Abstract
Echinococcosis is a major emerging zoonosis in central Asia. A study of the helminth fauna of foxes from Naryn Oblast in central Kyrgyzstan was undertaken to investigate the abundance of Echinococcus multilocularis in a district where a high prevalence of this parasite had previously been detected in dogs. A total of 151 foxes (Vulpes vulpes) were investigated in a necropsy study. Of these 96 (64%) were infected with E. multilocularis with a mean abundance of 8669 parasites per fox. This indicates that red foxes are a major definitive host of E. multilocularis in this country. This also demonstrates that the abundance and prevalence of E. multilocularis in the natural definitive host are likely to be high in geographical regions where there is a concomitant high prevalence in alternative definitive hosts such as dogs. In addition Mesocestoides spp., Dipylidium caninum, Taenia spp., Toxocara canis, Toxascaris leonina, Capillaria and Acanthocephala spp. were found in 99 (66%), 50 (33%), 48 (32%), 46 (30%), 9 (6%), 34 (23%) and 2 (1%) of foxes, respectively. The prevalence but not the abundance of E. multilocularis decreased with age. The abundance of Dipylidium caninum also decreased with age. The frequency distribution of E. multilocularis and Mesocestoides spp. followed a zero inflated negative binomial distribution, whilst all other helminths had a negative binomial distribution. This demonstrates that the frequency distribution of positive counts and not just the frequency of zeros in the data set can determine if a zero inflated or non-zero inflated model is more appropriate. This is because the prevalences of E. multolocularis and Mesocestoides spp. were the highest (and hence had fewest zero counts) yet the parasite distribution nevertheless gave a better fit to the zero inflated models.
Keywords: Fox, intestinal helminths, Echinococcus multilocularis, Kyrgyzstan, epidemiology, zero inflated, negative binomial
1. Introduction
Alveolar echinococcosis, caused by Echinococcus multilocularis, is one of the most pathogenic parasite zoonoses. E. multilocularis is widely endemic in the northern hemisphere including much of Europe, Turkey and Russia with central Asia in general and western China in particular representing major endemic areas (Bessonov et al., 1998; Vuitton et al., 2003; Eckert and Deplazes 2004; Craig, 2006; Shaikenov, 2006). In some communities in China 5% or more of the human population has alveolar echinococcosis (AE) (Budke et al., 2004).
There have also been studies which describe a high prevalence of E. multilocularis in dogs and the transmission dynamics of this parasite in dogs from both China (Budke et al, 2005a) and Kyrgyzstan (Ziadinov et al., 2008). In contrast, to date, there are few data on the abundance of E. multilocularis in foxes, the natural definitive hosts and no data analysing the frequency distribution of this parasite in these hosts from these geographical regions. Such data are important to assess the relative importance of foxes and/or dogs in maintaining the transmission cycle. In addition data can be used to better understand the epidemiology of E. multilocularis in the natural cycle between foxes and small mammals, the spill over to dogs and the transmission from either foxes or dogs to man. The role of the fox rather than the dog in maintaining the cycle has important consequences for control of this zoonotic parasite. If dogs act as an aberrant definitive host, frequent treatments of dogs may only marginally ameliorate the risk of AE in humans as the cycle within wildlife remains undisturbed. In contrast if dogs participate in the transmission cycle, frequent treatment will decrease the infection pressure to small mammals and should result in a greater reduction in the risk of human AE over time (Budke et al., 2005b). Mathematical models describing the transmission dynamics of Echinococcus spp. can also be used as a tool in developing control programmes (Budke et al., 2005b) and quantitative rather than purely qualitative data are of great benefit in such model development.
The analysis of the frequency distribution of parasites within hosts can give important clues to the ecology of the parasite population and information regarding the epidemiology of transmission (Shaw et al., 1998). In addition the use of general linear models utilising the appropriate parasite distribution results in fewer statistical errors than traditional ways of dealing with aggregation or other departures from normality such as logarithmic transformation of the data (Wilson et al., 1996). The negative binomial distribution has proved in the past to be a rigorous description of the frequency distributions of parasites in wildlife (Shaw et al., 1998). However, recently zero-inflated models have been suggested as an alternative that might improve upon the use of the negative binomial distribution and has been used to describe the distribution of helminth parasites of sheep (Denwood et al., 2008). Hence we wished to explore which were the most appropriate frequency distributions to describe populations of helminths in naturally infected foxes.
Information about fox parasites in general from Kyrgyzstan is very limited. Previous studies date back to the 1950s. Gagarin (1954) investigated 12 fox intestines from south Kyrgyzstan and described the presence of 9 helminths: Multiceps serialis (Taenia serialis), Mesocestoides lineatus, Toxocara canis, Toxoascaris leonina, Numidica numidica (Nematoda, Ascaroidea, Heterakidae – see Baylis and Daubney, 1926), Eucoleus aerophilus (Capillaria aerophila), Uncinaria stenocephala, Crenosoma vulpis and Physalopetra sibirica. Gagarin and Iksinov (1954) also reported 2 of 36 foxes from the Issyk Kyl region of Kyrgystan to be infected with Echinococcus granulosus. In neighboring Uzbekistan a study conducted in 1966 reported 29 helminth species of foxes including E. granulosus but not E. multilocularis (Matchanov, 1968). Such E. granulosus-infected foxes (5 of a sample of 110) had burdens ranging from 9 to 1550 parasites. Other studies from Kazakhstan and from Uzbekistan have reported E. multilocularis prevalences of 8.6% – 26% in red foxes (Vulpes vulpes) and 22%–30% in corsacs (Vulpes corsac) (Shaikenov, 2006).
The present study was aimed to obtain an understanding of the helminth fauna of foxes, the abundance of infection with E. multilocularis and information regarding the epidemiology and frequency distribution in the natural definitive host in Kyrgyzstan. The study was conducted in the same geographical location in which E. multilocularis had previously been detected in dogs at a prevalence of 18% (Ziadinov et al., 2008).
2. Materials and methods
Study area
The Atbashy region with a population of approximately 48,000 in 21 villages stretches over an area of 19,000 km2 and is situated in Naryn Oblast in central Kyrgyzstan with an altitude of between 2000 and 5500 metres. The regional centre is Atbashy with a population of 12,000. Livestock farming is the main occupation.
Sample collection
From December 2006 to February 2007red foxes (Vulpes vulpes) were collected from 11 villages. The sample collection, during the winter hunting season, was not random but consisted of the samples that hunters and landowners supplied. The animals had either been shot or killed by the dogs of hunters. All stomach and intestines were removed, frozen immediately by placing in a freezer at −20°C or frozen outside in the environment. The lower jaw of each fox was also collected. A total 151 of fox intestines were collected and were transported frozen to the laboratory in Bishkek. On arrival in Bishkek, all samples were first placed at −80°C for 1 week to ensure the death of all Echinococcus eggs.
For each fox basic information was collected from the suppliers. This included location where the fox was killed, sex of animal and approximate age (table 1). Age was confirmed by dental analysis (see below).
Table 1.
Age and sex distribution of foxes sampled.
| Age (years) | Number of male foxes | Number of female foxes |
|---|---|---|
| <1 | 26 | 34 |
| 1–2 | 14 | 15 |
| 2–3 | 19 | 12 |
| >3 | 17 | 14 |
| Total | 76 | 75 |
Parasite recovery and identification
Parasites were recovered from the intestinal tract using the sedimentation and counting technique (Deplazes & Eckert (1996) and Hofer et al. (2000). All parasites were identified on morphological structure at the Laboratory Parasitology of the Kyrgyz Medical Academy by light microscopy. E. multilocularis was identified based on typical morphological structures, especially the location of the genital pore. Where there were few worms, the whole sediment was counted. When worms were numerous, an aliquot was examined and the total count estimated from the proportion of total sediment the aliquot represented. A sample of 5 Taenia spp. was investigated for species identity by PCR/sequencing (Trachsel et al., 2007). Following enumeration all helminths were preserved in 70% ethanol. A sample of faeces from the rectum was also taken for microscopical identification of eggs.
Dental analysis
Teeth were examined histologically to estimate the age of foxes according to the method of Merkulov (1969). Initially, the teeth were decalcified with 10–12% nitric acid for approximately 1.5 days followed by washing in water. The teeth were dehydrated with 70% ethanol, dried at 22°C, mounted with colloid, sectioned and stained with haematoxilin.
Statistical analyses
The data were entered onto an Excel spreadsheet and imported into R (www.r-project.org). For analysis, a number of general linear models (GLM) or zero inflated generalised Poisson models (ZIGP) were used. The mean worm burden and 95% confidence intervals were calculated for age and gender of foxes and village of origin. Similarly, for prevalence data, the proportion of foxes infected with each parasite for each age group, gender and village location together with the exact 95% binomial confidence intervals was calculated. The data were analysed to explore any potential variations in parasite abundance and prevalence. For prevalence data, the family binomial was analysed (i.e. logistic regression) whilst for abundance models negative binomial (GLM) and zero inflated negative binomial models (ZIPG) were analysed. In each case, a backward selection model was used only retaining variables with a p<0.2 at each step. Origin of fox (i.e. village) was analysed as a random and fixed effect whilst gender and age of fox were analysed as fixed effects.
The statistical model for the best fit of parasite abundance was chosen initially on the likelihood ratio test. This was confirmed by Monte Carlo methods with 1000 Monte Carlo samples generated of size 151 (the number of foxes) of the same mean and constant of aggregation assuming the simple model is correct (Rüegg et al., 2008). Confidence intervals for the expected proportion infected for ranges of worm intensities were calculated using Monte Carlo methods.
Results
In total, we identified nine helminth species (table 2). Adults of eight species were found in the gastrointestinal tract, whilst Capillaria spp. eggs were recovered from the fox faeces. PCR/sequencing demonstrated the presence of T. hydatigena (one parasite) and T. polyacantha (4 parasites) with 99–100% sequence homology (accession no for T. polyacantha DQ408419.1, for T. hydatigena AB031352.1, FJ608745.1, GQ228819.1). The prevalences and abundances of helminths recovered are given in tables 2 and 4. E. multilocularis was found in 96 (63.6%) of the foxes. Infected animals had between 1 and 182,400 adults and the mean abundance was 8668 parasites per fox.
Table 2.
Prevalence of intestinal helminths found in 151 Kyrgyz red foxes 2006–2007 (rural area of Naryn Oblast) (sedimentation and counting technique of intestinal tracts).
| Intestinal helminths | Numbers positive (prevalence %) | Prevalence 95% confidence intervals (%) |
|---|---|---|
| Mesocestoides spp. | 99 (65.6) | 57.4–73.1 |
| Dipylidium caninum | 50 (33.1) | 25.7–41.2 |
| Taenia spp. | 48 (31.7) | 24.5–39.9 |
| Echinococcus multilocularis | 96 (63.6) | 55.4–71.3 |
| Toxocara canis | 46 (30.4) | 23.3–38.5 |
| Toxascaris leonina | 9 (5.9) | 2.8–11.0 |
| *Capillaria spp. | 34 (22.5) | 16.0–30.0 |
| Acanthocephala spp. | 2 (1.3) | 0.2–4.7 |
Eggs detected by coproscopy of a rectal fecal sample
Table 4.
Abundance and frequency distributions of intestinal helminths in Kyrgyz foxes
| Parasite | #Mean abundance (95% CIs) | k | #Proportion zero-inflated | Probability distribution |
|---|---|---|---|---|
| Echinococcus multilocularis | 8669(5442–15041) | 0.099 (0.078–0.12) | Negative binomial model | |
| 12842 (8728–18872) | 0.26 (0.20–0.35) | 0.32(0.24–0.42) | Zero-inflated negative binomial | |
| Dipylidium caninum | 5.40(3.28–9.00) | 0.10(0.07–0.13) | Negative binomial | |
| *Taenia spp. | 1.36 (0.94–2.04) | 0.19(0.11–0.27) | Negative binomial | |
| Toxascaris leonina | 0.72(0.30–2.30) | 0.03(0.012–0.042) | Negative binomial | |
| Toxocara canis | 2.01(1.33–2.76) | 0.30(0.18–0.50) | Negative binomial | |
| Mesocestoides sp. | 22.38(16.47–30.40) | 0.27(0.21–0.34) | Negative binomial | |
| 32.13(25.42–40.60) | 0.75(0.52–1.07) | 0.30(0.22–0.39) | Zero-inflated negative binomial |
For zero-inflated models, the mean abundance is given for the non zero-inflated proportion. The overall population abundance is calculated by dividing this figure by 1-proportion zero-inflated.
Taenia spp. represented at least two species and the distributions of the individual species could not be calculated as only a sub-sample of parasites had their species identity confirmed.
There was no significant variation of either prevalence or abundance of E. multilocularis with fox gender. A comparison of mixed models with village of origin as a random effect and comparing it with models with village as a fixed effect and models without this variable in the model suggested that the village of origin of fox had no significant effect on either prevalence or abundance. The results of the analysis suggested that prevalence varied with age only. The regression coefficient in the logistic model was −0.22 (+/− SE 0.11) (p=0.042) per year which is 0.82 (CIs 0.65–0.99) when converted to odds ratio. Analysis of E. multilocularis abundance using a negative binomial GLM failed to demonstrate any relationship with age, sex or origin of foxes. Further analysis using a zero-inflated model confirmed both the lack of association of abundance with age, but confirmed the association of the zero counts (increasing) with age and hence consistent with an age related decrease in prevalence (table 3).
Table 3.
GLM models – significant associations with prevalence or abundance of parasites in foxes
| Parasite | Model Fit | Significant parameters | Value and 95% CIs | Parameter type | P value |
|---|---|---|---|---|---|
| E. multilocularis | Binomial GLM (logistic) | Age | 0.82(0.65–0.99) | Odds ratio | 0.043 |
| E. multilocularis | Zero-inflated Negative binomial GLM | Age (zero-inflated) | 1.28 (1.01–1.62) | Odds ratio | 0.042 |
| k | 0.26(0.19–0.37) | ||||
| Dipylidium caninum | Negative binomial GLM | Age | 0.57 (0.37–0.81) | Incidence rate Ratio | <0.0001 |
| Presence of E. multilocularis | 3.81(1.35–10.16) | Incidence Rate Ratio | 0.008 | ||
| Presence of T. leonina | 0.13(0.02–0.80) | Incidence Rate Ratio | 0.028 | ||
| k | 0.13(0.08–0.17) |
Incidence Rate Ratio. This is the parameter in a negative binomial generalised linear model. For a continuous variable such as age it is the proportional change in parasite count for each year. Thus, for each 1 year increase in age the abundance of D. caninum parasites decreases by 57%. For a binomial variable such as presence of E. multilocularis it is the ratio of D. caninum parasite counts between those foxes infected with E. multilocularis and those non-infected.
The abundance of Dipylidium also decreased with age and had a statistical association with the presence of E. multilocularis and the absence of Toxascaris (Table 3). No other significant associations were found. Parasite abundances gave a better fit to a zero-inflated negative binomial model then the negative binomial model for E. multilocularis and Mesocestoides. For other helminths the negative binomial model was the best model description. Only two foxes were infected with Acanthocephala so no meaningful analysis could be undertaken with this parasite. Capillaria spp. eggs were found in the faeces of foxes, but no intestinal specimens were detected.
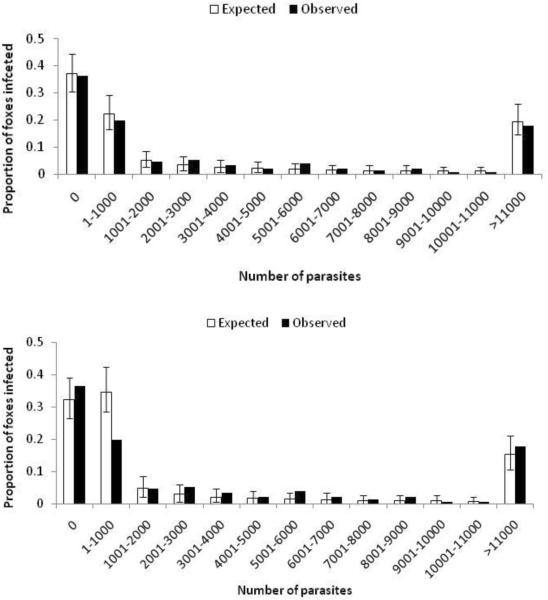
Analysis demonstrated that the distribution of E. multilocularis in foxes had a zero-inflated negative binomial distribution (p=0.00006, likelihood ratio test, p=0.001 by MonteCarlo methods) (table 4, figure 1). Likewise the abundance of Mesocestoides was zero-inflated (p=0.00003, likelihood ratio test, p=0.001 by Monte Carlo methods) with all other parasite abundances consistent with the negative binomial distribution (table 4).
Figure 1.
Goodness of fit of the observed intestinal worm burden of Echinococcus multilocularis to the expected worm burden (+/− 95% CIs) given the zero-inflated negative binomial distribution (top) and the negative binomial distribution (bottom).
Discussion
Helminths of foxes in Kyrgyzstan have been seldom studied. Gagarin and Iksinov (1954) claim to have found 2 of 36 foxes infected with E. granulosus but no criteria was given for confirming the species identity and is is possible that these were E. multilocularis. In addition E. multilocularis infection of the livers of a number of specimens of Microtus gregalis (the narrow headed vole which is native to central Asia, China, Mongolia and Russia) were found in the Bishkek region by Gagarin et al., 1957). E. multilocularis was also reported from M. gregalis and Ellobius talpinus (the northern mole vole with a range from central Asia to the Ukraine) by Tokobaev (1959) in Kyrgyzstan. This indicates that the parasite was known to be present in Kyrgyzstan over half a century ago. The prevalence we found in foxes of 63.6% (CIs 55.4–71.3%) was similar to that described in Zurich, Switzerland, by Hofer et al., (2000). The study of 110 foxes in Bukhara district in Uzbekistan (Matchanov et al., 1968) described 1 trematode species, 13 cestode species, 13 nematode species and 2 acanthocephala species. This is a considerably greater diversity of helminth fauna than the present study where we found evidence for, at least five cestode species, just three nematode species, one acanthocephala and no trematode species. This may be due to substantial differences in the climate (such as markedly lower temperatures) from central Kygystan (over 200m above sea level) compared to the Bukhara district in Uzbekistan (c 200 meters above sea level). Of interest though is the fact that E. granulosus was recovered from 5 foxes in the Uzbekistan study. Unfortunately, like the Gagarin and Iksinov (1954) study, Matchanov (1968) did not report the methods used to isolate the parasites from fox intestines or the criteria used to identify E. granulosus. Mesocestoides spp. was observed at comparable prevalence rate to those described in the Uzbekistan study and to a study in Spain (Gortazar et al., 1998). Toxocara canis is a common helminth of foxes with prevalences frequently reported of between 40–80% (for example: Reperant et al., 2006, Smith et al., 2003, Overgauuw and van Knapen 2000, Richards et al., 1995) and our observations indicate this parasite is also common in Kyrgyz foxes. Toxascaris,Dipylidium and Capillaria spp. were also found. Toxocara was found in 30% of foxes and we found no variation with age or sex of fox. However, this may be because the foxes in the present study were at least 6–9 months old as the study was conducted in winter. In a Swiss study, juvenile foxes sampled in spring or summer had higher Toxocara abundances compared to juveniles sampled in the winter (Reperant et al., 2007). Furthermore, worm burdens and prevalence were lower than in other studies (eg Ireland – Roddie et al., 2008). The Irish study also failed to demonstrate any relationship with age and worm burden. However a Danish study, which detected a similar prevalence to the Irish study, did detect higher parasite burdens in younger foxes compared to old foxes and a bias towards male foxes (Saeed and Kapel, 2006). We detected a sex bias with just one group of parasites: Taenia spp. The interpretation of this is uncertain as there were at least two species of Taenia, with very different means of transmission found in these foxes. T. hydatigena is a common parasite of dogs and the intermediate hosts are usually farm ungulates such as sheep. Thus, foxes are possibly infected through scavenging the carcasses of sheep. T. polyacantha is a common parasite of foxes which are infected by predation on small mammals such as rodents. Resources were only available to confirm the species identity of a small number of worms and hence it was not possible to analyse the patterns of Taenia infection at the species level.
The implications of the high prevalence of E. multilocularis in foxes as a public health issue are difficult to assess. Clearly, with such high abundance rates, there is the possibility for widespread contamination of the environment with worm eggs and potential transmission to humans. However, in Europe, where the prevalence and abundance in foxes is also very high, the numbers of human AE cases are quite low. Nevertheless, fox populations in Europe are increasing and invading urban areas (Deplazes et al. 2004). In Switzerland, this has been associated with an increase in the numbers of human AE cases diagnosed (Schweiger et al. 2007). Evidently, with the high Echinococcus biomass in foxes, the small mammal population in the area must also be infected to maintain the cycle, and this is the subject of ongoing studies. In China, dog contact is an established risk factor for human AE (Craig, 2006) and thus dogs must be seen as another potential conduit to the human population. Studies indicate that dogs have similar E. multilocularis prevalences in China (estimated 20 % with 95% CIs 13%-33%-Budke et al., 2005c) to those of dogs in the present study area (estimated 18% with 95% CIs 12%–30% - Ziadinov et al., 2008). However, there is a paucity of quantitative information regarding E. multilocularis infection of foxes from China, despite the natural cycle being the likely source of the spill over into dog populations. This present study gives abundance, prevalences and frequency distributions of fox infection in an area where there is concomitantly a high prevalence in dogs. Such a downstream affect of high dog prevalences may present a risk to public health in Kyrgyzstan in addition to China. Presently, the extent of human AE in central Kyrgyzstan has not been reported although this is the subject of ongoing investigations and likewise the risk factors for human AE are not yet known in Kyrgyzstan. Nevertheless, cystic echinococcosis which requires transmission through dogs, has increased substantially in Kyrgyzstan since the collapse of the Soviet Union (Torgerson et al., 2003; Torgerson et al. 2006). Depending on the transmission pathways to humans, preventive programs such as control of wild canids and prophylactic anthelmintic treatment of dogs, and health education could ameliorate the risk of AE.
A significant decrease in prevalence of infection in relation to age for E. multilocularis was detected in this sample of foxes, but no changes in mean abundance. The reasons for this may be associated with age-related behaviour of foxes or possibly immunity to reinfection. This does contrast somewhat with studies in Switzerland where there was a significant decrease with age in parasite abundance but not prevalence (Hofer et al., 2000). Different analytical techniques were used with the Swiss study using the Mann Whitney test to compare parasite abundances in contrast to the GLM techniques in the present study. Non parametric tests such as the Mann Whitney would be expected to be less powerful and hence significance would be likely if GLM or ZIGP techniques were used on the same data set. Nevertheless, the choice of analytical technique can affect the frequency of statistical errors and hence the interpretation of results (Wilson and Grenfell, 1997).
All parasite species recovered from the foxes' intestines had a highly overdispersed distribution similar to previous reports (eg Saeed et al, 2006 from Danish foxes). This was most marked with E. multilocularis and Mesocestoides spp. where a zero-inflated negative binomial distribution gave the best description of the abundance data. Such a distribution allows zero counts to arise from two distinct mechanisms: a binary process generating either a positive or zero count and a count process (including the possibility of a zero count). This may arise if the animals are exposed (generating the count process) or non-exposed (zero count). When parasites are able to develop following exposure it results in a non-zero count or a zero count if they do not develop. Other ways in which such processes may arise could depend on the method of diagnosis. Thus, the animal may have a zero count arising in two ways: the animal may have zero parasites in the intestine, or is infected but the diagnostic test gave a false negative result. As the data were based on necropsy, the latter process is unlikely in the present report. We have shown that a zero-inflated negative binomial model provides a superior description of the distribution or E. multilocularis and Mesocestoides abundance data than a negative binomial model alone. Similar zero-inflated models have been used frequently in the ecological literature where datasets with many zeros are commonplace (reviewed by Martin et al. 2005). In parasitology, there is a paucity of studies using zero-inflated models as an aid to describe patterns of infection. Examples include Denwood et al. (2008), Nødtvedt et al. (2002) and Walker et al. (2009). In the present study, only 2 of the 6 parasite species analysed had abundance data consistent with the zero-inflated model rather than the standard negative binomial model. Our studies on multi parasitism in foxes clearly demonstrate that the zero-inflated distribution, whilst a useful additional tool, is not ubiquitous for parasite abundance data and indeed for many studies the negative binomial model will suffice. It is also important to consider our results which suggest that the negative binomial without zero inflation was a better description of the distribution of Dipylidium, Toxocara and Toxascaris spp. These had lower prevalences and hence more zeros than E. multilocularis and Mesocestoides which were better described by the zero-inflated model. These two parasites with the zero-inflated data had higher mean abundances than the parasites with a negative binomial distribution and hence the magnitude of the positive counts also influences the choice of the best fitting model. This gives further evidence that zero inflation is not necessarily required when there are many zeros in the data. Taenia spp. also had a negative binomial distribution, but the interpretation of this is a challenge as there are at least two species with distinct intermediate host biology in this composite sample of parasites.
Our statistical analysis indicated that the likelihood ratio test performed satisfactorily for model selection with the present data. However, the p values indicate that the likelihood ratio test is less conservative than Monte Carlo simulation. For zero inflated models other tests such as the Vuong test (Vuong 1989) have been used for model selection (eg Tiwari et al., 2009) and for theoretical reasons such approaches may be more appropriate than the likelihood ratio test for this type of data. The Monte Carlo simulations we used to confirm model fit make no asymptotic assumptions and hence should be robust. Rüegg et al. (2008) used such Monte-Carlo methods to explore the fit of competing models based on binomial data and demonstrated that sometimes likelihood ratio tests may not select the most appropriate model with other types of data.
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation (SCOPES - IB74B0-110928 ), NIH (TWO1565-02) and INTAS (03-51-5661). We would like to express our thanks to the workers of the Atbashy regional veterinary department, Kyrgyzstan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baylis HA, Daubney R. A synopsis of the families and genera of nematode. The British Museum; London: 1926. p. 320. [Google Scholar]
- Bessonov AS. Echinococcus multilocularis infection in Russia and neighbouring countries. Helminthologia. 1998;35:73–79. [Google Scholar]
- Budke CM, Jiamin Q, Zinsstag J, Qian W, Torgerson PR. Utilization of DALYs in the estimation of the disease burden of echinococcosis for a high endemic region of the Tibetan plateau. Am. J. Trop.Med. Hyg. 2004;71:56–64. [PubMed] [Google Scholar]
- Budke CM, Craig PS, Jiamin Q, Torgerson PR. Modeling of the transmission of Echinococcus multilocularis and Echinococcus granulosus in dogs for a high endemic region of the Tibetan plateau. Int. J. Parasitol. 2005a;35:163–170. doi: 10.1016/j.ijpara.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Budke CM, Jiamin Q, Qian W, Torgerson PR. Economic effects of echinococcosis on a highly endemic region of the Tibetan plateau. Am. J. Trop.Med. Hyg. 2005b;73:2–10. [PubMed] [Google Scholar]
- Budke CM, Campos-Ponce M, Qian W, Torgerson PR. A canine purgation study and risk factor analysis for echinococcosis in a high endemic region of the Tibetan plateau. Vet. Parasitol. 2005c;127:49–55. doi: 10.1016/j.vetpar.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Craig PS. Epidemiology of human alveolar echinococcosis in China. Parasitol. Int. 2006;55:S221–S225. doi: 10.1016/j.parint.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Denwood MJ, Stear MJ, Matthews L, Reid SWJ, Toft N, Innocent GT. The distribution of the pathogenic nematode Nematodirus battus in lambs is zero-inflated. Parasitology. 2008;135:1225–1235. doi: 10.1017/S0031182008004708. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Eckert J. Diagnosis of the Echinococcus multilocularis infection in final hosts. Appl. Parasitol. 1996;37:245–252. [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanisation of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagarin VG. Materials on the helminth fauna of canids in south Kyrgyzstan. Works of the Institute Zoology and Parasitology of the Kyrgyz Academy of Sciences. 1954;2:117–121. [in Russian] [Google Scholar]
- Gagarin VG, Iksanov KI. Materials on the helminth fauna of carnivores and their veterinary public health importance. Works of the Institute Zoology and Parasitology of the Kyrgyz Academy of Sciences. 1954;2:115–117. [in Russian] [Google Scholar]
- Gagarin VG, Steshenko VM, Tokobaev MM. Role of rodents in the distribution of helminth zoonoses. Works of the Institute Zoology and Parasitology of the Kyrgyz Academy of Sciences. 1957;6:159–161. [in Russian] [Google Scholar]
- Gortazar C, Villafuerte R, Lucientes J, Fernandez-de-Luco D. Habitat related differences in helminth parasites of red foxes in the Ebro Valley. Vet. Parasitol. 1998;80:75–81. doi: 10.1016/s0304-4017(98)00192-7. [DOI] [PubMed] [Google Scholar]
- Hofer S, Gloor S, Müller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol. Let. 2005;8:1235–1246. doi: 10.1111/j.1461-0248.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- Matchanov N. The helminths of the small intestines of foxes from the Bukhara region. Materials of the Conference in the Memory of N. V. Badanina; Tashkent. Ministry of Agriculture of the Usbek SSR. Scientific Research Veterinary Institute; 1968. pp. 196–197. [in Russian] [Google Scholar]
- Merkulov GA. Course on pathological and histological techniques. Leningrad; Medgiz: 1969. p. 424. [in Russian] [Google Scholar]
- Nødtvedt A, Dohoo I, Sanchez J, Conboy G, DesCoteaux L, Keefe G, Leslie K, Campbell J. The use of negative binomial modelling in a longitudinal study of gastrointestinal parasite burdens in Canadian dairy cows. Can. J. Vet. Res. 2002;66:249–257. [PMC free article] [PubMed] [Google Scholar]
- Overgaauw PAM, Van Knapen F. Dogs and nematode zoonoses. In: MacPherson CNL, Meslin FX, Wandeler AI, editors. Dog Zoonoses and Public Health. CABI Publishing Oxon; New York: 2000. pp. 213–245. [Google Scholar]
- Reperant L, Hegglin D, Fischer C, Kohler L, Weber M, Deplazes P. Influence of urbanization on the epidemiology of intestinal helminths of the red fox (Vulpes vulpes) in Geneva, Switzerland. Parasitol. Res. 2007;101:605–611. doi: 10.1007/s00436-007-0520-0. [DOI] [PubMed] [Google Scholar]
- Roddie G, Holland C, Stafford P, Wolfe A. Contamination of fox hair with eggs of Toxocaracanis. J.Helminthol. 2008;82:293–296. doi: 10.1017/S0022149X08996954. [DOI] [PubMed] [Google Scholar]
- Richards DT, Harris S, Lewis JW. Epidemiological studies on intestinal helminth parasites of rural and urban red foxes (Vulpes vulpes) in the United Kingdom. Vet. Parasitol. 1995;59:39–51. doi: 10.1016/0304-4017(94)00736-v. [DOI] [PubMed] [Google Scholar]
- Ruegg SR, Heinzmann D, Barbour AD, Torgerson PR. Estimating the transmission dynamics of Theileria equi and Babesia caballi in horses using Monte Carlo techniques. Parasitology. 2008;135:555–565. doi: 10.1017/S0031182008004204. [DOI] [PubMed] [Google Scholar]
- Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed I, Maddox-Hyttel C, Monrad J, Kapel M. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–79. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Saeed IS, Kapel CMO. Population dynamics and epidemiology of Toxocara canis in Danish red foxes. J. Parasitol. 2006;92:1196–1201. doi: 10.1645/GE-720R.1. [DOI] [PubMed] [Google Scholar]
- Shaikenov B. Distribution and ecology of Echinococcus multiolocularis in central Asia. Parasitol. Int. 2006;55:S213–S219. doi: 10.1016/j.parint.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Shaw DJ, Grenfell BT, Dobson AP. Patterns of macroparasite aggregation in wildlife host population. Parasitology. 1998;117:597–610. doi: 10.1017/s0031182098003448. [DOI] [PubMed] [Google Scholar]
- Smith GC, Ganqadharan B, Taylor Z, Laurenson MK, Hide G, Hughs JM, Dinkel A, Romig T, Craig PS. Prevalence of zoonotic important parasites in red fox (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2003;118:133–142. doi: 10.1016/j.vetpar.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Tiwari A, VanLeeuwen JA, Dohoo IR, Keefe GP, Haddad JP, Scott HH, Whiting T. Risk factors associated with Mycobacterium avium subspecies paratuberculosis seropositivity in Canadian dairy cows and herds. Prev. Vet. Med. 2009;88:32–41. doi: 10.1016/j.prevetmed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Tokobaev MM. Helminths of rodents in Kyrgyzia. Works of the Institute Zoology and Parasitology of the Kyrgyz SSR Academy of Sciences. 1959;7:133–142. [in Russian] [Google Scholar]
- Vuitton A, Zhou H, Bresson-Hadni S, Wang Q, Piarroux M, Raoul F, Giraudoux P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. 2003;123:S87–107. [PubMed] [Google Scholar]
- Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1989;57:307–333. [Google Scholar]
- Torgerson P, Karaeva R, Corkeri N, Abdyjaparov T, Kuttubaev O, Shaikenov B. Human cystic echinococcosis in Kyrgyzstan: an epidemiological study. Acta Trop. 2003;85:51–61. doi: 10.1016/s0001-706x(02)00257-7. [DOI] [PubMed] [Google Scholar]
- Torgerson PR, Oguljahan B, Muminov AE, Karaeva RR, Kuttubaev OT, Aminjanov M, Shaikenov B. Present situation of cystic echinococcosis in Central Asia. Parasitol. Int. 2005;55:S207–212. doi: 10.1016/j.parint.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- Walker M, Hall A, Anderson RM, Basanesz MG. Density-dependent effect on the weight of female Ascaris lumbricoides infections of humans and its impact on patterns of egg production. Paras. Vect. 2009;2:11. doi: 10.1186/1756-3305-2-11. doi:10.1186/1756-3305-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT. Generalized linear modelling for parasitologists. Parasitol. Today. 1997;13:33–38. doi: 10.1016/s0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT, Shaw DJ. Analysis of aggregated parasite distributions: a comparison of methods. Functional Ecology. 1996;10:592–601. [Google Scholar]
- Ziadinov I, Mathis A, Trachsel D, Rysmukhambetova A, Abdyjaparov T, Kuttubaev O, Deplazes P, Torgerson P. Canine echinococcosis in Kyrgyzstan: using prevalence data adjusted for measurement error to develop transmission dynamics models. Int. J. Parasitol. 2008;38:1179–1190. doi: 10.1016/j.ijpara.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]