007;65:227–234. [PubMed] [Google Scholar]
- 8.Kanwal F, Spiegel BMR, Hays RD, Durazo F, Han S, Saab S, et al. Prospective validation of the short form Liver Disease Quality of Life instrument. Aliment Pharmacol Ther. 2008;28:1088–1101. doi: 10.1111/j.1365-2036.2008.03817.x. [DOI] [PubMed] [Google Scholar]
- 9.Kanwal F, Gralnek IM, Hays RD, Zeringue A, Durazo F, Han SB, et al. Health related quality of life predicts mortality in patients with advanced chronic liver disease. Clin Gastroenterol Hepatol. 2009;7:793–799. doi: 10.1016/j.cgh.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Ong SC, Mak B, Aung MO, Li SC, Lim SG. Health-related quality of life in chronic hepatitis B patients. Hepatology. 2008;47:1108–1117. doi: 10.1002/hep.22138. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 12.Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 13.Domingo SA, Lamarca R, Ferrer M, Garcia AJ, Alonso J, Felez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 14.Kanwal F, Hays RD, Kilbourne AM, Dulai GS, Gralnek IM. Are physician-derived disease severity indices associated with health-related quality of life in patients with end-stage liver disease? Am J Gastroenterol. 2004;99:1726–1732. doi: 10.1111/j.1572-0241.2004.30300.x. [DOI] [PubMed] [Google Scholar]
- 15.Saab S, Ibrahim AB, Shpaner A, Younossi ZM, Lee C, Durazo F, et al. MELD fails to measure quality of life in liver transplant candidates. Liver Transpl. 2005;11:218–223. doi: 10.1002/lt.20345. [DOI] [PubMed] [Google Scholar]
- 16.United Network for Organ Sharing. [Accessed December 2007];MELD calculator documentation. http://www.unos.org.
- 17.Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–482. [PubMed] [Google Scholar]
- 18.Ware J, Kosinski M, Keller S. A User’s Manual. Boston, MA: Health Institute; 1994. SF-36 Physical and Mental Health Summary Scales. [Google Scholar]
- 19.Yeo W, Mo FK, Koh J, Chan AT, Leung T, Hui P, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. 2006;17:1037–1038. doi: 10.1093/annonc/mdl065. [DOI] [PubMed] [Google Scholar]
- 20.Jones DE, Bhala N, Burt J, Goldblatt J, Prince M, Newton JL. Four year follow up of fatigue in a geographically defined primary biliary cirrhosis patient cohort. Gut. 2006;55:536–541. doi: 10.1136/gut.2005.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Freeman RB., Jr The Model for End-Stage Liver Disease comes of age. Clin Liver Dis. 2007;11:249–263. doi: 10.1016/j.cld.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 2001;7:213–219. doi: 10.1053/jlts.2001.22184. [DOI] [PubMed] [Google Scholar]
- 24.Beyer N, Aadahl M, Strange B, Kirkegaard P, Hansen BA, Mohr T, et al. Improved physical performance after orthotopic liver transplantation. Liver Transpl Surg. 1999;5:301–309. doi: 10.1002/lt.500050406. [DOI] [PubMed] [Google Scholar]