Abstract
Most human obesity is inherited as a polygenic trait which is largely refractory to medical therapy because obese individuals avidly defend their elevated body weight set-point. This set-point is mediated by an integrated neural network that controls energy homeostasis. Epidemiological studies suggest that perinatal and pre-pubertal environmental factors can promote offspring obesity. Rodent studies demonstrate the important interactions between genetic predisposition and environmental factors in promoting obesity. This review covers issues of development and function of neural systems involved in the regulation of energy homeostasis and the roles of leptin and insulin in these processes, the ways in which interventions at various phases from gestation, lactation and prepubertal stages of development can favorably and unfavorably alter the development of obesity n offspring. These studies suggest that early identification of obesity-prone humans and of the factors that can prevent them from becoming obese could provide an effective strategy for preventing the world wide epidemic of obesity.
Keywords: Obesity, Perinatal environment, Development, Leptin, Insulin, Exercise
Introduction
Over the last 20 years a world-wide epidemic of obesity has developed which affects both adult [1; 2; 3; 4; 5; 6] [7; 8; 9; 10] and childhood populations [10; 11; 12; 13]. The major adverse health impact of this epidemic is due to the association of obesity with co-morbidities such as diabetes, cardiovascular disease, hypertension, hyperlipidemia, psycho-social and other disorders that either shorten life or reduce the quality of life of affected individuals [14; 15; 16; 17; 18; 19]. Once obesity develops, available pharmacological and behavioral interventions have a dismal track record of producing long-term weight loss in affected individuals. Aside from labor- and resource-intensive weight loss programs, more than 90% of obese individuals who lose weight, regain it within 1–2 years [20; 21]. Such figures emphasize the importance of identifying factors that predispose individuals to become obese so that they can be prevented from becoming obese in the first place. Among the most important of these predisposing factors are genetic background and the perinatal environment. As much as 70% of human obesity is inherited, predominantly in a polygenic fashion [22; 23; 24]. While, many studies to be reviewed here point to alterations in the pre- and postnatal environment as major determinants of the predisposition of offspring to become obese, it is becoming increasingly clear that the impact of such perturbations are affected by the genetic background of the effected individual. Thus, the focus of this review will be on the interactions between genetic background and the perinatal environmental factors that determine the propensity of offspring to become obese. It will also cover the various issues involved in the neural control of energy homeostasis and the current state of our knowledge of how the brain and periphery interact to regulate this process.
Evidence for perinatal influences on offspring obesity in humans
Dorner [25] carried out the first epidemiological studies suggesting a causal link between perinatal metabolic factors and the development of metabolic diseases in offspring. They found an increased prevalence of obesity in offspring of mothers who were undernourished during the famine that occurred in Germany during World War II as compared to offspring from the same geographical regions who were in utero after the war. From this he proposed that abnormal levels of systemic hormones and neurotransmitters produced by either genetic defects or deficient environments occurring during brain development can act as teratogens producing abnormal brain organization leading to permanent dysfunctions of fundamental processes such as metabolism [26]. He later showed that men who were born during the food shortages during and after the war (1943–47) had a lower incidence of type 1 diabetes mellitus (T1DM), but a more than a 50% higher incidence of T2DM compared to comparable subjects born after the food shortages occurred [27]. In addition, Ravelli and colleagues[28; 29] periodically assessed the offspring of mothers who were undernourished (400–800 calories per day) during various phases of gestation as a result of the Dutch Hunger Winter that occurred in the western Netherlands after World War II. Although the outcomes varied somewhat depending upon the timing of follow up and the groups examined, they first found that 19 year old male offspring undernourished in utero during the first two trimesters were more than twice as obese as controls who were in utero after the rationing occurred [28]. However, when men and women were followed up at 50 years of age, gestationally undernourished females but not males had a higher body mass index (BMI) than controls [30]. On the other hand, those subjected to undernutrition during mid- to late gestation had lower birth weights and developed insulin resistance with impaired glucose tolerance at age 50 [29].
Based on these studies, Hales and Barker [31] modified Neel’s original idea of a “thrifty genotype” and proposed that perinatal undernutrition led to a “thrifty phenotype” which predisposed to the development of T2DM. Subsequently, Subsequently, Barker [32] proposed that human fetuses adapt to a limited supply of nutrients by permanently programming changes in their physiology and metabolism. This “Barker Hypothesis” is generally credited with giving birth to the field of fetal origins of adult diseases which has been extended to include coronary heart disease and the related disorders: stroke, diabetes and hypertension. Later retrospective studies of infants of T1DM mothers vs. those from mothers with gestational diabetes showed both increased birth weight and a higher incidence of overweight from 1–4 years of age [33]. Furthermore, maternal obesity and/or diabetes have been associated with increased birth weight and often an increase in body weight gain and obesity in their offspring [34; 35; 36] [37; 38; 39]. However, it is important to note that evidence linking increased birth weight, often thought to predispose to adult obesity, is controversial [40] and the association of intrauterine growth retardation to adverse outcomes in adulthood are not fully consistent across all studies [41]. Interestingly, the apparent paradox whereby either a nutritional surfeit or dearth during gestation can lead to offspring obesity is mirrored by the fact that insulin deficiency (TIDM) and insulin excess (T2DM) during pregnancy both increase the risk that offspring will develop obesity and T2DM [42; 43].
One possible reason for such apparent paradoxical findings in human retrospective epidemiological studies is that there is no way to separate the effects of genetic background from perinatal and later postnatal environmental factors. While up to two-thirds of human obesity is inherited in a polygenic fashion [22; 23; 24], environment clearly plays a critical role in determining the metabolic fate of such individuals. A variety of studies have shown correlations between maternal and/or paternal obesity or BMI and offspring obesity or BMI [44; 45; 46; 47]. However, not surprisingly, a maternal “obesogenic” environment appears to have an independent effect on the development of obesity and the metabolic syndrome in their offspring [13; 39; 48; 49; 50; 51; 52]. Breast feeding is one major way in which a mother can influence the nutritional status of her infant. Breast feeding has generally been considered to offer some protection against the development of offspring obesity [53; 54; 55; 56; 57; 58]. Such a conclusion is likely to be biased by the content of the control populations of non-breast fed infants fed formula since formula content has varied over the years and by geographic locations. For example, the possible overnutrition and adverse effects of formula feeding on offspring glucose and lipid metabolism, obesity and blood pressure [59] may be related to formula protein content [60]. Furthermore, breast milk from mothers with T1DM appears to predispose their offspring to become obese and glucose intolerant later in childhood [61]. Thus, many genetic, perinatal metabolic and nutritional factors contribute to the ultimate outcome of offspring. Sorting out the relative role of these factors in humans has been quite difficult for a variety of reasons that will be addressed below.
Questions about the regulation of energy homeostasis and obesity that affect gene x environmental interactions on offspring
Is there a thrifty genotype?
Neel [62] first proposed that there was a thrifty genotype that evolved to enable hunter-gatherer humans to better withstand the rigors imposed by unstable cycles of energy excess and scarcity. He postulated that such a genotype would predispose individuals to become obese and glucose intolerant in Western societies when food supplies were abundant. This hypothesis was modified by Hales and Barker [31] in an attempt to reconcile the finding that gestational undernutrition often predisposes to offspring T2DM. In theory, such a thrifty genotype or phenotype should confer a competitive survival advantage to individuals by maximizing their ability to ingest and store large amounts of energy during periods of food surfeit and minimizing their energy expenditure during periods of severe shortage. However, as pointed out by Speakman [63; 64], there is little evidence to support such a competitive advantage for either survival or reproduction, at least during periods of famine. In fact, there might even be a competitive disadvantage conferred by storage of too many calories as fat which would expose such individuals to increased risk of predation. In addition, there is little evidence to suggest that obese or obesity-prone humans are more efficient at holding onto stored calories than are obesity-resistant individuals during periods of energy deficit. While this might be the case, it appears that all individuals, regardless of their starting weight, maximize the conservation of energy stores when caloric intake is restricted [65]. Most tellingly, since at least one-third of most human populations are obesity-resistant, no one has answered the question of why there are so many of such individuals left in any given population. Thus, even if there is a thrifty genotype, it may be that this is due to genetic drift rather than true competitive selection pressure.
While it is unlikely that this argument will be settled by currently available information, it is possible that a sizable population of obesity-resistant humans has survived over the millennia because they were able to migrate away from famine-stricken areas and thus maintain a population of individuals with a lean genotype. Regardless of whether a thrifty genotype exists and confers a competitive survival advantage, the fact is that certain populations, such as that in the US, contain very high percentage of overweight and obese individuals [66; 67]. One hypothesis is that obesity-prone individuals have an inherited and/or acquired predisposition to ignore or minimize the impact of increasing levels of negative feedback signals such as leptin and insulin that reflect increasing adipose stores during the development and maintenance of obesity. In the human population there are several extreme examples of such impaired negative feedback that lead to extreme obesity. These include individuals who make little or no leptin [68] or have congenital deficiency of the leptin receptor [69]. Others lack the ligands or receptors that effectuate the powerful downstream catabolic effects of leptin signaling in the brain [70; 71].
Is there a set-point for body weight regulation?
Based on a number of studies in rodents, Keesey and colleagues [72; 73; 74; 75; 76; 77] have argued that there is a set-point about which body weight and adiposity are defended and that this set-point resides in central pathways that regulate energy homeostasis. But this concept has met with resistance by those [78] who argue that the defended body weight reflects a “settling point” which is reached when all of the internal and external hormonal, metabolic and behavioral are summated and integrated by the pathways that regulate overall energy homeostasis. By this line of reasoning, such a settling point should be infinitely changeable as long as the inputs to the system are capable of changing. However, this idea does not account for the apparent fixed upregulation of the defended body weight that occurs in the majority of obese humans [20; 21] and some strains of obesity-prone rodents [79; 80; 81]. In these instances, the defended body weight can be moved upward but, with rare exceptions, cannot be moved back downward in the absence of surgical intervention. Thus, the recidivism rate in the treatment of obesity is generally greater than 90% in the absence of surgical interventions [82]. Similarly, both outbred and selectively bred rats that are obesity-prone will maintain their elevated body weight set-point, even after several months of caloric restriction [79; 81; 83; 84; 85]. Likewise, experimentally induced overfeeding of humans and rodents, regardless of the starting body weight, results in compensatory reduction in intake and increase in energy expenditure which returns them to their initial level of body weight when overfeeding ceases [65; 76; 83; 86; 87].
It is important to note that this type of defense against overfeeding is seen when humans are paid to overeat a large number of calories or when rodents are either force-fed or provided with a highly palatable diet that causes them to massively overeat. Such overfeeding is often kept at persistently elevated levels for the duration of these studies such that subjects are persistently in a state of excess energy intake vs. expenditure. On the other hand, when an elevated obese set-point is established by chronic, more gradual, smaller caloric excess, intake and expenditure more rapidly come into a homeostatic balance which is avidly defended against caloric restriction. Thus, the rate and degree may determine whether an elevated set-point will be defended. Massive overfeeding stimulates catabolic and inhibits anabolic systems, while more gradual accretion of calories may fall below the threshold of detection required for catabolic systems to become activated. In addition, palatability and the rewarding properties of the diet engage reward pathways in the brain that appear to operate by different mechanisms than those activated by overfeeding [83].
Given the critical role of the central nervous system in regulating the defended body weight [88], the persistent upregulation of the defended body weight resulting from chronic, gradual mild to moderate excess intake is reminiscent of the neural plasticity that underlies the formation of long-term memories that occurs throughout life. However, current evidence suggests that environmental interventions during the formation of hypothalamic pathways involved in energy homeostasis regulation can alter neural plasticity to perpetuate long-term changes in the defended body weight [89; 90]. On the other hand, selective lesions in the distributed network of brain sites involved in the regulation of energy homeostasis can alter the defended body weight [91; 92]. While such brain lesions have been proposed and even attempted as a treatment for obesity in humans [93], the permanence and potential complications inherent in such surgical lesions makes their use more an act of desperation than a potential practical treatment for the current obesity epidemic.
A brief overview of energy homeostasis regulation
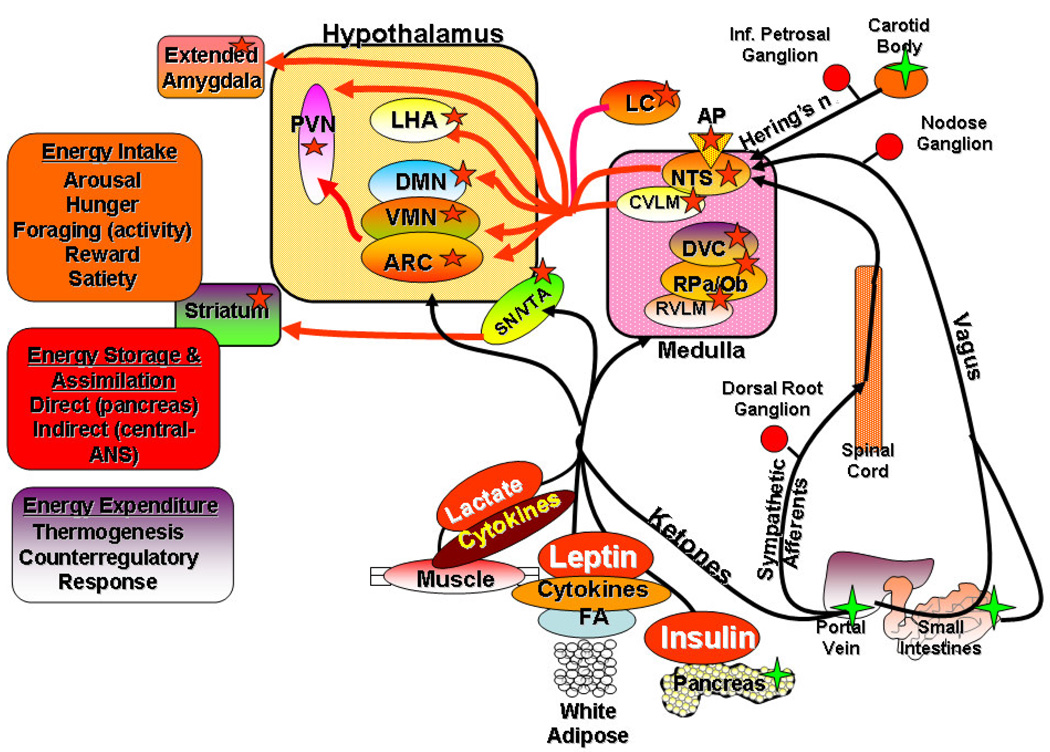
Energy homeostasis is the balance between intake on the one hand and expenditure on the other. Excess caloric intake over expenditure is stored primarily as adipose tissue and this storage depot is utilized as a primary fuel source during periods of energy deficit. The regulation of energy homeostasis reflects an ongoing dialogue between the internal and external environments and the brain. The brain both senses and integrates a multitude of hormonal, metabolic and hard wired neural signals from peripheral sensors and organs in its role as the primary regulator of both the control of individual meals and the long-term balance between intake and expenditure (Figure 1). Leptin is produced primarily in adipose depots in proportion to their size and acts as the prototypic signal for keeping the brain informed about the status of peripheral adipose stores [94; 95]. When intake exceeds expenditure, depot size increases and leptin levels rise to provide a tonic, negative feedback on anabolic, (increased intake, decreased expenditure) and a positive feedback on catabolic (decreased intake, increased expenditure) neural circuits. Insulin, although of pancreatic origin, indirectly reflects adipose levels [96; 97] and acts as an additional negative feedback signal [98]. Various gut neuropeptides are produced in response to both pre- and post-ingestive factors to modulate short-term regulation of individual meals [99; 100]. Many of these peptides act on receptors on vagal and sympathetic afferents whose central processes terminate in critical regulatory brainstem areas [100] (Figure 1). In addition, a variety of metabolic substrates such as glucose, fatty acids, ketone bodies and lactate, as well as cytokines produced in the periphery, signal the brain via these same autonomic afferents and by being transported across the blood-brain barrier [101; 102; 103] (Figure 1). These signals are sensed and integrated by a distributed network of “metabolic sensing” neurons located in key areas of the hindbrain and forebrain (Figure 1). These systems are complex and highly interconnected. In general, the midline nuclei of the hindbrain (nucleus tractus solitarius (NTS) and dorsal motor vagal complex) and hypothalamus (arcuate (ARC), ventromedial (VMN), paraventricular (PVN) and dorsomedial (DMN) nuclei) are involved in the metabolic aspects of energy sensing and responding [101; 102; 103]. The integrated signals from these nuclei are relayed to efferent neuroendocrine and autonomic areas of the hypothalamus (PVN and lateral hypothalamic area (LHA)) and hindbrain. In addition, neurons in areas of the brain involved in motivation, reward (ventral tegmental area, extended amygdala) and memory (hippocampus) are engaged by many of the same afferent signals from the periphery [101; 102; 103] (Figure 1). Metabolic sensing neurons in many of these areas express receptors for catabolic (leptin and insulin) and anabolic (ghrelin) hormones and transporters for a variety of metabolic factors from the periphery. These hormones provide phasic and tonic inputs that alter the metabolic impact and reward valence and salience of a given signal for production of memories and plastic change involved in energy homeostasis regulation [104; 105; 106]. Although the components of this network are widely distributed across both the periphery and central nervous system, site specific lesions of critical areas within this network (such as the ventromedial hypothalamus (VMH= ARC +VMN), LHA or DMN) can markedly alter the defended body weight [91; 92].
Figure 1.
Steps of neural maturation that can be influenced by leptin (Lep) and insulin (Ins). Precursor cells differentiate into either glia or neurons. The latter migrate from their birth place to the position they occupy in the mature nervous system and then send out axonal projections to target areas. Some of these axons establish connections with target neurons (cell bodies and/or dendrites). This interaction provides trophic support for and allows survival of the presynaptic neuron. Those neurons which do not establish functional contacts undergo cell death (apoptosis). While most new neuron formation (neurogenesis) occurs before E18 in the hypothalamus, it now appears that neurogenesis can continue well into adult life. Steps at which leptin and/or insulin have effects to promote various steps of neuronal maturation are labeled by red ovals.
Of all the neurons known to be involved in the regulation of energy homeostasis, those in the ARC are perhaps the best characterized and among the most important. ARC neurons expressing both neuropeptide Y (NPY) and agouti-related peptide (AgRP) project to the PVN, LHA and other brain areas where the release of these peptides provides an anabolic stimulus [88]. They also project locally onto neighboring neurons which express proopiomelanocortin (POMC). POMC neurons project to most of the same targets as do NPY/AgRP neurons. In these neurons, POMC is cleaved to α-melanocyte stimulating hormone (α-MSH) which acts on melanocortin-4 (MC4R) and −3 receptors to produce a catabolic response. Importantly, the main action of AgRP is as a functional antagonist (inverse agonist) at the MC3/4R’s [107]. The downstream targets of these neurons include those expressing thyroid hormone and corticotrophin releasing hormone in the PVN and orexin (hypocretin) and melanin concentrating hormone in the LHA [108; 109; 110]. Thyroid hormone is a critical regulator of thermogenesis, while corticotrophin releasing hormone is a primary mediator of the hypothalamo-pituitary-adrenal axis. In the LHA, orexin and melanin concentrating hormone neurons project widely throughout the neuraxis to areas mediating arousal, reward, motor activity and a host of other functions. Thus, these PVN and LHA neurons can be considered second order neurons by which ARC NPY/AgRP and POMC neurons effect the highly complex behavioral, physiological, hormonal and neural activities required for the regulation of energy intake, expenditure and storage (Figure 1). In addition, the LHA neurons have reciprocal connections with reward and memory areas of the brain, as well as downstream integration centers in the hindbrain [111].
Many of these same neurons involved in the regulation of energy homeostasis, including ARC NPY/AgRP and POMC neurons, are also the targets of neural, hormonal and metabolic signals produced in the periphery as a part of an ongoing dialogue between the brain and periphery. These were fist described as “glucosensing” because, as opposed to the vast majority of neurons that utilize glucose as a primary metabolic substrate to fuel their ongoing energy requirements [112], these specialized sensing neurons utilize glucose as a signaling molecule that regulates their membrane potential, neural activity and transmitter/peptide release[111; 113; 114]. In these neurons, various intracellular metabolic pathways modulate the production of ATP, AMP, nitric oxide, reactive oxygen species and other intracellular messengers from glucose. These metabolic products then act on ion channels, such as the ATP-dependent K+ channel, to alter membrane potential and neuronal activity [103]. Glucose excited (GE) neurons increase and glucose inhibited (GI) neurons decrease their activity as brain glucose levels rise and are affected oppositely as glucose levels fall [103]. In addition to glucose, many of these same neurons can utilize long chain fatty acids as signaling molecules [115; 116; 117; 118] and many also have receptors for hormones and peptides such as insulin, leptin and ghrelin [119]. Thus, the terms metabolic or nutrient sensors are probably the best descriptors for such neurons. ARC NPY/AgRP and POMC neurons, dopamine neurons in the ventral tegmental area and substantia nigra and noradrenergic neurons in the NTS are prototypic examples of such multimodal metabolic sensing neurons [103]. Again, it is important to emphasize that, although most of these neurons can play important independent roles in the regulation of energy homeostasis, their most important function is as part of a widely distributed network that includes both the brain, its neuroendocrine effector systems and peripheral target organs and the metabolic and peptide-hormone sensors they contain.
Development of systems regulating energy homeostasis
Progenitor cells arise in a number of sites throughout the nervous system differentiate during development into glial and neural elements (Figure 2). In the hypothalamus, new neuron formation (neurogenesis) takes place in the neuroepithelial lining of the third cerebral ventricle [120; 121; 122; 123; 124]. These new neurons migrate to their ultimate positions and then send axonal projections to their target areas. Those axons that form functional target connections survive while those that do not undergo apoptotic cell death [125; 126]. Among the many issues of using rodents as surrogate models for humans is that there are critical differences in the timing of brain development between humans and rodents. In fact, we know more about the development of the rodent than primate brain. In non-human primates, hypothalamic neurogenesis occurs during the first few months of gestation [127]. Limited human studies suggest that early hypothalamic neurogenesis occurs during weeks 9–10 of gestation [128; 129; 130; 131; 132]. In non-human primates the anabolic NPY/AgRP ARC to PVN projections develop during the late second trimester (by gestational day 100). The mature pattern fully develops by gestational day 170 [133]. In human fetuses, NPY immunoreactive fibers are detected in the ARC and in the PVN by 21 weeks of gestation [132]. On the other hand, previous data suggest that most rodent hypothalamic neurons differentiate into mature neurons at embryonic days 12–16 (E12–16) [134], where birth of the pup occurs at ~E22. However, it is now clear that some hypothalamic neurogenesis continues into early postnatal, and possibly into adult life in rodents [120; 123; 124]. It is unknown whether similar continued neurogenesis occurs in humans. Finally, rodent ARC-PVN projections are not completed until P12–14 in rodents [135; 136; 137] although manipulations such as caloric restriction during the post-weaning period (after 3 weeks of age) can still alter the development of these pathways [138]. Thus, while rodent studies suggest that various interventions made during the formation of the ARC-PVN pathways can alter the development of obesity, the marked differences between the developmental patterns of the rodent and primate brains make it difficult to decide when it might be most expeditious to intervene during human development to promote generation of neural pathways that prevent offspring from becoming obese.
Figure 2.
The distributed network of central metabolic sensing neurons (red stars) and the peripheral inputs that allow them to monitor peripheral metabolic activity and the status of adipose stores. Metabolic sensing neurons in the nucleus tractus solitarius (NTS) receive hardwired vagal and sympathetic afferents from peripheral sensing elements (green stars) in the portal vein, small intestines and carotid body. Neural projections from the NTS disseminate these neural signals to metabolic sensing neurons the hypothalamus (arcuate (ARC), ventromedial (VMN), dorsomedial (DMN), paraventricular (PVN) nuclei, lateral hypothalamic area (LHA)) and areas of the extended amygdala which also have transporters, receptors and sensing apparatus for metabolic substrates (glucose, lactate, ketone bodies, long chain free fatty acids (FA)), hormones (insulin, leptin) and cytokines generated in peripheral organs. Additional metabolic sensing neurons also reside in the caudal ventrolateral medulla (CVLM), rostral ventrolateral medulla (RVLM), dorsal vagal complex (DVC), raphe pallidus (RPa) and obscurus (Rob), substantia nigra (SN), area postrema (AP), locus coeruleus (LC), ventral tegmental area (VTA) and striatum. All of these metabolic sensing neurons integrate the neural, metabolic and hormonal signals from the periphery which results in alterations in membrane potential, action potential frequency and neurotransmitter/peptide release. Their efferent outputs regulate the behavioral, metabolic, hormonal and physiological functions involved in energy homeostasis (intake, expenditure, storage).
Finally, brain areas outside the hypothalamus involved in the regulation of energy homeostasis undergo different temporal patterns of development in rodents. For example, the pathways from the hindbrain to the hypothalamus are fully developed at birth [139; 140; 141; 142]. In addition brain areas involved in motivation and reward do not fully develop functionally until well into the postnatal period. For example, pathways in the medial prefrontal cortex involved in fear conditioning in rats develop fully only by P17–24 [143], while the dopamine innervation of the forebrain areas involved in reward and decision making (prefrontal cortex, basolateral and central nuclei of the amygdala) can still be altered by various interventions as late as P30–60 [144]. The cannabinoid and opiate systems in the prefrontal cortex and nucleus accumbens continue to develop from P29–49 [145]. Functionally, some of these same pathways in humans develop over a prolonged period from 8–27 years of age [146; 147]. Thus, even in humans, there exists a broad window of opportunity by which external interventions might be undertaken to favorably alter the development of neural systems involved in the regulation of energy homeostasis.
Issues involved in the study of development and function of systems regulating energy homeostasis
Before considering the various interventions that can affect the development of neural and other systems involved in the regulation of energy homeostasis and how they promote offspring obesity and diabetes, there are a number of important factors that must be taken into account regarding experimental methodologies that can markedly affect the outcome of such interventions.
How do neurohumoral and metabolic signals reach the brain?
The blood-brain barrier is comprised of tight junctions between vascular endothelial cells in brain microvessels. These tight junctions prevent diffusion of most substances into the brain. Instead, they undergo facilitated transport down a concentration barrier to enter the brain [148]. Leptin, insulin and most metabolic substrates (glucose, lactate, ketone bodies) are transported across this barrier [149], while many gut peptides such as peptide YY, glucagon-like peptide-1 and cholecystokinin and transmitter such as norepinephrine, epinephrine and serotonin are not [148]. The requirement for such transport, as well as rapid uptake by neurons and astrocytes, accounts for the fact that extracellular glucose levels in the brain are maintained at only 10–20% of blood levels across virtually the entire neuraxis [150; 151; 152]. However, there are several brain areas, known as the circumventricular organs, which have fenestrated blood vessels which do allow for free diffusion of substances into the surrounding neuropil [153; 154]. The median eminence and area postrema are two of the most important of these since the former lies just ventromedial to the ARC and the latter lies dorso-medial to the NTS. There is still considerable controversy as to whether ARC neurons are exposed to plasma levels of various peptides, hormones and metabolic substrates. On the one hand, there appears to be limited or no diffusion of substances such as glucose from the median eminence to the ARC over short periods of time because of the extensive network of tanycyte processes that effectively compartmentalize the ARC (and NTS) and wall them off from the median eminence and possibly the area postrema [151; 154; 155; 156; 157]. On the other hand, there are some fenestrated vessels that originate in the median eminence that extend up into the medial ARC [153; 158]. Despite these, it is unlikely that there is significant free diffusion of small molecules such as glucose from the median eminence into the ARC since extracellular glucose levels in the ARC are comparable to those of the adjacent VMN rather than those found in plasma [151]. However, ARC neurons do send axons into the median eminence and these axons are capable of uptake and retrograde transport to their cell bodies of substances that do not cross the blood-brain barrier [159]. ARC astrocytes also take up such substances [160] but such uptake is generally a slow process which occurs over several hours.
Another general assumption is that various substances found in the cerebrospinal fluid can enter the brain in a manner that mimics normal physiological delivery of these substances across the blood-brain barrier. Such an assumption led to the common practice of introducing various test substances into the ventricular system to assess their effects on energy homeostasis. However, because neurons in the ARC, VMN and NTS are separated from the ventricular system by tanycytes which express tight junctions similar to those found in brain microvessels [156; 161; 162], it is likely that many of those substance either do not enter those nuclei directly or that they may have unanticipated secondary effects due to requirement of primary uptake by the metabolically active tanycytes. These glia cells express glucose transporters (Glut-1 and - 2) and glucokinase [163; 164], the hexokinase responsible for glucosensing in many neurons [164; 165]. They also possess neurotransmitter transporters and receptors [166; 167] and type 2 deiodinase [168]. Tanycytes also accumulate substances such as insulin-like growth factor I [169]and β-endorphin [170] and are contacted by axon terminals of neurons expressing serotonin [171; 172] and opioids [173] and are reversibly destroyed by toxins such as alloxan [174]. Finally, tanycyte processes extend laterally and ventrally to subdivide the VMN and ARC into compartments which effectively walls off ARC neurons from most of the fenestrated vessels of the median eminence [155; 175].
Pharmacology vs. physiology
It is common practice to inject hormones and peptides either peripherally or into the brain to evaluate their potential roles in the regulation of energy homeostasis. In most cases, the levels reached using either route far exceed those seen during normal diurnal or feeding cycles. In the brain, with the exception of glucose and lactate, the actual extracellular levels of hormones such as leptin and insulin, peptides such as NPY, AgRP, α-MSH and ghrelin, metabolites such as free fatty acids are largely unknown due to technical issues involved in their measurement. Thus, we are left in the unenviable position of having to define “relevant” dosages by their behavioral or physiologic effects. Add to this the fact that hormones such as leptin and insulin are most often (but not always) injected in a bolus into the cerebrospinal fluid or brain producing a rapid increase in levels… a situation that virtually never occurs under physiological conditions where levels of such hormones change over much longer periods of time and likely exert tonic rather than phasic influences on neural function.
Thus, there are a number of factors that limit our ability to extrapolate data obtained from many studies carried out in rodents or other animal models to derive useful conclusions about their relevance to human beings and the impact of environmental and developmental factors upon the development of obesity and diabetes in offspring. Nevertheless, such animal models can still provide significant insights that might allow us to identify critical factors that affect human obesity and diabetes as long as they are evaluated with a full understanding of their limitations.
Perinatal and prepubertal factors that cause offspring obesity by altering the development of their energy homeostatic systems
Both the type and timing of external interventions can have a major impact on the development of neural systems involved in the regulation of energy homeostasis. There are two broad categories of possibly interrelated changes which affect offspring development during the perinatal and pre-pubertal periods; epigenetic changes in gene expression and physical alterations of organ development that are not necessarily dependent upon altered gene expression. Non-Mendelian, or epigenetic modifications of genes can occur by methylation or histone acetylation. Such alterations of gene structure can markedly alter gene expression regulating organ formation and neural pathways involved in the regulation of energy homeostasis. Prader-Willi syndrome exemplifies a disease caused by imprinting of one parental gene which affects both energy homeostasis and neural development. Such imprinting inhibits expression of genes at the 15q11-q13 locus which undergo histone methylation on the maternal chromosome [176]. The Prader-Willi syndrome is characterized by hypotonia, early life feeding difficulties followed by obsessive/compulsive food seeking, hyperphagia and obesity in association with short stature and hypogonadism [177]. In addition to such well described syndromic disorders, modifying the perinatal nutritional environment can also cause epigenetic changes that alter the phenotypic characteristics of offspring. For example, supplementing the maternal diet of yellow agouti mice with a diet high in methyl donors increases methylation of CpG islands on genes resulting in altered offspring coat color [178]. In addition to nutritional interventions, high levels of maternal licking and grooming and arched-back nursing alter offspring histone acetylation and transcription factor NGFI-A binding to a hippocampal glucocorticoid receptor gene promoter region. This affects both offspring glucocorticoid receptor expression and hypothalamic-pituitary-adrenal responses to stress. This effect can be reversed either by cross-fostering to dams exhibiting low levels of these behaviors or by central infusion of a histone deacetylase inhibitor [179]
Aside from epigenetic changes in gene expression, perinatal interventions can also alter the development of specific organ systems though changes in the metabolic and hormonal milieu of the perinatal environment. Barker [180] first proposed this idea that fetal or early life environmental perturbations can program organ development resulting in adverse health conditions in adult life. This aforementioned Barker hypothesis was later applied specifically to perinatal environmental effects leading to offspring type 2 diabetes [31]. While epigenetic changes in gene function might be involved in these processes, they are not necessarily required. Maternal caloric or protein undernutrition is a commonly studied intervention in rodents [29; 30; 181; 182; 183; 184; 185; 186; 187; 188; 189; 190; 191] which is used to mimic the observations that gestational undernutrition in humans increases the risk of offspring obesity and diabetes [25; 28; 29; 192]. On the other hand, maternal obesity during gestation and/or lactation can also predispose to offspring obesity and insulin resistance [184; 193; 194; 195; 196; 197]. Importantly, perinatal and prepubertal dietary manipulations have long lasting effects on the development and function of hypothalamic pathways and systems involved in the regulation of energy homeostasis [117; 138; 198; 199; 200; 201; 202; 203; 204];[195].
No concrete evidence provides mechanisms to explain the paradox that both perinatal undernutrition and overnutrition can produce offspring obesity. Hales and Barker [205] proposed that poor fetal and infant growth and the subsequent development of the metabolic syndrome are a direct result of early life undernutrition by which the fetus attempts to compensate for limited nutritional resources by increasing metabolic efficiency to aid survival both pre- and postnatally. On the other hand, while it might seem logical that perinatal overnutrition could lead to permanent offspring obesity, the mechanisms that produce such obesity are still a matter of current investigation. However, as will be seen below, because of their critical roles in the development of neural systems regulating energy homeostasis, increased levels of insulin and leptin in the pre- and early postnatal environments may well be the critical factors that increase the predisposition offspring of obese mothers to become obese.
Leptin and insulin play critical roles in the development of energy homeostatic systems (Figure 2)
A large number of factors can influence the development of neural systems that regulate energy homeostasis. Most of these have been studied in depth only in rodent brains. Leptin and insulin are among the most important of such factors because of their well documented roles in the ongoing regulation of energy homeostasis and because they also affect neuronal migration, survival (neurotropic) and process outgrowth (neurotrophic) of developing neurons [89; 90; 206; 207; 208; 209]. Mice lacking leptin (ob/ob) and mice (db/db) and rats (selectively bred “DIO”) with deficient leptin signaling have abnormal development of ARC-PVN axonal projections of anabolic NPY/AgRP and catabolic proopiomelanocortin (POMC) neurons involved in the regulation of energy homeostasis [89; 90]. Importantly, leptin replacement in ob/ob mice from P4–12, but not in adult life, can fully restore these pathways [89]. On the other hand, injection of insulin into the dam during the last week of gestation [198; 210; 211] or direct hypothalamic insulin injections in neonates at P2 or P8 alters hypothalamic development in association with adult onset obesity [212; 213]. It is unclear whether the alterations produced by differences in leptin and insulin signaling involve epigenetic modifications of genes or by directly altering the physical properties of the developing nervous system.
During gestation, leptin and/or its receptors are produced by the placenta in humans [214; 215; 216; 217; 218; 219], sheep [220; 221] and rodents [222; 223; 224; 225]. The degree to which leptin is transported into the fetal circulation is still a matter of some controversy. Similarly, maternal insulin or other hormones, amino acids and glucose may be transported across the placenta where they can alter the development of the brain and other organs in humans and rodents [226; 227; 228; 229; 230]. During early postnatal development, both leptin and insulin are secreted into maternal milk where they can be absorbed into the infant circulation to potentially affect brain and organ formation, metabolic function and obesity development [231; 232] [233; 234; 235; 236; 237; 238]. Although the data are conflicting and highly dependent upon the timing, duration, dosage and route of administration [232; 237; 239; 240], several studies suggest that early postnatal exposure of suckling neonates to high levels of leptin, prior to the normal increase seen during the second week of lactation[241], can predispose them to develop diet-induced obesity and/or hyperinsulinemia as adults [242; 243; 244; 245; 246; 247]. Thus, depending upon a number of factors, exposure of the developing fetus and neonate to high levels of leptin and/or insulin associated with maternal obesity may well be the critical pathogenic factors leading to the development of obesity in their offspring.
Gene x environment effects upon systems regulating energy homeostasis and the development of obesity
Most studies of perinatal factors that affect offspring obesity have been carried out either retrospectively in humans or in animal models where possible genetic predispositions towards obesity could not be (in the case of humans) or were not considered (in the case of rodents). With some exceptions [197], the importance of gene x environment interactions acting to predispose offspring to become obese has largely been ignored. We originally addressed this issue by developing substrains of rats which we selectively bred for their propensity to develop diet-induced obesity (DIO) or to be diet-resistant (DR) when fed a 31%, 25% sucrose high energy (HE) diet [248]. The subsequent DIO and DR phenotypes have been remarkably stable over more than 45 generations. While the genes underlying their phenotypes have not been identified, the DIO phenotype has been transmitted to the obesity-resistant inbred Fischer F344 rat strain to produce a small but obesity-prone, insulin resistant F.DIO rat [249]. Such studies and others suggest a polygenic transmission of the DIO phenotype with its associated hyperphagia, obesity, insulin resistance, hypertension and hyperlipidemia which develop only when they consume a diet with increased caloric, fat and sucrose content [250]. Importantly, their polygenic inheritance and phenotypic expression closely mimic many of the characteristics of a majority of obese humans [22; 24].
An inborn resistance to the behavioral and physiological effects of leptin on systems regulating energy homeostasis is a critical feature of the DIO rat. From as early as P10, they have reduced activation of signaling pathways downstream of the long, signaling form of the leptin receptor (Lepr-b) in the ARC [90]. This is associated with a reduced anorectic and thermogenic effect of leptin [251] and decreased expression of the Lepr-b gene [252; 253], binding of leptin to its receptor in the ARC, VMN and DMN [104]and the excitatory effects of leptin on their VMN neurons [201]. In keeping with leptin’s trophic effect on developing ARC-PVN AgRP and α-MSH neuronal projections, DIO rats, like ob/ob and db/db mice [89], show maldevelopment of these pathways [90]. DIO rats also have altered development of the VMN which includes reduced neuronal dendritic arborization [254] and overall size [255] of this nucleus which plays a critical role in the regulation of energy homeostasis [256]. In addition to inborn leptin resistance, DIO rats are also resistant to central anorectic effects of insulin [257]. This is paralled by reduced hypothalamic binding of insulin to its receptors in the ARC [104]. In addition to defective hormonal responses and neuropeptide pathway development, DIO rats also have altered hypothalamic norepinephrine, dopamine and serotonin metabolism before they become obese [255; 258; 259; 260]. Finally, DIO rats have increased sensitivity to the inhibitory effects of both glucose and long chain fatty acids on VMN neurons [117]. Their altered glucosensing at the neuronal level is paralleled by reduced physiological responses to the central effects of glucose [261; 262; 263]. Thus, the differential responsiveness of DIO and DR rats to the development of obesity and insulin resistance, along with their well characterized defects in leptin, insulin and glucose signaling make these two substrains ideal for the study of gene x environment interactions affecting the development of obesity and diabetes in their offspring.
We first used the DIO and DR rats to assess the effects of maternal obesity x genotype on the development of offspring obesity. Making DIO dams obese during gestation and lactation caused their adult offspring to become obese and insulin resistant, even when fed only a low fat diet from weaning [193]. Importantly, neither feeding DR dams HE diet nor making them obese on a highly palatable liquid diet during gestation and lactation altered the obesity resistance of DR offspring fed HE diet as adults [193]. In association with their increased predisposition to become obese, offspring of obese DIO dams also had a reduction in PVN, ARC and VMN norepinephrine reuptake transporters [195]. Since most norepinephrine is removed from the synapse by reuptake into axons terminals by these transporters, a reduced complement of these reuptake transporters should increase synaptic norepinephrine content, a condition which increases food intake and causes obesity in rats [195; 264]. In addition to the effects on norepinephrine pathways, maternal obesity in DIO and DR dams was associated with enlargement of the areal extent of both the VMN and DMN in their offspring suggesting that this particular developmental effect is independent of genetic background. Instead it might be due to the hyperinsulinemia and hyperleptinemia exhibited by both sets of obese dams [195]. Also, despite the fact that their offspring did not become obese as adults, offspring of obese DR dams had an increased complement of norepinephrine transporters in the PVN. Such an increase should reduce synaptic norepinephrine and possibly decrease ongoing feeding. These offspring of obese DR dams also had a generalized increase in serotonin reuptake transporters in all hypothalamic areas [195].
We have studied the responsiveness of individual neurons in the VMN because of the critical role of this nucleus in the regulation of both energy [256] and glucose homeostasis [265; 266; 267; 268]. Offspring of lean DIO dams have fewer VMN neurons that are either excited or inhibited by leptin than do offspring of lean DR dams. Maternal intake of HE diet during gestation and lactation reduces the number of leptin-excited neurons in both DIO and DR offspring. However, maternal obesity proportionally reduces a greater number of such leptin-excited neurons in the offspring of obese DIO dams [201]. The VMN is also in enriched in metabolic sensing neurons which respond to both glucose and long chain fatty acids. Whereas 3–4 week old offspring of lean DIO dams have twice as many VMN neurons that are inhibited by glucose (GI neurons) than do those of lean DR dams, this difference is eliminated in offspring of obese DIO dams [117]. Similarly, while offspring of lean DIO dams have more GI neurons that are either excited or inhibited by oleic acid than do lean DR offspring, maternal obesity enhances this difference in DIO offspring [117]. Thus, VMN neurons in DIO rats are generally less responsive to both hormonal and metabolic signals from the periphery than are those from DR rats and this difference is exaggerated by maternal intake of HE diet and the development of obesity in DIO dams.
Because outgrowth of ARC NPY/AgRP and POMC neurons to the PVN occurs postnatally in rodents, manipulation of the environment during this period can have major effects on the development of these pathways [89; 90; 137; 269]. It is likely that postnatal development of other neural pathways and/or metabolic outcomes of offspring can also be affected by gene x environment interactions during this period. For example, cross-fostering mice with an obesity-prone genotype to genetically lean dams at birth ameliorates their obesity, while fostering lean mice to obesity-prone dams makes the genetically lean mice obese[197]. Similarly, fostering offspring of lean DR dams to obese (but not lean) DIO dams causes the offspring to become obese and insulin resistant when fed HE diet as adults [233]. Their obesity is associated an anabolic profile in the VMH, i.e. significant increases in ARC AgRP and decreases in VMN Lepr-b and insulin receptor mRNA expression [233]. These alterations in peptides and receptors appear to be mediated by maternal milk content. Milk from obese DIO dams has very low levels of both poly- and mono-unsaturated fatty acids and, despite having relatively low plasma insulin levels during lactation, their milk has a marked increase in insulin levels [233] which can thereby increase offspring blood levels by direct absorption [234; 235]. Presumably because of it neurotrophic and neurotropic properties, increasing hypothalamic insulin levels by direct injections during this period cause altered hypothalamic neuronal morphology and density, particularly in the VMN [212]. Most importantly, such insulin-induced changes are associated with the development of obesity during adult life [213]. As an example, human babies breast fed by diabetic mothers have an increased incidence of obesity and glucose intolerance later in life [61]. Thus abnormal milk composition appears to be a major factor predisposing cross-fostered DR pups to become obese as adults. Disappointingly, fostering offspring of either lean or obese DIO dams to lean DR dams has essentially no protective effect on their propensity to become obese on HE diet as adults [233]. Thus, there is a genotype specific effect of altering the pre- and early postnatal environment on the propensity of selectively bred DIO and DR rats to become obese and on the underlying neuronal responsiveness of hypothalamic neurons to hormones and metabolic substrates that regulate energy homeostasis.
Exercise is another factor that can alter the predisposition to and maintenance of obesity in humans and rodents [101; 270; 271; 272]. In adult and juvenile rodents, exercise generally produces weight loss only in obese males. Unlike lean males and obese females which compensate for increased energy expenditure and weight loss by increasing their caloric intake, obese males fail to do so and lose weight [138; 273; 274; 275; 276]. However, this weight and adipose loss is maintained only as long as exercise continues in the adult [277]. We hypothesized that early onset exercise might have a more long-lasting effect on lowering body weight and adiposity in DIO rats by altering the structure and/or function of the neural pathways involved in energy homeostasis regulation. Thus, when male DIO rats were fed HE diet but also provided with a running wheel from four weeks of age, they gained less weight than did sedentary DIO rats on HE diet. Moreover, when their wheels were removed, they maintained their reduced body weight and adiposity gain for up to two and a half months [276]. Three, but not two weeks, of running wheel exposure was sufficient to maintain this post-exercise obesity protection despite continued intake of HE diet [276]. At least part of this exercise-induced effect was due to increased sensitivity to the anorectic effects of leptin in association with increased binding of leptin to its receptor in association with increased leptin-induced downstream signaling [138]. Importantly, this increased leptin sensitivity persisted for at least four weeks after exercise cessation, even though it had no effect on ARC-PVN neuropeptide pathway development [138]. On the other hand, caloric restriction of sedentary DIO rats fed HE diet during this same period not only caused an increase in obesity when the rats were subsequently allowed ad libitum intake, but also further inhibited outgrowth of outgrowth of α-MSH axons to the PVN [138]. These studies clearly demonstrate that relatively short-term postnatal interventions initiated prior to the onset of puberty can have long-term effects on the regulation of energy homeostasis and the development of obesity either by altering leptin sensitivity or neuronal pathway development. These findings further widen the window of opportunity during which interventions may act to alter the long-term propensity of an individual to develop obesity as an adult.
Summary and Conclusions
Obesity, with its associated increases in morbidity and mortality, has so far largely resisted medical and behavioral interventions n humans. Thus, early identification of obesity-prone individuals and initiation of steps to prevent obesity-prone individuals from becoming obese is the best strategy to fight the world-wide epidemic of obesity. Energy homeostasis is regulated by the brain and the development of neural systems that regulate energy homeostasis are affected by a number of perinatal and early pre-pubertal manipulations. Thus, this developmental period represents a critical window of opportunity time for initiation of interventions aimed at preventing obesity. To do this, we must first identify the individuals most likely to become obese based on their underlying genetic predisposition and the specific factors within the perinatal and prepubertal environments that are most likely to promote or prevent the development of obesity. Of these predisposing factors, both maternal undernutrition and maternal obesity loom large as the two extremes of conditions that predispose offspring to become obese. For undernutrition, the idea that early life undernutrition causes the fetus to compensate for limited nutritional resources by increasing metabolic efficiency to aid survival both pre- and postnatally [205] seems a logical hypothesis. For maternal obesity, I propose that elevated levels of maternal leptin and/or insulin during gestation and lactation may exert a critical, maladaptive effect on the neural systems that regulate energy homeostasis to increase the likelihood of their offspring becoming obese, particularly in genetically predisposed individuals. It remains unclear whether these important perinatal factors associated with maternal undernutrition and obesity exert their effects by epigenetic effects on gene expression or on the development of critical organs systems and neural pathways.
Thus, many challenges lie ahead for scientists in this field to move beyond our largely descriptive studies to those that identify the genetic, molecular, cellular and biochemical mechanisms by which the maternal environment so markedly alters the metabolic phenotype of offspring. While these are ambitious goals, the availability of animal models that mimic the human conditions associated with obesity development and maintenance provide hope that we will eventually reach these goals. Our own experience suggests that including a genetic predisposition for obesity as an experimental variable in studies in animal models is critical since a majority of human obesity is inherited as a polygenic trait [22; 24; 278; 279]. Furthermore, our studies suggest that a profitable area for exploration is the identification of specific factors produced by alterations of the perinatal nutritional and metabolic environments and physiological interventions such as exercise that increase the inhibitory feedback of signals such as leptin as a strategy for producing pharmacological interventions to prevent and/or treat early onset obesity.
Acknowledgements
The author is funded by the Research Service of the Department of Veterans Affairs and by grants from the NIDDK (RO1 DK30066; RO1 DK 53181).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr.Rev. 1999;56:106–114. doi: 10.1111/j.1753-4887.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 3.Keinan-Boker L, Noyman N, Chinich A, Green MS, Nitzan-Kaluski D. Overweight and obesity prevalence in Israel: findings of the first national health and nutrition survey (MABAT) Isr Med Assoc J. 2005;7:219–223. [PubMed] [Google Scholar]
- 4.Andersen LF, Lillegaard IT, Overby N, Lytle L, Klepp KI, Johansson L. Overweight and obesity among Norwegian schoolchildren: changes from 1993 to 2000. Scand J Public Health. 2005;33:99–106. doi: 10.1080/140349404100410019172. [DOI] [PubMed] [Google Scholar]
- 5.Al-Almaie SM. Prevalence of obesity and overweight among Saudi adolescents in Eastern Saudi Arabia. Saudi Med J. 2005;26:607–611. [PubMed] [Google Scholar]
- 6.World Health Organization (WHO); WHO fact sheet: Obesity and overweight.
- 7.Kim DM, Ahn CW, Nam SY. Prevalence of obesity in Korea. Obes Rev. 2005;6:117–121. doi: 10.1111/j.1467-789X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Castillo CP, Velasquez-Monroy O, Lara-Esqueda A, Berber A, Sepulveda J, Tapia-Conyer R, James WP. Diabetes and hypertension increases in a society with abdominal obesity: results of the Mexican National Health Survey 2000. Public Health Nutr. 2005;8:53–60. doi: 10.1079/phn2005659. [DOI] [PubMed] [Google Scholar]
- 9.van der Sande MA, Ceesay SM, Milligan PJ, Nyan OA, Banya WA, Prentice A, McAdam KP, Walraven GE. Obesity and undernutrition and cardiovascular risk factors in rural and urban Gambian communities. Am J Public Health. 2001;91:1641–1644. doi: 10.2105/ajph.91.10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y. Cross-national comparison of childhood obesity: the epidemic and the relationship between obesity and socioeconomic status. Internat. J.Epidemiol. 2001;30:1129–1136. doi: 10.1093/ije/30.5.1129. [DOI] [PubMed] [Google Scholar]
- 13.Boney C, Verma A, Tucker R, Vohr B. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. 2005;115 doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 14.Bjorntorp P. Abdominal obesity and the metabolic syndrome. Ann.Med. 1992;24:465–468. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- 15.Erermis S, Cetin N, Tamar M, Bukusoglu N, Akdeniz F, Goksen D. Is obesity a risk factor for psychopathology among adolescents? Pediatr Int. 2004;46:296–301. doi: 10.1111/j.1442-200x.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 16.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Inter.J.Obesity Rel.Metab.Disord. 1999;23:S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 17.Valencia-Flores M, Orea A, Castano VA, Resendiz M, Rosales M, Rebollar V, Santiago V, Gallegos J, Campos RM, Gonzalez J, Oseguera J, Garcia-Ramos G, Bliwise DL. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes.Res. 2000;8:262–269. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 18.Nowicki EM, Billington CJ, Levine AS, Hoover H, Must A, Naumova E. Overweight, obesity, and associated disease burden in the Veterans Affairs ambulatory care population. Military Med. 2003;168:252–256. [PubMed] [Google Scholar]
- 19.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, t.N.E.a.D.C.o.M.R.G. NEDCOM Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann.Intern.Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 20.Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med. 1994;97:354–362. doi: 10.1016/0002-9343(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Hill JO. Successful weight loss maintenance. Ann.Rev.Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 22.Bouchard C, Perusse L. Genetics of obesity. Ann.Rev.Nutr. 1993;13:337–354. doi: 10.1146/annurev.nu.13.070193.002005. [DOI] [PubMed] [Google Scholar]
- 23.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J.Nutr. 1997;127:943S–947S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 24.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256:51–54. [PubMed] [Google Scholar]
- 25.Dorner G. [Possible significance of prenatal and-or perinatal nutrition for the pathogenesis of obesity] Acta Biol Med Ger. 1973;30:K19–K22. [PubMed] [Google Scholar]
- 26.Dorner G. Hormone-dependent brain development. Psychoneuroendocrinology. 1983;8:205–212. doi: 10.1016/0306-4530(83)90057-4. [DOI] [PubMed] [Google Scholar]
- 27.Dorner G, Thoelke H, Mohnike A, Schneider H. High food supply in perinatal life appears to favour the development of insulin-treated diabetes mellitus (ITDM) in later life. Exp.Clin.Endocrinol. 1985;85:1–6. doi: 10.1055/s-0029-1210414. [DOI] [PubMed] [Google Scholar]
- 28.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 29.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 30.Ravelli AC, Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 years in men and women exposed to famine prenatally. Am.J.Clin.Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 31.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJ. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc.Royal Soc.Lond. 1995;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- 33.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int.J.Obes.Relat.Metab.Disord. 1997;21:451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- 34.Kliegman RM, Gross T. Perinatal problems of the obese mother and her infant. Obstet Gynecol. 1985;66:299–306. [PubMed] [Google Scholar]
- 35.Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers. N Engl J Med. 1988;318:461–466. doi: 10.1056/NEJM198802253180801. [DOI] [PubMed] [Google Scholar]
- 36.Kohlhoff R, Dorner G. Perinatal hyperinsulinism and perinatal obesity as risk factors for hyperinsulinaemia in later life. Exp Clin Endocrinol. 1990;96:105–108. doi: 10.1055/s-0029-1210995. [DOI] [PubMed] [Google Scholar]
- 37.Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm.Metab.Res. 1994;26:213–221. doi: 10.1055/s-2007-1001668. [DOI] [PubMed] [Google Scholar]
- 38.Weintrob N, Karp M, Hod M. Short- and long-range complications in offspring of diabetic mothers. J Diabetes Complications. 1996;10:294–301. doi: 10.1016/1056-8727(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 39.Philipson EH, Kalhan SC, Edelberg SC, Williams TG. Maternal obesity as a risk factor in gestational diabetes. Am J Perinatol. 1985;2:268–270. doi: 10.1055/s-2007-999967. [DOI] [PubMed] [Google Scholar]
- 40.Allison DB, Paultre F, Heymsfield SB, Pi-Sunyer FX. Is the intra-uterine period really a critical period for the development of adiposity? Inter.J.Obesity Rel.Metab.Disord. 1995;19:397–402. [PubMed] [Google Scholar]
- 41.Martorell R, Stein AD, Schroeder DG. Early nutrition and later adiposity. J.Nutr. 2001;131:874S–880S. doi: 10.1093/jn/131.3.874S. [DOI] [PubMed] [Google Scholar]
- 42.Berenson GS, Bao W, Srinivasan SR. Abnormal characteristics of young offspring of parents with non-insulin-dependent diabetes mellitus. The Bogalusa Heart Study. Am.J.Epidemiol. 1997;144:962–967. doi: 10.1093/oxfordjournals.aje.a008866. [DOI] [PubMed] [Google Scholar]
- 43.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–628. doi: 10.2337/diab.37.5.622. [DOI] [PubMed] [Google Scholar]
- 44.Maffeis C, Micciolo R, Must A, Zaffanello M, Pinelli L. Parental and perinatal factors associated with childhood obesity in north-east Italy. Int J Obes Relat Metab Disord. 1994;18:301–305. [PubMed] [Google Scholar]
- 45.Vogels N, Posthumus DL, Mariman EC, Bouwman F, Kester AD, Rump P, Hornstra G, Westerterp-Plantenga MS. Determinants of overweight in a cohort of Dutch children. Am J Clin Nutr. 2006;84:717–724. doi: 10.1093/ajcn/84.4.717. [DOI] [PubMed] [Google Scholar]
- 46.Rutters F, Nieuwenhuizen AG, Vogels N, Bouwman F, Mariman E, Westerterp-Plantenga MS. Leptin-adiposity relationship changes, plus behavioral and parental factors, are involved in the development of body weight in a Dutch children cohort. Physiol Behav. 2008;93:967–974. doi: 10.1016/j.physbeh.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Safer DL, Agras WS, Bryson S, Hammer LD. Early body mass index and other anthropometric relationships between parents and children. Int J Obes Relat Metab Disord. 2001;25:1532–1536. doi: 10.1038/sj.ijo.0801786. [DOI] [PubMed] [Google Scholar]
- 48.Fuentes RM, Notkola IL, Shemeikka S, Tuomilehto J, Nissinen A. Familial aggregation of body mass index: a population-based family study in eastern Finland. Horm Metab Res. 2002;34:406–410. doi: 10.1055/s-2002-33474. [DOI] [PubMed] [Google Scholar]
- 49.Modanlou HD, Dorchester WL, Thorosian A, Freeman RK. Macrosomia-- maternal, fetal, and neonatal implications. Obstet Gynecol. 1980;55:420–424. [PubMed] [Google Scholar]
- 50.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 51.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 40 Suppl. 1991;2:121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 52.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18:611–617. doi: 10.2337/diacare.18.5.611. [DOI] [PubMed] [Google Scholar]
- 53.Gillman MW, Rifas-Shiman SL, Camargo CA, Jr, Berkey CS, Frazier AL, Rockett HR, Field AE, Colditz GA. Risk of overweight among adolescents who were breastfed as infants. Jama. 2001;285:2461–2467. doi: 10.1001/jama.285.19.2461. [DOI] [PubMed] [Google Scholar]
- 54.Singhal A, Farooqi IS, O'Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75:993–999. doi: 10.1093/ajcn/75.6.993. [DOI] [PubMed] [Google Scholar]
- 55.Vohr BR, Lipsitt LP, Oh W. Somatic growth of children of diabetic mothers with reference to birth size. J Pediatr. 1980;97:196–199. doi: 10.1016/s0022-3476(80)80473-2. [DOI] [PubMed] [Google Scholar]
- 56.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramer MS. Do breast-feeding and delayed introduction of solid foods protect against subsequent obesity? J Pediatr. 1981;98:883–887. doi: 10.1016/s0022-3476(81)80579-3. [DOI] [PubMed] [Google Scholar]
- 58.von Kries R, Koletzko B, Sauerwald T, von Mutius E, Barnert D, Grunert V, von Voss H. Breast feeding and obesity: cross sectional study. BMJ. 1999;319:147–150. doi: 10.1136/bmj.319.7203.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP. Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Arch.Dis.Child. 2000;82:248–252. doi: 10.1136/adc.82.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koletzko B, von Kries R, Monasterolo RC, Subias JE, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Anton B, Gruszfeld D, Dobrzanska A, Sengier A, Langhendries JP, Cachera MF, Grote V. Infant feeding and later obesity risk. Adv Exp Med Biol. 2009;646:15–29. doi: 10.1007/978-1-4020-9173-5_2. [DOI] [PubMed] [Google Scholar]
- 61.Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25:16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 62.Neel V. Diabetes mellitus: a "thrifty" genotype rendered detrimental by progress. Am.J.Hum.Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 63.Speakman JR. Thrifty genes for obesity and the metabolic syndrome--time to call off the search? Diab Vasc Dis Res. 2006;3:7–11. doi: 10.3132/dvdr.2006.010. [DOI] [PubMed] [Google Scholar]
- 64.Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the 'drifty gene' hypothesis. Int J Obes (Lond) 2008;32:1611–1617. doi: 10.1038/ijo.2008.161. [DOI] [PubMed] [Google Scholar]
- 65.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N.Eng.J.Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 66.Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with Type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2009 doi: 10.1016/j.jdiacomp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9:322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 69.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O'Rahilly S, Farooqi IS. A POMC variant implicates beta- melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 72.Mitchel JS, Keesey RE. Defense of a lowered weight maintenance level by lateral hypothalamically lesioned rats: Evidence from a restriction-refeeding regimen. Physiol.Behav. 1977;18:1121–1125. doi: 10.1016/0031-9384(77)90020-8. [DOI] [PubMed] [Google Scholar]
- 73.Boyle PC, Storlien LH, Keesey RE. Increased efficiency of food utilization following weight loss. Physiol Behav. 1978;21:261–264. doi: 10.1016/0031-9384(78)90050-1. [DOI] [PubMed] [Google Scholar]
- 74.Keesey RE, Mitchell JS, Kemnitz JW. Body weight and body composition of male rats following hypothalamic lesions. Am.J.Physiol. 1979;237:R68–R73. doi: 10.1152/ajpregu.1979.237.1.R68. [DOI] [PubMed] [Google Scholar]
- 75.Keesey RE, Corbett SW. Metabolic defense of the body weight set-point. In: Stunkard AJ, Stellar E, editors. Eating and its disorders. New York: Raven Press; 1984. pp. 87–96. [PubMed] [Google Scholar]
- 76.Corbett SW, Wilterdink EJ, Keesey RE. Resting oxygen consumption in over- and underfed rats with lateral hypothalamic lesions. Physiol.Behav. 1985;35:971–977. doi: 10.1016/0031-9384(85)90268-9. [DOI] [PubMed] [Google Scholar]
- 77.Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am.J.Clin.Nutr. 1986;44:173–180. doi: 10.1093/ajcn/44.2.173. [DOI] [PubMed] [Google Scholar]
- 78.Wirtshafter D, Davis JD. Set points, settling points, and the control of body weight. Physiol.Behav. 1977;19:75–78. doi: 10.1016/0031-9384(77)90162-7. [DOI] [PubMed] [Google Scholar]
- 79.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am.J.Physiol. 2000;278:R231–R237. doi: 10.1152/ajpregu.2000.278.1.R231. [DOI] [PubMed] [Google Scholar]
- 80.Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1117–R1129. doi: 10.1152/ajpregu.00808.2007. [DOI] [PubMed] [Google Scholar]
- 81.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1577–R1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- 82.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 83.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am.J.Physiol. 2002;282:R46–R54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- 84.Levin BE, Keesey RE. Defense of differing body weight set-points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–R419. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- 85.Hill JO, Dorton J, Sykes MN, Digirolamo M. Reversal of dietary obesity is influenced by its duration and severity. Int.J.Obes. 1989;13:711–722. [PubMed] [Google Scholar]
- 86.Katzeff HL, Danforth E., Jr Decreased thermic effect of a mixed meal during overnutrition in human obesity. Am.J.Clin.Nutr. 1989;50:915–921. doi: 10.1093/ajcn/50.5.915. [DOI] [PubMed] [Google Scholar]
- 87.Bernstein IL, Lotter EC, Kulkosky PJ, Porte D, Jr, Woods SC. Effect of force-feeding upon basal insulin levels of rats. Proc.Soc.Exp.Biol.Med. 1975;150:546–548. doi: 10.3181/00379727-150-39075. [DOI] [PubMed] [Google Scholar]
- 88.Levin BE. Why some of us get fat and what we can do about it. J Physiol. 2007;583:425–430. doi: 10.1113/jphysiol.2007.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 90.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keesey RE, Powley TL. Body energy homeostasis. Appetite. 2008;51:442–445. doi: 10.1016/j.appet.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 93.Quaade F, Vaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir (Wien) 1974;30:111–117. doi: 10.1007/BF01405759. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc.R.Soc.Lond.B.Biol.Sci. 1953;611:221–235. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 96.Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46:1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woods SC, Seeley RJ, Porte J, D, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1387. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 99.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. [Review] [57 refs] Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 100.Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav. 2009;97:572–580. doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patterson CM, Levin BE. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology. 2008;87:65–70. doi: 10.1159/000100982. [DOI] [PubMed] [Google Scholar]
- 102.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiol Behav. 2006;89:486–489. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 104.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin and melanocortin binding associated with moderate fat diet and predisposition to obesity. Endocrinology. 2007;148:310–316. doi: 10.1210/en.2006-1126. [DOI] [PubMed] [Google Scholar]
- 105.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levin BE, Hamm MW. Plasticity of brain α-adrenoceptors during the development of diet-induced obesity in the rat. Obes Res. 1994;2:230–238. doi: 10.1002/j.1550-8528.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 107.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 108.Fliers E, Noppen NW, Wiersinga WM, Visser TJ, Swaab DF. Distribution of thyrotropin-releasing hormone (TRH)-containing cells and fibers in the human hypothalamus. J Comp Neurol. 1994;350:311–323. doi: 10.1002/cne.903500213. [DOI] [PubMed] [Google Scholar]
- 109.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 110.Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- 111.Levin BE, Kang L, Sanders NM, Dunn-Meynell AA. Role of neuronal glucosensing in the regulation of energy homeostasis. Diabetes 55. 2006;2 Suppl:S122–S130. [Google Scholar]
- 112.Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew O, Sakaruda O, Shinohara M. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J.Neurochem. 1977;23:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 113.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am.J.Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 114.Oomura Y, Kimura K, Ooyama H, Maeo T, Iki M, Kuniyoshi N. Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- 115.Jo YH, Su Y, Gutierrez-Juarez R, Chua S., Jr Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol. 2009;101:2305–2316. doi: 10.1152/jn.91294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol. 2009;297:R655–R664. doi: 10.1152/ajpregu.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Effects of maternal genotype and diet on offspring glucose and fatty acid sensing ventromedial hypothalamic nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1351–R1357. doi: 10.1152/ajpregu.00370.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Migrenne S, Cruciani-Guglielmacci C, Kang L, Wang R, Rouch C, Lefevre AL, Ktorza A, Routh VH, Levin BE, Magnan C. Fatty Acid signaling in the hypothalamus and the neural control of insulin secretion. Diabetes 55. 2006;2 Suppl:S139–S144. [Google Scholar]
- 119.Kang L, Routh VH, Kuzhikandathil EV, Gaspers L, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- 120.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 121.Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 122.Altman J, Bayer SA. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- 123.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 124.Cottrell EC, Cripps RL, Duncan JS, Barrett P, Mercer JG, Herwig A, Ozanne SE. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol. 2009;296:R631–R639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S. The effects of prenatal stress on the development of hypothalamic paraventricular neurons in fetal rats. Neuroscience. 1999;92:1079–1088. doi: 10.1016/s0306-4522(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 126.Botchkina GI, Morin LP. Organization of permanent and transient neuropeptide Y-immunoreactive neuron groups and fiber systems in the developing hamster diencephalon. J Comp Neurol. 1995;357:573–602. doi: 10.1002/cne.903570408. [DOI] [PubMed] [Google Scholar]
- 127.van Eerdenburg FJ, Rakic P. Early neurogenesis in the anterior hypothalamus of the rhesus monkey. Brain Res Dev Brain Res. 1994;79:290–296. doi: 10.1016/0165-3806(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 128.Bugnon C, Fellmann D, Bresson JL, Clavequin MC. [Immunocytochemical study of the ontogenesis of the CRF-containing neuroglandular system in the human hypothalamus (author's transl)] C R Seances Acad Sci III. 1982;294:491–496. [PubMed] [Google Scholar]
- 129.Burford GD, Robinson IC. Oxytocin, vasopressin and neurophysins in the hypothalamo-neurohypophysial system of the human fetus. J Endocrinol. 1982;95:403–408. doi: 10.1677/joe.0.0950403. [DOI] [PubMed] [Google Scholar]
- 130.Ackland J, Ratter S, Bourne GL, Rees LH. Characterisation of immunoreactive somatostatin in human fetal hypothalamic tissue. Regul Pept. 1983;5:95–101. doi: 10.1016/0167-0115(83)90117-9. [DOI] [PubMed] [Google Scholar]
- 131.Mai JK, Lensing-Hohn S, Ende AA, Sofroniew MV. Developmental organization of neurophysin neurons in the human brain. J Comp Neurol. 1997;385:477–489. [PubMed] [Google Scholar]
- 132.Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. Organization of human hypothalamus in fetal development. J Comp Neurol. 2002;446:301–324. doi: 10.1002/cne.10175. [DOI] [PubMed] [Google Scholar]
- 133.Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 134.Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23:257–291. doi: 10.1016/s0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 136.Grove KL, Allen S, Grayson BE, Smith MS. Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience. 2003;116:393–406. doi: 10.1016/s0306-4522(02)00668-1. [DOI] [PubMed] [Google Scholar]
- 137.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three-weeks of post-weaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296 doi: 10.1152/ajpregu.90859.2008. 90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- 140.Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. J.Comp.Neurol. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- 141.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rinaman L. Ontogeny of hypothalamic-hindbrain feeding control circuits. Dev Psychobiol. 2006;48:389–396. doi: 10.1002/dev.20146. [DOI] [PubMed] [Google Scholar]
- 143.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lehmann K, Grund T, Bagorda A, Bagorda F, Grafen K, Winter Y, Teuchert-Noodt G. Developmental effects on dopamine projections and hippocampal cell proliferation in the rodent model of postweaning social and physical deprivation can be triggered by brief changes of environmental context. Behav Brain Res. 2009;205:26–31. doi: 10.1016/j.bbr.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 145.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burnett S, Bird G, Moll J, Frith C, Blakemore SJ. Development during adolescence of the neural processing of social emotion. J Cogn Neurosci. 2009;21:1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept. 2008;149:11–14. doi: 10.1016/j.regpep.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J.Neurochem. 1979;33:439–445. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 150.Silver IA, Erecinska M. Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-and hyperglycemic animals. J.Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J. Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- 153.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, Levine J. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150:5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp.Br.Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 155.Krisch B, Leonhardt H. The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res. 1978;192:327–339. doi: 10.1007/BF00220750. [DOI] [PubMed] [Google Scholar]
- 156.Felten DL, Harrigan P, Burnett BT, Cummings JP. Fourth ventricular tanycytes: a possible relationship with monoaminergic nuclei. Brain Res Bull. 1981;6:427–436. doi: 10.1016/s0361-9230(81)80013-5. [DOI] [PubMed] [Google Scholar]
- 157.Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 158.Shaver SW, Pang JJ, Wainman DS, Wall KM, Gross PM. Morphology and function of capillary networks in subregions of the rat tuber cinereum. Cell Tissue Res. 1992;267:437–448. doi: 10.1007/BF00319366. [DOI] [PubMed] [Google Scholar]
- 159.Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia. 2005;52:228–233. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- 160.Cheunsuang O, Stewart AL, Morris R. Differential uptake of molecules from the circulation and CSF reveals regional and cellular specialisation in CNS detection of homeostatic signals. Cell Tissue Res. 2006;325:397–402. doi: 10.1007/s00441-006-0162-z. [DOI] [PubMed] [Google Scholar]
- 161.Krisch B, Leonhardt H, Buchheim W. The functional structural border between the CSF- and blood-milieu in the circumventricular organs (organum vasculosum laminae terminalis, subfornical organ, area postrema) of the rat. Cell Tiss.Res. 1978;195:485–497. doi: 10.1007/BF00233891. [DOI] [PubMed] [Google Scholar]
- 162.Cummings JP, Felten DL. A raphe dendrite bundle in the rabbit medulla. J Comp Neurol. 1979;183:1–23. doi: 10.1002/cne.901830102. [DOI] [PubMed] [Google Scholar]
- 163.Garcia MA, Carrasco M, Godoy A, Reinicke K, Montecinos VP, Aguayo LG, Tapia JC, Vera JC, Nualart F. Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J.Cell.Biochem. 2001;80:491–503. [PubMed] [Google Scholar]
- 164.Roncero I, Alvarez E, Vazques P, Blazquez E. Functional glucokinase isoforms are expressed in rat brain. J.Neurochem. 2000;74:1848–1857. doi: 10.1046/j.1471-4159.2000.0741848.x. [DOI] [PubMed] [Google Scholar]
- 165.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- 166.Diano S, Naftolin F, Horvath TL. Kainate glutamate receptors (GluR5–7) in the rat arcuate nucleus: relationship to tanycytes, astrocytes, neurons and gonadal steroid receptors. J.Endocrinol. 1998;10:239–247. doi: 10.1046/j.1365-2826.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 167.Berger UV, Hediger MA. Differential distribution of the glutamate transporters GLT-1 and GLAST in tanycytes of the third ventricle. J Comp Neurol. 2001;433:101–114. doi: 10.1002/cne.1128. [DOI] [PubMed] [Google Scholar]
- 168.Lechan RM, Fekete C. Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome. J Endocrinol Invest. 2004;27:105–119. [PubMed] [Google Scholar]
- 169.Fernandez-Galaz MC, Torres-Aleman I, Garcia-Segura LM. Endocrine-dependent accumulation of IGF-I by hypothalamic glia. Neuroreport. 1996;8:373–377. doi: 10.1097/00001756-199612200-00073. [DOI] [PubMed] [Google Scholar]
- 170.Bjelke B, Fuxe K. Intraventricular beta-endorphin accumulates in DARPP-32 immunoreactive tanycytes. Neuroreport. 1993;5:265–268. doi: 10.1097/00001756-199312000-00021. [DOI] [PubMed] [Google Scholar]
- 171.Sladek JR, Jr, Sladek CD. Localization of serotonin within tanycytes of the rat median eminence. Cell Tiss.Res. 1978;186:465–474. doi: 10.1007/BF00224935. [DOI] [PubMed] [Google Scholar]
- 172.Larsen PJ, Hay-Schmidt A, Vrang N, Mikkelsen JD. Origin of projections from the midbrain raphe nuclei to the hypothalamic paraventricular nucleus in the rat: a combined retrograde and anterograde tracing study. Neurosci. 1996;70:963–988. doi: 10.1016/0306-4522(95)00415-7. [DOI] [PubMed] [Google Scholar]
- 173.Beauvillain JC, Moyse E, Dutriez I, Mitchell V, Poulain P, Mazzuca M. Localization of mu opioid receptors on the membranes of nerve endings and tanycytes in the guinea-pig median eminence by electron microscopic radioautography. Neuroscience. 1992;49:925–936. doi: 10.1016/0306-4522(92)90368-c. [DOI] [PubMed] [Google Scholar]
- 174.Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes. 2004;53:1230–1236. doi: 10.2337/diabetes.53.5.1230. [DOI] [PubMed] [Google Scholar]
- 175.Rethelyi M. Diffusional barrier around the hypothalamic arcuate nucleus in the rat. Brain Res. 1984;307:355–358. doi: 10.1016/0006-8993(84)90494-3. [DOI] [PubMed] [Google Scholar]
- 176.Xin Z, Tachibana M, Guggiari M, Heard E, Shinkai Y, Wagstaff J. Role of histone methyltransferase G9a in CpG methylation of the Prader-Willi syndrome imprinting center. J Biol Chem. 2003;278:14996–15000. doi: 10.1074/jbc.M211753200. [DOI] [PubMed] [Google Scholar]
- 177.Gurrieri F, Accadia M. Genetic imprinting: the paradigm of Prader-Willi and Angelman syndromes. Endocr Dev. 2009;14:20–28. doi: 10.1159/000207473. [DOI] [PubMed] [Google Scholar]
- 178.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 180.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin signalling protein expression in 21 month-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2004;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- 182.Ozanne SE, Lewis R, Jennings BJ, Hales CN. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci (Lond) 2004;106:141–145. doi: 10.1042/CS20030278. [DOI] [PubMed] [Google Scholar]
- 183.Levin BE, Magnan C, Migrenne S, Chua SC, Jr, Dunn-Meynell AA. The F-DIO obesity-prone rat is insulin resistant prior to obesity onset. Am J Physiol. 2005;289:R704–R711. doi: 10.1152/ajpregu.00216.2005. [DOI] [PubMed] [Google Scholar]
- 184.Guo F, Jen K-L. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol.Behav. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 185.Buckley AJ, Keseru B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism. 2005;54:500–507. doi: 10.1016/j.metabol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 186.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 187.Kennedy GC. The development with age of hypothalamic restraint upon the appetite of the rat. J Endocrinol. 1957;16:9–17. doi: 10.1677/joe.0.0160009. [DOI] [PubMed] [Google Scholar]
- 188.Faust IM, Johnson PR, Hirsch J. Long-term effects of early nutritional experience on the development of obesity in the rat. J Nutr. 1980;110:2027–2034. doi: 10.1093/jn/110.10.2027. [DOI] [PubMed] [Google Scholar]
- 189.Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 190.Jones AP, Simson EL, Friedman MI. Gestational undernutrition and the development of obesity in rats. J.Nutr. 1984;114:1484–1492. doi: 10.1093/jn/114.8.1484. [DOI] [PubMed] [Google Scholar]
- 191.Jones AP, Assimon SA, Friedman MI. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol.Behav. 1986;37:381–386. doi: 10.1016/0031-9384(86)90194-0. [DOI] [PubMed] [Google Scholar]
- 192.Ravelli GP, Belmont L. Obesity in nineteen-year-old men: family size and birth order associations. Am J Epidemiol. 1979;109:66–70. doi: 10.1093/oxfordjournals.aje.a112660. [DOI] [PubMed] [Google Scholar]
- 193.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am.J.Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 194.Levin BE. Metabolic imprinting on genetically predisposed neural circuits perpetuates obesity. Nutrition. 2000;16:909–915. doi: 10.1016/s0899-9007(00)00408-1. [DOI] [PubMed] [Google Scholar]
- 195.Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am.J.Physiol. 2002;283:R1087–R1093. doi: 10.1152/ajpregu.00402.2002. [DOI] [PubMed] [Google Scholar]
- 196.Gorski J, Levin BE. Effects of cross fostering on body weight, adiposity and insulin sensitivity in selectively bred obesity-prone and resistant rats. Obes Res. 2004;12:A103. [Google Scholar]
- 197.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res.Devel.Brain Res. 1995;88:127–131. doi: 10.1016/0165-3806(95)00078-r. [DOI] [PubMed] [Google Scholar]
- 199.Plagemann A, Harder T, Rake A, Melchior K, Rittel F, Rohde W, Dorner G. Hypothalamic insulin and neuropeptide Y in the offspring of gestational diabetic mother rats. NeuroReport. 1998;9:4069–4073. doi: 10.1097/00001756-199812210-00012. [DOI] [PubMed] [Google Scholar]
- 200.Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 2009;117:85–93. doi: 10.1042/CS20080393. [DOI] [PubMed] [Google Scholar]
- 201.Irani BG, Le Foll C, Dunn-Meynell AA, Levin BE. Ventromedial nucleus neurons are less sensitive to leptin excitation in rats bred to develop diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R521–R527. doi: 10.1152/ajpregu.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Plagemann A, Rake A, Harder T, Melchior K, Rohde W, Dorner G. Reduction of cholecystokinin-8S-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Neurosci Lett. 1998;258:13–16. doi: 10.1016/s0304-3940(98)00823-4. [DOI] [PubMed] [Google Scholar]
- 203.Heidel E, Plagemann A, Davidowa H. Increased response to NPY of hypothalamic VMN neurons in postnatally overfed juvenile rats. Neuroreport. 1999;10:1827–1831. doi: 10.1097/00001756-199906230-00005. [DOI] [PubMed] [Google Scholar]
- 204.Plagemann A, Harder T, Janert U, Rake A, Rittel F, Rohde W, Dorner G. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev.Neurosci. 1999;21:58–67. doi: 10.1159/000017367. [DOI] [PubMed] [Google Scholar]
- 205.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 206.Udagawa J, Hashimoto R, Hioki K, Otani H. The role of leptin in the development of the cortical neuron in mouse embryos. Brain Res. 2006;1120:74–82. doi: 10.1016/j.brainres.2006.08.116. [DOI] [PubMed] [Google Scholar]
- 207.Puro DG, Agardh E. Insulin-mediated regulation of neuronal maturation. Science. 1984;225:1170–1172. doi: 10.1126/science.6089343. [DOI] [PubMed] [Google Scholar]
- 208.Recio-Pinto E, Lang FF, Ishii DN. Insulin and insulin-like growth factor II permit nerve growth factor binding and the neurite formation response in cultured human neuroblastoma cells. Proc.Natl.Acad.Sci.,USA. 1984;81:2562–2566. doi: 10.1073/pnas.81.8.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tanaka M, Sawada M, Yoshida S, Hanaoka F, Marunouchi T. Insulin prevents apoptosis of external granular layer neurons in rat cerebellar slice cultures. Neurosci.Lett. 1995;199:37–40. doi: 10.1016/0304-3940(95)12009-s. [DOI] [PubMed] [Google Scholar]
- 210.Jones AP, Dayries M. Maternal hormone manipulations and the development of obesity in rats. Physiol.Behav. 1990;47:1107–1110. doi: 10.1016/0031-9384(90)90359-c. [DOI] [PubMed] [Google Scholar]
- 211.Jones AP, Olster DH, States B. Maternal insulin manipulations in rats organize body weight and noradrenergic innervation of the hypothalamus in gonadally intact male offspring. Dev.Brain Res. 1996;97:16–21. doi: 10.1016/s0165-3806(96)00128-9. [DOI] [PubMed] [Google Scholar]
- 212.Plagemann A, Harder T, Rake A, Janert U, Melchior K, Rohde W, Dorner G. Morphological alterations of hypothalamic nuclei due to intrahypothalamic hyperinsulinism in newborn rats. Internat.J.Devel.Neurosci. 1999;17:37–44. doi: 10.1016/s0736-5748(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 213.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp.Clin.Endocrinol. 1992;99:91–95. doi: 10.1055/s-0029-1211143. [DOI] [PubMed] [Google Scholar]
- 214.Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, Hauguel-de Mouzon S. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47:847–850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- 215.Lea RG, Howe D, Hannah LT, Bonneau O, Hunter L, Hoggard N. Placental leptin in normal, diabetic and fetal growth-retarded pregnancies. Mol Hum Reprod. 2000;6:763–769. doi: 10.1093/molehr/6.8.763. [DOI] [PubMed] [Google Scholar]
- 216.Lepercq J, Challier JC, Guerre-Millo M, Cauzac M, Vidal H, Hauguel-de Mouzon S. Prenatal leptin production: evidence that fetal adipose tissue produces leptin. J Clin Endocrinol Metab. 2001;86:2409–2413. doi: 10.1210/jcem.86.6.7529. [DOI] [PubMed] [Google Scholar]
- 217.Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, Nakao K, Fujii S. Possible role of placental leptin in pregnancy: a review. Endocrine. 2002;19:65–71. doi: 10.1385/ENDO:19:1:65. [DOI] [PubMed] [Google Scholar]
- 218.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 219.Yura S, Sagawa N, Mise H, Mori T, Masuzaki H, Ogawa Y, Nakao K. A positive umbilical venous-arterial difference of leptin level and its rapid decline after birth. Am J Obstet Gynecol. 1998;178:926–930. doi: 10.1016/s0002-9378(98)70525-3. [DOI] [PubMed] [Google Scholar]
- 220.Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. J Physiol. 2009;587:1145–1152. doi: 10.1113/jphysiol.2008.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Muhlhausler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biol Reprod. 2002;67:493–499. doi: 10.1095/biolreprod67.2.493. [DOI] [PubMed] [Google Scholar]
- 222.Mercer JG, Moar KM, Hoggard N, Strosberg AD, Froguel P, Bailleul B. B219/OB-R 5'-UTR and leptin receptor gene-related protein gene expression in mouse brain and placenta: tissue-specific leptin receptor promoter activity. J Neuroendocrinol. 2000;12:649–655. doi: 10.1046/j.1365-2826.2000.00501.x. [DOI] [PubMed] [Google Scholar]
- 223.Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression, materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur.J.Endocrinol. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- 224.Pankevich DE, Mueller BR, Brockel B, Bale TL. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol Behav. 2009;98:94–102. doi: 10.1016/j.physbeh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boskovic R, Feig DS, Derewlany L, Knie B, Portnoi G, Koren G. Transfer of insulin lispro across the human placenta: in vitro perfusion studies. Diabetes Care. 2003;26:1390–1394. doi: 10.2337/diacare.26.5.1390. [DOI] [PubMed] [Google Scholar]
- 227.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jansson T, Cetin I, Powell TL, Desoye G, Radaelli T, Ericsson A, Sibley CP. Placental transport and metabolism in fetal overgrowth -- a workshop report. Placenta. 2006;27 Suppl A:S109–S113. doi: 10.1016/j.placenta.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 229.Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, Powell TL, Jansson T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87:1743–1749. doi: 10.1093/ajcn/87.6.1743. [DOI] [PubMed] [Google Scholar]
- 230.Osmond DT, King RG, Brennecke SP, Gude NM. Placental glucose transport and utilisation is altered at term in insulin-treated, gestational-diabetic patients. Diabetologia. 2001;44:1133–1139. doi: 10.1007/s001250100609. [DOI] [PubMed] [Google Scholar]
- 231.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity. 2006;14:1371–1377. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 232.Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T, Palou A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes (Lond) 2007;31:1199–1209. doi: 10.1038/sj.ijo.0803585. [DOI] [PubMed] [Google Scholar]
- 233.Gorski J, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 234.Koldovsky O, Illnerova H, Macho L, Strbak V, Stepankova R. Milk-borne hormones: possible tools of communication between mother and suckling. Physiol Res. 1995;44:349–351. [PubMed] [Google Scholar]
- 235.Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;146:2575–2582. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- 236.Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009;52:249–252. doi: 10.1016/j.appet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 237.Sanchez J, Priego T, Palou M, Tobaruela A, Palou A, Pico C. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology. 2008;149:733–740. doi: 10.1210/en.2007-0630. [DOI] [PubMed] [Google Scholar]
- 238.Stocker CJ, Wargent E, O'Dowd J, Cornick C, Speakman JR, Arch JR, Cawthorne MA. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1810–R1818. doi: 10.1152/ajpregu.00676.2006. [DOI] [PubMed] [Google Scholar]
- 239.Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul.Pept. 2000;92:1–7. doi: 10.1016/s0167-0115(00)00142-7. [DOI] [PubMed] [Google Scholar]
- 240.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 241.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J.Clin.Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lins MC, de Moura EG, Lisboa PC, Bonomo IT, Passos MC. Effects of maternal leptin treatment during lactation on the body weight and leptin resistance of adult offspring. Regul Pept. 2005;127:197–202. doi: 10.1016/j.regpep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 243.Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006;95:830–837. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]
- 244.de Oliveira Cravo C, Teixeira CV, Passos MC, Dutra SC, de Moura EG, Ramos C. Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm Metab Res. 2002;34:400–405. doi: 10.1055/s-2002-33473. [DOI] [PubMed] [Google Scholar]
- 245.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 246.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 247.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Mogami H, Ogawa Y, Fujii S. Neonatal exposure to leptin augments diet-induced obesity in leptin-deficient Ob/Ob mice. Obesity (Silver Spring) 2008;16:1289–1295. doi: 10.1038/oby.2008.57. [DOI] [PubMed] [Google Scholar]
- 248.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 249.Levin BE, Dunn-Meynell AA, McMinn JE, Alperovich M, Cunningham- Bussel A, Chua SC., Jr A new obesity-prone, glucose-intolerant rat strain (F.DIO) Am J Physiol Regul Integr Comp Physiol. 2003;285:R1184–R1191. doi: 10.1152/ajpregu.00267.2003. [DOI] [PubMed] [Google Scholar]
- 250.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R610–R618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 251.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1782–R1791. doi: 10.1152/ajpregu.00749.2006. [DOI] [PubMed] [Google Scholar]
- 252.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol. 2003;285:E949–E957. doi: 10.1152/ajpendo.00186.2003. [DOI] [PubMed] [Google Scholar]
- 253.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling prior to obesity onset. Am J Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 254.Labelle DR, Cox JM, Dunn-Meynell AA, Levin BE, Flanagan-Cato LM. Genetic and dietary effects on dendrites in the rat hypothalamic ventromedial nucleus. Physiol Behav. 2009;98:511–516. doi: 10.1016/j.physbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Levin BE. Reduced paraventricular nucleus norepinephrine responsiveness in obesity-prone rats. Am.J.Physiol. 1996;270:R456–R461. doi: 10.1152/ajpregu.1996.270.2.R456. [DOI] [PubMed] [Google Scholar]
- 256.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body- weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 257.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 258.Wilmot CA, Sullivan AC, Levin BE. Effects of diet and obesity on brain α1- and α2-noradrenergic receptors in the rat. Brain Res. 1988;453:157–166. doi: 10.1016/0006-8993(88)90154-0. [DOI] [PubMed] [Google Scholar]
- 259.Levin BE. Reduced norepinephrine turnover in organs and brains of obesity-prone rats. Am J Physiol. 1995;268:R389–R394. doi: 10.1152/ajpregu.1995.268.2.R389. [DOI] [PubMed] [Google Scholar]
- 260.Hassanain M, Levin BE. Dysregulation of hypothalamic serotonin turnover in diet-induced obese rats. Brain Res. 2002;929:175–180. doi: 10.1016/s0006-8993(01)03387-x. [DOI] [PubMed] [Google Scholar]
- 261.Levin BE. Intracarotid glucose-induced norepinephrine response and the development of diet-induced obesity. Int J Obesity. 1992;16:451–457. [PubMed] [Google Scholar]
- 262.Levin BE, Planas B. Defective glucoregulation of brain α2-adrenoceptors in obesity-prone rats. Am J Physiol. 1993;264:R305–R311. doi: 10.1152/ajpregu.1993.264.2.R305. [DOI] [PubMed] [Google Scholar]
- 263.Dunn-Meynell AA, Govek E, Levin BE. Intracarotid glucose infusions selectively increase Fos-like immunoreactivity in paraventricular, ventromedial and dorsomedial nuclei neurons. Brain Res. 1997;748:100–106. doi: 10.1016/s0006-8993(96)01280-2. [DOI] [PubMed] [Google Scholar]
- 264.Leibowitz SF, Roissin P, Rosenn M. Chronic norepinephrine injection into the hypothalamic paraventricular nucleus produces hyperphagia and increased body weight in the rat. Pharmacol.Biochem.Behav. 1984;21:801–808. doi: 10.1016/s0091-3057(84)80022-2. [DOI] [PubMed] [Google Scholar]
- 265.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 267.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamic glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 268.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J.Clin.Invest. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56:1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 270.Klem ML, Wing RR, McGuire MT, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am.J.Clin.Nutr. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 271.McGuire MT, Wing RR, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes.Res. 1999;7:334–341. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 272.Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, Hill JO. Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am.J.Clin.Nutr. 1999;69:1189–1193. doi: 10.1093/ajcn/69.6.1189. [DOI] [PubMed] [Google Scholar]
- 273.Mayer J, Marshall NB, Vitale JJ, Christensen JH, Mashayekhi MB, Stare FJ. Exercise, food intake and body weight in normal rats and genetically obese adult mice. Am J Physiol. 1954;177:544–548. doi: 10.1152/ajplegacy.1954.177.3.544. [DOI] [PubMed] [Google Scholar]
- 274.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol. 2004;286:R771–R778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 275.Levin BE, Dunn-Meynell AA. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int J Obes (Lond) 2006;30:722–727. doi: 10.1038/sj.ijo.0803192. [DOI] [PubMed] [Google Scholar]
- 276.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R290–R301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 277.Applegate EA, Upton DE, Stern JS. Exercise, detraining: effect on food intake, adiposity and lipogenesis in Osborne-Mendel rats made obese by a high fat diet. J Nutr. 1984;114:447–459. doi: 10.1093/jn/114.2.447. [DOI] [PubMed] [Google Scholar]
- 278.Bouchard C, Tremblay A. Genetic effects in human energy expenditure components. Int.J.Obes. 1990;14 Suppl-55 [PubMed] [Google Scholar]
- 279.Chagnon YC, Perusse L, Bouchard C. Familial aggregation of obesity, candidate genes and quantitative trait loci. Curr Opin Lipidol. 1997;8:205–211. doi: 10.1097/00041433-199708000-00003. [DOI] [PubMed] [Google Scholar]