Abstract
Serotonin is an influential monoamine neurotransmitter that signals through a number of receptors to modulate brain function. Among different serotonin receptors, the serotonin 1A (5-HT1A) receptors has been tied to a variety of physiological and pathological processes, notably in anxiety, mood, and cognition. 5-HT1A receptors couple not only to the classical inhibitory G-protein-regulated signaling pathway, but also to signaling pathways traditionally regulated by growth factors. Despite the importance of 5-HT1A receptors in brain function, little is known about how these signaling mechanisms link 5-HT1A receptors to regulation of brain physiology and behavior. Following a brief summary of the known physiological and behavioral effects of 5-HT1A receptors, this article will review the signaling pathways regulated by 5-HT1A receptors, and discuss the potential implication of these signaling pathways in 5-HT1A receptor-regulated physiological processes and behaviors.
Keywords: serotonin, 5-HT1A receptors, G-protein, MAPK, Akt, behavior
1. Introduction
Serotonin (5-Hydroxytryptamine, 5-HT) is a monoamine neurotransmitter that plays important roles in physiological functions such as sleep, feeding, sexual behavior, temperature regulation, pain, and cognition, as well as in pathological states including mood disorders, anxiety disorders, psychosis, and pain disorders. Serotonergic neurons originate in the dorsal and median raphe nucleus of the brainstem. Ascending projections from these neurons reach throughout the brain to release serotonin in a paracrine manner, allowing serotonin released from one terminal to activate receptors on a number of post-synaptic cells and modulate a variety of neuronal activities [4].
There are at least 16 different types of serotonin receptors, and these are broadly grouped into sub-families based on their primary signaling mechanisms [5]. 5-HT1 receptors (A, B, D, E, and F) classically couple to the inhibitory G proteins (Gi/o) that inhibit adenylyl cyclase/protein kinase A (PKA) signaling cascade. 5-HT2 (A, B, C) receptors couple to Gq protein that stimulates the phospholipase C (PLC)/protein kinase C (PKC) signaling cascade and enhance intracellular calcium signaling. 5-HT3 receptors (A, B, C) are unique among serotonin receptors as they are ligand-gated ion channels. 5-HT4, 6, and 7 receptors couple to the stimulatory G protein (Gs) and activate adenylyl cyclase/PKA. Signaling of 5-HT5 (A and B) receptors is less clear, but evidence suggests that it may be linked to Gi/o proteins and inhibition of adenylyl cyclase/PKA [4, 6]. Further diversity of serotonin receptors is derived from transcriptional modifications, such as mRNA editing [7] and alternative splicing [8]. Among these subtypes of serotonin receptors, the 5-HT1A receptors were the first to be cloned and are widely studied for their roles in regulation of mood, anxiety, and cognition [1-3]. Below, we will summarize the known functions of 5-HT1A receptors, placing an emphasis on the regulation of signal transduction pathways by 5-HT1A receptors in neurons and brain.
2. Characterization and localization of 5-HT1A receptors
5-HT1A receptors are among the best characterized serotonin receptors due to the early discovery of its encoding gene and the availability of selective ligands. The gene for the human 5-HT1A receptors was cloned in 1987 as a single intronless gene, and was identified as a G protein-coupled receptor (GPCR) based on sequence homology to the β2-adrenergic receptor gene [9]. Subsequent binding studies identified the gene as encoding for the 5-HT1A receptors [10].
In the mammalian brain, 5-HT1A receptors are divided into two distinct classes based on their location. The 5-HT1A autoreceptors are located on the soma and dendrites of serotonergic neurons in the raphe nucleus [11-16]. Activation of 5-HT1A autoreceptors suppresses firing of serotonergic neurons and thus reduces activity-dependent serotonin release [17]. The 5-HT1A heteroreceptors are located on non-serotonergic neurons, primarily of the limbic areas, such as on the dendrites and soma of glutamatergic pyramidal neurons [16]; axon terminals of GABAergic [18, 19], and cholinergic [20] neurons. Activation of 5-HT1A heteroreceptors on these distinct neurons usually reduces neuronal excitability and firing [21, 22]. The heterorecepters are particularly enriched in the hippocampus, where immunohistochemistry and radioligand binding have demonstrated high receptor levels in the stratum radiatum of CA1 and the granule cell layer of the dentate gyrus, and at moderate levels in CA3 [11-15]. 5-HT1A heteroreceptors are also strongly expressed in the entorhinal cortex, frontal cortex, and lateral septum, and moderately expressed in the amygdala, superior colliculus, piriform cortex, and interpeduncular nucleus, as well as in several hypothalamic and thalamic nuclei [11-15]. The broad distribution of 5-HT1A receptors suggests that they have a variety of functions in brain.
3. Functions of 5-HT1A receptors in neuronal activity
3.1 Synaptic physiology and plasticity
In neurons, activation of 5-HT1A receptors activates the G protein-coupled inwardly-rectifying potassium (GIRK) channels, an action that profoundly hyperpolarizes neurons [23, 24] and decreases firing [25-28]. 5-HT1A receptors also reduce calcium currents and evoked calcium influx [29-31]. The combination of these two effects allows serotonin to rapidly and effectively silence neuronal transmission.
5-HT1A receptors also play a role in regulation of post-synaptic activity. Activation of 5-HT1A receptors reduces N-Methyl-D-Aspartate (NMDA) receptor mediated currents in the prefrontal and visual cortex [25, 32-34]. In cortical pyramidal neurons, this action of 5-HT1A receptors involves down-regulation of surface NR2B subunit of NMDA receptors [33]. Activation of 5-HT1A receptors also reduces α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor currents [35] as well as surface expression of GluR2/3 subunits of AMPA receptors [36]. Through these actions, 5-HT1A receptor activation reduces excitatory postsynaptic potentials (EPSPs) in several brain regions [28, 31]. 5-HT1A receptors also have effects on prolonged changes in the strength of synapses, known as synaptic plasticity. In the visual cortex and the dentate gyrus of the acute brain slices, 5-HT1A receptor agonist 8-Hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT) blocks long term potentiation (LTP) [37] [26], one of the best studied forms of synaptic plasticity induced by robust activation of a synapse, whereas the 5-HT1A receptor antagonist NAN-190 reverses the in vivo inhibitory effect of antidepressants fluvoxamine and milnacipran on LTP in area CA1 of the hippocampus [38, 39].
However, the effect of 5-HT1A receptors in synaptic plasticity may depend on the type of activation in specific brain regions, as direct activation of 5-HT1A receptors in the dentate gyrus of the hippocampus results in increased glutamatergic output of granule cells [40]. Conversely, recordings in the intact dentate gyrus reveal decreased LTP when 5-HT1A autoreceptors are activated, and thus reducing release of serotonin in the dentate gyrus, or 5-HT1A heteroreceptors in the dentate gyrus are blocked [41]. The direct effect of 5-HT1A receptors in the dentate gyrus is thought to be a result of silencing inhibitory interneurons [41]. Thus, the effects of 5-HT1A receptors on synaptic plasticity may also be tied to state-dependent alterations in GABAergic tone [42, 43].
While it seems clear that 5-HT1A receptors can profoundly affect synaptic physiology and plasticity through changes in membrane potential and alteration of excitatory and inhibitory tones, the signaling mechanisms mediating the effect of 5-HT1A receptors to the induction or long-term maintenance of synaptic plasticity are not completely understood, and remain to be elucidated.
3.2 Neurogenesis and neuroprotection
Adult neurogenesis is increasingly recognized as an important process in the maintenance of normal neuronal function [44], and 5-HT1A receptors have been shown to regulate neurogenesis in the subgranular zone of the dentate gyrus. Activation of 5-HT1A receptors increases proliferation of neuronal progenitors [45] and promotes development of neural precursors into adult neurons [46], whereas 5-HT1A receptor antagonists decrease neurogenesis in the dentate gyrus [47]. This effect of 5-HT1A receptors is not prevented by serotonin depletion, suggesting that this is a direct function of 5-HT1A heteroreceptors [48]. The effect of 5-HT1A receptors on neurogenesis may have important roles in maintaining normal contextual memory formation that requires ongoing neurogenesis [49], as well as mediating antidepressant action as it may be mediated by neurogenesis [50].
5-HT1A receptors also have important function in neuroprotection in both neuronal cell cultures [51-59] and in the mammalian brain [60, 61]. In animal models of ischemia [60-63] and Parkinson’s disease [64], 5-HT1A receptor agonists have shown promise as potential neuroprotective therapies. The neuroprotective effect of 5-HT1A receptors is dependent on the activities of the growth factor-associated signaling molecules mitogen-activated protein kinase (MAPK) and Akt [65-67], and involves inhibition of NMDA receptor-mediated excitotoxicity by reducing calcium influx and glutamate release [57, 58, 63].
4. Functions of 5-HT1A receptors in Behaviors
4.1 Anxiety
5-HT1A receptors are particularly influential in anxiety-related behaviors [68]. Systemic administration of 5-HT1A receptor agonist 8-OH-DPAT and partial agonists buspirone and gepirone generally decreases anxiety in rodents, as observed in the elevated plus maze and social interaction tests [69]. The effects of 5-HT1A receptor agonists on anxiety in rodents appear to be ligand-specific. The structurally similar ligands buspirone and gepirone are consistently anxiolytic [69-71], although gepirone may only be effective after chronic treatment [72], while mixed results have been found with 8-OH-DPAT [69, 71, 73]. The anxiolytic effect of buspirone after local injection to the hippocampus is task-specific since it reduces anxiety-like behaviors in the elevated plus maze and the open field [70], but not in the social interaction test [74]. Buspirone has demonstrated clinical efficacy for generalized anxiety disorder [75, 76], but it remains to be determined how the ligand-, temporal-, spatial-, and task-specific regulation of anxiety by 5-HT1A receptor agonists determines their therapeutic implication in anxiety disorders.
Some of these questions have been addressed using genetically modified animals. 5-HT1A receptor knockout mice exhibit increased anxiety-like behaviors in the elevated plus maze, elevated zero maze, open field test, and novel object exploration [77-79]. The impaired performance of these mice in anxiety-related tasks is likely due to an enhanced fear response in aversive environments [80], but not due to changes in exploration or behavioral inhibition [81]. Furthermore, restoring 5-HT1A receptor function to the forebrain of 5-HT1A knockout mice rescues anxiety-like behaviors, suggesting a crucial role for heteroreceptors in regulation of anxiety and fear [82]. This rescue does not occur if forebrain 5-HT1A receptors are restored after postnatal day 20, whereas elimination of forebrain 5-HT1A receptors after postnatal day 80 has no effect on anxiety [82], further suggesting that 5-HT1A receptor signaling early in life plays a crucial role in the development of the brain’s fear and anxiety systems [83].
4.2 Depression
5-HT1A receptors also regulate mood-related behaviors, particularly those related to depression. Sub-chronic administration of the 5-HT1A receptor agonists 8-OH-DPAT and azapirones reduces depressive behaviors in the forced swim test [71, 84] and tail suspension test [85]; and chronic administration of 8-OH-DPAT reduces depressive behavior in the novelty-suppressed feeding test [50]. In the learned helplessness model of depression, systemic injection of 5-HT1A agonists reversed escape deficits induced by inescapable stress [86], and this effect can be observed after direct injection of an agonist into the septum or after ascending serotonergic fibers are destroyed [87, 88], suggesting that the antidepressant-like effect is due to action of 5-HT1A heteroreceptors. However, one study reports reduced escape deficits in the learned helplessness model after intra- dorsal raphe infusion of 5-HT1A receptor agonist [89], suggesting that 5-HT1A autoreceptors may also contribute to an antidepressant effect.
5-HT1A receptors are likely important mediators of antidepressant responses. In animal studies, 5-HT1A receptor antagonists alone do not alter depressive behaviors, but they prevent the antidepressant effect of desipramine in the forced swim test [90] and fluoxetine in the tail suspension test [85], and 5-HT1A receptor knockout mice fail to respond to fluoxetine in the novelty-suppressed feeding test [50]. It is also thought that 5-HT1A autoreceptor desensitization is a necessary step for the chronic effect of antidepressants in animals and in humans [91]. In accordance with these finding, 5-HT1A receptor knockout mice have intrinsic antidepressant-like behavioral phenotypes [77, 78]. However, a recent study suggests that autoreceptor desensitization alone is not sufficient for the response of antidepressant to occur, but a sufficient basal serotonergic tone at a low autoreceptor level is necessary for a better response to antidepressant [92].
Evidence from human studies also supports a role of 5-HT1A receptors in the pathology and treatment of depression. Well-replicated postmortem and brain imaging studies have shown that 5-HT1A receptors are reduced in crucial areas of brain in depressed subjects, including the dorsal raphe nucleus, hippocampus, amygdala, and prefrontal cortex [91, 93]. Additionally, postmortem brains of suicide subjects have decreased 5-HT1A coupling to G-proteins and reduced activity of signaling molecules associated with 5-HT1A receptors [94]. A single nucleotide polymorphism of the 5-HT1A receptor gene that alters expression levels of the receptors has been associated with both susceptibility to depression and responsiveness to antidepressants [91]. Although it has not determined whether the diminished function of 5-HT1A receptors in depressed patients is a cause or an effect of the disease, the results from these studies, combined with the strong evidence from animal studies, suggest that 5-HT1A receptors play an important role in mood regulation. A better understanding of this role, and of the signaling molecules mediating it, may yield more effective and faster acting antidepressant treatments.
4.3 Learning and Memory
Many studies have investigated the role of 5-HT1A receptors in learning and memory. The most consistent effect has been shown in passive avoidance learning, a paradigm in which animals are trained to avoid natural behavioral responses to stressful stimuli by associating those responses with an aversive stimulus, such as a shock. Systemic administration of several 5-HT1A receptor agonists suppresses performance [95-99], whereas 5-HT1A receptor antagonists facilitates learning [100], suggesting that 5-HT1A receptors play a role in controlling inhibitory learning. Regulation of passive avoidance learning is primarily a function of heteroreceptors, as the 5-HT1A receptor agonist 8-OH-DPAT is effective both when injected into the entorhinal cortex [101], and after endogenous serotonin is depleted [97, 98].
In fear conditioning, a hippocampus- and amygdala- dependent paradigm in which animals learn to associate a cue or a context with an aversive stimulus, both systemic and local 8-OH-DPAT injections into the hippocampus or the medium raphe nucleus inhibit contextual fear conditioning [102, 103]. In agreement, 5-HT1A receptor knockout mice have enhanced contextual fear conditioning [81, 104], an effect that can be reversed by selectively expressing and activating 5-HT1A receptors in the dentate gyrus [104].
While there is a consensus that 5-HT1A receptor activation inhibits learning in the passive avoidance and fear conditioning paradigms, both paradigms depend on learning being induced by activation of fear circuits in the brain [105]. Given the fact that 5-HT1A receptors alter these fear circuits and regulate anxiety [80], it is difficult to determine if the effects of 5-HT1A receptors in these learning paradigms are a reduction of anxiety or suppression of learning. However, the effect of 5-HT1A receptors on cognition does not appear to be solely due to the receptor’s strong effect on anxiety. In the Morris Water Maze, a spatial memory task that is not intertwined as strongly with anxiety and fear, 5-HT1A receptor agonists also show inhibitory effects on learning and memory [106-109].
5. 5-HT1A receptor-regulated Signal transduction pathways
5.1 Canonical 5-HT1A receptor-regulated signaling pathway
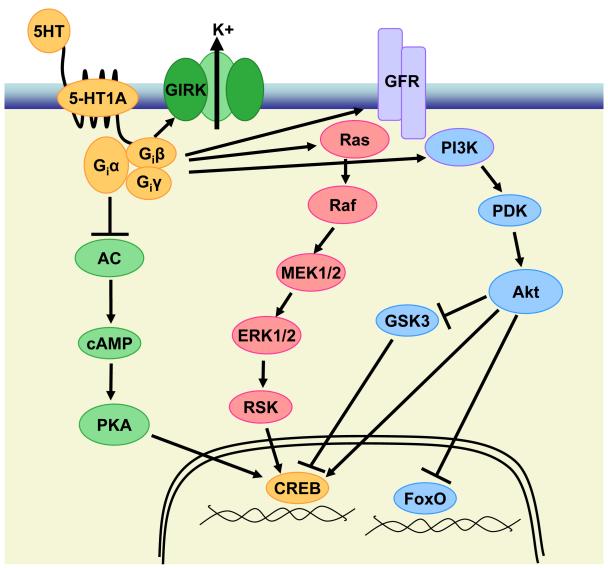
Early studies identified that 5-HT1A receptors couple to the inhibitory G-proteins (Gi/o) [112]. Agonist binding to the receptor exchanges GDP for GTP on the alpha subunit of Gi/o (Giα/Goα) [110], active of which primarily functions to inhibit adenylyl cyclase, resulting in decreased cyclic adenosine monophosphate (cAMP) production and PKA activity [111] (Figure 1). Experiments in mammalian hippocampal membranes demonstrated that serotonin inhibited forskolin-stimulated cAMP accumulation through 5-HT1A receptors [112][113]. This effect has been duplicated in hippocampal and cortical neuron cultures [114], and in cells expressing 5-HT1A receptor gene [21, 115-118]. In the brain, only 5-HT1A heteroreceptors have been shown to couple to Giα-induced inhibition of adenylyl cyclase, as 5-HT1A autoreceptors located in the dorsal raphe nucleus do not inhibit adenylyl cyclase [119]. The spatial preference may account for the expression of signaling components in specific brain areas. For example, 5-HT1A receptors primarily couple to Giα3 in the dorsal raphe nucleus and to Goα in the hippocampus [120]. In addition, the desensitization properties of 5-HT1A autoreceptors in the dorsal raphe nucleus are far more sensitive than are heteroreceptors in the limbic areas [121-123], which may also affect its coupling to the signaling pathway.
Figure 1.
5-HT1A Receptor-regulated Signal Transduction Pathways.
5-HT, serotonin; 5-HT1A, serotonin 1A receptors; Gi, inhibitory Guanine nucleotide binding protein; AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; GIRK, G-protein coupled inward rectifying potassium channel; GFR, growth factor receptor; PKA, protein kinase A; CREB, cAMP response element binding protein; MEK1/2, MAP and ERK kinase 1/2; ERK 1/2, extracellular signal-regulated kinase 1/2; RSK, ribosomal S6 kinase; PI3K, phosphotidylinositol-3 kinase; PDK, phosphoinositide dependent kinase; GSK3, glycogen synthase kinase-3; FoxO, forkhead box O transcription factors
In the passive avoidance paradigm, the 8-OH-DPAT-induced decrease in PKA activity in the hippocampus causes increased protein phosphatase-1 activity and a reduction of training-induced phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), and this signaling effect is accompanied by an impairment of performance [124]. Therefore, inhibition of adenylyl cyclase and PKA activity may mediate 5-HT1A receptor-regulated behaviors.
Activation of 5-HT1A receptors also activates G protein-coupled inward rectifying postassium channels (GIRKs) [125] in the hippocampus [23, 126, 127] and in the dorsal raphe nucleus [119, 128]. Given that 5-HT1A receptors do not couple to inhibition of adenylyl cyclase in the raphe nucleus [119], the robust activation of inward potassium currents in the dorsal raphe nucleus by 5-HT1A receptor agonists is unlikely to be an adenylyl cyclase-dependent response. Instead, activation of GIRKs is primarily mediated by G protein βγ subunits upon receptor activation [129]. The ability of 5-HT1A receptors to activate GIRK-induced hyperpolarizing currents allows them to have a strong effect on neuronal firing and excitability [128], a physiological process that may be linked to 5-HT1A receptor-regulated behaviors [104].
Despite of the well-established coupling of 5-HT1A receptors to the Giα/oα-mediated adenylyl cyclase-cAMP-PKA and the Gβγ-mediated GIRK pathways, the functions of these canonical signaling mechanisms in 5-HT1A receptor-regulated neuronal activity and behaviors have not been studied in detail. The complex signal transduction mechanisms in brain require combined pharmacological, biochemical, and molecular techniques to elucidate the role of each component within the signaling pathway, which may have limited the in vivo approaches to define their roles in 5-HT1A receptor-regulated functions in neurons and in brain as a whole. Additionally, signaling mechanisms other than the canonical pathways may have substantial effects in mediating functions of 5-HT1A receptors, among which the signaling pathways traditionally associated with growth factor receptors have been increasingly recognized for their association with 5-HT1A receptors.
5.2 5-HT1A receptors and Mitogen Activated Protein Kinases (MAPK) signaling pathway
MAPKs are known for their roles in growth and survival [130], and they are critical regulators of development and plasticity in the central nervous system [131, 132]. The MAPK family includes extracellular signal-regulated kinases 1 and 2 (ERK1/2, also known as p42 and p44 MAPK), p38-MAPK, and c-Jun N-terminal kinase (JNK) [133, 134]. Of these MAPKs, ERK is particularly affected by 5-HT1A receptors. ERK is traditionally activated by growth factor tyrosine kinase receptors. These receptors activate the small molecule GTPase Ras, which activates Raf1, which in turn phosphorylates and activates MAPK/ERK kinase 1 and 2 (MEK1/2) [135] (Figure 1). MEK is a direct upstream protein kinase regulator of ERK, activation of which phosphorylates and activates ERK [131]. Activation of this pathway leads to changes in downstream protein kinases, such as the Ribosomal S6 kinase (RSK) [136], and transcription factors such as Myc [137] and the oncogene Elk1 [137]. Phosphorylation of proteins by ERK in neurons results in receptor and ion channel activation, gene expression, and neuroplasticity [132], all of which may alter behaviors. One interesting example is activation of the transcription factor CREB by the ERK substrate serine/threonine protein kinase RSK [136, 138, 139]. CREB is a widely studied transcription factor for its gene expression function and the underlying roles in stress, anxiety, and depression [140], its regulation by the ERK signaling pathway suggests that ERK may have important impact in mood-related behaviors. The behavioral effects of the MEK/ERK signaling pathway have been reported in several studies, with MEK inhibitors causes diverse behavioral changes in animals, ranging from hyperactivity, reduced or increased anxiety, and depressive-like behavior [141-144], and MEK inhibitors also block the behavioral effect of antidepressants [145]. The diverse effect of MEK inhibitors may be due to the multiple regulators and substrates linked to MEK/ERK, and to further dissect the behavioral effects of this signaling pathway, it is important to understand the specific regulators to each component of this signaling pathway.
5-HT1A receptors were first reported to activate ERK by phosphorylation in non-neuronal cells expressing 5-HT1A receptors [146, 147]. This effect of 5-HT1A receptors is sensitive to pertussis toxin-induced inhibition of G-proteins [66, 146-149], suggesting that G-protein-coupled signaling mechanism is involved in the initiation of ERK activation by 5-HT1A receptors. As in growth factor-regulated ERK activation, 5-HT1AR-induced ERK activation is mediated by the small GTPases Ras and Raf [66, 146, 149, 150] and active MEK [66], a signal cascade that requires the calmodulin-dependent endocytosis of receptors as an intermediate step [150]. Additionally, activation of ERK by 5-HT1A receptors in non-neuronal cells can be mediated by the phosphatidylinositol 3-kinase (PI3K) and phosphatidylcholine-specific phospholipase C (PLC) in a G-protein-dependent manner [66, 146, 147], but the details of signal transduction from G-protein-dependent PI3K to ERK during 5-HT1A receptor activation are not completely known.
Despite consistent findings in cell systems with heterologous expression of 5-HT1A receptors, effects of 5-HT1A receptors on ERK activity vary in cells of neuronal origin. In hippocampal-derived differentiated HN2-5 cells, 5-HT1A agonists increase ERK phosphorylation and activity, an effect that is dependent on the small GTPases Ras and Raf, MEK and calcium mobilization [54, 56]. However, this effect of 5-HT1A receptors was not found in primary culture of hippocampal neurons [151] or fetal rhombencephalic neurons [65], and in differentiated raphe neurons, 5-HT1A receptors are coupled to a Gβγ subunit-dependent decrease in MEK activity and ERK phosphorylation [152]. Many factors may affect the response of ERK to 5-HT1A receptor activation in cells. For example, high receptor density in cell culture seems to be required for 5-HT1A receptor-induced activation of ERK [153], but the preferred coupling of 5-HT1A receptors to a specific G protein subtype and its availability in the tested cells may also affect the response [154]. Although determinants that associate 5-HT1A receptors to ERK remain to be identified, results from these studies suggest that 5-HT1A receptor-mediated regulation of ERK in neurons could be highly selective. This can be particularly important in the brain because of the diversity of brain regions and neuron types that are home to 5-HT1A receptors.
Indeed, several studies have suggested that activation of ERK by 5-HT1A receptor activation is not a universal response in brain. Consistent findings have shown that 5-HT1A receptor agonists rapidly but transiently increase phosphorylation of ERK in the hypothalamus [155-158], and this effect of 5-HT1A receptors is likely an intermediate step for 5-HT1A receptor-induced elevation of oxytocin, adrenocorticotropin (ACTH), and prolactin [155]. In contrast, 5-HT1A receptor activation decreases ERK phosphorylation in the hippocampus [155-157, 159]. Although the underlying significance of this negative coupling of ERK to 5-HT1A receptors in the hippocampus is unclear, ERK is known as a crucial regulator in cognition and an important mediator of synaptic plasticity [131, 132]. Inhibition of hippocampal ERK activity could potentially play a role in 5-HT1A receptor-mediated alterations in synaptic plasticity or in 5-HT1A receptor-induced disruption of cognition. Findings for regulation of ERK phosphorylation by 5-HT1A receptor activation in other brain areas, such as the cerebral cortex, amygdala, and dorsal raphe nucleus, are less consistent. In the frontal cortex, 5-HT1A receptor agonists are reported to increase ERK phosphorylation in some studies [157, 158], but have no effect in other studies [159]. In acute prefrontal cortical slices, activation of neither 5-HT1A nor NMDA receptors alone affects ERK, but simultaneous activation of both receptors results in a decrease in ERK phosphorylation [25], suggesting that crosstalk between different neurotransmitters, receptors, and signaling mechanisms coordinates the regulation of ERK in the cortex. Detailed studies in defined cortical areas and neuron types are warranted to further understanding of a relation between 5-HT1A receptors and ERK signal transduction in the cortex. In contrast to studies in differentiated raphe neurons showing a decrease in ERK activity by 5-HT1A receptor activation [152], systemic treatment with 5-HT1A receptor agonist results in a transient increase in ERK activity in the dorsal raphe nucleus [155, 156], whereas selective activation of 5-HT1A autoreceptors may also indirectly affect ERK activity through regulation of serotonin release in other brain areas [157]. Therefore, regulation of ERK activity by 5-HT1A receptors in brain is divergent and complicated. Experiments focusing on localized 5-HT1A receptor activation or using transgenic mice with spatial- and temporal- 5-HT1A receptor modification will be useful in further delineating specific regulation of ERK by 5-HT1A receptors in brain.
With the prominent effect of 5-HT1A receptors in regulating anxiety, mood, and cognition, and the evidence showing its brain region-selective effect on ERK, further investigation of the role of ERK in mediating 5-HT1A receptor-regulated neuronal activity and behaviors may help define the specific function of MAPK signaling pathway in brain, as well as the therapeutic potentials of modulating this 5-HT1A receptor-regulated signaling pathway.
5.3 5-HT1A receptors and the Akt signaling pathway
Another growth factor-regulated signaling pathway, the PI3K and Akt pathway, can also be regulated by 5-HT1A receptors. When tyrosine kinase receptors are activated by growth factors, they recruit PI3K to activate phosphoinositide-dependent kinase (PDK), which phosphorylates and activates Akt [160] (Figure 1). Akt is a well-known regulator of cell survival as activation of Akt by growth factors mediates insulin-stimulated growth responses and promotes survival against apoptotic stimuli [160]. In brain, Akt has been increasingly recognized as a crucial mediator in neurotrophin and neurotransmitter actions [161, 162]. As with ERK, Akt is a protein kinase that phosphorylates a variety of substrates, such as downstream protein kinases and transcription factors.
Glycogen synthase kinase 3 (GSK3) is a protein kinase that is primarily phosphorylated and inactivated by Akt [163] and several other protein kinases, such as PKC [164] and PKA [165]. GSK3 is a potential molecular target in several psychiatric disorders, particularly mood disorders, as the mood stabilizer lithium is a selective inhibitor of GSK3 [166, 167]. Inhibition of GSK3 by pharmacological or genetic means mimics the effects of antidepressants [168, 169] and anti-manic drugs [169, 170], whereas impaired regulation of GSK3 results in behavioral abnormalities reminiscent of states of mania and depression [171, 172]. Another relevant group of Akt substrates is the Forkhead box O transcription factors (FoxOs). In response to growth factors, active Akt phosphorylates and inactivates FoxOs by exporting them out of the nucleus [173]. In both invertebrate and animal brain, FoxOs can be phosphorylated and inactivated by serotonin via the PI3K/Akt-dependent mechanism [174, 175], and the brain FoxO3a subtype can be inactivated by the antidepressant imipramine [175] and down-regulated by lithium [176]. In addition, mice with FoxO deficiency exhibit antidepressive and anxiolytic behavioral phenotypes [175]. Therefore, regulation of protein substrates by Akt in brain plays a critical role not only in neuronal growth and survival, but also in the maintenance of neuronal activity and behavior.
In non-neuronal cells, activation of heterologously expressed 5-HT1A receptors increases Akt phosphorylation that represents the active state of Akt [66, 67, 177]. Similar to regulation of ERK, regulation of Akt by 5-HT1A receptors is sensitive to Gi/o and is mediated by PI3K and Ras [66]. Additionally, activation of Akt by 5-HT1A receptors can be inhibited by cAMP and restored after inactivation of PKA [67], suggesting that 5-HT1A receptor-induced inhibition of adenylyl cyclase-cAMP-PKA signaling pathway is also involved in activation of Akt. This finding are in line with other studies showing that cAMP can induce dephosphorylation and inactivation of Akt via PKA-dependent activation of protein phosphatases [178].
5-HT1A receptor agonists have consistently shown to increase Akt phosphorylation in neuronal cells, including hippocampal derived HN2-5 cells [54], primary hippocampal neurons [151, 179], and primary fetal rhombencephalic neurons [65]. As in non-neuronal cells, 5-HT1A receptor-induced Akt activation in neurons is sensitive to pertussis toxin and is dependent on PI3K [151]. Therefore, regulation of Akt and its down-stream targets is potentially a signal transduction mechanism that mediates the physiological and behavioral functions of 5-HT1A receptors.
Regulation of Akt by 5-HT1A receptors in the mammalian brain has not yet been reported; however, some indirect evidence does suggest an effect of 5-HT1A receptors in regulating Akt. For example, systemic treatment of mice with the 5-HT1A receptor agonist 8-OH-DPAT robustly increased the N-terminal serine phosphorylation of GSK3, a major Akt-regulated mechanism [163], in several brain regions [180], and the serotonin-induced increase in GSK3 phosphorylation can be blocked by a 5-HT1A receptor antagonist [180]. Additionally, in the mouse brain, enhancing synaptic serotonin resulted in increased phosphorylation of Akt, an effect that was blocked by intracerebroventricular injection of a PI3K inhibitor [175]. However, none of these studies directly examined if 5-HT1A receptors regulate Akt in the mammalian brain, and it is also not known if an effect of 5-HT1A receptors on Akt is brain region-specific. Additional studies are also needed to determine if regulation of Akt signaling pathway by 5-HT1A receptors has an impact in 5-HT1A receptor-regulated neuronal activity and behavior.
Taken together, increasing evidence suggests that 5-HT1A receptors are linked to not only the conventional Gi/o-mediated signaling pathway, but also MAPK and Akt signaling pathways that are associated with neuronal development and survival. Additional studies are needed to elucidate brain region- and cell type-specific signaling mechanisms regulated by 5-HT1A receptors as they may diversely mediate the physiological and behavioral functions of this major serotonin receptor. A better understanding of the signal transduction mechanisms associated with 5-HT1A receptors may lead to discovery of novel drug targets for the treatment of pathological conditions associated with abnormal activity of 5-HT1A receptors.
Acknowledgements
Support for this work was provided by NIH grants MH073723 and MH 86622 (XL) and NS61788 (fellow, AP). The authors thank Ms. Johanna Gandy for critical comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].King MV, Marsden CA, Fone KC. Trends Pharmacol Sci. 2008;29(9):482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [2].Ogren SO, Eriksson TM, Elvander-Tottie E, D’Addario C, Ekstrom JC, Svenningsson P, Meister B, Kehr J, Stiedl O. Behav Brain Res. 2008;195(1):54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- [3].Akimova E, Lanzenberger R, Kasper S. Biol Psychiatry. 2009;66(7):627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [4].Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Cell Tissue Res. 2006;326(2):553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- [5].Hoyer D, Martin G. Neuropharmacology. 1997;36(4-5):419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- [6].Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Pharmacol Ther. 2001;92(2-3):179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- [7].Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Nature. 1997;387(6630):303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- [8].Bender E, Pindon A, van Oers I, Zhang YB, Gommeren W, Verhasselt P, Jurzak M, Leysen J, Luyten W. J Neurochem. 2000;74(2):478–489. doi: 10.1046/j.1471-4159.2000.740478.x. [DOI] [PubMed] [Google Scholar]
- [9].Kobilka BK, Frielle T, Collins S, Yang-Feng T, Kobilka TS, Francke U, Lefkowitz RJ, Caron MG. Nature. 1987;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- [10].Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. Nature. 1988;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- [11].Zhou FC, Patel TD, Swartz D, Xu Y, Kelley MR. Brain Res Mol Brain Res. 1999;69(2):186–201. doi: 10.1016/s0169-328x(99)00101-1. [DOI] [PubMed] [Google Scholar]
- [12].Pazos A, Palacios JM. Brain Res. 1985;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- [13].Pompeiano M, Palacios JM, Mengod G. J Neurosci. 1992;12(2):440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. J Comp Neurol. 1996;365(2):289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- [15].Chalmers DT, Watson SJ. Brain Res. 1991;561(1):51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- [16].Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. J Comp Neurol. 2000;417(2):181–194. [PubMed] [Google Scholar]
- [17].Sprouse JS, Aghajanian GK. Synapse. 1987;1(1):3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- [18].Freund TF, Gulyas AI, Acsady L, Gorcs T, Toth K. Proc Natl Acad Sci U S A. 1990;87(21):8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Halasy K, Miettinen R, Szabat E, Freund TF. Eur J Neurosci. 1992;4(2):144–153. doi: 10.1111/j.1460-9568.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- [20].Cassel JC, Jeltsch H. Neuroscience. 1995;69(1):1–41. doi: 10.1016/0306-4522(95)00241-a. [DOI] [PubMed] [Google Scholar]
- [21].Sprouse JS, Aghajanian GK. Neuropharmacology. 1988;27(7):707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- [22].Mauk MD, Peroutka SJ, Kocsis JD. J Neurosci. 1988;8(1):1–11. doi: 10.1523/JNEUROSCI.08-01-00001.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Andrade R, Nicoll RA. J Physiol. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Araneda R, Andrade R. Neuroscience. 1991;40(2):399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- [25].Zhong P, Yuen EY, Yan Z. Mol Cell Neurosci. 2008;38(2):290–299. doi: 10.1016/j.mcn.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sakai N, Tanaka C. Brain Res. 1993;613(2):326–330. doi: 10.1016/0006-8993(93)90921-9. [DOI] [PubMed] [Google Scholar]
- [27].Tada K, Kasamo K, Ueda N, Suzuki T, Kojima T, Ishikawa K. J Pharmacol Exp Ther. 1999;288(2):843–848. [PubMed] [Google Scholar]
- [28].Manahan-Vaughan D, Anwyl R, Rowan MJ. Br J Pharmacol. 1994;112(4):1083–1088. doi: 10.1111/j.1476-5381.1994.tb13194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bayliss DA, Umemiya M, Berger AJ. J Physiol. 1995;485(Pt3):635–647. doi: 10.1113/jphysiol.1995.sp020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Penington NJ, Kelly JS. Neuron. 1990;4(5):751–758. doi: 10.1016/0896-6273(90)90201-p. [DOI] [PubMed] [Google Scholar]
- [31].Cheng LL, Wang SJ, Gean PW. Eur J Neurosci. 1998;10(6):2163–2172. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- [32].Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. J Biol Chem. 2008;283(25):17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. J Neurosci. 2005;25(23):5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Edagawa Y, Saito H, Abe K. Brain Res. 1999;827(1-2):225–228. doi: 10.1016/s0006-8993(99)01300-1. [DOI] [PubMed] [Google Scholar]
- [35].Cai X, Gu Z, Zhong P, Ren Y, Yan Z. J Biol Chem. 2002;277(39):36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- [36].Schiapparelli L, Del Rio J, Frechilla D. J Neurochem. 2005;94(4):884–895. doi: 10.1111/j.1471-4159.2005.03193.x. [DOI] [PubMed] [Google Scholar]
- [37].Edagawa Y, Saito H, Abe K. Eur J Pharmacol. 1998;349(2-3):221–224. doi: 10.1016/s0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- [38].Tachibana K, Matsumoto M, Togashi H, Kojima T, Morimoto Y, Kemmotsu O, Yoshioka M. Neurosci Lett. 2004;357(2):91–94. doi: 10.1016/j.neulet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- [39].Kojima T, Matsumoto M, Togashi H, Tachibana K, Kemmotsu O, Yoshioka M. Brain Res. 2003;959(1):165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- [40].Levkovitz Y, Segal M. J Neurosci. 1997;17(14):5591–5598. doi: 10.1523/JNEUROSCI.17-14-05591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sanberg CD, Jones FL, Do VH, Dieguez D, Jr., Derrick BE. Learn Mem. 2006;13(1):52–62. doi: 10.1101/lm.126306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wigstrom H, Gustafsson B. Acta Physiol Scand. 1985;125(1):159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- [43].Moser EI. J Neurosci. 1996;16(3):1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kempermann G, Wiskott L, Gage FH. Curr Opin Neurobiol. 2004;14(2):186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [45].Gould E. Neuropsychopharmacology. 1999;21(2 Suppl):46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- [46].Banasr M, Hery M, Printemps R, Daszuta A. Neuropsychopharmacology. 2004;29(3):450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- [47].Radley JJ, Jacobs BL. Brain Res. 2002;955(1-2):264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- [48].Huang GJ, Herbert J. Neuroscience. 2005;135(3):803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- [49].Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- [51].Ahlemeyer B, Krieglstein J. Brain Res. 1997;777(1-2):179–186. doi: 10.1016/s0006-8993(97)01109-8. [DOI] [PubMed] [Google Scholar]
- [52].Suchanek B, Struppeck H, Fahrig T. Eur J Pharmacol. 1998;355(1):95–101. doi: 10.1016/s0014-2999(98)00469-5. [DOI] [PubMed] [Google Scholar]
- [53].Ahlemeyer B, Glaser A, Schaper C, Semkova I, Krieglstein J. Eur J Pharmacol. 1999;370(2):211–216. doi: 10.1016/s0014-2999(99)00136-3. [DOI] [PubMed] [Google Scholar]
- [54].Adayev T, El-Sherif Y, Barua M, Penington NJ, Banerjee P. J Neurochem. 1999;72(4):1489–1496. doi: 10.1046/j.1471-4159.1999.721489.x. [DOI] [PubMed] [Google Scholar]
- [55].Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, Le PT. Brain Res Dev Brain Res. 2004;150(2):79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [56].Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. Biochim Biophys Acta. 2003;1640(1):85–96. doi: 10.1016/s0167-4889(03)00023-5. [DOI] [PubMed] [Google Scholar]
- [57].Lee HJ, Ban JY, Cho SO, Seong YH. Pharmacol Res. 2005;51(3):261–268. doi: 10.1016/j.phrs.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [58].Madhavan L, Freed WJ, Anantharam V, Kanthasamy AG. J Pharmacol Exp Ther. 2003;304(3):913–923. doi: 10.1124/jpet.102.044370. [DOI] [PubMed] [Google Scholar]
- [59].Fricker AD, Rios C, Devi LA, Gomes I. Brain Res Mol Brain Res. 2005;138(2):228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [60].Ramos AJ, Rubio MD, Defagot C, Hischberg L, Villar MJ, Brusco A. Brain Res. 2004;1030(2):201–220. doi: 10.1016/j.brainres.2004.10.019. [DOI] [PubMed] [Google Scholar]
- [61].Salazar-Colocho P, Del Rio J, Frechilla D. Brain Res. 2008;1199:159–166. doi: 10.1016/j.brainres.2007.12.032. [DOI] [PubMed] [Google Scholar]
- [62].Alessandri B, Tsuchida E, Bullock RM. Brain Res. 1999;845(2):232–235. doi: 10.1016/s0006-8993(99)01948-4. [DOI] [PubMed] [Google Scholar]
- [63].Mauler F, Fahrig T, Horvath E, Jork R. Brain Res. 2001;888(1):150–157. doi: 10.1016/s0006-8993(00)03074-2. [DOI] [PubMed] [Google Scholar]
- [64].Bezard E, Gerlach I, Moratalla R, Gross CE, Jork R. Neurobiol Dis. 2006;23(1):77–86. doi: 10.1016/j.nbd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [65].Druse M, Tajuddin NF, Gillespie RA, Le P. Brain Res Dev Brain Res. 2005;159(1):18–28. doi: 10.1016/j.devbrainres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- [66].Hsiung SC, Tamir H, Franke TF, Liu KP. J Neurochem. 2005;95(6):1653–1666. doi: 10.1111/j.1471-4159.2005.03496.x. [DOI] [PubMed] [Google Scholar]
- [67].Hsiung SC, Tin A, Tamir H, Franke TF, Liu KP. J Neurosci Res. 2008;86(10):2326–2338. doi: 10.1002/jnr.21676. [DOI] [PubMed] [Google Scholar]
- [68].Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Neuropsychopharmacology. 1999;21(2 Suppl):52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- [69].Dunn RW, Corbett R, Fielding S. Eur J Pharmacol. 1989;169(1):1–10. doi: 10.1016/0014-2999(89)90811-x. [DOI] [PubMed] [Google Scholar]
- [70].Kostowski W, Plaznik A, Stefanski R. Eur J Pharmacol. 1989;168(3):393–396. doi: 10.1016/0014-2999(89)90803-0. [DOI] [PubMed] [Google Scholar]
- [71].Kostowski W, Dyr W, Krzascik P, Jarbe T, Archer T. Pharmacol Toxicol. 1992;71(1):24–30. doi: 10.1111/j.1600-0773.1992.tb00515.x. [DOI] [PubMed] [Google Scholar]
- [72].Motta V, Maisonnette S, Morato S, Castrechini P, Brandao ML. Psychopharmacology (Berl) 1992;107(1):135–139. doi: 10.1007/BF02244978. [DOI] [PubMed] [Google Scholar]
- [73].Critchley MA, Njung’e K, Handley SL. Psychopharmacology (Berl) 1992;106(4):484–490. doi: 10.1007/BF02244819. [DOI] [PubMed] [Google Scholar]
- [74].Andrews N, Hogg S, Gonzalez LE, File SE. Eur J Pharmacol. 1994;264(3):259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- [75].Davidson JR, DuPont RL, Hedges D, Haskins JT. J Clin Psychiatry. 1999;60(8):528–535. doi: 10.4088/jcp.v60n0805. [DOI] [PubMed] [Google Scholar]
- [76].Goldberg HL, Finnerty RJ. Am J Psychiatry. 1979;136(9):1184–1187. doi: 10.1176/ajp.136.9.1184. [DOI] [PubMed] [Google Scholar]
- [77].Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Proc Natl Acad Sci U S A. 1998;95(24):14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Proc Natl Acad Sci U S A. 1998;95(25):15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Proc Natl Acad Sci U S A. 1998;95(18):10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Biol Psychiatry. 2000;48(12):1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- [81].Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Neuropsychopharmacology. 2006;31(1):101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- [82].Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Nature. 2002;416(6879):396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- [83].Leonardo ED, Hen R. Neuropsychopharmacology. 2008;33(1):134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- [84].Wieland S, Lucki I. Psychopharmacology (Berl) 1990;101(4):497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- [85].Miyata S, Hirano S, Kamei J. Neuropsychopharmacology. 2004;29(3):461–469. doi: 10.1038/sj.npp.1300354. [DOI] [PubMed] [Google Scholar]
- [86].Giral P, Martin P, Soubrie P, Simon P. Biol Psychiatry. 1988;23(3):237–242. doi: 10.1016/0006-3223(88)90034-0. [DOI] [PubMed] [Google Scholar]
- [87].Martin P, Beninger RJ, Hamon M, Puech AJ. Behav Brain Res. 1990;38(2):135–144. doi: 10.1016/0166-4328(90)90011-3. [DOI] [PubMed] [Google Scholar]
- [88].Martin P, Tissier MH, Adrien J, Puech AJ. Life Sci. 1991;48(26):2505–2511. doi: 10.1016/0024-3205(91)90605-b. [DOI] [PubMed] [Google Scholar]
- [89].Maier SF, Grahn RE, Watkins LR. Behav Neurosci. 1995;109(3):404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- [90].Detke MJ, Wieland S, Lucki I. Psychopharmacology (Berl) 1995;119(1):47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- [91].Savitz J, Lucki I, Drevets WC. Prog Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. Neuron. 65(1):40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stockmeier CA. J Psychiatr Res. 2003;37(5):357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- [94].Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. J Neurochem. 2003;87(1):182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- [95].Rowan MJ, Cullen WK, Moulton B. Psychopharmacology (Berl) 1990;100(3):393–398. doi: 10.1007/BF02244613. [DOI] [PubMed] [Google Scholar]
- [96].Carli M, Tranchina S, Samanin R. Eur J Pharmacol. 1992;211(2):227–234. doi: 10.1016/0014-2999(92)90533-a. [DOI] [PubMed] [Google Scholar]
- [97].Mendelson SD, Quartermain D, Francisco T, Shemer A. Eur J Pharmacol. 1993;236(2):177–182. doi: 10.1016/0014-2999(93)90587-8. [DOI] [PubMed] [Google Scholar]
- [98].Misane I, Johansson C, Ogren SO. Br J Pharmacol. 1998;125(3):499–509. doi: 10.1038/sj.bjp.0702098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Winsauer PJ, Rodriguez FH, Cha AE, Moerschbaecher JM. J Pharmacol Exp Ther. 1999;288(1):335–347. [PubMed] [Google Scholar]
- [100].Madjid N, Tottie EE, Luttgen M, Meister B, Sandin J, Kuzmin A, Stiedl O, Ogren SO. J Pharmacol Exp Ther. 2006;316(2):581–591. doi: 10.1124/jpet.105.092262. [DOI] [PubMed] [Google Scholar]
- [101].Ardenghi P, Barros D, Izquierdo LA, Bevilaqua L, Schroder N, Quevedo J, Rodrigues C, Madruga M, Medina JH, Izquierdo I. Behav Pharmacol. 1997;8(8):745–751. doi: 10.1097/00008877-199712000-00010. [DOI] [PubMed] [Google Scholar]
- [102].Stiedl O, Misane I, Spiess J, Ogren SO. J Neurosci. 2000;20(22):8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Almada RC, Borelli KG, Albrechet-Souza L, Brandao ML. Behav Brain Res. 2009;203(2):279–287. doi: 10.1016/j.bbr.2009.05.017. [DOI] [PubMed] [Google Scholar]
- [104].Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Nat Neurosci. 2007;10(7):896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Neuropharmacology. 2007;52(1):215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- [106].Carli M, Samanin R. Br J Pharmacol. 1992;105(3):720–726. doi: 10.1111/j.1476-5381.1992.tb09045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].McNaughton N, Morris RG. Pharmacol Biochem Behav. 1992;43(1):167–171. doi: 10.1016/0091-3057(92)90653-w. [DOI] [PubMed] [Google Scholar]
- [108].Kant GJ, Wylie RM, Chu K, Ghosh S. Pharmacol Biochem Behav. 1998;59(3):729–735. doi: 10.1016/s0091-3057(97)00553-4. [DOI] [PubMed] [Google Scholar]
- [109].Koenig J, Cosquer B, Cassel JC. Hippocampus. 2008;18(1):99–118. doi: 10.1002/hipo.20368. [DOI] [PubMed] [Google Scholar]
- [110].Birnbaumer L. Biochim Biophys Acta. 2007;1768(4):772–793. doi: 10.1016/j.bbamem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cooper DM, Londos C. Horiz Biochem Biophys. 1982;6:309–333. [PubMed] [Google Scholar]
- [112].De Vivo M, Maayani S. J Pharmacol Exp Ther. 1986;238(1):248–253. [PubMed] [Google Scholar]
- [113].Schoeffter P, Hoyer D. Br J Pharmacol. 1988;95(3):975–985. doi: 10.1111/j.1476-5381.1988.tb11728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dumuis A, Sebben M, Bockaert J. Mol Pharmacol. 1988;33(2):178–186. [PubMed] [Google Scholar]
- [115].Harrington MA, Shaw K, Zhong P, Ciaranello RD. J Pharmacol Exp Ther. 1994;268(3):1098–1106. [PubMed] [Google Scholar]
- [116].Raymond JR, Albers FJ, Middleton JP, Lefkowitz RJ, Caron MG, Obeid LM, Dennis VW. J Biol Chem. 1991;266(1):372–379. [PubMed] [Google Scholar]
- [117].Liu YF, Albert PR. J Biol Chem. 1991;266(35):23689–23697. [PubMed] [Google Scholar]
- [118].Raymond JR, Olsen CL, Gettys TW. Biochemistry. 1993;32(41):11064–11073. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- [119].Clarke WP, Yocca FD, Maayani S. J Pharmacol Exp Ther. 1996;277(3):1259–1266. [PubMed] [Google Scholar]
- [120].Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Mol Pharmacol. 2006;70(3):1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- [121].Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. J Neurosci. 2001;21(6):2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L. Neuropharmacology. 2000;39(1):110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- [123].Chaput Y, Blier P, de Montigny C. J Neurosci. 1986;6(10):2796–2801. doi: 10.1523/JNEUROSCI.06-10-02796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Moyano S, Del Rio J, Frechilla D. Neuropsychopharmacology. 2004;29(12):2216–2224. doi: 10.1038/sj.npp.1300504. [DOI] [PubMed] [Google Scholar]
- [125].Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. Neuron. 1997;19(3):687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- [126].Oleskevich S. J Neurophysiol. 1995;74(5):2189–2193. doi: 10.1152/jn.1995.74.5.2189. [DOI] [PubMed] [Google Scholar]
- [127].Colino A, Halliwell JV. Nature. 1987;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- [128].Clarke WP, De Vivo M, Beck SG, Maayani S, Goldfarb J. Brain Res. 1987;410(2):357–361. doi: 10.1016/0006-8993(87)90338-6. [DOI] [PubMed] [Google Scholar]
- [129].Kovoor A, Lester HA. Neuron. 2002;33(1):6–8. doi: 10.1016/s0896-6273(01)00572-4. [DOI] [PubMed] [Google Scholar]
- [130].Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Science. 1999;286(5443):1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- [131].Samuels IS, Saitta SC, Landreth GE. Neuron. 2009;61(2):160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sweatt JD. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [133].Raman M, Cobb MH. Curr Biol. 2003;13(22):R886–888. doi: 10.1016/j.cub.2003.10.053. [DOI] [PubMed] [Google Scholar]
- [134].Rubinfeld H, Seger R. Mol Biotechnol. 2005;31(2):151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- [135].Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Science. 1995;267(5198):682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- [136].Sturgill TW, Ray LB, Erikson E, Maller JL. Nature. 1988;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- [137].Chuang CF, Ng SY. FEBS Lett. 1994;346(2-3):229–234. doi: 10.1016/0014-5793(94)00480-3. [DOI] [PubMed] [Google Scholar]
- [138].Xing J, Ginty DD, Greenberg ME. Science. 1996;273(5277):959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- [139].Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Mol Cell Biol. 1998;18(4):1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Blendy JA. Biol Psychiatry. 2006;59(12):1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [141].Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. J Neurosci. 2003;23(19):7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ailing F, Fan L, Li S, Manji S. J Psychiatr Res. 2008;43(1):55–63. doi: 10.1016/j.jpsychires.2008.01.018. [DOI] [PubMed] [Google Scholar]
- [143].Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, McEwen B, Jay TM, Svenningsson P. Neuropharmacology. 2009;56(1):37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- [144].Creson TK, Hao Y, Engel S, Shen Y, Hamidi A, Zhuo M, Manji HK, Chen G. Bipolar Disord. 2009;11(4):339–350. doi: 10.1111/j.1399-5618.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- [145].Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. Biol Psychiatry. 2007;61(5):661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- [146].Garnovskaya MN, van Biesen T, Hawe B, Casanas Ramos S, Lefkowitz RJ, Raymond JR. Biochemistry. 1996;35(43):13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- [147].Cowen DS, Sowers RS, Manning DR. J Biol Chem. 1996;271(37):22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- [148].Millan MJ, Newman-Tancredi A, Duqueyroix D, Cussac D. Eur J Pharmacol. 2001;424(1):13–17. doi: 10.1016/s0014-2999(01)01127-x. [DOI] [PubMed] [Google Scholar]
- [149].Garnovskaya MN, Mukhin Y, Raymond JR. Biochem J. 1998;330(Pt 1):489–495. doi: 10.1042/bj3300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. J Biol Chem. 1999;274(8):4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- [151].Cowen DS, Johnson-Farley NN, Travkina T. J Neurochem. 2005;93(4):910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kushwaha N, Albert PR. Eur J Neurosci. 2005;21(3):721–732. doi: 10.1111/j.1460-9568.2005.03904.x. [DOI] [PubMed] [Google Scholar]
- [153].Mendez J, Kadia TM, Somayazula RK, El-Badawi KI, Cowen DS. J Neurochem. 1999;73(1):162–168. doi: 10.1046/j.1471-4159.1999.0730162.x. [DOI] [PubMed] [Google Scholar]
- [154].Lin SL, Setya S, Johnson-Farley NN, Cowen DS. Br J Pharmacol. 2002;136(7):1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Sullivan NR, Crane JW, Damjanoska KJ, Carrasco GA, D’Souza DN, Garcia F, Van de Kar LD. Naunyn Schmiedebergs Arch Pharmacol. 2005;371(1):18–26. doi: 10.1007/s00210-004-1005-7. [DOI] [PubMed] [Google Scholar]
- [156].Crane JW, Shimizu K, Carrasco GA, Garcia F, Jia C, Sullivan NR, D’Souza DN, Zhang Y, Van de Kar LD, Muma NA, Battaglia G. Brain Res. 2007;1183:51–59. doi: 10.1016/j.brainres.2007.07.101. [DOI] [PubMed] [Google Scholar]
- [157].Buritova J, Berrichon G, Cathala C, Colpaert F, Cussac D. Neuropharmacology. 2009;56(2):350–361. doi: 10.1016/j.neuropharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [158].Newman-Tancredi A, Martel JC, Assie MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D. Br J Pharmacol. 2009;156(2):338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chen J, Shen C, Meller E. Eur J Pharmacol. 2002;452(2):155–162. doi: 10.1016/s0014-2999(02)02297-5. [DOI] [PubMed] [Google Scholar]
- [160].Sale EM, Sale GJ. Cell Mol Life Sci. 2008;65(1):113–127. doi: 10.1007/s00018-007-7274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Yao R, Cooper GM. Science. 1995;267(5206):2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- [162].Beaulieu JM, Gainetdinov RR, Caron MG. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- [163].Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- [164].Goode N, Hughes K, Woodgett JR, Parker PJ. J Biol Chem. 1992;267(24):16878–16882. [PubMed] [Google Scholar]
- [165].Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Mol Cell Biol. 2000;20(24):9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Klein PS, Melton DA. Proc Natl Acad Sci U S A. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].De Sarno P, Li X, Jope RS. Neuropharmacology. 2002;43(7):1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- [168].Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Biol Psychiatry. 2004;55(8):781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [169].Gould TD, Einat H, Bhat R, Manji HK. Int J Neuropsychopharmacol. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- [170].Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Proc Natl Acad Sci U S A. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. J Neurosci. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Polter AM, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon LL, Bartolucci AA, Li X, Jope RS. Neuropsychopharmacology. doi: 10.1038/npp.2010.43. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- [174].Liang B, Moussaif M, Kuan CJ, Gargus JJ, Sze JY. Cell Metab. 2006;4(6):429–440. doi: 10.1016/j.cmet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [175].Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, Peng SL, Jope RS, Li X. Biol Psychiatry. 2009;65(2):150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Mao Z, Liu L, Zhang R, Li X. Biol Psychiatry. 2007;62(12):1423–1430. doi: 10.1016/j.biopsych.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [177].Dizeyi N, Hedlund P, Bjartell A, Tinzl M, Austild-Tasken K, Abrahamsson PA. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- [178].Kim S, Jee K, Kim D, Koh H, Chung J. J Biol Chem. 2001;276(16):12864–12870. doi: 10.1074/jbc.M001492200. [DOI] [PubMed] [Google Scholar]
- [179].Chen S, Owens GC, Crossin KL, Edelman DB. Mol Cell Neurosci. 2007;36(4):472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [180].Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. Neuropsychopharmacology. 2004;29(8):1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]