Abstract
Background
c-erbB2, a proto-oncogene coding epidermal growth factor receptor-like receptor, also as a chemosensitivity/prognosis marker for gynecologic cancer, may be involved in initiation of growth of rat primordial follicles. The aim of the present study is to investigate the role and signal pathway of c-erbB2 in onset of rat primordial follicle development.
Methods
The expression of c-erbB2 mRNA and protein in neonatal ovaries cultured 4 and 8 days with/without epidermal growth factor (EGF) were examined by in situ hybridization, RT-PCR and western blot. The function of c-erbB2 in the primordial folliculogenesis was abolished by small interfering RNA transfection. Furthermore, MAPK inhibitor PD98059 and PKC inhibitor calphostin were used to explore the possible signaling pathway of c-erbB2 in primordial folliculogenesis.
Results
The results showed that c-erbB2 mRNA was expressed in ooplasm and the expression of c-erbB2 decreased after transfection with c-erbB2 siRNA. Treatment with EGF at 50 ng/ml significantly increased c-erbB2 expression and primary and secondary follicle formation in ovaries. However, this augmenting effect was remarkably inhibited by c-erbB2 siRNA transfection. Furthermore, folliculogenesis offset was blocked by calphostin (5 × 10(-4) mmol/L) and PD98059 (5 × 10(-2) mmol/L), but both did not down-regulate c-erbB2 expression. In contrast, the expressions of p-ERK and p-PKC were decreased obviously by c-erbB2 siRNA transfection.
Conclusions
c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways, suggesting an important role of c-erbB2 in rat primordial follicle initiation and development.
Background
Folliculogenesis is a complex process consisting of sequential and ordered follicular development and growth. Although much is known about the events and regulation of the later stages of ovarian follicular development, the early follicular development is very poorly understood. More recently, attention has focused on regulation of the initiation of follicular growth (follicle activation) [1,2]. The initiation of follicular growth and progression beyond the primary follicle stage requires locally produced factors and peptides, which can occur without gonadotrophins [3-6]. The local growth factors such as epidermal growth factor (EGF), stem cell factor (SCF), basic fibroblast growth factor (bFGF) and serum anti-Mullerian have been known to be necessary to induce primordial follicle development and initiate folliculogenesis [7-12].
There is accumulating evidence implicating EGF as a key regulator of primordial follicle development in mammals. EGF has been shown, as mitogen for cultured granulosa cells, to stimulate oocyte growth during the primordial to primary follicle transition in vitro [8,13], and EGF receptor has been demonstrated in oocytes from primordial and primary follicles in many kind of species [14-16]. Furthermore, EGF triggered primordial follicle development by stimulating proliferation of granulosa cells [17]. However, the molecular mechanism by which EGF triggers primordial follicle development has not been fully clarified. c-erbB2, a member of the EGF receptor family, encoding a transmembrane EGF receptor [18,19], is expressed in primordial germ cells, granulosa cells, luteal cells and oocytes [20,21]. c-erbB2 is also reported as a marker for chemosensitivity and prognosis of breast and ovarian cancer [22,23].
Recently, we focused on characterizing the effect of c-erbB2 on oocyte maturation and found that c-erbB2 induced oocyte maturation via activation of mitogen-activated protein kinase (MAPK) [24]. MAPKs are known as extracellular-signal-regulated kinases (ERKs), and ERK1/2 are known as classical MAP kinases. In the present study, we tested the expression of c-erbB2 mRNA and protein translation and investigated the role and signaling pathway of c-erbB2 in primordial follicle development. In addition, we explore the molecular mechanism of EGF effect on primordial folliculogenesis.
Methods
Animals and reagents
Animal use was approved by the Committee of Nanchang University for Animal Research. Sprague Dawley rats (obtained from the Animal Care of Medical College of Nanchang University) were used for all the experiments. EGF, PD98059 (a MAPK inhibitor) and calphostin (a PKC inhibitor) were purchased from Sigma (St. Louis MO).
Histology and organ culture
Ovaries from 2-day-old rats were collected fresh or cultured for 4 and 8 days, with 20 ovaries in each group. Fresh ovaries were fixed in Bouins solution for 1-2 h, embedded in paraffin, sectioned (3-5 × 10-3 mm), and were stained with hematoxylin and eosin. The number of follicles at each developmental stage was counted in two serial sections from the largest cross-section through the center of the ovary [25]. Normally, two ovaries were in each treatment group as a replicate and 150-200 follicles were present in an ovary cross-section. Experiments were repeated three times (therefore, n = 6 for each treatment group). Follicles were classified as either primordial (stage 0), or as one of the developing preantral stages (stage 1-4) as described previously [25]. Briefly, primordial follicles consist of one oocyte partially or completely encapsulated by flattened squamous pregranulosa cells. Developing (stage 1-4) follicles contain successively more cuboidal granulosa cells in layers around the oocyte. Whole ovaries were cultured on sponge in 0.5 ml of Waymouth MB 752/1 medium supplemented with 0.1% BSA (Sigma, St. Louis, MO), 0.1% albumax (Gibco BRL, Gaithersburg, MD). Ovaries were cultured at 37°C with 5% CO2 in 4-well plates (Nunc plate; Applied Scientific, South San Francisco, CA), ovaries were randomly assigned to treatment groups with 1-3 ovaries per well. The medium was changed every 2 days. During organ culture, ovaries were treated with EGF (50 ng/ml) and c-erbB2 small interfering RNA (siRNA, 0.1 mmol/L) alone or in combinations. In addition, ovaries were challenged with PD98059 (5 × 10-2 mmol/L) or calphostin (5 × 10-4 mmol/L).
In situ hybridization
Localization of c-erbB2 mRNA was determined by in situ hybridization. The c-erbB2 multiphase oligonucleotide probes digoxigenin-labeled were as follows: 1)5'-G G A A GGACGTCTTCCGCAAGAATAACCAACTGGCT-3';2)5'-G C T T T G T A C A C ACTGTACCTTGGGACCAGCTCTTC-3';3)5'-C G G A C C T C T C C T A C A T G C CCATCTGGAAGTACCCG3'.Before hybridization, the liquids and containers have been strictly treated with 0.1% DEPC. Slides were deparaffinized and rehydrated with 3% H2O2, and subjected to enzymatic digestion with pepsin for 2-3 min(diluted with 3% citric acid, fresh), and then incubated in pre-hybridization solution at 37°C for 2 h. After discarding the prehybridization solution, the slides were transferred to hybridization solution overnight with water, covering the specimens on special coverslips of the situ hybridization. The next morning the coverslips were opened and washed three times in 2 × SSC (standard saline Citrate, containing Nacl 3 mol/L, sodium citrate 0.3 mol/L), 0.5 × SSC, 0.2 × SSC, and then were incubated with the incubating solution at 37°C for 30 min. Slides were exposed to biotinylated mouse anti-digoxigenin IgG for 60 min. Finally, the immunoreactions were detected by using SABC (Strept-Avidin-Biotin Complex) system. Slides were counterstained with haematoxylin before observation. As negative control we used pre-hybridization solution which without probe to replace hybridization solution with probe solution.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Expression of mRNA for c-erbB2 was assayed by RT-PCR. Ovaries from the same culture well were pooled to make single RNA sample. RNA was extracted using the Trizol reagent (Sigma, St. Louis, MO). Total RNA from each sample was reverse transcribed into cDNA using a standard oligo-dT RT protocol. cDNA samples were used as template for polymerase chain reaction (PCR) analysis. The 2 × EasyTaq PCR Supermix kit (TRansGen Biotec) was used according to the manufacturer's instructions. The c-erbB2 primers forward: 5'-CAGTGTGTCAACTGCAGTCA-3', reverse: 5'-CAGGAGTGGGTGCAGTTGAT-3'. The housekeeping reference gene GAPDH primers forward: 5'-GCAAGTTCAACGGCACAG-3', reverse:5'-AGGTGG AAG AATGGGAGTTGCT- 3'. The protocol was 94°C for 4 min, then 35 cycles of 95°C for 15 sec, 55°C for 60 sec and 72°C for 2 min. Fluorescent detection data were analyzed and normalized for c-erbB2 mRNA levels to GAPDH mRNA levels. The identities of the PCR products were confirmed by direct sequencing (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd)
Western blotting analysis
Tissue protein extracts were electrophoretically separated under reduced conditions using NuPAGE 4-12% Bis-Tris gels (Invitrogen; Paisley, UK). Standard Mark (Invitrogen) was used as the molecular weight standard. Proteins were then electrotransferred to nitrocellulose membranes (BIORAD; Munich, Germany) and the immunoblots were subsequently blocked for 2 h at room temperature in TBST (TBS containing 0.1% Tween 20) containing 5% nonfat dry milk. The membranes were incubated overnight at 4°C with antibodies against PCNA, ErbB2, p-ERK1/2, p-PKC or β-actin (Sigma). The β-actin bands were used as an internal control for equal loading. After rinsing with TBST, the membranes were incubated for 30 min at room temperature with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (Amersham; Aylesbury, UK). Finally, the membranes were stained with DAB according to the manufacturer's instructions and analyzed with Gel image analysis system.
Designing and transfecting of c-erbB2 siRNA
EASY siRNA kit was purchased from Shanghai Chemical Technology Co., Ltd. target to c-erbB2 gene (NM_017003). The c-erbB2 siRNA was as follows: sense, 5'-CAGUACCUUCUACCGUUCAtt-3', antisence, 5'-UGAACGGUAGAAGGUA CUGtt-3'. Transfection was followed on the manufacturer's instructions. Briefly, 3 × 10-3 ml 2 × 10-2 mM of siRNA and 2 × 10-3 ml of liposomes (METAFECTENE) were each added to in 5 × 10-2 ml free of serum and antibiotics medium respectively, and the two solutions were combined without any mixture procedures and incubated at room temperature for 15-20 min. After incubation, siRNA-lipid complexes were added to culture flasks (containing 0.5 ml medium and ovaries) and swirl flasks and incubated at 37°C in CO2 incubator. The final siRNA concentration of transfection was 0.1 mmol/L. Ovaries were cultured with/without 0.1 mmol/L targeting siRNA for 12 h. The medium was replaced after 12 h transfection with fresh medium containing no siRNA, and ovaries were cultured for 24 h and then collected to detect gene expression and protein translation by using RT-PCR and western-blot. Ovaries without transfection were used as the control. The negative control was the group transfected with negative siRNA. In addition, ovaries were processed for morphometric assessment of the development of primordial follicles.
Statistics
The experiment was repeated three times. All data were presented as the means ± SEM and analyzed by ANOVA and Duncan's new multiple range tests. p < 0.05 was considered significantly difference.
Results
Expression of c-erbB2 in the ovaries during the initiation of growth of primordial follicle
To examine the expression of c-erbB2, in situ hybridization and RT-PCR were performed. Hybridization histochemistry demonstrated that c-erbB2 mRNA was expressed in ooplasm from primordial follicles of 2 day postnatal ovaries to cultured 8 days of ovaries. Moreover, c-erbB2 mRNA expression increased with prolonged culture, especially in proliferating cumulus cells of cultured ovaries. To investigate more direct actions of EGF, ovaries were incubated in the absence or presence of EGF before RNA collection and analysis. After treatment with EGF (50 ng/ml), the ovaries showed more intense labeling for c-erbB2 mRNA than the control (Fig. 1).
Figure 1.
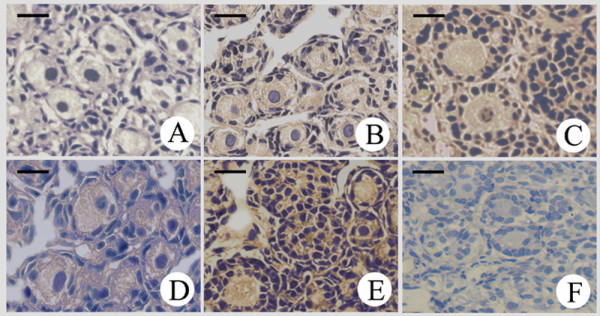
In situ hybridization detection of c-erbB2 mRNA in the ovaries. (A), Control (ovary of 2-day-old rat); (B), Ovary of 2-day-old rat cultured for 4 days; (C), Ovary of 2-day-old rat cultured for 8 days; (D), Ovary of 2-day-old rat cultured for 4 days with 50 ng/ml EGF; (E), Ovary of 2-day-old rat cultured for 8 days with 50 ng/ml EGF; (F), Negative control. Scale bar: 2.5 × 10-2 mm.
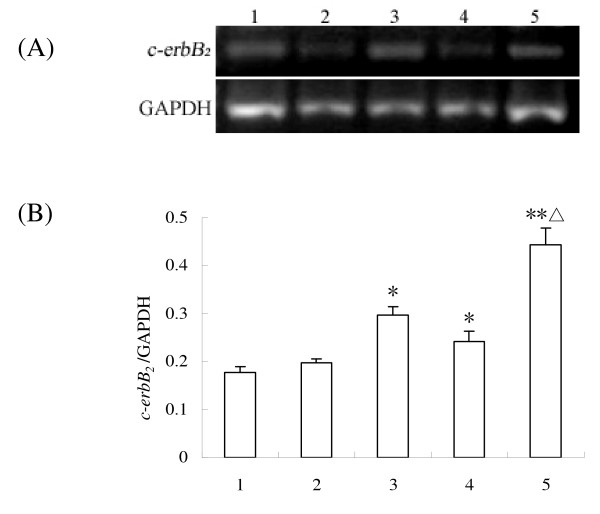
After RT-PCR, cDNA was amplified from RNA extracted from cultured ovaries to assess c-erbB2 expression in response to different treatments (Fig. 2). As previously discussed, the neonatal ovary is primarily composed of early-stage primordial follicles, which can initiate growth when cultured in vitro. As shown in Fig. 2, c-erbB2 mRNA appeared more abundant with primordial follicles that had initiated growth and EGF treatment increased c-erbB2 mRNA expression compared to controls (P < 0.05), and the results were consistent with in situ hybridization analysis.
Figure 2.
RT-PCR detection of c-erbB2 mRNA in the ovaries. (A), The expressions of c-erbB2 mRNA (322 bp) by RT-PCR; (B), Semiquantitative analysis of the RT-PCR result; (1), Control (ovary of 2-day-old rat); (2), Ovary of 2-day-old rat cultured for 4 days; (3), Ovary of 2-day-old rat cultured for 8 days; (4), Ovary of 2-day-old rat cultured for 4 days with 50 ng/ml EGF; (5), Ovary of 2-day-old rat cultured for 8 days with 50 ng/ml EGF. Data are presented as means ± SEM (n = 3). *, P < 0.05; **, P < 0.01 vs control group; Δ, P < 0.05 vs group 3.
Western blotting detection of PCNA protein in the ovaries
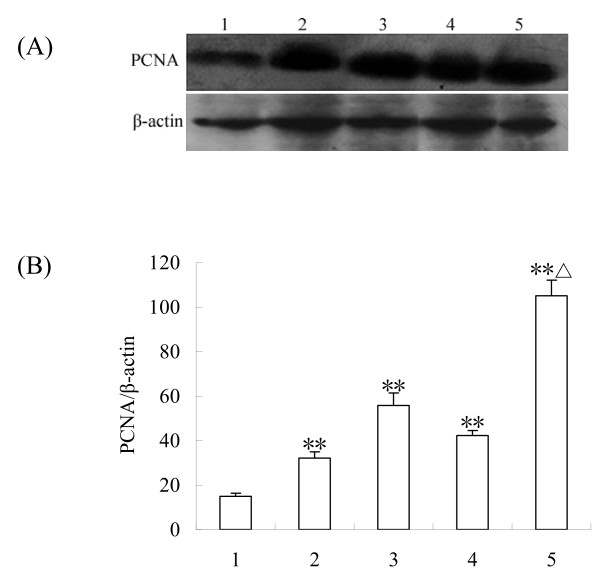
To identify the growth of primordial follicle, the expression of proliferating cell nuclear antigen (PCNA) protein was detected by western blotting analysis. PCNA was expressed in the rat ovary, with a positive band in most of the samples. Differences in the intensity of the band from different group of ovaries were observed, depending on the cultured days. Our data indicated that PCNA protein levels increased with the cultured days, and EGF further enhanced PCNA protein levels by promoting primordial follicle development (Fig. 3).
Figure 3.
Western blotting detection of PCNA protein in the ovaries. (A), The expressions of. PCNA protein by western blot; (B), Semiquantitative analysis of the western-blot result. (1), Control (ovary of 2-day-old rat); (2), Ovary of 2-day-old rat cultured for 4 days; (3), Ovary of 2-day-old rat cultured for 8 days; (4), Ovary of 2-day-old rat cultured for 4 days with 50 ng/ml EGF; (5), Ovary of 2-day-old rat cultured for 8 days with 50 ng/ml EGF. Data are presented as means ± SEM (n = 3). **, P < 0.01 vs control group; Δ, P < 0.05 vs group 3.
Effect of c-erbB2 siRNA on primordial follicle development
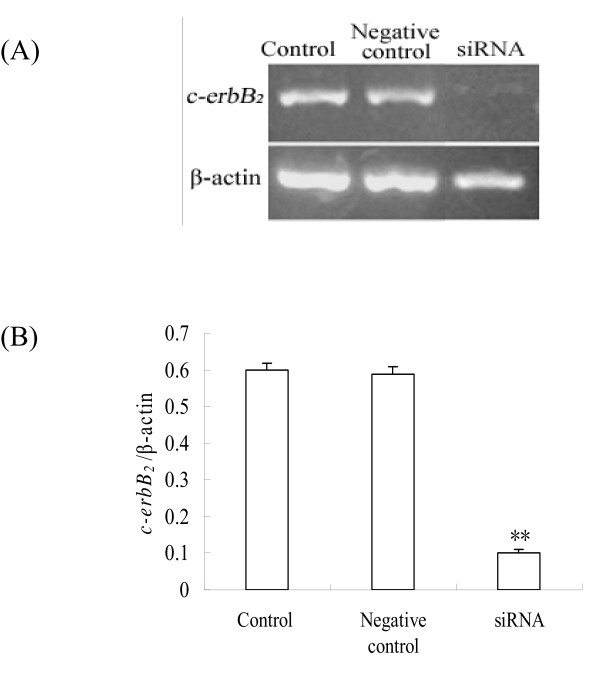
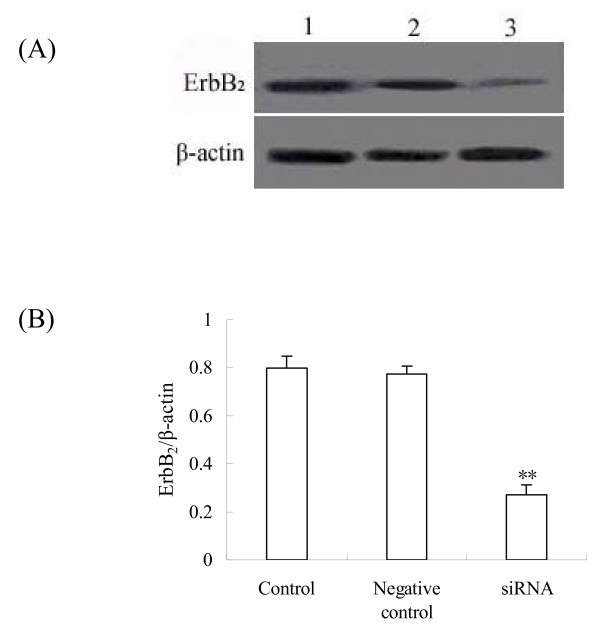
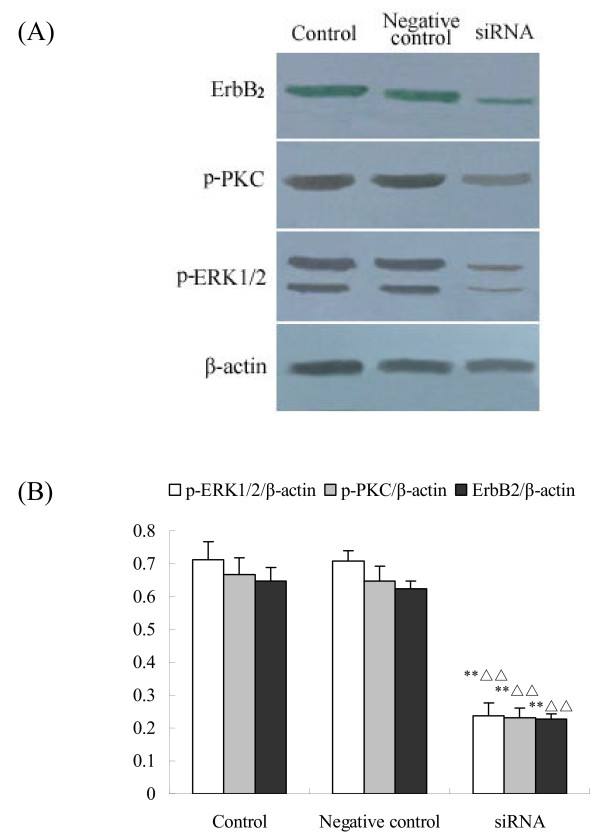
To clarify whether c-erbB2 pathway was involved in initiation of growth of primordial follicle, we synthesized in vitro three siRNAs targeting the c-erbB2 mRNA and transferred them into the newborn rats' ovary to examine the effect of c-erbB2 on primordial follicle development. The siRNA with maximal effect was used in the present study (data not shown). The specificity of the c-erbB2 siRNA effect was verified by examining the levels of c-erbB2 mRNA in ovaries exposed to c-erbB2 siRNA. Although nontargeting control siRNA did not affect the basal transcript level of the gene, c-erbB2 siRNA specifically and appreciably knocked down the levels of c-erbB2 mRNA in ovaries cultured for 4 days. Meanwhile, ErbB2 protein expression was also reduced (Fig. 4 and 5).
Figure 4.
Effect of c-erbB2 siRNA on c-erbB2 mRNA expression. (A), Expression of c-erbB2 mRNA (322 bp) measured by RT-PCR in cultured ovaries after c-erbB2 siRNA transfection. (B), Semiquantitative analysis of the RT-PCR results. Control is the group without transfection; Negetive control is the group transfected with negative control siRNA. Data are presented as means ± SEM (n = 3). **, P < 0.01.
Figure 5.
Effect of c-erbB2 siRNA on ErbB2 protein expression. (A), Expression of c-erbB2 protein in cultured ovaries after siRNA transfection by Western blot. (B), Semiquantitative analysis of the western-blot result. Control is the group without transfection; Negetive control is the group transfected with negative control siRNA. Data are presented as means ± SEM (n = 3). **, P < 0.01 vs control group.
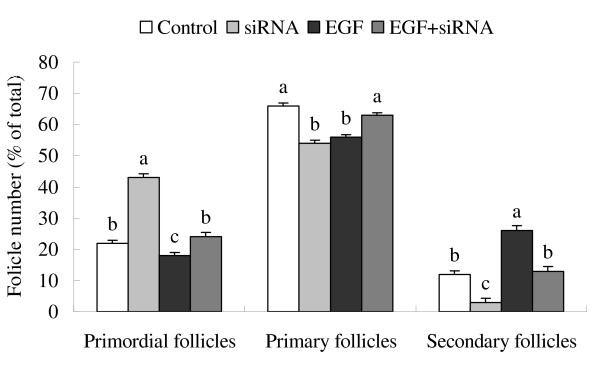
A primordial follicle is composed of an oocyte surrounded by flattened pregranulosa cells, and can initiate the growth spontaneously when cultured in vitro. Statistical analysis revealed that, compared with the control (cultured for 8 days without treatment), siRNA treatment significantly inhibited the growth of primordial follicle and reduced the percentage of secondary follicles. The highest percentage of secondary follicles was observed after 8 day culture with 50 ng/ml EGF. However, EGF-stimulated increase of secondary follicles was obviously inhibited by concurrent treatment with c-erbB2 siRNA (Fig. 6). These data indicate that c-erbB2 siRNA can block spontaneous and EGF-induced activation of primordial follicles by suppressing the expression of c-erbB2.
Figure 6.
Effect of c-erbB2 siRNA on the initiation of primordial follicle growth. (A), Ovaries were cultured for 8 days with treatments: A, control; B, 0.1 mmol/L siRNA targeted c-erbB2; C, 50 ng/ml EGF; D, 0.1 mmol/L siRNA+50 ng/ml EGF. (B), The number of follicles was counted in serial cross-sections. Percentage of the number of each category over the total number was plotted. Data are presented as means ± SEM (n = 5). Bars with different superscripts are statistically different (P < 0.01).
Expressions of ErbB2, p-ERK and p-PKC protein after c-erbB2 siRNA transfection
To investigate the signal pathway of c-erbB2, siRNA was transfected into the cultured neonatal rat ovaries in vitro by liposome. After 8 day culture, western blot analysis was performed to measure the expressions of ErbB2, p-ERK and p-PKC protein after c-erbB2 siRNA transfection. As shown in Fig. 7, the expression of ErbB2, p-ERK and p-PKC protein were remarkably inhibited by c-erbB2 siRNA, compared with the control.
Figure 7.
Effect of c-erbB2 siRNA on the expressions of ErbB2, p-ERK and p-PKC protein. (A), The expressions of ErbB2, p-ERK and p-PKC protein in the ovaries by western blot; (B), Semiquantitative analysis of the western-blot result. Control is the group without transfection; Negetive control is the group transfected with negative control siRNA. Data are presented as means ± SEM (n = 3). **, P < 0.01 vs control group; ΔΔs, P < 0.01 vs negative control.
Effect of PD98059 and calphostin on primordial follicle development
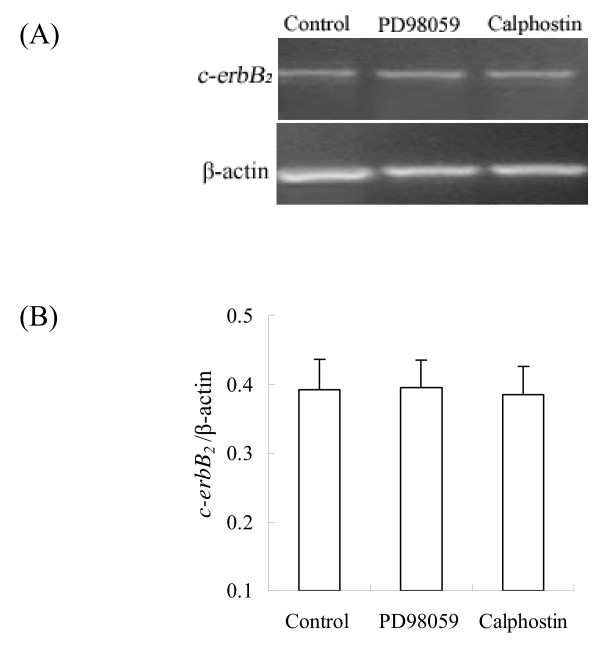
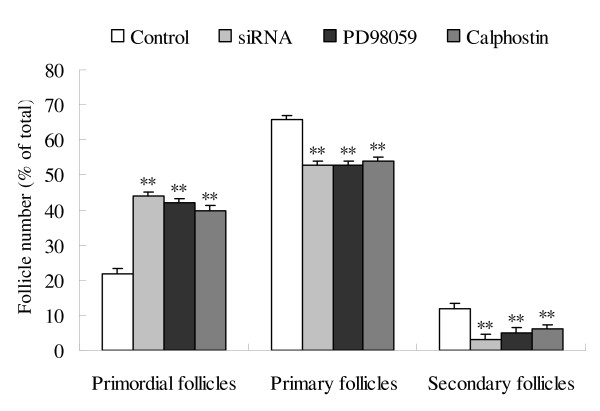
The expression of c-erbB2 mRNA were examined by RT-PCR. The results showed that PD98059 and calphostin did not significantly affect the expression of c-erbB2 mRNA (Fig. 8). Compared with the control, c-erbB2 siRNA, PD98059 and calphostin significantly inhibited the primordial to primary follicle transition of primordial follicles after 8 days culture. The number of primordial follicles was markedly increased and the number of primary follicles and secondary follicles was obviously decreased (Fig. 9). These data suggest that MAPK and PKC pathways are involved in initiation of growth of rat primordial follicles.
Figure 8.
Effect of MAPK inhibitor PD98059 and PKC inhibitor calphostin on the expression of c-erbB2 mRNA. (A), The expression of c-erbB2 mRNA (322 bp) in the ovaries by RT-PCR; (B), Semiquantitative analysis of the RT-PCR results. Data are presented as means ± SEM (n = 3).
Figure 9.
Effect of c-erbB2 siRNA, PD98059 and calphostin on the initiation of primordial follicle growth. (A), Ovaries were cultured for 8 days with treatments: A, control; B, 0.1 mmol/L siRNA; C, 5 × 10-2 mmol/L PD98059; D, 5 × 10-4 mmol/L calphostin. (B), The number of follicles was counted in serial cross-sections. Percentage of the number of each category over the total number was plotted. Data are presented as means ± SEM (n = 5) **, P < 0.01 vs control.
Discussion
Follicles form when some of primordial germ cells are enveloped by a single layer of flattened pre-granulosa cells (pre-GC). When some follicles leave the resting pool and start the initiation of follicular growth (follicle activation), the granulosa cells (GC) become cuboidal and begin to express markers of cell proliferation, such as PCNA. EGF is essential to initiate growth of primordial follicles [17,26]. Our previous results suggested that c-erbB2 played an important role in the regulation of functions of granulosa cells and maturation of oocytes [24,27]. Interestingly, c-erbB2 also mediates spermatogonial proliferation in newt testis [28]. Therefore, we hypothesized that EGF might stimulate the initiation of primordial follicles growth via the c-erbB2 pathway.
In the present study, we examined the expression of c-erbB2 during primordial folliculogenesis and investigated the influence of EGF on c-erbB2 expression as well as the effects of c-erbB2 down-regulation on the initiation of primordial follicle growth and on the activating role of EGF. ErbB2 protein plays the role of epidermal growth factor receptor (EGFR) and hasn't a specific ligand. ErbB receptor has distinct signaling properties depending on its dimerization. ErbB2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling [29]. We investigated the relation between c-erbB2 and MAPK or PKC signaling pathways during primordial folliculogenesis. Our study revealed that expression of c-erbB2 mRNA was present in oocytes of primordial follicles, and also appeared in cuboidal granulosa cells after initiation of follicular growth. The expression of c-erbB2 mRNA increased in proliferated multilayer granulosa cells after prolonged culture. EGF promoted PCNA protein expression and follicular growth by initiating primordial follicle development. In addition, EGF promoted the expression of c-erbB2 mRNA. Therefore, we conjecture that EGF and c-erbB2 might be involved in the onset of primordial follicle development.
To further understand the action of c-erbB2 during primordial folliculogenesis, we used the synthetic siRNA for c-erbB2 to transfect ovarian cells in organ culture. We observed the condition of the growth initiation of primordial follicles through inducing c-erbB2 gene silencing. In the current experiment, most of the primordial follicles in the control group developed to the primary follicles, whereas the number of primary follicles and secondary follicles was significantly decreased by c-erbB2 siRNA. Furthermore, c-erbB2 siRNA blocked the promoting effect of EGF on the initiation of primordial follicle growth. ErbB2, an orphan receptor tyrosine kinase, which can dimerize with other ligand-activated members of the EGF receptor family, might be a selecting marker for initiation of follicular growth. We observed that c-erbB2 siRNA inhibited the expression of ErbB2 protein. These results suggest that c-erbB2 plays an important role on the initiation of primordial follicle growth and mediates the regulating role of EGF as a key signal molecule.
A variety of signaling pathways, including the MAPK and PKC regulating systems, are involved in the initiation of the growth of primordial follicles [30,31]. Phosphorylated MAPK (active) exists in some proliferating granulosa cells, and the activity of MAPK constantly increases during he process of oogonium proliferation [32]. The PKC family has been implicated in various functional responses on the regulation of cell development including cell growth, cell cycle progression, cell survival, apoptosis and cell differentiation. As a potent and selective inhibitor of MAP kinase kinase (MEK), PD-98059 blocks activation of MEK binding to the ATP site of dephosphorylation MEK, thereby inhibites phosphorylation and activation of MAP kinase1, 2 (ERK1/2) [33]. Calphostin C, a potent inhibitor of protein kinase C, inhibits phorbol dibutyrate binding to PKC [34]. In this study, both PD98059 and calphostin significantly inhibited the development of primordial follicles, suggesting that MAPK and PKC signal pathways are involved in the initiation of primordial follicle growth. However, the upstream and downstream relationship between MAPK or PKC and c-erbB2 is still unclear during primordial folliculogenesis. Therefore, we investigated the MAPK and PKC pathways as possible mediators for the expression of c-erbB2 using calphostin and PD98059. Both inhibitors did not change the expression of c-erbB2 mRNA, while the expression levels of PKC and MAPK protein were significantly decreased by c-erbB2 siRNA transfection in primordial follicles. These results indicated that c-erbB2 might be an upstream activator of MAPK and PKC, which regulated the initiation of primordial follicle growth at least in part via the activation of MAPK and PKC signal pathways (Fig.10).
Figure 10.
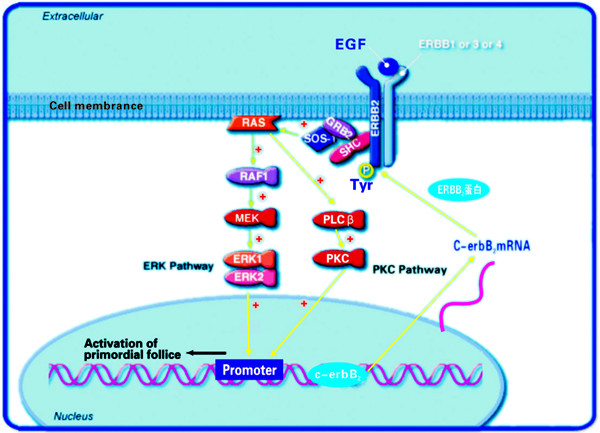
EGF signal transduction figure.
A complex signal network system composed of a variety of autocrine, paracrine and endocrine factors regulates the growth of primordial follicles via intercellular communications, and it has been demonstrated that the growth of primordial follicles was associated with precise spatiotemporal expression of multiple genes and interactions between these genes [3,4,16,17,26,35,36]. The signaling pathways, such as PI3K and mTORC1 pathways, regulate the activation of primordial follicles and the early development of ovarian follicles [37,38]. However, the exact mechanism by which the various factors regulate the growth of primordial follicles has not yet been fully understood. c-erbB2 might have roles in the growth of primordial follicles beyond that of mediating EGF signaling. It also might regulate proliferation of granulosa cells and cumulus cells, which have close signaling communication with oocytes, to govern initiation of follicular growth, development, and steroidogenesis. Futher research of c-erbB2 functions may provide novel information for understanding the mechanism of the follicular initiation and development.
Conclusions
In conclusion, we showed that EGF promoted the initiation of primordial follicle development and the expression of c-erbB2 in ovaries, whereas the promoting effect of EGF was blocked by c-erbB2 siRNA transfection. In addition, the initiation of primordial follicle growth was inhibited by MAPK or PKC inhibition. The expression of ErbB2, p-ERK and p-PKC protein and primordial follicle development were inhibited by c-erbB2 siRNA transfection. These results indicated that c-erbB2 played an important role in primordial follicle initiation and development and the effect of c-erbB2 might be mediated by a mechanism involving the PKC and MAPK pathways.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZLP carried out all the experiments. ZDL, HJ, XLQ, XAX, DXY and TDF performed statistical analysis and drafted the paper. ZYH designed the study and amended the paper. All authors read and approved the final manuscript.
Contributor Information
Zheng Li-Ping, Email: stone91021@163.com.
Zhang Da-Lei, Email: zhangdalei@ncu.edu.cn.
Huang Jian, Email: jian2004fei@126.com.
Xu Liang-Quan, Email: xlq2005002@sina.com.
Xu Ai-Xia, Email: xuax1027@126.com.
Du Xiao-Yu, Email: clsde507@sina.com.
Tang Dan-Feng, Email: tangdanfeng0509@yahoo.cn.
Zheng Yue-Hui, Email: yuehuizheng@163.com.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.39260035) and Jiangxi province major sciences and technologies supporting program.
References
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol. 2000;163:53–60. doi: 10.1016/S0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Fair T. Follicular oocyte growth and acquisition of developmental competence. Anim Reprod Sci. 2003;78:203–216. doi: 10.1016/S0378-4320(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–69. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82-83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Tajima K, Orisaka M, Yata H, Goto K, Hosokawa K, Kotsuji F. Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc Res Tech. 2006;69:450–458. doi: 10.1002/jemt.20304. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Ohsumi K, Tsuji Y, Harauma N, Miyata Y, Fukuda A, Hosoi Y, Iritani A, Morimoto Y. Growing porcine oocyte-granulosa cell complexes acquired meiotic competence during in vitro culture. J Reprod Dev. 2007;53:379–384. doi: 10.1262/jrd.18132. [DOI] [PubMed] [Google Scholar]
- Silva JRV, van den Hurk R, de Matos MHT, dos Santos RR, Pessoa C, de Moraes MO, de Figueiredo JR. Influences of FSH and EGF on primordial follicle during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–1704. doi: 10.1016/j.theriogenology.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Tamura M, Sasano H, Suzuki T, Fukaya T, Funayama Y, Takayama K, Takaya R, Yajima A. Expression of epidermal growth factors and epidermal growth factor receptor in normal cycling human ovaries. Hum Reprod. 1995;10:1891–1896. doi: 10.1093/oxfordjournals.humrep.a136203. [DOI] [PubMed] [Google Scholar]
- Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/S0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2000;67:1593–1604. doi: 10.1095/biolreprod.102.005470. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Heath DA, Lundy T, Fidler AE, Quirke L, O'Connell A, Smith P, Groome N, Tisdall DJ. Control of early ovarian follicular development. J Reprod Fertil Suppl. 1999;54:3–16. [PubMed] [Google Scholar]
- Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, Levinson A, Ullrich A. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Carpenter CD, Ingraham HA, Cochet C, Walton GM, Lazar CS, Sowadski JM, Rosenfeld MG, Gill GN. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem. 1991;266:5750–5755. [PubMed] [Google Scholar]
- Kierszenbaum AL, Tres LL. Primordial germ cell-somatic cell partnership: a balancing cell signaling act. Mol Reprod Dev. 2001;60:277–280. doi: 10.1002/mrd.1088. [DOI] [PubMed] [Google Scholar]
- Maguire HC, Greene MI. Neu (c-erbB-2), a tumor marker in carcinoma of the female breast. Pathobiology. 1990;58:297–303. doi: 10.1159/000163601. [DOI] [PubMed] [Google Scholar]
- Cirisano FD, Karlan BY. The role of the HER-2/neu oncogene in gynecologic cancers. J Soc Gynecol Investig. 1996;3:99–105. doi: 10.1016/1071-5576(96)00001-9. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Zheng LP, Li F, Wu L, Dai YC. c-erbB(2) and c-myb induce oocyte maturation via activation of mitogen-activated protein kinase and maturation promoting factor. Acta Physiologica Sinica. 2008;60:97–104. [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/en.140.9.4262. [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Thuel B. Control of oocyte growth and maturation by follicular cells and molecules present in follicular fluid. A review. Reprod Nutr Dev. 1998;38:345–362. doi: 10.1051/rnd:19980401. [DOI] [PubMed] [Google Scholar]
- Jin X, Fang L, Zheng YH, Zhong ZS, Kuang HB. Effects of c-erbB(2) on the hCG-induced progesterone production of rat luteal cells. Acta Physiologica Sinica. 2000;52:17–21. [PubMed] [Google Scholar]
- Abé K, Eto K, Abé S. Epidermal growth factor mediates spermatogonial proliferation in newt testis. Reprod Biol Endocrinol. 2008;6:6–7. doi: 10.1186/1477-7827-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Han CS, Zhang XS, Yuan JX, Hu ZY, Liu YX. Signal transduction of stem cell factor in promoting early follicle development. Mol Cell Endocrinol. 2005;229:3–10. doi: 10.1016/j.mce.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Serafica MD, Goto T, Trounson AO. Transcripts from a human primordial follicle cDNA library. Hum Reprod. 2005;20:2074–2091. doi: 10.1093/humrep/dei030. [DOI] [PubMed] [Google Scholar]
- Yu HQ, Sun QY, Chen DY, Bou S. The expression and activation of MAPK and p90rsk during folliculogenesis in rat. Chinese Journal of Zoology. 2002;37:1–7. [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. Bio chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Bio chem. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Rivera GM, Yang MY. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci. 2004;82-83:109–126. doi: 10.1016/j.anireprosci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Ojeda SR. Role of neurotrophic factors in early ovarian development. Semin Reprod Med. 2009;27:24–31. doi: 10.1055/s-0028-1108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Zheng WJ, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397 –410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]