Abstract
Previous work suggests a role for dopamine D3-like receptors in psychostimulant reinforcement. The development of new compounds acting selectively at dopamine D3 receptors has opened new possibilities to explore the role of these receptors in animal models of psychostimulant dependence. Here we investigated whether the dopamine D3 partial agonist CJB090 (1–10 mg/kg, i.v) and the D3 antagonist PG01037 (8–32 mg/kg, s.c.,) modified methamphetamine (0.05 mg/kg/injection) intravenous self-administration under fixed- (FR) and progressive- (PR) ratio schedules in rats allowed limited (short access, ShA; 1h sessions 3 days/week) or extended access (long access, LgA; 6h sessions 6 days/week). Under a FR1 schedule, the highest dose of the D3 partial agonist CJB090 selectively reduced methamphetamine self-administration in LgA but not in ShA rats, whereas the full D3 antagonist PG01037 produced no effect in either group. Under a PR schedule of reinforcement, the D3 partial agonist CJB090 reduced the maximum number of responses performed (“breakpoint”) for methamphetamine in LgA rats at the doses of 5 and 10 mg/kg and also it produced a significant reduction in the ShA group at the highest dose. However, the D3 full antagonist PG01037 only reduced PR methamphetamine self-administration in LgA rats at the highest dose of 32 mg/kg with no effect in the ShA group. The results suggest that rats might be more sensitive to pharmacological modulation of dopamine D3 receptors following extended access to methamphetamine self-administration, opening the possibility that D3 receptors play a role in excessive methamphetamine intake.
Keywords: methamphetamine, self-administration, partial agonist, dopamine, D3 receptor, dependence
INTRODUCTION
Psychostimulants are reinforcing drugs with high addictive potential. Much of the experimental attention specifically devoted over the past years to the study of psychostimulant dependence has focused on cocaine and amphetamine. Methamphetamine is the N-methyl-analog of amphetamine with significant pharmacological differences (Fleckenstein, et al., 2007) and has a particularly powerful addictive potential, a problem that has reached epidemic proportions over the last two decades (Degenhardt, et al., 2008; Derlet and Heischober, 1990; Maxwell and Rutkowski, 2008; McKetin, et al., 2008).
Preclinical animal models used for the study of psychostimulant dependence have begun to incorporate methamphetamine as a drug for investigation. Traditionally, studies to explore the neuropharmacological basis of the acute reinforcing effects of drugs of abuse and to investigate the preclinical effects of compounds with potential therapeutic value have used models of limited daily access to drug self-administration. Using these models, rats are allowed access to less than 3 hours of drug self-administration per day, thus producing highly stable levels of intake and patterns of responding between daily sessions. Based on conceptual grounds that addiction is characterized by a compulsion to take drugs and a loss of control in limiting intake (American Psychiatric Association, 2000), animal models of intravenous drug self administration with extended drug access that result in a progressive increase in drug intake (escalation) have been developed. These models are critical in our pursuit of examining the neurobiological mechanisms that underlie this component of addiction (Koob, 2008). As such, extended access models have been widely used to study various components of psychostimulant addiction including increased motivation to seek the drug and the loss of control over intake, which results in compulsive use (Kitamura, et al., 2006; Ahmed and Koob, 1998; Wee, et al., 2007; Orio, et al., 2009).
Previous preclinical work suggests a primary role for the mesolimbic dopamine system that projects to the basal forebrain in the acute rewarding effects of psychostimulants (Koob, 1992; Koob, et al., 1994) and altered dopamine neurotransmission in advanced states of the addiction cycle (Ahmed and Koob, 2004; Wee, et al., 2007). However, pharmacological attempts to modify the course of psychostimulant addiction in humans have met with very limited clinical success, and thus far, the use of receptor nonselective dopamine agonists and antagonists in treatment of addiction also has essentially failed (Withers, et al., 1995; Gorelick, et al., 2004).
Much evidence stemming from different experimental approaches supports the potential for compounds with partial agonistic properties at the level of dopamine receptors in psychostimulant dependence (Pulvirenti, et al., 1998; Pulvirenti and Koob, 2002; Pulvirenti and Koob, 1994; Wee, et al., 2007). Partial agonists have lower intrinsic activity at receptors than full agonists (Hoyer and Boddeke, 1993), allowing them to act either as functional agonists or as functional antagonists, depending on the endogenous levels of the naturally occurring neurotransmitter. Under normal conditions, and especially in conditions of dopamine depletion, partial agonists show functional agonist activity; in the presence of a full agonist, partial agonists show functional antagonist properties.
The development of new compounds acting selectively at dopamine D3 receptors, over the past decade, has stimulated attention on the potential use of these compounds for treatment of psychostimulant dependence (Heidbreder, 2008; Heidbreder, et al., 2005). For example, the dopamine D3 partial agonist BP897 reduced relapse to cocaine intake produced by drug-associated environmental stimuli (Pilla, et al., 1999), blocked amphetamine– and cocaine-induced conditioned locomotor activity (Aujla, et al., 2002; Le, et al., 2002) and the D3 receptor agonist PD-128907 reduced responding for cocaine. This hypothesis was translated into clinical studies where both the D3 partial agonist BP897 and the D2 partial agonist aripiprazole underwent Phase I clinical studies (N.I.D.A. Medications Development, 2004). More recently, GlaxoSmithKline has initiated clinical trials for substance dependence, with an initial focus on nicotine, using a novel D3-selective antagonist (see http://clinicaltrials.gov).
In order to extend previous preclinical studies to incorporate the recently developed models of transition from moderate to excessive drug intake, the present study was specifically designed to test the effects of the D3 receptor partial agonist CJB090 and the D3 receptor antagonist PG01037 on methamphetamine self-administration in animals given limited and extended access. We tested the effects of these compounds on methamphetamine maintained behavior under a fixed-ratio and a progressive-ratio schedule of reinforcement. Results based on these studies suggest that compounds acting at dopamine D3 receptors may be useful for reducing compulsive methamphetamine intake associated with the development of addiction and in this model the D3-selective partial agonist, CJB090 was more effective than the D3 antagonist PG01037.
MATERIALS AND METHODS
Animals
Forty-two adult male Wistar rats (Charles River, Hollister, CA) weighting 225–250 g were used at the start of the experiment. Rats were housed 2 or 3 per cage with food and water available ad libitum, and maintained in a 12 h light/dark cycle, with lights on at 6 am. Self-administration sessions took place during the dark cycle, the active cycle of the rats. Seven rats in the ShA group and nine rats in the LgA group did not complete the experiments due to catheter failure, health complications or because they did not acquire self-administration behavior. Two rats were re-catheterized into the left jugular vein. All procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines (NIH Publication no. 85-23, revised 1996).
Intravenous surgery
Rats were anesthetized with 2–3% of isofluorane mixed in oxygen and implanted with a sterilized silastic catheter (0.64 ID × 1.19 OD mm; Dow Corning Co. Midland, MI) into the right jugular vein under aseptic conditions. The distal end of the catheter was threaded under the skin to the back of the rat and exited the skin via a metal guide cannula (22G, Plastic One, Inc, Roanoke, VA). Immediately after surgery, Flunixin® (2.5 mg/kg, s.c.) was given as analgesic. The rats were subjected to antibiotic therapy with Timentin® (20 mg, i.v, once daily) during 10 days after the surgeries, and Sulfamethoxazole-Trimethoprim Oral Suspension (TMS, Hi-Tech Pharmacal Co., Inc., Amityville, New York) in the drinking water (0.48 mg/ml) when health complications appeared during the study. Catheters were flushed daily with heparinized saline (30 USP units/ml) and tested eventually for patency using methohexital sodium (Brevital®, 10 mg/ml, 2 mg/rat).
Methamphetamine self-administration procedure
After at least 5-days of post-operatory recovery, rats were trained to self-administer methamphetamine in a 1 h session under a fixed ratio 1 (FR1) schedule of reinforcement. At the beginning of each session rats were presented with 2 response levers into the chamber. Responding on the front lever resulted in the delivery of 0.1 ml of drug over 4 sec and the illumination of a light above the lever that lasted throughout a time-out period of 20 sec. Responding on the back lever was recorded but had no programmed consequences. Rats were trained to self-administer a high methamphetamine dose of 0.1 mg/kg/injection during two days and then the dose of methamphetamine was reduced to 0.05 mg/kg/injection. After 8 days of baseline period the rats were divided into two groups balanced by the number of methamphetamine injections per session during the last three baseline sessions. One group of rats were exposed to methamphetamine (0.05 mg/kg/injection) during daily 6 h sessions (long access, LgA), whereas the other group of rats was exposed to intermittent 1 h session/day 3 days per week (short access, ShA) during a total of 15–19 sessions. The ShA group has been used in this study as a control for the extended access (LgA) group since we have previously observed that an intermittent limited access to methamphetamine leads to a very stable pattern of methamphetamine self-administration (Mandyam, et al., 2007). The dose of methamphetamine (0.05 mg/kg/infusion) used in this study was carefully selected based on our previous studies (Kitamura, et al., 2006; Wee, et al., 2007) and accounts for a highly stable self-administration behavior in rats. This dose of methamphetamine is within the range of the descending limb of the methamphetamine dose-response self-administration curve in rats, and is appropriate to measure reliable and stable dose-dependent effects (Kitamura, et al., 2006; Wee, et al., 2007).
CJB090 and PG01037 treatments on methamphetamine self-administration
CJB090 was tested in a FR1 schedule after 18 methamphetamine sessions. CJB090 was injected immediately before self-administration (0.0, 1.0, 5.0 and 10.0 mg/kg, i.v.) following a within-subject Latin square design. In a different cohort of animals, PG01037 (0.0, 8.0, 16.0 and 32.0 mg/kg, s.c.) was injected 30 min before self-administration and following a Latin square design. Then, the effects of CJB090 and PG01037 were tested under a progressive ratio (PR) schedule of reinforcement. PR is a schedule tailored to specifically measure the relative reinforcing properties of a drug. In the PR schedule the number of lever presses required to receive a single i.v. methamphetamine infusion was progressively raised following an ascending series of lever press/injection: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, …etc, and ended when the rat failed to achieve the response requirement within 1 h (Richardson and Roberts, 1996). The break points measure the maximum amount of “work” each individual is willing to perform under a PR schedule for a single dose of reinforcer. Test sessions under FR or PR schedules were always separated by baseline sessions (FR1) (one session per day) to maintain methamphetamine intake under extended or limited conditions and assure a stable baseline in order to exclude a possible remaining pharmacological effect of the compound tested in the last test session. For the PR testing, all rats were injected with vehicle and tested in additional PR sessions before and after the Latin Square design. Performances under the PR schedule in vehicle-injected rats did not differ across the study (one-way ANOVA, p>0.05, n.s.). The doses and routes of administration of CJB090 and PG01037 were chosen based on published studies (Collins, et al., 2005; Martelle, et al., 2007). For CJB090, the effective doses in the primate were 0.3–3.0 mg/kg intravenous. Extrapolation to the rat based on interspecies drug scaling (Mordenti and Chappell, 1989) resulted conservatively in doses of 1,5 and 10 mg/kg and the intravenous route was retained. For PG01037, the I.C. 50 doses for blocking l-Dopa and apomorphine-induced abnormal involuntary movements in the rat were 7.4 mg/kg i.p. and 18.4 mg/kg i.p., respectively.
Drugs
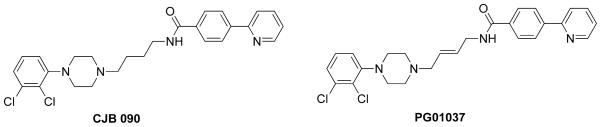
Methamphetamine hydrochloride (National Institute on Drug Abuse, Baltimore, MD) was dissolved in sterile 0.9% saline and filtered for self-administration. Dose of methamphetamine (0.05 mg/kg/injection) was calculated as a function of rat body weight and updated every 3–4 days. CJB090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide) and PG01037 (N-(4-(-(2,3-dichlorophenyl)-piperazin-1-yl)-trans-but-2-enyl)-4-pyridine-2-ylbenzamide) (Fig. 1) were obtained from N.I.D.A.-Medicinal Chemistry Section (Baltimore, MD) and were synthesized as described in (Newman, et al., 2003; Grundt, et al., 2005). Both CJB090 and PG01037 were dissolved in 5% 2-hydroxyl-β-cyclodextrin in saline and filtered. These compounds were formulated as the HCl salts and doses reflect these.
Figure 1. Molecular structure of D3 receptor ligands.
CJB090, a dopamine D3 partial agonist. PG01037, a dopamine D3 antagonist.
Data analyses
Data were analyzed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). All data are presented as means ± S.E.M. Two-way analysis of variance (ANOVA) with repeated measurements was used to compare methamphetamine intake between groups and the effects of CJB090 or PG01037 on methamphetamine self-administration under FR or PR schedules. Additionally, one-way ANOVA followed by Dunnett’s Multiple Comparison test (comparison only with control animals) or Newman-Keuls post hoc test were conducted on simple main effects or when a significant interaction was found. Appropriate pairings were compared using Bonferroni post-hoc test (after two-way ANOVA) which includes pair-wise multiple-comparison correction.
RESULTS
Methamphetamine self-administration under a FR1 schedule in LgA and ShA rats
CJB090 group
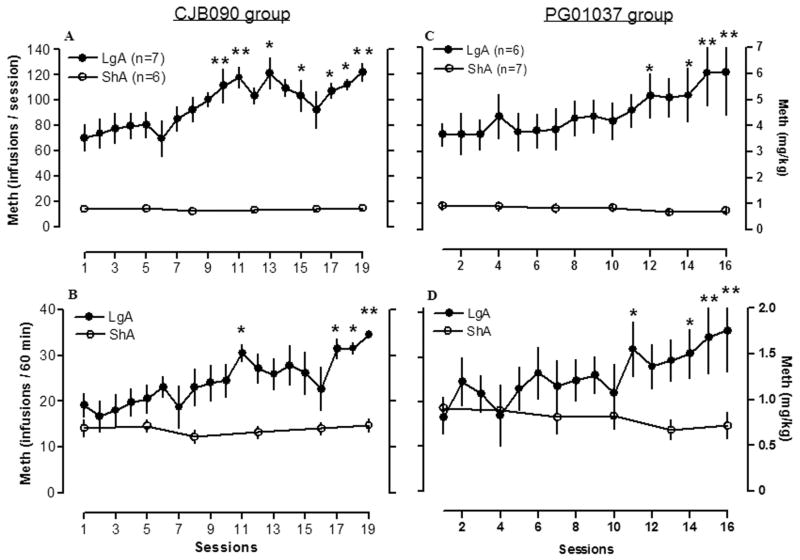
Methamphetamine intake (in milligram per kilogram) for the CJB090 group (future D3 partial agonist group) of animals is illustrated in Figure 2a (entire sessions) and Figure 2b (first hour sessions). Data analyses of methamphetamine intake during the first hour of the session by ShA rats and LgA rats (n=6–7) revealed a main effect of daily session (F5,55 =4.45, p<0.001), main effect of access (F1,55 =22.41, p<0.001), and an overall interaction (F5,55 =4.94, p<0.001). Methamphetamine self-administration in the first hour of the session by LgA rats significantly increased in sessions 11, 17, 18 and 19 and no effect was observed in the ShA group (F5,30 =13.63, n.s.) (Fig. 2b). Within a 6 h session, there was an interaction (access × session) (F5,55 =4.45, p<0.01) and main effects of daily session (F5,55 =4.3, p<0.01) and access (F1,55 =184.8, p<0.001). Additional analysis revealed that methamphetamine self-administration by LgA was increased starting in session 10 until session 19 (Fig. 2a).
Figure 2. Methamphetamine self-administration under a FR1 schedule by LgA and ShA rats.
Methamphetamine self-administration (0.05 mg/kg/injection) gradually increased over days in rats given extended access to the drug (6h/day daily; LgA, filled circles), whereas methamphetamine intake remained stable over days in rats with limited access to the drug (1h/day every 2–3 days, ShA, opened circles). Left panels (A, B) are data from animals in the D3 partial agonist (CJB090) study, and right panels (C, D) are data from animals in the D3 antagonist (PG01037) study. Top panels (A, C) are data from the entire sessions (6h for LgA rats and 1h for ShA rats), and bottom panels (B, D) are data from the first hour of the sessions. Data are expressed as the mean ± SEM of the number of methamphetamine injections on the left axis and mg/kg on the right axis. *p<0.05, **p<0.01, compared to respective session 1.
PG01037 group
Methamphetamine intake for the PG01037 cohort of animals (future D3 antagonist group) is illustrated in Fig. 2c (entire sessions) and Fig. 2d (first hour sessions). There was a significant interaction between group and daily sessions in methamphetamine self-administration between LgA and ShA rats (n=6–7) in the first hour of the sessions (F5,55 = 5.65; p<0.001) and within the overall session (F5,55 = 5.24; p<0.001), and main effects of daily session (F5,55 =3.80; p<0.01) and access (F1,55 = 22.40; p<0.001) within the 6h sessions. LgA rats significantly increased the rate of methamphetamine self-administration in sessions 11, 14, 15 and 16 during the first hour (Fig. 2d), and the increase was significant from session 12 throughout all self-administration sessions (Fig. 2c). No change in the rate of methamphetamine self-administration was observed in the ShA group (F5,30 =16.46, n.s.) (Fig. 2c, d).
Effect of CJB090 on methamphetamine self-administration under FR1 and PR schedules of reinforcement
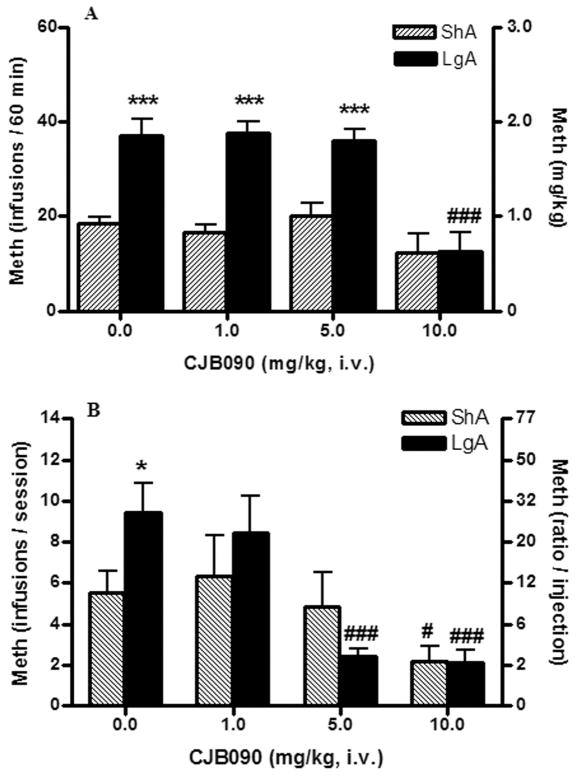
Under a FR1 schedule of reinforcement, the D3 partial agonist CJB090 reduced responding for methamphetamine during one hour session at the highest dose of 10 mg/kg selectively in LgA rats (F3,15= 24.61; p<0.001) and no effect was observed in the ShA group at any dose tested (F3,18= 2.03; n.s.) (Fig 3, upper panel). Two-way ANOVA revealed an interaction (access × session) between LgA and ShA rats (F3,33 = 7.71; p<0.001), and a main effect of access (F1,33 = 26.77; p<0.001) and dose (F3,33 = 20.21; p<0.0001).
Figure 3. Effect of CJB090 on methamphetamine self-administration under FR1 and PR schedules of reinforcement.
The dopamine D3 partial agonist CJB090 was injected i.v. immediately before a methamphetamine (0.05mg/kg/injection) test session. Data on the top graph (A) correspond to test sessions under FR1 schedule of reinforcement and data on the bottom graph (B) are from sessions under a PR schedule. Sessions under a FR1 schedule lasted 1h and sessions under a PR schedule ended when rats failed to achieve methamphetamine reinforcement within 1h (see methods for details). Filled bars are methamphetamine extended access rats (6h/session daily, LgA, n=7) and stripped bars are methamphetamine limited access rats (1h/session every 2–3 days, ShA, n=6). Data are expressed as the mean ± SEM of the number of methamphetamine injections per session on the left axis (A and B) and methamphetamine intake in mg/kg (A) or ratio/injection (B) on the right axis. Different between access conditions: *p<0.05, ***p<0.001. Different to vehicle-injected rats in the same access condition: #p<0.05, ###p<0.001.
Under a PR schedule of reinforcement (Fig. 3, bottom panel), LgA and ShA vehicle-injected rats achieved statistically different break points (t-test, p<0.05). Two-way ANOVA found an interaction (dose × access) (F3,33 = 4.03; p=0.015), and a main effect of the dose (F3,33 = 15.57; p<0.0001). Additional analysis revealed that CJB090 reduced methamphetamine break points in LgA rats at doses of 5mg/kg and 10 mg/kg (F3,18 = 15.37; p<0.0001) and there was also a reduction in responding for methamphetamine in the ShA group at 10 mg/kg of CJB090 (F3,15 = 7.14; p<0.05).
Effect of PG01037 on methamphetamine self-administration under FR1 and PR schedules of reinforcement
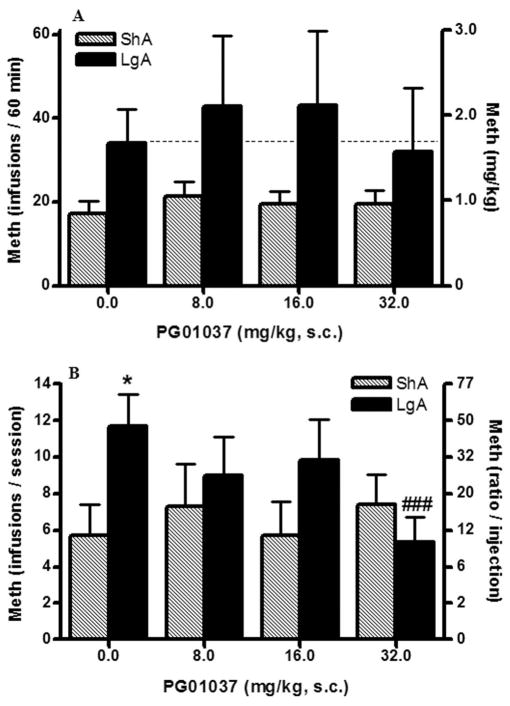
When the D3 antagonist PG01037 was tested under a FR1 schedule, no interaction or main effect was found between groups (F3,27 = 0.89; p=0.5, n.s.) (Fig. 4, upper panel).
Figure 4. Effect of PG01037 on methamphetamine self-administration under FR1 and PR schedules of reinforcement.
The dopamine D3 antagonist PG01037 was injected s.c. 30 min before a methamphetamine (0.05mg/kg/injection) test session. Data on the top graph (A) correspond to test sessions under FR1 schedule of reinforcement and data on the bottom graph (B) are from sessions under a PR schedule. Sessions under a FR1 schedule lasted 1h and sessions under a PR schedule ended when rats failed to achieve methamphetamine reinforcement within 1h (see methods). Filled bars are methamphetamine extended access rats (6h/session daily, LgA, n=5–6) and stripped bars are methamphetamine limited access rats (1h/session every 2–3 days, ShA, n=6–7). Data are expressed as the mean ± SEM of the number of methamphetamine injections per session on the left axis (A and B) and methamphetamine intake in mg/kg (A) or ratio/injection (B) on the right axis. Different between access conditions: *p<0.05. Different to vehicle-injected rats in the same access condition: ###p<0.001.
Under a PR schedule (Fig. 4, bottom panel), two-way ANOVA revealed an interaction (access × dose) (F3,27 = 8.18; p<0.001) and a main effect of dose (F3,27 = 3.38; p<0.05). Additional analysis revealed that LgA and ShA vehicle-injected rats achieved statistically different break points after vehicle injection (t-test, p<0.05) and PG01037 reduced methamphetamine break points at the highest dose selectively in the LgA group (F3,15 = 3.90; p<0.05) with no effect observed in the ShA group (F3,18 = 37.46; p>0.05, n.s.).
DISCUSSION
Dopamine D3 receptor subtypes originally were hypothesized to play a pivotal role in modulating the reinforcing and drug-seeking effects induced by psychostimulants (Caine and Koob, 1993; Pilla, et al., 1999). However, recent studies suggest that dopamine D3 receptors may be implicated in the motivation to self-administer drugs under schedules with high response requirements -rather than be involved in the direct reinforcing effects of drugs of abuse- and strongly modulate the influence of environmental stimuli on drug-seeking behaviour (Heidbreder, 2008; Le, et al., 2005a). Until recently, extensive pharmacological investigations have been hampered by the lack of highly D3 receptor selective compounds that can be used in vivo. Indeed, another complicating factor is that although functional coupling of D3 receptors to Gαi/o-proteins has been established (Newman-Tancredi, et al., 1999; Cussac, et al., 1999), the question of which G-protein signaling pathways are recruited by D3 receptor activation in vivo remains unanswered. Nevertheless, the fact that several D3 antagonists and partial agonists have demonstrated efficacy in animal models of drug abuse without the concomitant motor side effects associated with nonselective D2 antagonists (Achat-Mendes, et al., 2009; Micheli, et al., 2007), supports further pursuit of the D3 receptor as a potential target for medication development (for review see (Heidbreder, 2008; Le, et al., 2007).
In an early study, pre-treatment with D2/D3 agonists in rats decreased dose-dependently cocaine self-administration under a fixed ratio schedule following limited access to the drug (Caine and Koob, 1993), which was interpreted as a potentiation of the reinforcing effects of cocaine. Consistent with these results, the D3 antagonist SB-277011-A inhibited cocaine-triggered reinstatement of cocaine seeking and blocked enhancement of electrical brain stimulation reward by cocaine (Vorel, et al., 2002). D3 partial agonists such as BP 897 and CJB090 reduced cue-induced reinstatement of cocaine seeking and cocaine self-administration in rats and non-human primates, respectively (Martelle, et al., 2007; Pilla, et al., 1999). The D3 antagonists SB-277011-A and NGB 2904 also inhibited cocaine-associated cue-induced reinstatement of drug-seeking behavior (Di, et al., 2003; Gal and Gyertyan, 2006; Gilbert, et al., 2005) In addition, compounds selective for D3 receptors and characterized as partial agonists in vitro (Pilla 1999, Grundt et al., 2007), can attenuate the discriminative stimulus and reinforcing effects of cocaine while not producing cocaine-like effects (Martelle, et al., 2007; Pilla, et al., 1999; Beardsley, et al., 2001).
The discovery of new compounds selectively acting at dopamine D3 receptors together with the hypothesis that partial agonist activity, as opposed to full antagonist activity at dopamine D3 receptors, may be of potential use for treatment of several aspects of methamphetamine addiction, led us to study the effects of two new compounds acting at D3 receptors in an animal model of compulsive methamphetamine intake. Thus, we tested the effects of the D3 receptor compounds, CJB090, a partial agonist, and PG01037, a full antagonist, in rats given limited and extended access to methamphetamine self-administration. Our results suggest a role for dopamine D3 receptor subtypes in reducing excessive methamphetamine intake associated with compulsive psychostimulant use in advanced stages of the methamphetamine addiction process.
The present results confirm that animals given extended access to methamphetamine showed increased rates of self-administration (Kitamura, et al., 2006; Wee, et al., 2007) and increased responding under PR schedules, which may indicate high motivation to seek the drug and/or high reinforced activity of methamphetamine in the LgA group (Wee, et al., 2007; Paterson and Markou, 2003; Orio, et al., 2009). In this regard, this animal model with extended session duration has been hypothesized to mimic the transition from moderate to excessive drug intake associated with addiction, since human abusers progressively increase drug consumption during the development of the addiction process (American Psychiatric Association, 2000, (Koob, 2008). In the present study we used an intermittent short access group as a control for the extended access group, which has been used previously in our studies (Mandyam, et al., 2007). However, this group represents both short access and intermittent access and it is not clear exactly which of the controlling factors (short duration of exposure or more intermittent exposure) is responsible for the effects observed. Previous work has shown that daily short access to methamphetamine can result in a modest escalation in intake over time whereas intermittent short access did not lead to an escalation in intake (Mandyam et al., 2007).
The D3 partial agonist CJB090 reduced methamphetamine self-administration under a fixed ratio schedule and the maximum number of responses performed for a single injection of the drug under a progressive ratio schedule in the LgA group at medium and high doses. The D3 full antagonist PG01037, in contrast, reduced responding only under a progressive ratio schedule and then only at the highest dose tested. These results support the hypothesis that compounds acting at dopamine D3 receptors with partial agonist activity may be more useful in reducing excessive methamphetamine intake associated with extended access compared with full antagonists. More specifically, partial agonists may block the increased DA transmission during methamphetamine self-administration and correct the low dopaminergic tone in LgA rats, which was has been observed in dependent subjects in absence of methamphetamine (Hoefer, et al., 2006; Kitamura, et al., 2006; Volkow, et al., 2009). This dual action makes DA partial agonists possibly preferable as anti-addiction drugs than full antagonists (Koob, et al., 2009).
The doses of partial agonist used in the present study correspond reasonably well with the doses in the literature if one considers interspecies pharmacokinetic differences as elaborated by the well known principle of interspecies drug scaling (McCann and Ricaurte, 2001; Mordenti and Chappell, 1989). Smaller animals require higher doses of drug, on a mg/kg basis, to achieve the same effect. For methamphetamine the elimination half life in the rat is 70 min versus 12 h in humans (Cook, et al., 1993; Melega, et al., 1995). To model the plasma levels of methamphetamine observed in humans (range of 1–3 microM) where humans received a dosing of 0.5 mg/kg every 3 hours (Melega, et al., 2007), these pharmacokinetic variables needed to be taken into account and rats received an intravenous dosing every 15 min. demonstrating a 12 fold difference between rat and human (Cho, et al., 2001). Using functional endpoints, approximately 0.32 mg/kg intramuscular methamphetamine produced a peak locomotor stimulating dose in monkeys (Crean, et al., 2006) whereas the peak locomotor stimulating effect of methamphetamine in the rat is approximately 3 mg/kg subcutaneous (Schindler, et al., 2002). Regarding toxic doses, 4–6 mg/kg methamphetamine in a monkey risks a lethal outcome (Madden, et al., 2005) and the lethal dose in humans is approximately 14 mg/kg (Baselt, 1982) whereas the LD 90 in the rat is 100 mg/kg (Derlet, et al., 1990). In summary, there is approximately a 10 fold+ difference in dosing between human/monkey and rat, thus providing a rationale for the dose parameters used in the present series of studies.
Although CJB090-induced reduction in methamphetamine self-administration and break points in the LgA group are likely due to D3 receptor blockade, one cannot exclude the possibility that high doses of CJB090 may also affect D2 receptors. The D3 partial agonist CJB090 has approximately 50-fold selectivity at the D3 receptor compared with the D2L receptor (Grundt, et al., 2005). Also, the doses of CJB090 (5 and 10 mg/kg, i.v.) that were effective in reducing excessive methamphetamine intake are equivalent to those used in a previously reported study in rhesus monkeys (Martelle, et al., 2007), nevertheless, there is the possibility that off-target effects, such as D2 partial agonist activity (Grundt, et al., 2007) could play a role in the behaviors observed. This possibility may account for the reduction in methamphetamine self-administration observed in ShA rats at the highest dose of CJB090
Therefore, the present results, although not unequivocally attributable to action on D3 receptors, confirm that CJB090, a drug with a preferential D3 partial agonist profile differentially modifies methamphetamine-seeking behavior following extended exposure to the drug. The present results are similar to the different sensitivity shown by LgA and ShA rats to the partial agonist aripiprazole (Wee et al., 2007) where, the nonselective D2 partial agonist aripiprazole reduced responding by methamphetamine LgA and ShA rats under both a progressive- and a fixed-ratio schedule, although LgA rats were more sensitive to these effects (Wee, et al., 2007). These results further support the hypothesis that following transition from moderate to excessive drug intake sensitivity to pharmacological intervention changes.
Additionally, unlike D2 receptor blockade, selective compounds acting at D3 receptors appear not to interfere with normal on going activities, showing a lack of effect on conditioned responses to natural reinforcers and the absence of aversive-like, sedative, or locomotor impairing effects (Le, et al., 2005b; Duarte, et al., 2003b; Duarte, et al., 2003a; Spiller, et al., 2008; Pak, et al., 2006). This adds to the potential of this class of compounds for a possible use in human addicts and represents an important finding of heuristic value from a clinical perspective.
PG01037 has been shown to be a selective full D3 receptor antagonist in vivo (Collins, et al., 2005; Collins, et al., 2008; Collins, et al., 2009; Grundt, et al., 2005; Grundt, et al., 2007). In the present study PG01037 did not modify methamphetamine self-administration under a FR1 schedule but reduced methamphetamine break points in the LgA group, at a dose (32 mg/kg, s.c.) that has been shown to be D3 selective in rats (Collins, et al., 2005; Collins, et al., 2008; Collins, et al., 2009). In previous studies the full D3 antagonist SB277011-A was shown to blunt the motivation to self-administer cocaine under PR schedule or a FR schedule with a high response requirement (FR10) (Xi, et al., 2005). One hypothesis would be that D3 receptor antagonists decrease specifically the motivation to take methamphetamine, which is particularly observable under conditions of high response requirement (“price”) to obtain the drug (such as performances under PR schedules) and/or they are effective only when low methamphetamine brain concentrations are reached (i.e. in PR conditions compared to FR1 conditions). However, methamphetamine self-administration by ShA rats -which is sustained at lower methamphetamine concentrations than LgA rats- was not reduced by PG01037, which appears to exclude a simple version of this hypothesis. Alternatively, adaptations in dopamine D3 receptors may be hypothesized to take place in advanced states of the addictive process that may contribute to the excessive methamphetamine intake observed in LgA rats. This hypothesis is supported by the elevated levels of D3 receptor mRNA and binding in brains of long-term cocaine abusers (Segal, et al., 1997; Staley and Mash, 1996) and in animals treated chronically with cocaine (Le, et al., 2002). The upregulation of dopamine receptors with chronic administration of psychostimulants appears to be selective for D3 receptors, since no changes in other dopamine receptor subtype densities or binding were observed in animal brains after cocaine or methamphetamine (Le, et al., 2002; Wee, et al., 2007).
The full D3 antagonist PG01037 also binds to the 5-HT1a receptor. Although PG01037 shows a 65-fold selectivity at D3 compared with 5-HT1a receptors (Kumar, et al., 2009), it is possible that at least some of the effects observed here might be due to additional activity at serotonin terminals. This is a possibility that remains to be explored since no information is available to date regarding intrinsic activity of PG01037 at 5-HT1a receptors (Kumar, et al., 2009). However, it is noteworthy that the lowest effective dose reported here (32 mg/kg) is the same dose that was necessary in previous behavioral studies to antagonize D3-mediated effects (Collins, et al., 2005; Collins, et al., 2008; Collins, et al., 2009). Also, the effects of PG01037 on L-dopa-induced involuntary movements in rats unilaterally lesioned in the medial forebrain bundle was unaffected by treatment with the selective 5-HT1a antagonist WAY 100635.
The exact neuropharmacological mechanism by which dopamine D3 receptors could modulate the excessive psychostimulant intake in extended access animals remains unclear. D3 receptors are located in both presynaptic and postsynaptic cells (Sokoloff, et al., 1990) and the anti-addiction properties of D3-acting compounds are hypothesized to be mediated by blockade of D3 postsynaptic receptors, whereas blockade of D3 presynaptic receptors may augment DA release and enhance synaptic DA (Spiller, et al., 2008). The NAc appears to be a primary area through which D3 ligands may act (Gainetdinov, et al., 1996; Gilbert, et al., 1995). Thus, local administration of SB177011-A into the NAc blocked reinstatement of stress-induced cocaine-seeking behavior (Xi, et al., 2004). However, other areas such as the amygdala and the somatosensory cortex have been also proposed (Levesque, et al., 1992; Frances, et al., 2004; Le, et al., 2002).
Partial dopamine agonists may be particularly effective for the treatment of psychostimulant addiction in humans for several reasons. Clinical experience has shown that chronic treatment with direct dopamine agonists is usually accompanied by side effects which may be particularly undesirable and harmful in the context of addiction therapy. For example, a tendency towards impulsive behavior and emergence of psychotic reactions, two side effects associated with repeated administration of dopamine agonists in patients (Weintraub, et al., 2006; Wolters, 1999), are likely to be important limiting factors in addiction therapy. In contrast, partial agonists appear to be much less likely to produce these untoward side effects (Hamidovic, et al., 2008; Voronin, et al., 2008). Also, in conditions of high functional dopamine activity like in the case of psychostimulant intoxication, partial dopamine agonists, due to their high affinity for dopamine receptors but low intrinsic activity, are likely to produce an antagonist-like effect. Although partial agonists now represent an important component of opiate and nicotine addiction therapy (Jimenez-Ruiz, et al., 2009; Gowing, et al., 2009), the relative lack of clinical effective compounds acting on dopamine receptors, especially on D3 receptors which are probably a critical target, has not allowed appropriate clinical investigation. The importance of drug design efforts aiming at partial agonists selectively active on dopamine receptor subtypes is therefore of paramount importance.
In summary, the present study further supports the involvement of D3 receptors in psychostimulant abuse and addiction and suggests that a D3 partial agonist is particularly effective in blocking the increased motivation for self-administration of methamphetamine observed with extended access. Further studies will be necessary to explore the effects of drugs with different agonist, partial agonist or antagonist profiles within the D3 agonist-antagonist continuum. Specifically, follow-up studies with more selective D3 partial agonists (Grundt, et al., 2007; Newman, et al., 2009) are planned to further investigate these observations and potentially clarify the roles of intrinsic activity and D3-selectivity in the attenuation of methamphetamine self administration. These studies provide a rationale to continue the exploration of highly selective D3 partial agonists as pharmacotherapeutic compounds for the treatment of methamphetamine addiction.
Acknowledgments
This study was supported by N.I.D.A. grant DA10072 (G.F.K.), N.I.D.A.-Intramural Research Program, NIH (A.H.N.) and the Pearson Center for Alcoholism and Addiction Research. L.O. thanks Spanish MICINN, F.E.C.Y.T. and Fulbright Program for postdoctoral Fulbright Award (FU-2006-0200). This is publication number 20493 of the Scripps Research Institute.
Footnotes
Disclosure/Conflicts of Interest
The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology (Berl) 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology (Berl) 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. IV-TR. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Aujla H, Sokoloff P, Beninger RJ. A dopamine D3 receptor partial agonist blocks the expression of conditioned activity. Neuroreport. 2002;13:173–176. doi: 10.1097/00001756-200201210-00039. [DOI] [PubMed] [Google Scholar]
- Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. 2 Davis, Calif: Biomedical Publications; 1982. [Google Scholar]
- Beardsley PM, Sokoloff P, Balster RL, Schwartz JC. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav Pharmacol. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truccone A, Haji-Abdi F, Newman AH, Grundt P, Rice KC, Husbands SM, Greedy BM, Enguehard-Gueiffier C, Gueiffier A, Chen J, Wang S, Katz JL, Grandy DK, Sunahara RK, Woods JH. Proerectile effects of dopamine D2-like agonists are mediated by the D3 receptor in rats and mice. J Pharmacol Exp Ther. 2009;329:210–217. doi: 10.1124/jpet.108.144048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol Pharmacol. 1999;56:1025–1030. doi: 10.1124/mol.56.5.1025. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Roxburgh A, Black E, Bruno R, Campbell G, Kinner S, Fetherston J. The epidemiology of methamphetamine use and harm in Australia. Drug Alcohol Rev. 2008;27:243–252. doi: 10.1080/09595230801950572. [DOI] [PubMed] [Google Scholar]
- Derlet RW, Albertson TE, Rice P. Antagonism of cocaine, amphetamine, and methamphetamine toxicity. Pharmacol Biochem Behav. 1990;36:745–749. doi: 10.1016/0091-3057(90)90071-o. [DOI] [PubMed] [Google Scholar]
- Derlet RW, Heischober B. Methamphetamine. Stimulant of the 1990s? West J Med. 1990;153:625–628. [PMC free article] [PubMed] [Google Scholar]
- Di CP, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Duarte C, Biala G, Le BC, Hamon M, Thiebot MH. Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacology (Berl) 2003a;166:19–32. doi: 10.1007/s00213-002-1310-0. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003b;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Frances H, Le FB, Diaz J, Smirnova M, Sokoloff P. Role of DRD3 in morphine-induced conditioned place preference using drd3-knockout mice. Neuroreport. 2004;15:2245–2249. doi: 10.1097/00001756-200410050-00021. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS. In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. Eur J Pharmacol. 1996;308:261–269. doi: 10.1016/0014-2999(96)00300-7. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gilbert DB, Millar J, Cooper SJ. The putative dopamine D3 agonist, 7-OH-DPAT, reduces dopamine release in the nucleus accumbens and electrical self-stimulation to the ventral tegmentum. Brain Res. 1995;681:1–7. doi: 10.1016/0006-8993(95)00247-n. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM. Cochrane Database Syst Rev. 2009. Buprenorphine for the management of opioid withdrawal; p. CD002025. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de WH. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets. 2008;7:410–421. doi: 10.2174/187152708786927822. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L. Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacol Biochem Behav. 2006;83:403–409. doi: 10.1016/j.pbb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- Jimenez-Ruiz C, Berlin I, Hering T. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–1338. doi: 10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr. 1994;145:1–18. [PubMed] [Google Scholar]
- Koob GF, Kenneth LG, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 Dopamine Receptor Selective Antagonist PG01037 on L-Dopa Dependent Abnormal Involuntary Movements in Rats. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le FB, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Le FB, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005a;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Le FB, Goldberg SR, Sokoloff P. Dopamine D3 receptor ligands for the treatment of tobacco dependence. Expert Opin Investig Drugs. 2007;16:45–57. doi: 10.1517/13543784.16.1.45. [DOI] [PubMed] [Google Scholar]
- Le FB, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005b;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, Henriksen SJ, Fox HS. Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30:350–359. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992–2007. Drug Alcohol Rev. 2008;27:229–235. doi: 10.1080/09595230801919460. [DOI] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. Caveat emptor: editors beware. Neuropsychopharmacology. 2001;24(3):333–6. doi: 10.1016/S0893-133X(00)00171-8. [DOI] [PubMed] [Google Scholar]
- McKetin R, Kozel N, Douglas J, Ali R, Vicknasingam B, Lund J, Li JH. The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev. 2008;27:220–228. doi: 10.1080/09595230801923710. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Fabio RD, Donati D, Gentile G, Gribble A, Hamprecht D, Tedesco G, Terreni S, Tarsi L, Lightfoot A, Stemp G, Macdonald G, Smith A, Pecoraro M, Petrone M, Perini O, Piner J, Rossi T, Worby A, Pilla M, Valerio E, Griffante C, Mugnaini M, Wood M, Scott C, Andreoli M, Lacroix L, Schwarz A, Gozzi A, Bifone A, Ashby CR, Jr, Hagan JJ, Heidbreder C. 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- Mordenti, Chappell . The Use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics and New Drug Development. New York: Pergamon Press; 1989. pp. 42–96. [Google Scholar]
- National Institute on Drug Abuse. Medications Development. 2004. [Google Scholar]
- Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl)arylcarboxamides as novel dopamine D(3) receptor antagonists. Bioorg Med Chem Lett. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, Ho D, Luedtke RR. N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. J Med Chem. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Pasteau V, Gavaudan S, Millan MJ. G protein activation by human dopamine D3 receptors in high-expressing Chinese hamster ovary cells: A guanosine-5′-O-(3-[35S]thio)- triphosphate binding and antibody study. Mol Pharmacol. 1999;55:564–574. [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Piercy M, Koob GF. Characterization of the effects of the partial dopamine agonist terguride on cocaine self-administration in the rat. J Pharmacol Exp Ther. 1998;286:1231–1238. [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci. 1994;15:374–379. doi: 10.1016/0165-6147(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Being partial to psychostimulant addiction therapy. Trends Pharmacol Sci. 2002;23:151–153. doi: 10.1016/s0165-6147(00)01991-x. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, Gaal J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology (Berl) 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin K, Randall P, Myrick H, Anton R. Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm--possible influence of self-control. Alcohol Clin Exp Res. 2008;32:1954–1961. doi: 10.1111/j.1530-0277.2008.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers NW, Pulvirenti L, Koob GF, Gillin JC. Cocaine abuse and dependence. J Clin Psychopharmacol. 1995;15:63–78. doi: 10.1097/00004714-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Wolters EC. Dopaminomimetic psychosis in Parkinson’s disease patients: diagnosis and treatment. Neurology. 1999;52:S10–S13. [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]