Abstract
Neural stem or progenitor cells (NSC/NPCs) able to generate the different neuron and glial cell types of the cerebellum have been isolated in vitro, but their identity and location in the intact cerebellum are unclear. Here, we use inducible Cre recombination in GFAPCreERT2 (GCE) mice to irreversibly activate reporter gene expression at P2, P5 and P12 in cells with GFAP promoter activity and analyze the fate of genetically tagged cells in vivo. We show that cells tagged at P2-P5 with βgal or EGFP reporter genes generate at least 30% of basket and stellate GABAergic interneurons in the molecular layer (ML) and that they lose their neurogenic potential by P12, after which they generate only glia. Tagged cells in the cerebellar white matter (WM) were initially GFAP/S100β+ and expressed the NSC/NPCs proteins LeX, Musashi1 and Sox2 in vivo. One week after tagging, reporter+ cells in the WM up-regulated the neuronal progenitor markers Mash1, Pax2 and Gad-67. These Pax2+ progenitors migrated throughout the cerebellar cortex, populating the ML and leaving the WM by P18. These data suggest that a pool of GFAP/S100β+ glial cells located in the cerebellar WM generate a large fraction of cerebellar interneurons for the ML within the first postnatal 12 days of cerebellar development. This restricted critical period implies that powerful inhibitory factors may restrict their fate potential in vivo at later stages of development.
Keywords: cerebellum, astrocytes, neural stem cells, neural differentiation, progenitor cell, tissue specific stem cell, transgenic mouse, GABA interneurons
INTRODUCTION
The cerebellum, which contains just seven morphologically distinct neuronal subtypes, serves as an excellent model to investigate the mechanisms and signals that instruct cell fate decisions and neural circuit formation during brain development 1, 2. The proper maturation of the cerebellar architecture and circuitry requires the spatiotemporally controlled birth and migration of the various neuronal and glial subtypes. It is, therefore, critical to identify the different populations of cerebellar precursors and establish their fate at precise epochs of development.
The earliest cerebellar precursor cells are segregated into two distinct regions, the neuroepithelium of the 4th ventricle and the anterior rhombic lip in the mouse brain. The neuroepithelium of the 4th ventricle gives rise to the neurons of the cerebellar nuclei, the Purkinje cells and GABAergic interneurons, whereas the rhombic lip gives rise to excitatory glutamatergic neurons. Cerebellar neurons are generated according to a specific temporal sequence: the neurons of the deep cerebellar nuclei (DN) and Purkinje cells between embryonic day 10.5 (E10.5) and E13.5, and the Golgi neurons and other GABAergic interneurons of the internal granular layer (IGL) in late embryogenesis 3–7. The stellate and basket interneurons of the molecular layer (ML) are generated postnatally from highly proliferative precursors residing in the white matter (WM) of the cerebellum, identified by their expression of the paired box transcription factor Pax-2 8. These cells then migrate through the WM underlying the cerebellar lobules and take up residence in the ML 8–11.
In contrast, the murine precursors of all glutamatergic neurons of the cerebellum, including the granule neurons and those resident in the DN, express the neurogenic bHLH transcription factor Math1. Beginning from E12.5 Math1+ cells migrate from the anterior rhombic lip to the cerebellar primordium, spreading tangentially across its surface to form the external granule layer (EGL) 12–17. From P0 through P20, they exit the cell cycle and migrate down the fibers of the Bergmann glia to populate the IGL 18–21.
Despite thorough study of this region, the identity and potential of neural precursor cells in the postnatal cerebellum is not well known. Specifically, it is uncertain whether all postnatally-generated inhibitory and excitatory cerebellar cell types are exclusively derived from precursors genetically committed to a neurogenic cell fate during embryonic development, or whether glial precursors/neural stem cells (NSCs) may still contribute to these lineages. The former hypothesis is supported by the embryonic expression of neuronal fate determinants such as Pax-2 and Math1 by highly mitotic cell populations in the ventricular zone and the rhombic lip, respectively, and the continued expression of these genes in the neonatal brain 8, 13. However, these studies do not exclude that a population of non-committed cells might persist in these areas.
Recent studies suggest that there may be a multipotent progenitor or NSC/NPCs population in the perinatal and even adult cerebellum. For example, neurospheres containing all the major cerebellar cell types can be generated from non-neuronal prominin1-expressing cells isolated in vitro from the infant and mature rodent cerebellum 22, 23. Furthermore, heterotopic cell transplantation studies demonstrate that all varieties of cerebellar GABAergic neurons derive from a common progenitor 24.
The present study reports that a glial cell population identified in the developing cerebellum in vivo functions as neurogenic precursor. To study these cells and their progeny we used the GCE transgenic mice, carrying a tamoxifen-inducible Cre recombinase (CreErT2) under the control of the human GFAP promoter. The CreErT2 protein lacks a nuclear localization sequence and therefore it remains in the cytoplasm unless it is bound by the drug tamoxifen, which targets the protein to the nucleus and enables recombination at loxP sites 25, 26. We used this system to mark with reporter genes cells with GFAP promoter activity at specific time points in the early postnatal period and studied their fate. Control experiments demonstrated that Cre recombination occurs at the time of tamoxifen injection. Our results indicate that non-neuronal cells expressing the glial markers GFAP and S100β in the cerebellar WM are source of GABAergic interneurons during a temporally restricted window, limited to the first postnatal 12 days of cerebellar development. These precursor express nestin, LeX and Sox2, proteins common to both embryonic radial glia and juvenile and adult NSCs of the subventricular zone and dentate gyrus 27–32.
MATERIALS AND METHODS
Generation of Mice, Genotyping and Breeding strategy
The GFAPCreERT2 (GCE) mice were generated as previously described and back-crossed to C57/B6 mice to the F6 generation 32. All results in this paper pertain to line 505; however, some experiments were performed in at least another line (line 516) with identical results. GCE transgenic mice carry a Cre recombinase-estrogen receptor type 2 fusion protein (CreErT2) placed under control of the human GFAP promoter, which is active in radial glia, astrocytes and adult neural stem cells 32, 33. Genotyping was done by PCR using primers to the Cre gene (5′-GCAACGAGTGATGAGGTTCGCAAG-3′) (forward) and (5′-TCCGCCGCATAACCAGTGAAACAG-3′) (reverse) to generate a band of 307 bp 32. GCE mice were crossed with either the R26R LacZ reporter mice 34 (available from The Jackson Laboratory, Bar Harbor, ME) or the CAG-EGFP reporter mice 35 in order to conduct fate mapping experiments. All animal experiments comply with Institutional and National policies and guidelines.
Induction of Cre Recombination via Tamoxifen Treatment
In order to induce Cre recombination in GFAP promoter expressing cells, GCE mice crossed with reporter lines were administered either tamoxifen or its metabolite 4-hydroxy-tamoxifen at a dosage of 33 mg/kg (two i.p. administration separated by 4 hr) for mice P5 and younger or 60 mg/kg (i.p., single administration) for older animals from a 2 mg/ml stock solution prepared in autoclaved sunflower seed oil and stored at −20 °C. For the analyses of cell proliferation, 2-bromodeoxyuridine (BrdU) was injected (50 μg/gr, i.p.) after the last tamoxifen injection.
Tissue Preparation and Immunohistochemistry
Mice were deeply anesthetized and sacrificed via transcardial perfusion with 4% paraformaldehyde (PFA) and the brains were then post fixed for one to three hours depending on the age. Samples were then transferred to 20% sucrose overnight for cryoprotection and then embedded in OCT and stored at −80 °C. Serial 10 μm cryosections were obtained as previously described 32. For immunohistochemistry, sections were blocked in PBS containing 0.1% Tween-20/0.2% Triton (PBS-T) containing 10% goat serum (10% GS/PBS-T) or 10% donkey serum (10% DS/PBS-T), and then incubated in primary antibody in 5% GS/PBS-T or DS/PBS-T. We used the following primary antibodies: βgal (Cappel or Abcam), GFP (Molecular Probes), NeuN (Chemicon), Gad-67 (Chemicon), Hu (Molecular Probes), GFAP (Sigma), DCX (Santa Cruz), Nestin (Chemicon and Iowa Hybridoma Bank), BrdU (Accurate Chemical), LeX (BD Bioscience), PCNA (Accurate); Sox2 (Chemicon), Musashi1 (Chemicon), NG2 and PDGFRα (both from Dr. William Stallcup, Burnham Institute for Medical Research, La Jolla, CA). Sections were washed thoroughly and then reacted to the secondary antibody of the appropriate species. The secondary antibodies used were as follows: Alexa 488, Alexa 594, Alexa 350 (Molecular Probes), FITC and RRX (Jackson Labs). BrdU staining was done sequentially. First immunohistochemistry for all antigens other than BrdU was performed as described above. Section were post-fixed for 15 minutes at room temperature in 4% PFA and then incubated for 45 minutes in 1.5 N HCl, followed by immunohistochemistry for BrdU.
Cell Counting and Confocal analysis
Unbiased estimates of cell number were obtained via a Zeiss Axioskope 2 Mot Plus (Carl Zeiss, Thornwood, NY, USA) attached to a motorized stage and connected to a computer running the Stereoinvestigator Software (MicroBrightfield, Colchester, VT, USA). Serial sagittal sections (one every 400 μm) were used for all counts. Contours of the EGL, ML, IGL, interlobule WM, deep cerebellar nuclei and SEZ of the 4th ventricle were drawn. Nuclear profiles of stained cells were counted using the optical fractionator probe with a 40× oil-immersion objective. Sampling grids sized from 100 μm × 100 μm to 300 μm × 300 μm, depending on the cerebellar layer under analysis, were randomly superimposed over the sections using Neurolucida. Tri-dimensional sampling boxes (50 μm × 50 μm × 6 μm) with 3 out of 6 exclusion borders 36, 37 were automatically placed by StereoInvestigator at each grid intersection point. The total number of cells was calculated by the formula:
where ΣQ is the total number of nuclei counted, t the mean section thickness, h the height of the optical dissector, asf is the area sampling fraction, and ssf is the section sampling fraction 37. The density for each cell type was calculated by dividing the total number of cells by the total volume sampled. Sampling grids and magnifications were adjusted for each staining in order to obtain a relatively constant number of cells sampled and obtain a coefficient of error ≤ 0.5). This systematic yet unbiased method provides an estimate of cell density and number that is independent from cell size, shape, orientation, tissue shrinkage and spatial distribution of the cells 38. Cells were assessed and counted for double immunostaining using a dual 594 and 488 exposure filter. Image acquisition and Z-stack analysis was performed on an ApoTome equipped Axiovert 200M with Axiovision 4.5 software (Carl Zeiss, Thornwood, NY, USA).
RESULTS
Cre recombination is tamoxifen inducible in early postnatal GCE mice
To characterize the cells with GFAP promoter activity in the postnatal cerebellum, GFAP-CreERT2 (GCE) mice were crossed to R26R or CAG-EGFP reporter lines. Mice were administered tamoxifen to induce Cre activity which activates the β-galactosidase (βgal) and EGFP gene products by excising a transcriptional Stop sequence in the R26R and CAG-EGFP reporter lines, respectively. In confirmation of previous data 32, X-gal staining (Supplemental Fig. 1 A) and reporter immunoreactivity (not shown) revealed no detectable reporter gene expression in GCE;R26R mice without tamoxifen treatment. Three days after two tamoxifen injections at postnatal day 5 (P5) in GCE;R26R double transgenic mice (dosage, 33 mg/kg), X-gal staining was present mostly in cells in the Purkinje layer (PL), the internal granular layer (IGL) and the white matter (WM) (Supplemental Fig. 1 B). This pattern of reporter gene expression was stronger, but essentially unaltered, after 5 daily tamoxifen injections at P5-P9 and analysis at P14 (Supplemental Fig. 1 C).
Shortly after recombination, reporter gene expression is induced in populations of glial cells mostly expressing astrocyte lineage markers
Cells targeted by Cre recombination were assessed by immunocytochemical analyses at P7-P8, two-three days after the P5 tamoxifen treatment, to allow enough time for translation of the reporter protein after reporter gene induction. The LacZ+ cells were NeuN-negative Bergmann glia in the Purkinje cell layer (Supplemental Fig. 1 B, thick arrows) and small NeuN-negative cells in the EGL, IGL and WM (Supplemental Fig. 1 B, thin and open arrows), that appeared to co-localize GFAP protein (Supplemental Fig. 1 C, red arrows). Because of the poor cellular resolution of X-gal staining, we identified recombined cells by immunofluorescence for the βgal and EGFP reporters together with a panel of lineage markers and systematically analyzed their phenotype by nonbiased random sampling using stereological methods. At P7-P8, we did not detect a single NeuN/βgal or NeuN/EGFP double positive cell in any of the brains analyzed (Supplemental Fig. 1 D & Table 1), suggesting that Cre recombination was strictly targeted to non-neuronal cells at P5. To control for nonspecific induction of Cre recombination, we identified the embryonically generated Purkinje neurons by their location and their Gad-67 and calbindin immunoreactivity; Purkinje cells did not express βgal either at P8 (Supplemental Fig. 1 E–G) or at P35 (Supplemental Fig. 1 H,I). Identical expression patterns were found in at least two GCE lines.
Table 1.
GFAP promoter-positive cells induced to express reporter gene at P5 are astroglial in phenotype, but generate both neurons and glia
| Percentage of reporter+ cells that express the indicated markers (± s.e.m.) at P7-P8 | ||||||
|---|---|---|---|---|---|---|
| GFAP | NG2 | PDGFRα | GAD-67 | Rip | NeuN | |
| SEZ IV ventricle | 93 ± 1.0 | ND | ND | 0 | 0 | 0 |
| Deep cerebellar WM | 90 ± 1.0 | ND | ND | 0 | 0 | 0 |
| Interlobule WM | 87.8 ± 4.8# | 1.1 ± 0.2 | 2.2 ± 0.2 | 0 | 0# | 0# |
| PL & IGL | 83.0 ± 5.8# | <0.5 | <1.0 | 0 | 0# | 0# |
| DN | 69.5 ± 1.0 | ND | ND | 0 | 0 | 0 |
| Percentage of EGFP+ cells in the WM that express the indicated markers (± s.e.m.) at P7 | ||||
|---|---|---|---|---|
| GFAP | S100β | GFAP&S100β | undetermined | Total |
| 50.0 ± 5.0 | 12.34 ± 4.9 | 36.93 ± 4.3 | <1 | 100 |
| Percentage of βgal+ cells that express the indicated markers (± s.e.m.) at P35 | ||||
|---|---|---|---|---|
| GFAP | NeuN | GAD-67 | RIP | |
| Molecular layer | 34.0 ± 1.8 | < 0.1 | 50.2 ± 5.2 | 0 |
| WM | 68.0 ± 7.1# | 0# | 0 | 12.4 ± 5.3# |
Reporter expression was induced by tamoxifen injections at P5 in GCE;R26R or GCE; CAG-EGFP double-transgenic mice and animals were analyzed at P7-P8 or at P35, as indicated. Single and double-stained cells were systematically analyzed via confocal microscopy and stereological sampling methods in the indicated regions throughout the cerebellar anlage in parasagittal serial sections. Approximately 6 sections were analyzed per brain (N= 3 to 5 brains).
These values were already reported in Ganat et al.(2006) and are reported here for completeness. 0 = no double-stained cells observed. ND=not determined.
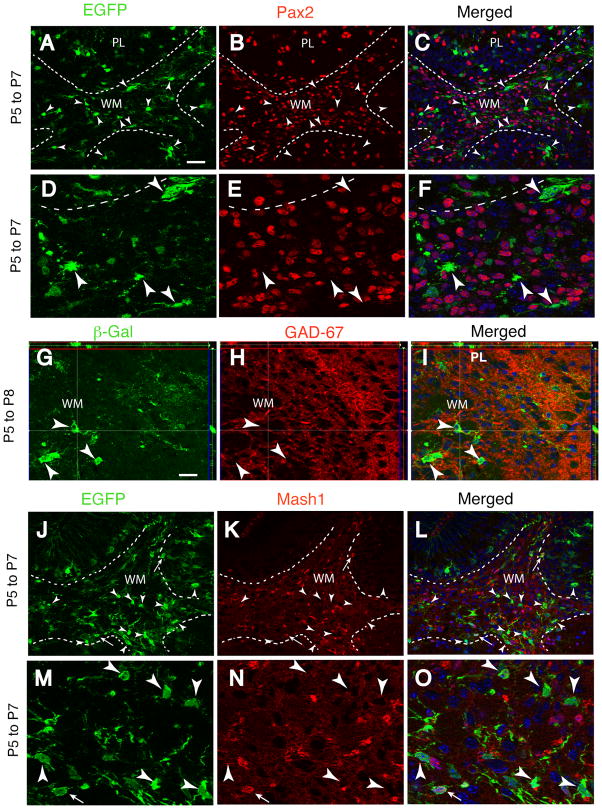
We next sought to determine if neuronal or oligodendrocyte progenitors were targeted by Cre recombinase activity after tamoxifen treatment. The GABA synthetic enzyme Gad-67 and the homeodomain transcription factor Pax2 are both expressed by proliferative precursors of GABAergic neurons 8, 39 and are both highly enriched in the cerebellar WM at the P3-P7 stage of development (Fig. 1A–I, Supplemental Fig. 2 A–C). However, only 2% of the EGFP+ cells in the WM expressed Pax2 two days after recombination, and conversely, only 0.6% of the Pax2+ cells in WM expressed the reporter protein (Fig. 1A–F; Table 2). Reporter+ cells in the PL and IGL were also negative for Pax2 (Supplemental Fig. 2 A–C; Table 2). Similarly, none of the reporter+ cells in WM, PL and IGL expressed Gad-67 at P7 (Fig. 1G–I; Table 1; Supplemental Fig. 1 E–G). We also investigated the colocalization of the reporter protein with Ascl1 (previoulsy Mash1), a bHLH proneural transcription factor thought to identify the earliest stages of commitment of NSCs to neuronal lineages 40, 41 which is expressed in the developing cerebellum 42. The Mash1+ lineage in the cerebellum is restricted to GABA neuron progenitors and oligodendrocytes 43. Similarly to Pax2, Mash1 is highly expressed in the cerebellar WM, yet reporter+ cells in this region did not express Mash1 at P7, 2 days post-recombination (Fig. 1J–O, arrowheads), consistent with the idea that Cre targeting does not occur in GABA neuron progenitors. However, a few EGFP+/Mash1+ cells located at the interface between the lobular WM and the IGL were Mash1+ (Fig. 1J–O, thin arrows). Together, these data suggest that cells tagged with reporter expression in WM are non-neuronal, but a small proportion of reporter+ cells have begun to enter the early stages of neuronal or oligodendrocyte differentiation.
Fig. 1. Reporter expression is not induced in neuronal progenitors.
Characterization of reporter-tagged cells at P7 or P8 in GCE;CAG-EGFP or GCE;R26R mice injected with tamoxifen at P5. Broken lines delimit the WM from adjacent regions of the cerebellar cortex in sagittal sections 200–400 μm from the midline. Double immunohistochemistry for EGFP or βgal (green, A,D,G,J,M), Pax2 (red, B,E), GAD-67 (red, H), Mash1 (red, K,N) and merged images (C,F,I,L,O). (D–F) and (M–O) are 3× higher magnifications of (A–C) and (J–L), respectively. Two or three days after targeting, reporter-expressing cells (arrowheads) do not express these progenitors markers in the cortex or WM. However, occasional reporter+ cells express Mash1 protein at the cortex-WM boundary (thin arrows in J–O), suggesting recent acquisition of neuronal identity. Images are representative 1 μm optical sections acquired via Apotome; side panels in G–I illustrate imaging in the Z-plane at the levels of the cross-hairs. Scale bar, 20 μm for A–C and J–L, 10 μm for G–I.
Table 2.
GFAP+ cells lineages traced via reporter gene expression at P5 are initially negative for Pax2 but subsequently generate Pax2+ cells
| P7 | P12 | ||||||
|---|---|---|---|---|---|---|---|
| EGL | PL&IGL | WM | EGL | ML | PL&IGL | WM | |
| Pax2+ cell density (cells/100 μm3) | 2.53 ± 0.34 | 17.38 ± 0.14 | 351.87 ± 73.6 | <2 | 5.6 ± 0.33 | 5.44 ± 0.87 | 18.18 ± 1.0 |
| EGFP/Pax2 cell density (cells/100 μm3) | 0 | 0 | 2.12 ± 1.06 | <0.05 | 1.81 ± 0.55 | 1.9 ± 0.07 | 21.80 ± 1.83 |
| % EGFP expressing Pax2 | 0 | 0 | 2.33 ± 0.66 | <2 | 77.08 ± 6.25 | 15.67 ± 2.85 | 64.76 ± 1.24 |
| % Pax2 expressing EGFP | 0 | 0 | 0.56 ± 0.18 | <2 | 24.23 ± 6.68 | 26.19 ± 2.38 | 54.45 ± 3.44 |
EGFP reporter expression was induced by tamoxifen injections at P5 in GCE;CAG-EGFP double-transgenic mice and animals were analyzed at P7 and P12. Single and double-stained cells were systematically analyzed via stereological methods. Data were obtained through counting 750 and 326 Pax2+ cells at P7 and P12, respectively, and 319 and 504 EGFP+ cells at P7 and P12, respectively in 2 brains per condition. 0 = no double-stained cells observed; for the EGL at P12, approximate values are given, as a precise estimate could not be obtained given the low density of the cells.
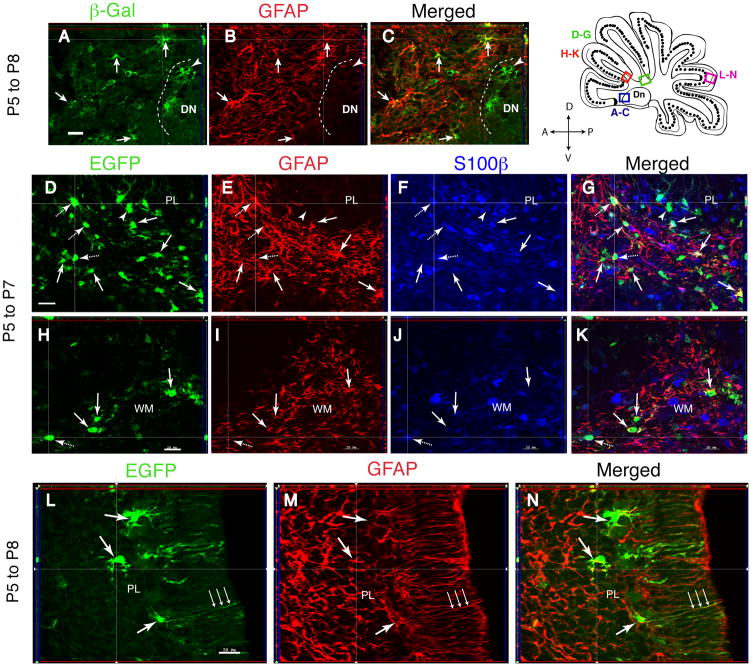
In accordance with these data, at P7-P8 the reporter protein is found almost exclusively in cells positive for markers of the astrocyte lineage. In the deep WM, βgal/GFAP-double positive cells had an irregularly shaped, small soma and many ramified processes, typical of astrocytes (Fig. 2A–C, arrows). In the PL, reporter+ cells with typical morphology of Bergmann glia extended GFAP+/Blbp+/nestin+ processes into the ML (Fig. 2L–N; Supplemental Fig. 2 D–I). Between 82% and 93% of the βgal-expressing cells in the various cerebellar compartments co-localized GFAP protein (Table 1). By contrast, in the DN, fewer (69.5%) βgal+ cells were GFAP+ (Fig. 2A–C; Supplemental Fig. 3 A–C; Table 1). To determine if GFAP negative cells expressed other markers of astrocytes, animals tamoxifen-injected at P5 were analyzed at P7 by triple staining for EGFP, GFAP and S100β. Counts of the triple-stained sections in confocal stacks of images revealed that in the WM, 50 ± 5.0 % EGFP+ cells expressed GFAP only, 36.9 ± 4.3 % expressed GFAP and S100β, 12.3 ± 4.9 % expressed only S100β, and only 0.8% were devoid of any detectable staining (Fig. 2D–K; Table 1).
Fig. 2. Reporter expression is induced in cells of astroglial identity.
Characterization of reporter-tagged cells using astrocytic markers at P7 or P8 in GCE;CAG-EGFP or GCE;R26R mice injected with tamoxifen at P5. (A–K), cerebellar WM; (L–N) cerebellar cortex. (A–C), βgal and GFAP double immunostaining; (D–K), EGFP, GFAP, and S100β triple immunostaining; (L–N), EGFP and GFAP double immunostaining. Arrows, reporter/GFAP double-labeled cells; dashed arrows, triple labeled cells; arrowheads, reporter+ cells that are negative for markers. Shown are single 1 μm optical sections in the sagittal plane 200–400 μm from the midline. Scale bars, 27 μm for D–G and 20 μm for all other panels.
We next analyzed reporter protein colocalization with immunoreactivity for NG2 and PDGFRα, which are expressed in oligodendrocyte progenitors 44–48. We found that in the interlobule WM of mice tamoxifen-treated at P5 and analyzed at P7, 1% and 2% of the EGFP+ cells expressed NG2 and PDGFRα, respectively, and that in the IGL/PL, less than 0.5% and 1% of EGFP+ cells expressed the NG2 and PDGFRα proteins (Table 1; Supplemental Figure 4). This was not due to a failure to observe NG2- and PDGFRα-expressing precursors in the cerebellum, which were very abundant in both the IGL and the WM; for example, the density of PDGFRα+ cells in the WM was 559,000 cells/mm3, but only 6.8% of these cells expressed EGFP (Supplemental Figure 4). In conclusion, reporter gene expression is observed, two-three days after recombinase induction, in astrocyte-like cells that express varying combinations of GFAP, Blbp, nestin or S100β immunoreactivity and in a small percentage (2% or less) of neuronal and oligodendrocyte progenitors, but not in neurons.
Temporally regulated genesis of stellate and basket interneurons from GFAP promoter expressing cells
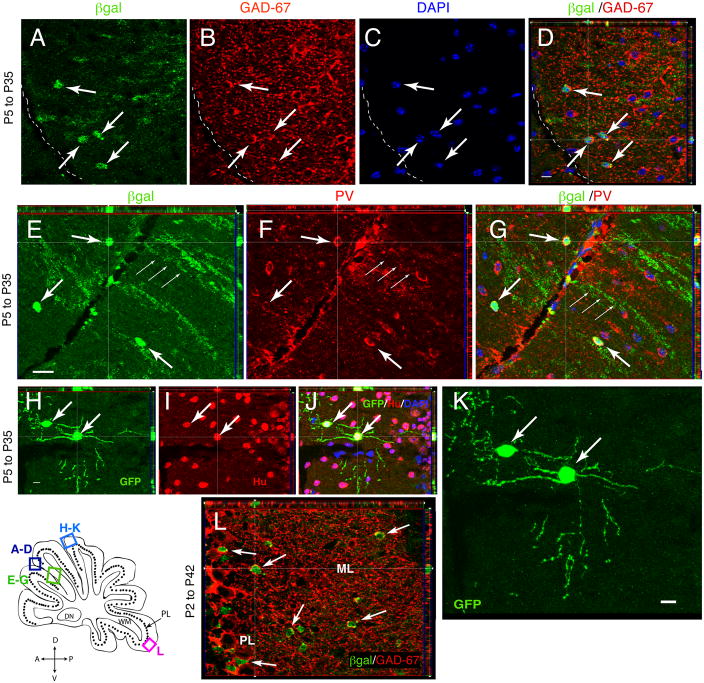
We next assessed the lineage of these glial cells with GFAP promoter activity in the early postnatal cerebellum. The GCE mice carrying a βgal or EGFP reporter gene were tamoxifen-injected at P5 and the reporter+ cells phenotypes were identified by their size, morphology and cell type-specific markers 4 weeks later, at P35. At this time, βgal+ cells with the typical phenotype of stellate interneurons were observed in the ML. GABAergic interneurons do not co-localize NeuN 49, so the identity of the βgal+ cells as stellate interneurons was substantiated by their co-expression of the neuronal markers Hu, Parvalbumin (PV) and Gad-67 (Fig. 3A–J). The βgal+ cells expressed the GABA synthetic enzyme Gad-67 (Fig. 3A–D), the calcium binding protein PV (Fig. 3E–G) and neuronal marker Hu (Fig. 3H–J). These cells had a round soma with approximately 6 moderately branched dendrites (Fig. 3H–K). The βgal/Gad-67 cells comprised 50% of the total number of βgal+ cells in the ML at P35 (Table 1). Conversely, 12.4% ± 3.5 (N= 3 brains) of Gad-67+ cells in the ML were βgal+; it is likely that at least three times as many Gad-67+ cells in the ML were derived from GFAP lineage at P5, since we estimated the targeting efficiency as less than 30% of the GFAP+ cells (Fig. 2). After injection at P5, the βgal+ cells colocalizing Gad-67, PV and Hu were restricted to the external portion of the ML (Fig. 3A–G), suggesting that GFAP-lineage cells generate stellate interneurons at this age, but not basket cells which are situated more closely to the PL 1, 50.
Fig. 3. Stellate and basket interneurons arise from P2–P5 glial ancestors 4 weeks after targeting.
Characterization of reporter-tagged cells 4–5 weeks after recombination at P35 (A–K) or P42 (L) in GCE;CAG-EGFP or GCE;R26R mice. Shown are single representative 1 μm optical sections imaged via ApoTome. Sagittal sections from the cerebellum 200–400 μm from the midline were double immunostained for βgal and Gad-67 (A–D), βgal and Parvalbumin (PV) (E–G) or EGFP and Hu (H–J), with merged images in (D,G,J), demonstrating colocalization of the two markers in the exterior portion of the ML. (K) Reconstructed image of the same view as in (H–J) generated from a 6 μm Z stack of 1 μm optical slices, demonstrating the morphology of two EGFP+/Hu+ interneurons in the outer half of the ML. (L) βgal/Gad-67 double immunostaining, demonstrating colocalization of the two markers in GCE; R26R mice given tamoxifen from P2 to P6 and analyzed at P42. Notice that βgal/Gad-67 double-positive cells now fill the entire extent of the ML. Scale bar in D, H and K, 10 μm.
To determine if GFAP-lineage cells generate basket cells at an earlier developmental epoch, we injected tamoxifen from P2 to P6 and analyzed the tissue 4–5 weeks later. Earlier targeting gave rise to Gad-67+/βgal+ cells scattered throughout the entire extent of the ML, suggesting that at P2 the cells with GFAP promoter activity are able to generated both basket and stellate neurons (Fig. 3L). Additionally, the percentage of Gad-67-immunoreactive cells that were βgal+ was now substantially higher, 30% ± 2.7 (N=3). Thus, a large percentage of GABAergic interneurons of the ML are derived from the postnatal GFAP lineage. In contrast to the ML Gad-67+ interneurons, βgal+ cells in the PL and IGL were Gad-67− negative (Supplemental Fig. 1 H–I; Table 1).
Finally, some reporter+ cells at P35 acquired glial identities in the GCE line. Four weeks after a P5 tamoxifen injection, approximately 12% of the βgal+ cells in WM were labeled by the Rip antibody, a marker for early and late oligodendrocytes (Table 1). In the ML and WM, 34% and 68% of the βgal+ cells, respectively, maintained GFAP/S100β+ astroglial identity and developed a mature astrocyte morphology.
Given that cells with GFAP promoter activity no longer gave rise to basket interneurons by P5, a time point that precedes the peak of proliferation for basket cell neuronal progenitors 11, 39, we hypothesized that GFAP lineage precursors may also lose their potential to generate stellate neurons at some point in their development. To test this hypothesis, we injected tamoxifen at P12-P14 and harvested tissue at P35 or P90. Neither time point revealed genesis of neurons in the cerebellum from the GFAP lineage, as revealed by Hu, Gad-67 and NeuN double staining in either the molecular or granule layers (Supplemental Fig. 5 A–C). Reporter-positive cell fates were restricted to GFAP+ Bergmann glia and parenchymal astrocytes (Supplemental Fig. 5 D–F).
GFAP-positive cells in WM give rise to GABAergic neuron precursors in the cerebellum
To elucidate the origin of the glial ancestors giving rise to GABAergic cells in ML, we injected tamoxifen at P5 and analyzed tissue at P8, P12, P15 and P18 using markers for proliferating, migratory precursors of ML interneurons 8, 39. As noted above, at P7-P8, 2–3 days after inducing recombination, no reporter+ cells co-expressed Gad-67, Mash1 and Pax2 in the EGL, PL and IGL and 2% expressed Pax2 in the cerebellar WM (Fig. 1; Table 2).
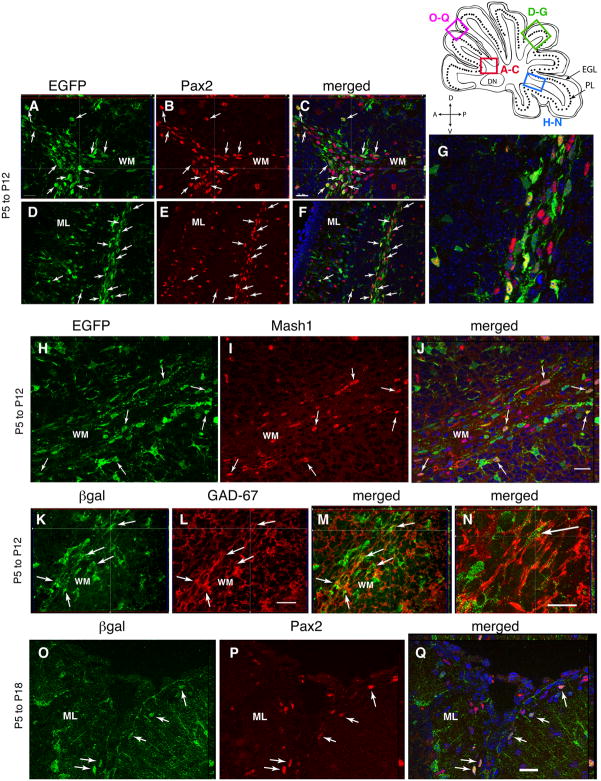
One week after recombination (P12), however, a large number of reporter+ cells had acquired Pax2, Gad-67 and Mash1 immunoreactivity in the subcortical WM underlying the cerebellar lobules (Fig. 4A–G). In the lobular WM, approximately 65% of the EGFP colocalized Pax2, and conversely, 54% of Pax2+ cells were EGFP+ at this time (Table 2). Similarly, an abundance of EGFP/Mash1 (Fig. 4H–J) and βgal/Gad-67 (Fig. 4K–N) double-positive cells were observed in the WM at P12. The reporter+ cells co-localizing Pax2, Mash1 and Gad-67 possessed an elongated morphology and single leading and lagging labeled processes, suggesting that they were migratory cells. Some of EGFP/Pax2 double positive cells were observed in other layers, but at much lower density—the density of EGFP/Pax2 positive in the ML and PL/IGL was 1.8 and 1.9 cells/100 μm3, respectively, as compared to a value of 21.8 cells/100 μm3 in the WM (Fig. 4D–G; Table 2). As most of the EGFP/Pax2 and βgal/Gad-67 double positive cells were restricted to the cerebellar WM at the P12 stage, we hypothesize that these cells represent GABAergic neuronal progenitors that arise locally from the tagged cell population.
Fig. 4. Glial cells give rise to Pax2+, Mash1+ and Gad-67+ interneurons precursors in the cerebellar WM that migrate to the ML.
Early markers for GABA interneurons in GCE;CAG-EGFP or GCE;R26R mice injected with tamoxifen at P5 and analysed at P12 (A–N) or P18 (O–Q). Double-immunostaining for EGFP (A,D,H) or βgal (K,O) together with Pax2 (B,E,P), Mash1 (I) or Gad-67 (L) and merged images (C,F,G,J,M,N,Q) in sagittal sections 200–400 μm from the midline. Blue shows DAPI nuclear staining. (G,N) are 4× and 3× higher magnifications of (F,M), respectively. Note that at P12, EGFP+ or βgal+ cells (arrows) with migratory morphology restricted to the WM expressed Pax2 (A–G), Mash1 (H–J) and Gad-67 (K–N), whereas by P18, βgal/Pax2 double stained neurons (arrows) are visible in the ML, suggesting that by this time they have already migrated into the cerebellar cortex. All panels show representative 1 μm optical sections. Scale bar in C, 20 μm for A–C and 40 μm for D–F; scale bar in J,L,Q, 20 μm; scale bar in N, 10 μm.
At P15, 10 days after recombination, βgal/Gad-67+ cells still populated the WM, but now they began to appear in the IGL (not shown). By P18, both βgal/Gad-67 and EGFP/Pax2 cells were absent from the cerebellar WM; a few could still be identified in the IGL/PL and had a distinct migratory morphology (Supplementary Fig. 6 G–J). The βgal/Gad-67+ and βgal/Pax2+ GABA interneurons had now settled at the outer edge of the cerebellar ML, some of them presumably still migrating to their final destination (Fig. 4O–Q; Supplementary Fig. 6 A–F). These cells had adopted the distinct round soma of ML interneurons and were encircled by GAD-67 immunoreactivity (Supplementary Fig. 5 K–N). However, no Mash1 immunoreactivty was discernible in either these reporter+ ML interneurons or anywhere in the ML (data not shown), suggesting that this transcription factor is rapidly downregulated as the cells exit the WM compartment.
Recombinase-targeted GFAP promoter positive cells in the cerebellar WM express markers of neural stem cells
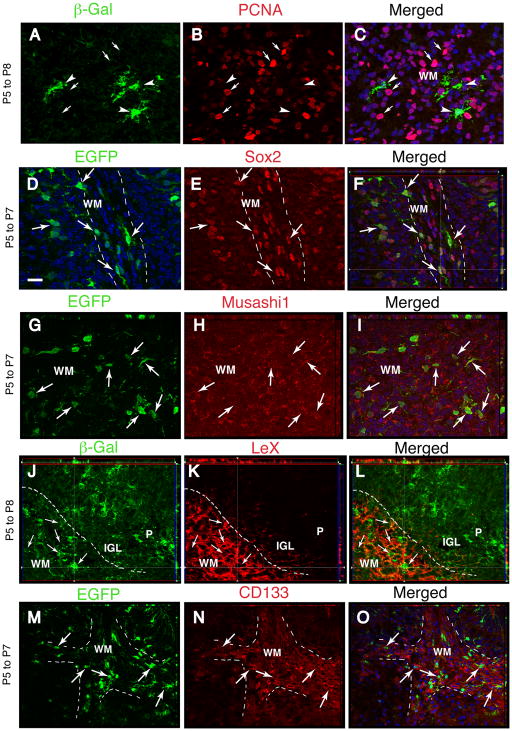
Because of the neurogenic potential of the glial cells targeted by the GFAP promoter, we ascertained whether there are cells with a biomarker profile of NSCs in the cerebellar WM. Some astrocyte-like cells have molecular and physiological properties of NSC/NPCs, particularly at immature stages of postnatal development 31, 32, 51, 52. For example, essentially all parenchymal astrocytes in the cerebral cortex express the NSC marker LeX in the first two weeks after birth 32. Furthermore, NSCs in the adult SVZ and dentate gyrus express GFAP and antigens typical of embryonic radial glia, including the intermediate filament nestin, the RNA-binding protein Musashi1, the transcription factor Sox2 and the cell surface glycoproteins LeX and CD133 22, 27, 53–56. In the cerebellar WM, virtually all βgal+ and EGFP+ cells at P7-P8 expressed Sox2 (Fig. 5D–F), Musashi1 (Fig. 5G–I), nestin (not shown), and LeX (Fig. 5J–L), but expressed low levels of CD133 (Fig. 5M–O). Interestingly, we did not detect LeX in the cerebellar cortex, including the Bergmann glia (Fig. 5J–L) or deep nuclei (Supplemental Fig. 3 D–F). Reporter+ Bergmann glial cells expressed nestin (Supplemental Fig. 2 G–I), Musashi1 and Sox2 (not shown).
Fig. 5. Reporter expression is induced in relatively quiescent cells expressing NSC/NPC markers in the cerebellar WM.
Double-immunostaining in deep (A–C and G–I) or interlobular WM (D–F, J–O) for βgal or EGFP reporters and PCNA or NSC/NPC markers (Sox2, Musashi1, LeX, CD133, as indicated) in sagittal sections from the cerebellum 200–400 μm from the midline. Mice were analyzed at P7-P8 after tamoxifen injections at P5. Broken lines delimit the lobular WM from adjacent regions of the cerebellar cortex. Merged images are shown in C,F,I, L,O. Blue shows DAPI nuclear staining. Arrows, double-labeled cells; arrowheads, βgal+ cells that are negative for PCNA; small arrows in A–C, PCNA-positive nuclei. All panels illustrate single representative 1 μm optical sections. Scale bar, 20 μm.
To further characterize the WM βgal+ cell population, we double-stained the sections with an antibody to Proliferating Cells Nuclear Antigen (PCNA). Most, but not all, βgal+ cells in the deep cerebellar WM (Fig. 5A–C) were negative for PCNA, and few of them incorporated BrdU (data not shown). The data suggest that reporter expression in WM is triggered in a cell population that is slowly proliferating, possibly in an extended G1 phase. In contrast, Bergmann glial cells demonstrated both PCNA immunostaining and BrdU incorporation (Supplemental Fig. 2 M–O) as already described 57.
In summary, reporter+ cells in WM, which are the likely source of ML interneurons, were slowly proliferating cells that displayed a GFAP/LeX/nestin/Musashi1/Sox2 and occasional CD133+ phenotype, while reporter+ Bergmann glia displayed a GFAP/Blbp/nestin/Sox2/Musashi1+ phenotype (see schematic outline in Supplemental Fig. 7).
DISCUSSION
Using mice expressing a temporally-inducible form of Cre recombinase, we tagged cells with GFAP promoter activity and glial phenotype in the first postnatal week and demonstrated that they generate both GABAergic interneurons and glia in the postnatal murine cerebellum. By inducing reporter gene expression in these glial cells at successive ages, we established that the birth of these differing cell types is temporally regulated during development. At P2-P5, a population of GFAP+/S100β+ astroglial cells located in the cerebellar WM appears to generate GABAergic interneurons for the ML (see Supplemental Fig. 7 for an outline). After P5, these cells cease generating basket interneurons for the ML, while still producing stellate interneurons. After P12, the in vivo fate of GFAP lineage cells is restricted to Bergmann glia and parenchymal astrocytes.
Our characterization of the reporter+ cells two-three days after recombination suggests that cells with GFAP promoter activity in the WM are a heterogeneous population that share characteristics of both glial and neural stem cells: 87% of these cells express GFAP protein, 12.3% express S100β, another astrocytic marker, and 2% or less express NG2 and the PDGFRα, markers for oligodendrocyte progenitors. It is likely that the S100β+ astroglia express GFAP protein below the limits of immunocytochemical detection, as do embryonic radial glia and parenchymal astrocytes in the neocortex 58, consistent with increasing evidence that astrocytes do not express uniform levels of marker proteins 59. Cre-targeted cells in the P5 cerebellar WM express markers typical of neural stem cells including nestin, Sox2, LeX and Musashi1; in this regard, immature targeted astroglia are similar to embryonic radial glia and NSCs in the postnatal SVZ and dentate gyrus of the hippocampus 27, 30, 60–62. We observed that 2% of the cells reporter-tagged using the GFAP promoter express the PDGFRα and are likely to be oligodendrocyte progenitors, which is similar to earlier fate mapping data of astroglial cells using the GLAST promoter 63. These cells may represent intermediate cellular phenotypes, which is also consistent with the idea that a subset of astroglial cells in the hippocampal parenchyma express NG2 64. These data suggest either that a small subset of oligodendrocyte progenitor cells have GFAP promoter activity, or that some oligodendrocyte precursor cells have arisen from astroglial cells in the 2 days elapsed from tamoxifen-induced targeting and analysis.
Our fate mapping data provide evidence that these GFAP+/S100β+ WM glial cells generate mitotic progenitors for GABAergic interneurons. We observed that approximately 50% of the Pax2+ cells in the WM at P12 and 30% of the ML interneurons at P35 are reporter+ and thus derive from GFAP promoter+ cells in the P5 cerebellum. When considering the incomplete targeting of the Cre transgene to all GFAP-expressing cells, early postnatal glial precursors may serve as the principal source of ML GABAergic interneurons in the cerebellum. Evidence for progressive neuronal differentiation of the tagged population is that while more than 99% of the reporter+ cells in WM expressed glial markers two days after recombination, 2% already co-localized Pax2, a gene expressed by GABAergic neuron precursors, and a small subset at the boundary between the cerebellar WM and the IGL expressed the neurogenic gene Mash1. Hence, the first step in the differentiation cascade appears to be the upregulation of Pax2 and Mash1 proteins in GFAP promoter+ glial cells. By P12, 7 days after targeting, 65% of the reporter+ cells express Pax2 in the WM. It is highly unlikely that the 2% reporter+/Pax2+ cells at P7 can give rise to such a high number of reporter+/Pax2+ cells by proliferation alone in 5 days. Rather, we suggest that 2 days after recombination cells are already transitioning from a glial to a Pax2+ phenotype, and that this progressive transformation into neuronal progenitors continues over the subsequent 5 days, which together with the proliferation of the Pax2+ cells, can account for the large number of reporter+/Pax2+ cells at P12.
While about 10% of these cells have already reached the ML at P12, reporter+ cells expressing Pax2 and Gad-67 are still largely restricted to the WM, strongly suggesting they are derived from this region. These cells assume a migratory morphology and are widely dispersed through the interlobule WM (Fig. 4). By P18, most of the Gad-67+/Pax2+/reporter+ cells reach their final destination in the ML and are no longer found in the WM, consistent with our data that neurogenesis from WM glia ceases by P12. In contrast to Pax2, Mash1 protein expression is confined to cells in the WM (Fig. 4), suggesting that this proneural gene is transiently expressed by GABA neuron precursors. To our knowledge, this is the first report implicating Mash1 in the early determination of ML GABA interneurons in the cerebellum.
These data indicate that not all cerebellar interneurons are specified during embryogenesis, but rather build on recent reports suggesting that postnatally generated cerebellar neurons originate de novo from a more naïve cell type active over the course of cerebellar development. The cerebellar WM is thought to contain mitotic progenitors for the ML interneurons in the first two weeks after birth 8–10, 39. Weisheit and colleagues have used a Pax2-GFP mouse line to study the Pax2/Gad-67 expressing population of interneuron progenitors. Their studies show that Pax2 is turned on near the final mitosis of these cells and conclude that they are derived from an earlier, but yet unidentified, precursor 11. We suggest that these precursors are the GFAP+/S100β+/Sox2+ cells in the WM which undergo a fate transition from glial to neuronal, presumably by upregulating Mash1, leading to their commitment and differentiation into Pax2+/Gad-67+ progenitors that migrate to the ML.
Because we are unable to target precise anatomical regions with the GCE mice, we cannot entirely exclude that other regions of the cerebellum may represent a source of ML interneurons, and the SEZ lining the 4th ventricle remains another possible candidate. However, we observe neither a highly mitotic cell population nor a stream of migratory cells expressing neuroblast markers, Pax2 or Gad-67, as one would expect of a germinal niche for interneurons (Supplemental Fig. 3). Similarly it is unlikely that Bergmann glia represent a source of GABA interneurons; although these cells, as already reported, express Sox2 65, they never express Pax2 or Mash1.
Because it is technically unfeasible to track the fate of single targeted cells within the cerebellum using this mouse model, we are unable to determine if the reporter+ neuronal and glial precursors represent lineally separate populations or are derived from an initially homogenous population whose fate is determined by the developmental niche in which they operate. Nor do we know whether all cells with GFAP promoter activity in the WM are neural precursors, although our observation that as many as 65% express Pax2 protein in the WM at P12 does suggest that a relatively high proportion is neurogenic. Future studies involving clonal analyses of temporally and spatially discrete populations of GFAP lineage cells, or the transplantation of GFAP+/S100β+ cells, may be required to more completely discern the lineage and potential of these pools of precursors.
This work extends a number of in vitro studies and transplantation experiments, which suggest the existence of a putative cerebellar NSC/NPCs in both the neonate and adult rodent 22, 23, 65,66. The present data are the first demonstration that populations of NSC/NPCs may indeed exist perinatally in vivo, in that the GFAP+/S100β+ glial cells are able to generate several neuronal subtypes in addition to glial cells and possess an antigenic profile similar to radial glia. In contrast to our in vivo results, neurosphere-generating NSC/NPCs can be isolated throughout life, even in the adult cerebellum 23. This discrepancy is likely due to different environmental cues present in vitro and in vivo, which would further suggest that environmental cues in the cerebellum restrict the fate of endogenous NSC/NPCs. However, it cannot be excluded that the NSC/NPCs isolated in vitro are a different population from the cells with GFAP promoter activity identified here. Future studies will probe the mechanisms that regulate the potential of GFAP+ progenitors and seek to determine if they possess capacity for self-renewal in vivo.
CONCLUSIONS
We demonstrate that GFAP+/S100β+ cells in the cerebellar WM at P2-P5 upregulate neuronal progenitor genes and migrate to the ML, acquiring basket/stellate interneuron phenotypes in the ensuing 10 days. These cells lose their neurogenic capacity by P12. Powerful in vivo cues govern the fate of glial NSC/NPCs; our animal model may provide an experimental system for their identification and therapeutic intervention.
Supplementary Material
Acknowledgments
We thank Dr. Paul Lombroso for making available the ApoTome system for this work. We thank Dr. Angelique Bordey for critical reading of a prior version of this paper, reagents and important suggestions. We are grateful to members of the Vaccarino laboratory for helpful discussions. This work was supported by P01 NS 35476.
Footnotes
Author Contributions: John Silbereis: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Elise Cheng: Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript
Yosif M. Ganat: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript
Laura R. Ment: Manuscript writing, Financial support, Final approval of manuscript
Flora M. Vaccarino: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Financial support, Final approval of manuscript
References
- 1.Ramon y Cajal S. Histology of the Nervous System. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 2.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Berlin, Heidelberg, New York: Springer; 1974. p. xii.p. 348. [Google Scholar]
- 3.Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Bayer SA. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978;179:23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- 5.Gould BB, Rakic P. The total number, time or origin and kinetics of proliferation of neurons comprising the deep cerebellar nuclei in the rhesus monkey. Exp Brain Res. 1981;44:195–206. doi: 10.1007/BF00237341. [DOI] [PubMed] [Google Scholar]
- 6.Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino M. Molecular machinery governing GABAergic neuron specification in the cerebellum. Cerebellum. 2006;5:193–198. doi: 10.1080/14734220600589202. [DOI] [PubMed] [Google Scholar]
- 8.Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(sici)1097-4695(19991105)41:2<281::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 10.Milosevic A, Goldman JE. Potential of progenitors from postnatal cerebellar neuroepithelium and white matter: lineage specified vs. multipotent fate. Mol Cell Neurosci. 2004;26:342–353. doi: 10.1016/j.mcn.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Weisheit G, Gliem M, Endl E, et al. Postnatal development of the murine cerebellar cortex: formation and early dispersal of basket, stellate and Golgi neurons. Eur J Neurosci. 2006;24:466–478. doi: 10.1111/j.1460-9568.2006.04915.x. [DOI] [PubMed] [Google Scholar]
- 12.Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Arie N, Bellen HJ, Armstrong DL, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 15.Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- 16.Lin JC, Cai L, Cepko CL. The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain. J Neurosci. 2001;21:159–168. doi: 10.1523/JNEUROSCI.21-01-00159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Altman J, Bayer S. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Function. Boca Raton, FL: CRC Press; 1997. p. 783. [Google Scholar]
- 19.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 20.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 21.Komuro H, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein C, Butt SJ, Machold RP, et al. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132:4497–4508. doi: 10.1242/dev.02037. [DOI] [PubMed] [Google Scholar]
- 24.Leto K, Carletti B, Williams IM, et al. Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. J Neurosci. 2006;26:11682–11694. doi: 10.1523/JNEUROSCI.3656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feil R, Brochard J, Mascrez B, et al. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzger D, Clifford J, Chiba H, et al. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 28.Graham V, Khudyakov J, Ellis P, et al. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 29.Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bani-Yaghoub M, Tremblay RG, Lei JX, et al. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 32.Ganat YM, Silbereis J, Cave C, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner M, Kisseberth WC, Su Y, et al. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Gen. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 36.Gundersen HJ, Bagger P, Bendtsen TF, et al. The new stereological tools: disector, fractionator, nucleator, and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 37.West MJ. New Stereological Methods for Counting Neurons. Neurobiology of Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 39.Yamanaka H, Yanagawa Y, Obata K. Development of stellate and basket cells and their apoptosis in mouse cerebellar cortex. Neurosci Res. 2004;50:13–22. doi: 10.1016/j.neures.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Fode C, Ma Q, Casarosa S, et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes & Development. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Q, Sommer L, Cserjesi P, et al. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. Journal of Neuroscience. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zordan P, Croci L, Hawkes R, et al. Comparative analysis of proneural gene expression in the embryonic cerebellum. Dev Dyn. 2008;237:1726–1735. doi: 10.1002/dvdy.21571. [DOI] [PubMed] [Google Scholar]
- 43.Kim EJ, Battiste J, Nakagawa Y, et al. Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci. 2008;38:595–606. doi: 10.1016/j.mcn.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 45.Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- 46.Nishiyama A, Lin XH, Giese N, et al. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Fruttiger M, Karlsson L, Hall AC, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 49.Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- 50.Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 51.Doetsch F, Caille I, Lim DA, et al. Subventricular Zone Astrocytes are Neural Stem Cells in the Adult Mammalian Brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 52.Laywell E, Rakic P, Kukekov VG, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis P, Fagan BM, Magness ST, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 54.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Sakakibara S, Nakamura Y, Satoh H, et al. Rna-binding protein Musashi2: developmentally regulated expression in neural precursor cells and subpopulations of neurons in mammalian CNS. J Neurosci. 2001;21:8091–8107. doi: 10.1523/JNEUROSCI.21-20-08091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imura T, Nakano I, Kornblum HI, et al. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- 57.Kamei Y, Inagaki N, Nishizawa M, et al. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23:191–199. doi: 10.1002/(sici)1098-1136(199807)23:3<191::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 58.Malatesta P, Hack MA, Hartfuss E, et al. Neuronal or Glial Progeny: Regional Differences in Radial Glia Fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 59.Kimelberg HK. The problem of astrocyte identity. Neurochem Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko Y, Sakakibara S, Imai T, et al. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 62.Miyagi S, Nishimoto M, Saito T, et al. The Sox2 regulatory region 2 functions as a neural stem cell-specific enhancer in the telencephalon. J Biol Chem. 2006;281:13374–13381. doi: 10.1074/jbc.M512669200. [DOI] [PubMed] [Google Scholar]
- 63.Mori T, Tanaka K, Buffo A, et al. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 64.Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 66.Snyder EY, Deitcher DL, Walsh C, et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.