Cdo activates Akt via indirect interaction with APPL1 during myoblast differentiation, and this complex likely mediates some of the promyogenic effect of cell–cell interaction. The promyogenic function of Cdo involves a coordinated activation of p38MAPK and Akt via interaction with scaffold proteins, JLP and Bnip-2 for p38MAPK and APPL1 for Akt.
Abstract
Cell–cell interactions between muscle precursors are required for myogenic differentiation; however, underlying mechanisms are largely unknown. Promyogenic cell surface protein Cdo functions as a component of multiprotein complexes containing other cell adhesion molecules, Boc, Neogenin and N-cadherin, and mediates some of signals triggered by cell–cell interactions between muscle precursors. Cdo activates p38MAPK via interaction with two scaffold proteins JLP and Bnip-2 to promote myogenesis. p38MAPK and Akt signaling are required for myogenic differentiation and activation of both signaling pathways is crucial for efficient myogenic differentiation. We report here that APPL1, an interacting partner of Akt, forms complexes with Cdo and Boc in differentiating myoblasts. Both Cdo and APPL1 are required for efficient Akt activation during myoblast differentiation. The defective differentiation of Cdo-depleted cells is fully rescued by overexpression of a constitutively active form of Akt, whereas overexpression of APPL1 fails to do so. Taken together, Cdo activates Akt through association with APPL1 during myoblast differentiation, and this complex likely mediates some of the promyogenic effect of cell–cell interaction. The promyogenic function of Cdo involves a coordinated activation of p38MAPK and Akt via association with scaffold proteins, JLP and Bnip-2 for p38MAPK and APPL1 for Akt.
INTRODUCTION
Differentiation of skeletal muscle progenitors is a multistep process that involves cell cycle withdrawal, expression of muscle-specific genes and formation of multinucleated myofibers by cell fusion (Molkentin and Olson, 1996). This process is coordinated by the myogenic basic helix-loop-helix factors (MyoD, Myf5, myogenin, and MRF4) and the myocyte enhancer factor 2 (MEF2) that form the core transcriptional network regulating myogenesis (Pownall et al., 2002; Bergstrom et al., 2002; Sartorelli and Caretti, 2005; Tapscott, 2005). These transcription factors auto- and cross-activate the expression of one another, resulting in a positive feedback network that amplifies and maintains the myogenic phenotype (Ludolph and Konieczny, 1995). Therefore activities of these transcription factors must be regulated tightly to ensure efficient cell differentiation and maintenance of the differentiated state of cells. However, the molecular mechanisms how these factors receive and interpret information from the extracellular environment are not well understood. Differentiation of skeletal muscle cells requires specific, but poorly understood, cell–cell recognition and adhesion. Cell adhesion molecules (CAMs), such as Cadherins and Immunoglobulin (Ig)/Fibronectin type III (FNIII) family proteins have been implicated in the determination and differentiation of myogenic precursors (Krauss et al., 2005). Recently, one of the odorant receptors MOR23 has been shown to be required for myotube formation and muscle regeneration, via regulation of muscle cell adhesion and migration (Griffin et al., 2009).
Cdo (CAM-related/down-regulated by oncogenes), a member of the Ig/FNIII family of cell surface receptors, appears to be an integral component of a positive feedback network that controls myogenesis (Cole et al., 2004). Cdo plays a promygenic role in vivo and in vitro. Cdo deficient mice exhibit delayed skeletal muscle development, and Cdo−/−primary myoblasts differentiate inefficiently in vitro (Cole et al., 2004). Overexpression of Cdo in C2C12 myoblasts enhances differentiation, whereas knockdown of Cdo by RNAi decreases differentiation of these cells (Takaesu et al., 2006). Cdo appears to function as a component of multiprotein complexes that include other cell adhesion molecules, Boc (brother of Cdo), Cadherins and Neogenin (Kang et al., 2002; Kang et al., 2003, 2004). Netrins have been shown to regulate multiple biological processes such as cell adhesion, migration, axon guidance, survival, and differentiation (Srinivasan et al., 2003; Kang et al., 2004; Cirulli and Yebra, 2007; Round and Stein, 2007; Fitamant et al., 2008). Among Netrins, Netrin-3 is mainly expressed in C2C12 myoblasts and the recombinant chicken Netrin-2, related to the mouse Netrin-3, enhances myotube formation of C2C12 cells and activates focal adhesion kinase (FAK) and extracellular signal–regulated kinase (ERK) in primary myoblasts in a Neogenin/Cdo-dependent manner (Kang et al., 2004, Bae et al., 2009a). During myoblast differentiation, multiple signals emanate from Cdo multiprotein complexes, including activation of the p38MAPK (P38 mitogen-activated protein kinase) pathway via direct binding of the Cdo cytoplasmic tail to scaffold proteins, JLP (a scaffold protein for the p38MAPK pathway) and Bnip-2 (a scaffold-like protein for the small GTPase, Cdc42), and a nonreceptor tyrosine kinase Abl (Takaesu et al., 2006; Kang et al., 2008; Bae et al., 2009b). Such signals positively regulate the activity of myogenic bHLH (basic helix loop helix) transcription factors, such as MyoD, at least in part via the phosphorylation of the ubiquitously expressed E-protein binding partners of the myogenic bHLH factors. Recently, we have shown that the p38MAPK pathway emanating from Cdo multiprotein complexes regulates neuronal differentiation of neural precursor cells (Oh et al., 2009), suggesting a conserved signaling pathway initiated by Cdo to regulate differentiation of several different cell lineages. Therefore identifying additional components and regulators of Cdo-containing complexes is important to elucidate a mechanism of differentiation.
APPL (adaptor protein containing PH [pleckstrin homology]) domain, PTB [phosphotyrosine binding] domain, and leucine zipper motif) was first identified as an interacting partner of Akt in a yeast two-hybrid screen (Mitsuuchi et al., 1999) and later as an interacting protein of DCC (deleted in colorectal cancer; therefore APPL1 is also called DIP13). DCC is a member of Ig/FNIII superfamily receptors related to Neogenin and functions as a receptor for Netrins. APPL1 is required for apoptosis induced by DCC in the absence of ligands (Liu et al., 2002). In addition, APPL1 has been reported to bind to various transmembrane receptors including TrkA (Lin et al., 2006; Varsano et al., 2006), AdipoR (adiponectin receptors; Mao et al., 2006) and FSHR (follicle-stimulating hormone receptor; Nechamen et al., 2007), mostly via its PTB domain and plays a key role in signaling pathways involving these receptors. One way APPL1 regulates signaling pathways triggered by TrkA and FSHR is by bridging the receptor and Akt as a scaffold protein and activating Akt. In addition, APPL1 has been shown to be required for nerve growth factor–mediated activation of ERK1/2, adiponectin-stimulated p38MAPK and AMP-activated protein kinase (AMPK; Lin et al., 2006; Mao et al., 2006; Varsano et al., 2006). APPL1 is highly expressed in both differentiated C2C12 myotubes and mouse skeletal muscle,, and interacts with Akt2 in mouse skeletal muscle (Mao et al., 2006; Saito et al., 2007). Moreover, APPL1 is required for the insulin-mediated Akt activation in C2C12 cells (Mao et al., 2006).
Two key signaling pathways, p38MAPK and Akt, have been proposed to play essential roles in myogenesis (Tamir and Bengal, 2000; Gonzalez et al., 2004; Simone et al., 2004; Serra et al., 2007). p38MAPK is required for the expression of muscle-specific genes and myoblast differentiation (Cuenda and Cohen, 1999; Puri et al., 2000; Lluis et al., 2006) via phosphorylation of MEF2 isoforms and the myogenic bHLH hetrodimeric partner E47 (Wu et al., 2000; de Angelis et al., 2005; Lluis et al., 2005; Perdiguero et al., 2007). Several lines of evidence suggest that the Akt signaling pathway also plays important roles in myoblast differentiation (Fujio et al., 2001; Vandromme et al., 2001). Akt is activated during myoblast differentiation, and overexpression of Akt enhances myoblast differentiation, whereas inhibition of Akt by expression of a dominant-negative form of Akt blocks myotube formation. In addition, ectopic expression of a constitutively active form of Akt can rescue the suppression of myogenesis caused by inhibition of the PI3-kinase (Jiang et al., 1999). Furthermore, activation of Akt is essential for cell survival in myogenesis mediated by insulin-like growth factor (IGF), a peptide growth factor that has been shown to be a major activator of the PI3-kinase/Akt pathway in myoblast differentiation (Lawlor and Rotwein, 2000a,b). It appears that both, Akt and p38MAPK pathways activate and reinforce each other's activity to induce efficient myogenic differentiation (Li et al., 2000; Wu et al., 2000; Gonzalez et al., 2004). The importance of a reciprocal reinforcement between these two signaling pathways is evident in rhabdomyosarcoma, an often aggressive soft tissue tumor occurring during childhood. Defects in both PI3-kinase/Akt and p38MAPK signaling pathways have been observed in rhabdomyosarcoma cell lines and these defects result in a failure of the exit from the cell cycle and terminal myogenic differentiation (Puri et al., 2000; Xu and Wu, 2000).
We report here that both, APPL1 and Cdo are expressed in developing premuscle cells and Cdo regulates Akt activation through indirect interaction with APPL1 during myoblast differentiation. Overexpression of APPL1 in C2C12 myoblasts enhances myoblast differentiation, whereas APPL1-depleted C2C12 cells by RNAi differentiate less efficiently. The cytoplasmic tail of Cdo appears to be required for complex formation with APPL1, and their association is induced by high cell density. Overexpression or knockdown of APPL1 increases or decreases Akt activation in C2C12 cells, respectively. Cells expressing a Cdo mutant deficient in complex formation with APPL1 exhibit a decrease in Akt activation and myoblast differentiation, when compared with the wild-type Cdo-expressing cells. Cdo−/− primary myoblasts and Cdo-depleted C2C12 cells exhibit a decrease in Akt activation during differentiation. The defective differentiation of Cdo-depleted C2C12 cells are fully rescued by overexpression of AKT-DD (a constitutively active form of Akt), whereas overexpression of APPL1 fails to do so. Taken together, Cdo activates two key promyogenic kinases, p38MAPK and Akt via association with the scaffold-type of proteins, JLP and Bnip-2 for p38MAPK and with APPL1 for Akt during myogenic differentiation.
MATERIALS AND METHODS
Cell Culture and Expression Vectors
C2C12, 293T, Cos7 and primary myoblasts derived from Cdo+/+ and Cdo−/− mice were cultured as previously described (Takaesu et al., 2006). To induce differentiation of C2C12 myoblasts, cells at near confluence were switched to DMEM containing 2% horse serum (differentiation medium, DM) and myotube formation was observed normally at about 2–3 d of differentiation. The efficiency of the myotube formation in stable and transient differentiation assays was quantified as previously described (Bae et al., 2009b). Student's t test was used for statistical analysis of myotube formation. To generate C2C12 cells that stably overexpress Cdo, APPL1, or RNAi for Cdo and APPL1, cells were transfected with the indicated expression vectors and FuGene6 (Roche Diagnostics, Indianapolis, IN) and cultures were selected in puromycin-containing medium.
The human APPL1 gene was amplified by RT-PCR of mRNAs purified from human embryonic kidney fibroblast cells. Full-length (aa1-709), BAR-PH (aa1-499), BAR (aa1-273) and PH-PTB (aa265-709) domains of APPL1 were inserted into mammalian expression vector pcDNA-hemagglutinin (HA) or pcDNA-SRT (spin-reorientation transitions; Lee et al., 2001). Initially we tested four different APPL1 RNAi oligonucleotides, and two of them (5′-GGGAGGCAGGCGUACAAAU-3′ and 5′-AUUUGUACGCCUGCCUCCC-3′; 5′-GCGGGAGAAGUGAAAGUAA-3′ and 5′-UUACUUUCACUUCUCCCGC-3′) displayed the effective knockdown in HeLa cells. Two mouse equivalent RNAi oligonucleotides were tested for their knockdown ability, and from these RNAi oligonucleotides we have inserted the following sequence into pSuper-puro vector: 5′-GGGAGGCAGGCGTACAAAT-3′. Cdo deletion mutants (CdoΔ986-1048, CdoΔ1035-1160, CdoΔ1160-1256), the glutathione S-transferase (GST)-Cdo intracellular construct, and the sequence for the pSuper/Cdo-RNAi construct were described previously (Bae et al., 2009b). To generate a constitutively active mutant of AKT, we have mutated two active sites of human AKT, threonine308 and serine473, to aspartic acids (AKT-DD), as previously described (Flores et al., 2008).
Western Blot Analysis, Antibodies, and Immunoprecipitation
Western blot analyses were carried out as previously described (Bae et al., 2009b). Briefly, cells were lysed in lysis buffer (10 mM Tris-HCl, pH 7.2, 150 mM NaCl/1% Triton X-100/1 mM EDTA) containing proteinase inhibitor (Roche Diagnostics), followed by SDS-PAGE. APPL1 rabbit polyclonal antibody was raised against C-terminal PTB domain of human APPL1. Sera were affinity-purified using Sulfolink beads (Pierce, Rockford, IL). Other antibodies used in this study were as follows: anti-Cdo (Zymed, South San Francisco, CA), anti-SRT (Lee et al., 2001), anti-HA (Roche Diagnostics), anti-pan-cadherin (Sigma-Aldrich, St. Louis, MO), anti-β-tubulin (Zymed), anti-p38MAPK (Sigma-Aldrich), anti-pp38MAPK, anti-Akt, anti-pAkt (Cell Signaling, Beverly, NA), anti-MHC (MF20; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-Troponin T (Sigma-Aldrich), anti-Boc (R&D Systems, Minneapolis, MN), anti-N-cadherin (Zymed), anti-Neogenin (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-GFP (Zymed). To study formation of Cdo-APPL1 complexes, coimmunoprecipitation was performed as described previously (Bae et al., 2009b).
For analysis of dissected hindlimbs by immunoblotting, hindlimb muscles from Cdo+/+ and Cdo−/− mice at E15.5 (embryonic day 15.5) embryos and P1 (postnatal day 1), P3, and P5 mice were pulverized and solubilized with lysis buffer. Lysates were then analyzed by immunoblotting as described above.
Immunocytochemistry, Immunohistochemistry, and Microscopy
GFP-Cdo plasmids were transfected in C2C12 cells with FuGene6 (Roche Diagnostics). The following day, media was replaced with DM, and cells were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) after 48 h of transfection. After blocking, fixed cells were incubated with anti-APPL1 or anti-EEA1 (BD Bioscience, San Jose, CA) antibody. Fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) was used to detect primary antibody. Cells were examined under confocal laser scan microscope (LSM510 META, Zeiss, Thornwood, NY). For the results shown in Figure 6, C2C12/pSuper or C2C12/Cdo RNAi cells in 12-well plates were cotransfected with 100 ng of green fluorescent protein (GFP) expression vector and 900 ng of the indicated DNA constructs for 2 d and induced to differentiate by switching to DM. Cells were then fixed with 3% paraformaldehyde in PBS, followed by blocking with 5% goat serum in PBS. Cells were incubated with anti-MHC (Developmental Studies Hybridoma Bank) or anti-GFP (Zymed) antibody. FITC- or Texas Red–conjugated secondary antibodies (Jackson ImmunoResearch) were used to detect primary antibody. Immunostaining for pp38MAPK and MHC was performed as described previously (Bae et al., 2009b). Images were obtained on a Zeiss LSM-510 Meta confocal microscope. Quantification of the fluorescent signal for pp38MAPK was performed with ImageGauge software (Fuji Film, Tokyo, Japan). Immunohistochemistry was performed on 8-μm paraffin sections that were rehydrated, treated with 10 μg/ml proteinase K/50 mM Tris-HCl, pH 8.0 for 10 min, and incubated with anti-APPL1 antibody in 5% goat serum containing PBS. After extensive washing, sections were incubated with biotinylated goat anti-rabbit and streptavidin-horseradish peroxidase conjugates (Jackson ImmunoResearch).
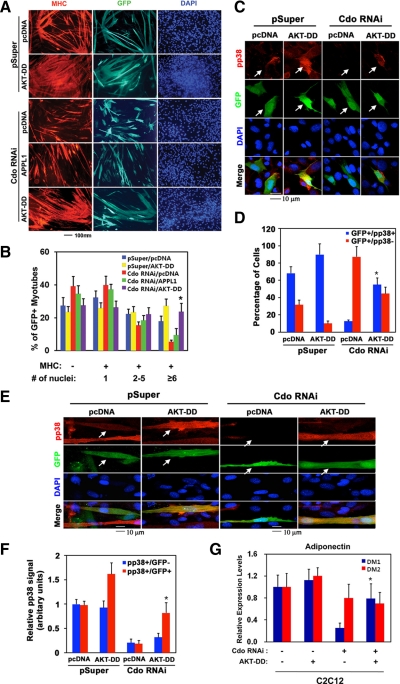
Figure 6.
The full rescue of the defective differentiation of Cdo-depleted C2C12 cells by overexpression of AKT-DD, but not APPL1. (A) C2C12/pSuper and C2C12/Cdo RNAi cells were transiently transfected with pcDNA, APPL1, or AKT-DD expression vector, plus GFP expression vector to mark transfected cells. These cell lines were induced to differentiate for 3 d, fixed, and immunostained with an MHC antibody, followed by DAPI staining. (B) Quantification of myotube formation by cell lines shown in A. Values represent means of triplicate determinations ±1 SD. The experiment was repeated with similar results. Significant difference from control, *p < 0.01. (C) C2C12/pSuper and C2C12/Cdo RNAi cells were transiently transfected with pcDNA or AKT-DD expression vector, plus GFP expression vector to mark transfected cells. Confluent cultures were then fixed and stained with antibodies to pp38 (red) and visualized for GFP expression (green). Cell nuclei were visualized by staining with DAPI (blue). White arrows indicate transfected cells. (D) Quantification of cultures shown in C. GFP+ cells were scored as positive or negative for pp38 staining. Values represent of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01. (E) C2C12/pSuper and C2C12/Cdo RNAi cells were transiently transfected with pcDNA or AKT-DD expression vector, plus GFP expression vector to mark transfected cells. Cultures were incubated in DM for 48 h and then fixed and stained with antibodies to pp38 (red) and visualized for GFP expression (green). Nuclei were visualized by DAPI staining (blue). White arrows indicate transfected cells. (F) Quantification of cultures shown in E. The intensity of the immunofluorescent pp38 signals in GFP+ and GFP− cells was quantified, with the values obtained from control vector–transfected C2C12/pSuper cultures set to 1.0. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01. (G) RNAs of the control and AKT-DD–expressing C2C12 cells from differentiation day 1 (DM1) and DM2 were subjected to qRT-PCR for Adiponectin expression. The expression level of the control cells at DM1 was set as 1 and the relative expression levels are plotted. Values represent means of triplicate determinations ±1 SD. The experiment was repeated twice with similar results. Significant difference from control, *p < 0.01.
RNA Extraction and Quantitative RT-PCR
Total RNA was extracted using easy-Blue reagent (iNtRON Biotechnology, Sungnam, Korea). The cDNAs were reverse-transcribed from 1 μg of total RNA using oligo-dT primer and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). The PCR reactions were performed in 20 μl using 5% of the reverse transcription (RT) reaction, 250 nM of each primer, and SYBR Premix Ex Taq (Takara, Tokyo, Japan). The PCR reactions were carried out on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Expression levels of Gapdh were used to normalize the expression levels of each sample. Primer sequences used for PCR were as follows: MHC: 5′-CCAAGGGCCTGAATGAGGAG-3′ and 5′-GCAAAGGCTCCAGGTCTGAG-3′; Troponin T: 5′-TGCACTAAAAGACCGCATTG-3′ and 5′-TCTTCTTGGCGTCATCCTCT-3′; Adiponectin: 5′-GCAGAGATGGCACTCCTGGA-3′ and 5′-CCCTTCAGCTCCTGTCATTCC-3′; and Gapdh: 5′-TGATGACATCAAGAAGGTGAAG-3′ and 5′-TCCTTGGAGGCCATGTAGGCCAT-3′.
TUNEL Assay
To monitor cell death, C2C12/pSuper or C2C12/APPL1 RNA interference (RNAi) cells that had been cultured in growth medium or DM were fixed, and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) was performed with a kit according to the manufacturer's instructions (Invitrogen) as previously described (Bae et al., 2009a).
Yeast Two-Hybrid Assay
cDNA fragments encoding APPL1 were ligated to the Gal4 DNA-binding domain in the pGBT9 vector, and a cDNA encoding the Cdo intracellular domain was ligated to the Gal4 activation domain in the pGAD vector. Yeast strain AH109 (Clontech, Palo Alto, CA) was sequentially transformed with these vectors, and β-galactosidase (β-Gal) activity was detected by colony lift filter assay as previously described (Bae et al., 2009b).
RESULTS
APPL1 Is Expressed in Developing Premuscles and Cultured Myoblasts
To investigate roles of APPL1 in myogenesis and the relationship between Cdo and APPL1 in this process, we analyzed the expression pattern of APPL1 in developing muscles. Paraffin sections of forelimb and pretrapezius premuscles from E13.5 embryos were subjected to immunohistochemistry with anti-APPL1 antibodies. As shown in Figure 1A, APPL1 was expressed ubiquitously as seen by the brown staining; however, the strongest immunoreactivity was observed in the developing forelimb and trapezius premuscles from E13.5 embryos. Cdo has previously been shown to be expressed in developing premuscles of E13.5 embryos (Mulieri et al., 2000). APPL1 was ubiquitously expressed in extracts of dissected hindlimb muscles of E15.5 embryos and P1, P3, and P5 mice, whereas Cdo expression was altered during muscle development. It was expressed at highest levels in the developing hindlimb at E15.5 and drastically down-regulated in the P5 hindlimb muscle extracts (Figure 1B). This expression pattern of Cdo is consistent with its proposed role as a regulator of myogenesis. In addition, APPL1 and Cdo were also coexpressed in differentiating C2C12 myoblasts and APPL1 levels increased during differentiation (Figure 1C).
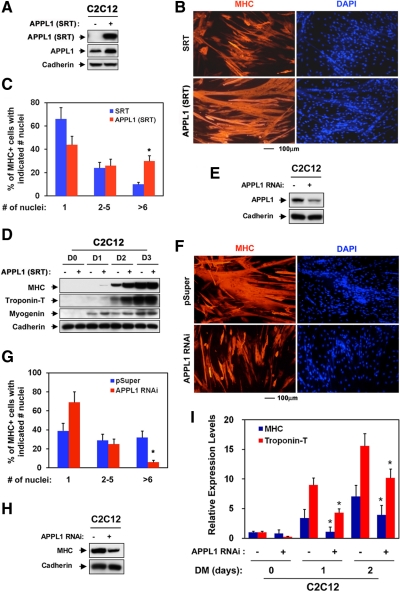
Figure 1.
Expression of APPL1 in developing premuscles and cultured myoblasts. (A) Transverse sections of premuscle mass of trapezius and forelimb obtained from E13.5 embryos were stained with an antibody against APPL1. (B) Extracts of hindlimb muscles from E15.5 embryo and P1, P3 and P5 mice were probed with antibodies to APPL1 and Cdo, and Akt as a loading control. (C) Lysates of C2C12 cells that were at near confluence (0) or in DM for indicated times were immunoblotted with antibodies to APPL1, Cdo, and MHC and Cadherin as a loading control.
APPL1 Promotes Myoblast Differentiation
To explore a function of APPL1 in myogenesis, we modulated APPL1 levels by stable expression of the SRT-tagged APPL1 (APPL1 [SRT]) or APPL1 RNAi in C2C12 cells and analyzed effects of altered APPL1 levels on myoblast differentiation. Forced expression of the APPL1 (SRT) in C2C12 cells generally resulted in a modest twofold increase of APPL1 (Figure 2A). The control and APPL1 (SRT)-expressing C2C12 cells were induced to differentiate for 2 d in DM (D2), fixed, and immunostained with anti-myosin heavy chain (MHC) antibodies followed by DAPI staining. Photomicrographs of these cells revealed that overexpression of APPL1 resulted in the formation of larger myotubes with more nuclei per myotube, in comparison to control cells (Figure 2, B and C). In addition, APPL1 (SRT)-expressing C2C12 cells showed an enhanced expression of differentiation markers, such as myogenin, MHC, and Troponin T, in comparison to control cells (Figure 2D). These data suggest that the increase in APPL1 levels resulted in enhanced myoblast differentiation on a morphological as well as a biochemical level. In converse experiments, the stable expression of APPL1 RNAi in C2C12 cells resulted in the reduction of APPL1 protein levels to 30%, relative to the control cells (Figure 2E). These cells were further analyzed for their differentiation ability by inducing differentiation for 3 d. As shown in Figure 2, F and G, these cells formed fewer myotubes with fewer nuclei, compared with control vector transfected cells. The formation of big myotubes with more than six nuclei was specifically affected by the APPL1 knockdown (Figure 2G). APPL1 RNAi-expressing cells exhibit a significant reduction of MHC levels at D3, compared with the control cells (Figure 2H). To further analyze the effect of APPL1 on expression of differentiation markers in early differentiation stage, RNAs of C2C12/APPL1 RNAi cells from the differentiation time course were analyzed for MHC and Troponin T by quantitative RT-PCR (qRT-PCR). As shown in Figure 2I, the expression of these differentiation markers during the early differentiation stage was significantly decreased. These data suggest that APPL1 appears to be required for both biochemical and morphological differentiation of myoblasts. However the myoblast fusion seems to be more affected by the APPL1 deficiency than the expression of muscle-specific proteins.
Figure 2.
APPL1 promotes myoblast differentiation. (A) Lysates of cell lines shown in B were immunoblotted with antibodies to SRT, APPL1, and to Cadherin as a loading control. (B) Photomicrographs of C2C12 cells that stably express SRT-tagged APPL1 (APPL1 [SRT]) or control (pcDNA-SRT) vectors were cultured in DM for 2 d, fixed, and stained with an antibody to MHC. (C) Quantification of myotube formation shown in B. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01. (D) Lysates of cell lines shown in B were immunoblotted with antibodies to MHC, Troponin T, or Myogenin and to Cadherin as a loading control. (E) Lysates of cell lines shown in F were immunoblotted using antibodies recognizing APPL1 to reveal the level of RNAi-mediated depletion and Cadherin as a loading control. (F) Photomicrographs of C2C12 cells stably transfected with APPL1 RNAi or control (pSuper) vectors were cultured in DM for 3 d, fixed, and immunostained with an antibody to MHC. (G) Quantification of myotube formation by cell lines shown in F. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01. (H) Lysates of control and APPL1 RNAi cell lines shown in F were immunoblotted using antibodies recognizing MHC and Cadherin as a loading control. (I) RNAs of the control and APPL1 RNAi cell lines from the different differentiation time course were subjected to qRT-PCR for MHC and Troponin T expression. The expression level of the control cells at the D0 condition was set as 1, and the relative expression levels were plotted. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01.
APPL1 Forms Complexes with Cdo
Because both Cdo and APPL1 were expressed in early developing muscles and C2C12 myoblasts and promoted myoblast differentiation, we next asked whether Cdo and APPL1 interact functionally. To test this possibility, we first examined if these proteins physically interact with each other. 293T cells were transiently transfected with Cdo and APPL1 (SRT) constructs, and lysates were subjected to immunoprecipitation analysis with SRT antibodies followed by immunoblotting with antibodies recognizing SRT or Cdo. As shown in Figure 3A, Cdo coprecipitates with APPL1 (SRT). To assess whether Cdo and APPL1 interact endogenously in myoblasts, cell lysates of proliferating as well as differentiating C2C12 myoblasts from a 3-d time course were immunoprecipitated with anti-Cdo antibodies and analyzed by immunoblotting with antibodies to Cdo or APPL1. In proliferating C2C12 cells cultured in growth medium (G), APPL1 was poorly coprecipitated with Cdo (Figure 3B). When cells were confluent (DM, day 0), the expression of APPL1 and its binding to Cdo was drastically increased and the interaction stayed constant during the 3 d-time course of differentiation (Figure 3B). These results suggest that Cdo and APPL1 interact with each other in myoblasts and cell density appears to be an important factor for the expression of APPL1 and the interaction between Cdo and APPL1. APPL1 has been shown to be associated with a distinct endosomal compartment involved in the regulation of multiple signaling pathways, and APPL1 endosomes mature into EEA1-positive early endosomes (Miaczynska et al., 2004). Therefore we assessed whether Cdo colocalized with APPL1 and/or EEA1 in endosomal compartments of C2C12 cells. Because no available antibodies to Cdo can be used for immunocytochemistry of the endogenous Cdo protein, C2C12 cells were transiently transfected with GFP-tagged Cdo, and 1 d later, these cells were switched into DM. At differentiation day 1 (D1), cells were fixed and immunostained with APPL1 or EEA1 antibodies followed by rhodamine-conjugated secondary antibodies. The confocal images were taken close to the basal plane of the cells. Cdo-GFP was detected along the plasma membrane and in the cytoplasm as a punctuated pattern. A fraction of APPL1 colocalized with Cdo-GFP mainly in the vicinity of the plasma membrane and most likely at the contact site of two cells (marked with arrows in the middle panel, Figure 3C). However Cdo-GFP did not appear to colocalize significantly with the EEA1-positive endosomal compartments (Figure 3C, bottom panel).
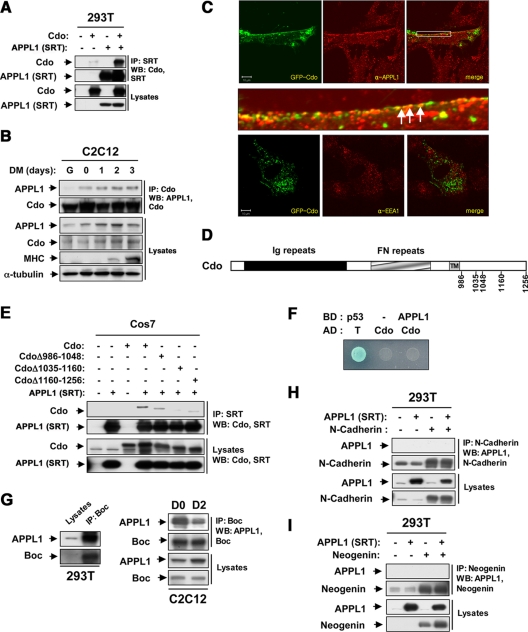
Figure 3.
APPL1 forms complexes with Cdo and Boc, but not with Neogenin and N-cadherin. (A) Lysates of 293T cells transiently transfected with Cdo, APPL1 (SRT), or control (−) expression vectors as indicated were immunoprecipitated (IP) with an antibody to SRT. The precipitates and total lysates were immunoblotted (WB) with antibodies against Cdo and SRT (APPL1). (B) Lysates of C2C12 cells that were proliferating in growth medium (G) at near confluence (0) or in DM for the indicated times were immunoprecipitated with antibodies to Cdo and immunoblotted with antibodies to APPL1 or Cdo. Total lysates were also immunoblotted with antibodies to APPL1, Cdo, or MHC to monitor differentiation, and to α-tubulin as a loading control. (C) C2C12 cells were transiently transfected with Cdo-GFP and fixed after 48 h and immunostained with antibodies to APPL1 (top and middle panels) or EEA1 (bottom panel). The enlarged image of the boxed area in the top panel is shown in the middle panel. Size bar, 10 μm. (D) Schematic representation of rat Cdo, the amino acid numbers indicating the deletion constructs. (E) Lysates of Cos7 cells transiently transfected with Cdo, Cdo deletion mutants, APPL1 (SRT), or control (−) expression vectors as indicated were immunoprecipitated with SRT antibodies and then immunoblotted with antibodies to Cdo or SRT. Total lysates were also immunoblotted with Cdo or SRT antibodies. (F) Yeast cells transformed with the indicated vectors were tested for expression of β-Gal, indicative of interaction. AD, Gal4 activation domain; BD, Gal4 DNA-binding domain. The set of p53 and SV40 large T antigen (T) served as a positive control. (G) Lysates of 293T and C2C12 cells at D1 and D2 were subjected to immunoprecipitation with Boc antibodies and immunoblotted with antibodies to Boc and APPL1. (H) 293T cells were transiently transfected with APPL1 (SRT) and N-cadherin, and lysates were immunoprecipitated with N-cadherin antibodies, followed by immunoblotting with N-cadherin and APPL1. (I) The transiently transfected 293T cells with Neogenin and APPL1 (SRT) were lysed and subjected to immunoprecipitation with Neogenin antibodies, followed by immunoblotting with Neogenin and APPL1 antibodies.
To assess the structural requirement for association between Cdo and APPL1, we have transiently transfected three Cdo intracellular mutants that harbor deletions in the cytoplasmic tail (Figure 3D) and analyzed the ability of these mutants to coprecipitate with APPL1. All three Cdo mutants showed a reduction in APPL1 binding; however, the Cdo mutant lacking the midportion (CdoΔ1035-1160) of the cytoplasmic tail precipitated with APPL1 most inefficiently (Figure 3E). Previously we have shown that this Cdo mutant (CdoΔ1035-1160) behaved as a loss-of-function mutant in myoblast differentiation (Takaesu et al., 2006), suggesting APPL1 may be a regulator of the Cdo-mediated myoblast differentiation via association with this protein. Next we asked whether Cdo and APPL1 interacted directly by utilizing a yeast two-hybrid system. Although the positive control proteins, p53 and SV40 large T antigen (T)-expressing yeast formed colonies on the selective plate, the yeast expressing both APPL1 and the Cdo intracellular region failed to grow, suggesting that Cdo and APPL1 most likely interact indirectly (Figure 3F). We have further analyzed a potential interaction between APPL1 and Neogenin, Boc, or N-cadherin that are components of Cdo multiprotein complexes (Kang et al., 2002, 2003, 2004). APPL1 was coimmunoprecipitated endogenously with Boc in 293T as well as in C2C12 cells at D0 and D2 (Figure 3G). Interestingly, the interaction between APPL1 and Boc decreased in differentiating C2C12 cells, even though the level of APPL1 increased. To further ask whether Neogenin and N-cadherin interacted with APPL1, we transiently transfected 293T cells with APPL1 (SRT) and N-cadherin or Neogenin. The lysates were subjected to immunoprecipitation with antibodies to N-cadherin or Neogenin, followed by Western blotting. As shown in Figure 3, H and I, N-cadherin and Neogenin did not interact with either the endogenous or the transfected APPL1, suggesting that these proteins are not interacting with APPL1. Because the interaction between Cdo and APPL1 appeared to be indirect, we then asked whether Boc and APPL1 interacted directly by using a yeast two-hybrid system. However we failed to detect any interaction between APPL1 and the Boc intracellular region in yeasts (data not shown). Taken together, these data suggest that APPL1, Cdo, and Boc may form complexes, and this interaction is mediated by a yet-to-be-identified protein.
Complex Formation of Cdo and APPL1 Is Mediated by the Region Encompassing Amino Acids 273-486 of APPL1
To investigate the role of Cdo and APPL1 complexes, we have generated HA-tagged APPL1 (APPL1 [HA]) deletion mutants according to its domain structure (Figure 4A). 293T cells were transiently cotransfected with the Cdo expression vector and the APPL1 (HA) construct of either the full-length or the deletion mutants and 2 d later, lysates were subjected to coimmunoprecipitation with anti-Cdo antibodies, followed by immunoblot analysis. As shown in Figure 4B, the APPL1 mutant 1-499 encompassing the BAR and PH domain and the mutant 265-709 containing the PH and PTB domains were coimmunoprecipitated with Cdo. However the mutant 1-273 containing the BAR domain alone did not precipitate with Cdo, suggesting that the 274-485 region containing the PH domain and the surrounding regions of APPL1 is required for complex formation with Cdo (Figure 4B). To explore the functional consequence of these deletion mutants, C2C12 cells stably expressing the full-length APPL1 protein or deletion mutants were induced to differentiate for 3 d and were assessed for their differentiation ability. Cells expressing APPL1 mutants 1-499 (which can bind to Cdo, but lacks PTB domain) and 265-709 differentiated as efficiently as the full-length APPL1-expressing cells, as seen by the formation of bigger myotubes and an enhanced expression of MHC, compared with the control cells (Figure 4, C–E). However expression of the mutant 265-709 containing both complex forming region with Cdo and the PTB domain displayed more efficient differentiation with larger myotubes that contained six or more nuclei and elevated levels of MHC, compared with the full-length APPL1-expressing cells. In addition, overexpression of the APPL1 mutant 1-273 did not show any effect on myotube formation as well as MHC expression, compared with the vector-expressing cells (Figure 4, C–E). These data suggest that the complex formation of Cdo with APPL1 is required for Cdo-mediated efficient myoblast differentiation.
Figure 4.
The complex formation of Cdo and APPL1 is mediated by the region encompassing amino acids 273-486 of APPL1. (A) Schematic representation of the domain structure of APPL1 and the deletion constructs of APPL1. (B) 293T cells were transiently transfected with the HA-tagged full-length or deletion mutants of APPL1 and Cdo or control (−) expression vectors as indicated. Cells were harvested after 48 h, immunoprecipitated using an antibody to Cdo, and analyzed by immunoblotting with antibodies to HA or Cdo. Total lysates were also immunoblotted with antibodies to HA or Cdo. (C) C2C12 cells were stably transfected with HA-tagged APPL1 (APPL1 [HA[) and APPL1 mutants, or control (pcDNA) expression vectors. These cell lines were cultured in DM for 2 d, fixed, and immunostained with an antibody to MHC followed by DAPI stain. (D) Quantification of myotube formation by cell lines shown in C. Values represent means of triplicate determinations ±1 SD. The experiment was repeated three times with similar results. Significant difference from control, *p < 0.01. (E) Lysates of cell lines shown in C were immunoblotted with antibodies to HA to reveal the level of ectopic expression of APPL1 and APPL1 mutants. In addition, lysates were immunoblotted with antibodies to MHC and β-tubulin as a loading control.
Cdo and APPL1 Are Required for Akt Activation in Myoblast Differentiation
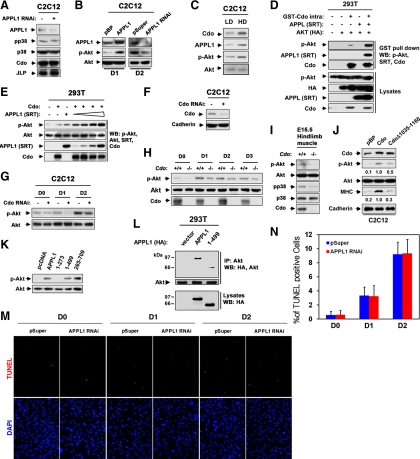
Because APPL1 promotes myogenic differentiation and forms complexes with Cdo, whose promyogenic properties are exerted mainly through p38MAPK, we examined the possibility that APPL1 may be involved in regulation of p38MAPK activity in myoblast differentiation. C2C12 cells were transfected stably with APPL1 RNAi, and lysates after 2 d of differentiation were subjected to immunoblot analysis with antibodies to APPL1, phosphorylated-p38MAPK (pp38), p38MAPK, Cdo and JLP (a scaffold protein interacting with Cdo and p38MAPK). As shown in Figure 5A, APPL1 knockdown did not alter the level of pp38 and Cdo as well as JLP, suggesting APPL1 is not required for Cdo-mediated p38MAPK activation. Because APPL1 is implicated in Akt activation in other cell systems and Akt plays an essential role in myoblast differentiation, we generated stable C2C12 cells with either increased or reduced levels of APPL1 and analyzed the phosphorylation of Akt by immunoblotting. The antibody to a phosphorylated form of Akt (p-Akt) reveals higher or lower immunoreactivity in response to either the increased or reduced APPL1 protein level, respectively (Figure 5B). Because Cdo and APPL1 associate efficiently in confluent C2C12 cells, we analyzed whether cell density regulates Akt activities. C2C12 cells were harvested from either 50% (LD) or 100% (HD) confluent cultures and analyzed with antibodies recognizing Cdo, APPL1, p-Akt, or Akt. As shown in Figure 5C, cells at high cell density express enhanced levels of Cdo, APPL1, and p-Akt. Next we explored whether Cdo forms a complex with APPL1 and Akt by using a GST-Cdo intracellular region fusion construct (GST-Cdointra) in GST pulldown experiments. 293T cells were transiently cotransfected with GST-Cdointra, Akt, and APPL1 or the control vector, and lysates were subjected to precipitation with glutathione beads. As shown in Figure 5D, p-Akt was coprecipitated with GST-Cdointra most likely mediated via the endogenous APPL1, and this association was strongly increased when APPL1 was overexpressed. We then asked whether Cdo and APPL1 collaborate in the regulation of Akt activities by cotransfecting 2.5 μg of Cdo expression vector with an increasing amount of APPL1 (0.25, 0.5, 1, or 2.5 μg). As a control, 2.5 μg of either Cdo or APPL1 were cotransfected with the control vector. Two days later, cells were harvested and analyzed by immunoblotting with antibodies recognizing p-Akt, Akt, APPL1, or Cdo. Ectopic expression of Cdo or APPL1 alone increased phosphorylation of endogenous Akt. The p-Akt levels were further increased by cotransfection of APPL1 and Cdo in a dose-dependent manner (Figure 5E). These data suggest that Cdo and APPL1 collaborate in the activation of Akt. To confirm Cdo's role in Akt activation during myoblast differentiation, C2C12 cells stably expressing control or Cdo RNAi (Figure 5F) as well as primary myoblasts isolated from Cdo+/+ and Cdo−/− mice were induced to differentiate and analyzed for Akt activation by immunoblotting. C2C12 cells that expressed Cdo RNAi displayed lower levels of p-Akt after 1 d in DM than did control cells (Figure 5G). Similarly, a reduction in Akt phosphorylation was observed in Cdo−/− primary myoblasts after 1 d in DM, relative to the control Cdo+/+ cells (Figure 5H). To investigate the in vivo role of Cdo in Akt activation, we analyzed extracts of dissected E15.5 hindlimb muscles by immunoblotting with antibodies against p-Akt, Akt, pp38, p38, or Cdo. Cdo was easily detected in extracts from Cdo+/+ hindlimb muscles (Figure 5I). As expected, pp38 levels were decreased in extracts from Cdo−/− hindlimb muscles. Furthermore, Cdo−/− hindlimb muscle extracts showed a reduction in p-Akt levels, relative to the Cdo+/+ extracts (Figure 5I). Next, we examined whether Cdo and APPL1-containing complex regulates Akt phosphorylation in myogenic differentiation. C2C12 cells stably expressing the full-length or a Cdo mutant (CdoΔ1035-1160, lacking the ability to associate with APPL1) were analyzed for their ability to activate Akt. Overexpression of Cdo resulted in enhanced levels of p-Akt as well as MHC expression, relative to the control vector–expressing cells. Even though CdoΔ1035-1160–expressing cells failed to enhance MHC expression, Akt activation was partially reduced, compared with the full-length Cdo-expressing cells, but it was higher, in relation to the control vector–transfected cells (Figure 5J). These data suggest that this domain might be required for full activation of Akt by Cdo, which is required for efficient myogenic differentiation. Because the deletion of this domain in Cdo did not abrogate Akt activation, there might be other pathways for Cdo to activate Akt besides association with APPL1 through this domain. C2C12 cells stably expressing the full-length or deletion mutants of APPL1 shown in Figure 4E were also analyzed for p-Akt levels. Akt activation correlated well with their ability to induce differentiation. Like the full-length APPL1, the APPL1 mutants, 1-499 and 265-709, showed a strong induction of Akt activation (Figure 5K). Interestingly, APPL1 mutant 265-709–expressing cells resulted in higher Akt activation, compared with the full-length APPL1-expressing cells. However the 1-273 APPL1 mutant failed to activate Akt phosphorylation, compared with the control full-length APPL1 (Figure 5K). Because it has been reported that APPL1 can interact with Akt via its PTB domain, we further analyzed whether APPL1 mutant 1-499 can interact with Akt, thereby activating Akt, when it is complexed with Cdo. We have transfected 293T cells with the full-length or the 1-499 mutant APPL1, followed by immunoprecipitation with an Akt antibody and analyzed interaction of Akt with APPL1. As shown in Figure 5L, both APPL1 proteins were detected in the precipitate, suggesting the 1-499 mutant APPL1 can form a complex with Akt. Because APPL1 has been shown to homodimerize via the N-terminal BAR domain (Li et al., 2007), it is possible that the PTB domain mutant form complexes with the endogenous APPL1 thereby with Akt. However the level of the 1-499 mutant in the precipitate was decreased, compared with that of the full-length APPL1, suggesting the PTB domain of APPL1 is required for the efficient interaction between APPL1 and Akt. Taken together, these data suggest that indirect association of APPL1 with Cdo is required for APPL1 to activate Akt in myoblast differentiation. Because Akt has been proposed to regulate cell survival and cell survival is required for the efficient myoblast differentiation, we asked whether the impaired myoblast differentiation of APPL1 knockdown cells was due to a decrease in cell survival. The control and APPL1-deficient C2C12 cells were induced to differentiate and fixed at various time points of differentiation, and cell death was analyzed by TUNEL and DAPI staining. As shown in Figure 5, M and N, these cells did not display any difference in cell death. Taken together, Cdo/APPL1/Akt pathway may be specifically involved in myoblast differentiation rather than regulating cell survival.
Figure 5.
Cdo and APPL1 are required for Akt activation in myoblast differentiation. (A) Confluent cultures of C2C12 cells stably expressing APPL1 RNAi were immunoblotted recognizing antibodies to pp38, p38, Cdo, JLP, or APPL1 to show depletion of APPL1. (B) C2C12 cells stably expressing APPL1 or control pBabePuro (pBP) expression vectors, and APPL1 RNAi or control (pSuper) vectors were cultured to confluency and induced to differentiate for 1 d (D1) or for 2 d (D2), respectively. Cell lysates were immunoblotted with antibodies to p-Akt, Akt, or APPL1 to show the level of overexpression or knockdown of APPL1 protein. (C) C2C12 cells were cultured either to 50% confluency (LD) or 100% confluency (HD) in growth medium, and lysates were immunoblotted with antibodies to Cdo, APPL1, p-Akt, or Akt. (D) 293T cells were transiently transfected with GST-Cdointra, HA-Akt, SRT-APPL1, or control pcDNA (−) expression vector as indicated. Lysates were pulled down with glutathione-Sepharose beads and analyzed by immunoblotting with antibodies to Cdo, SRT, HA, and p-Akt. (E) 293T cells were transiently cotransfected with 2.5 μg of Cdo, and varying amounts of APPL1 (0.25, 0.5, 1.25, and 2.5 μg) constructs, and 2.5 μg of Cdo, APPL1, or control, pcDNA (−) vector-transfected cells serve as controls. Lysates were analyzed after 48 h by immunoblotting with antibodies to p-Akt, Akt, SRT, or Cdo. (F) C2C12 cells stably transfected with control pSuper or Cdo RNAi were immunoblotted with antibodies to Cdo or Cadherin as a loading control. (G) Cell lines shown in F were cultured to near confluency and induced to differentiate for up to 2 d. Lysates were analyzed with antibodies to p-Akt or Akt. (H) Primary myoblasts isolated from Cdo+/+ or Cdo−/− mice were harvested at various differentiation time points. Lysates were immunoblotted with antibodies against p-Akt, Akt, or Cdo as control for the genotype of Cdo. (I) Extracts of hindlimb muscles from the control wild-type or Cdo−/− embryos at E15.5 were analyzed by immunoblotting with antibodies to p-Akt, Akt, pp38, p38, or Cdo as control. (J) C2C12 cells stably expressing Cdo, Cdo mutant (Δ1035-1160), or control pBP were cultured in DM for 2 d, and lysates were immunoblotted with antibodies to Cdo, p-Akt, Akt, MHC (indicative of differentiation response), or Cadherin as a loading control. p-Akt, Akt, MHC, and Cadherin loading control signals were quantified by densitometry; ratio reported under each lane in arbitrary units with C2C12/Cdo was set to 1. (K) Lysates of C2C12 cells stably expressing APPL1, APPL1 mutants, or control pcDNA (cell lines from Figure 4C) were immunoblotted with antibodies against p-Akt or Akt. (L) 293T cells were transiently transfected with HA-tagged APPL1, and lysates were subjected to coimmunoprecipitation with an antibody to Akt followed by Western blotting with antibodies to Akt and HA. (M) Control and APPL1 RNAi C2C12 cells from various differentiation time points were analyzed for cell death by TUNEL staining (red), followed by DAPI (blue) staining. (N) Quantification of TUNEL/DAPI staining shown in M. Values represent means of triplicate determinations ±1 SD. The experiment was repeated twice with similar results.
Expression of a Constitutively Active Akt Rescues the Defective Differentiation of Cdo-depleted C2C12 Cells
We next investigated whether the defective differentiation response displayed by Cdo depleted myoblasts by RNAi was caused by defects in Akt activation. To do so, the control or Cdo RNAi-expressing C2C12 cells were transiently transfected with APPL1 or AKT-DD (a constitutively active form of AKT) expression vector or a control vector, plus a GFP expression vector to mark transfectants. The defect in the differentiation response of Cdo-depleted C2C12 cells were rescued by overexpression of AKT-DD. About 73% of pcDNA-transfected C2C12/pSuper cells were MHC-positive (32% mononucleate; ∼22% containing 2–5 nuclei; ∼18% containing six or more nuclei; Figure 6, A and B). Overexpression of AKT-DD in control cells increased slightly the percentage of MHC-positive cells to 76%; however, it enhanced specifically the formation of larger myotubes with more than six nuclei to 27%. pcDNA-transfected C2C12/Cdo RNAi cells exhibited a reduction in differentiation, the percentage of MHC-positive cells decreasing to ∼61%, with ∼40% mononucleate, ∼16% containing two-to-five nuclei, and ∼5% containing six or more nuclei. APPL1-transfected C2C12/Cdo RNAi cells showed only a slight increase in MHC-positive myotubes, the percentage of MHC-positive cells increasing to 65%, with 18% containing 2–5 nuclei and ∼9% containing six or more nuclei, whereas AKT-DD–transfected C2C12/Cdo RNAi cells resulted in a full rescue of differentiation, the percentage of MHC-positive cells increasing to 72% and distribution of transfectants displaying one (26%), 2–5 (18%), and six or more nuclei (23%) being nearly identical between these cultures and the double control-vector cultures (Figure 6, A and B). These data suggest that Akt promotes myotube formation and the defect in efficient activation of Akt may be the cause of the defective differentiation of Cdo-deficient C2C12 cells.
Because activities of both p38MAPK and Akt are required for the efficient myoblast differentiation and Cdo-depleted C2C12 myoblasts exhibited defects in activation of both p38MAPK and Akt, we next examined the possibility that overexpression of AKT-DD in Cdo RNAi-expressing C2C12 cells restores p38MAPK activation, thereby rescuing differentiation of these cells. The control or Cdo RNAi-expressing C2C12 cells were transiently transfected with an AKT-DD expression vector or a control vector, plus a GFP expression vector to mark transfectants. Cells from a near-confluent condition (D0) and cultures in DM for 48 h (D2) were subjected to analysis for production of pp38 by immunocytochemistry with anti-pp38 antibodies. Under the D0 culture condition, ∼68% of C2C12/pSuper/pcDNA cells were positive for pp38 signals, and AKT-DD overexpression in C2C12/pSuper cells resulted in an increase in pp38 expression to ∼90%. In contrast, only ∼10% of C2C12/Cdo-RNAi/pcDNA cells were positive for pp38. Overexpression of AKT-DD in C2C12/Cdo-RNAi cells resulted in an increase in pp38-positive cells to 55% (Figure 6, C and D). Under the D2 culture condition, C2C12/pSuper/pcDNA cells formed small, multinucleated myotubes and displayed a robust pp38 signal, whereas C2C12/Cdo RNAi/pcDNA cells were mainly mononucleate and had evidently weaker pp38 signal. Overexpression of AKT-DD in the control C2C12/pSuper cells appeared to enhance pp38 signal. In addition, AKT-DD–transfected C2C12/Cdo RNAi cells formed small, multinucleated myotubes and these GFP-positive myotubes displayed a robust pp38 signal (Figure 6E). To quantify this, we scored the intensity of the immunofluorescent pp38 signal in untransfected (GFP-) versus transfected (GFP+) cells on the same coverslips, with the average pp38 signal in untransfected cells set to 1.0. Control vector–transfected cells also had a pp38 signal of ∼1.0, whereas AKT-DD–expressing control cells displayed an increase in a pp38 signal to 1.6. Both untransfected or control vector–transfected C2C12/Cdo RNAi cells had a pp38 signal of ∼0.2, whereas the signal for AKT-DD–transfected C2C12/Cdo RNAi was ∼0.8 (Figure 6F). In these cells, we did not see any alteration in the p38MAPK signal (data not shown). Taken together, these data suggest that forced activation of Akt by overexpression of AKT-DD in Cdo-deficient C2C12 cells rescues defective differentiation of these cells and restores activation of p38MAPK in these cells. To address how p38MAPK was activated in AKT-DD–expressing C2C12/Cdo RNAi cells, we analyzed the expression of well-known p38MAPK activators, such as Adiponectin, Insulin, and their receptors in these cells. RNAs of the AKT-DD–expressing or the control C2C12/Cdo RNAi cells at D1 or D2 were subjected to qRT-PCR for the expression of Adiponectin, Insulin, and their receptors. We did not observe any changes in expression of Insulin, Insulin receptor, and Adiponectin receptor 1 (data not shown). Interestingly control C2C12/Cdo RNAi cells displayed a transient reduction in Adiponectin expression at D1, whereas AKT-DD–expressing C2C12/Cdo RNAi cells showed a comparable expression of Adiponectin to the control cells. These data suggest that the Cdo/Akt pathway may be required for expression of Adiponectin in the early stage of myoblast differentiation.
DISCUSSION
Skeletal myogenesis is an excellent model system to study molecular mechanisms of cell differentiation, involving cell cycle exit, cell survival, and tissue-specific gene expression (Pownall et al., 2002; Tapscott, 2005; Krauss et al., 2005). p38MAPK and Akt signaling pathways have been shown to play crucial roles in myogenesis via regulation of activities of myogenic bHLH transcription factors (Wu et al., 2000; Gonzalez et al., 2004). These signaling pathways are regulated by a variety of extracellular promyogenic signals, including IGFs and cell–cell interactions (Rommel et al., 2001; Charge and Rudnicki, 2004; Krauss et al., 2005). Cell–cell interactions of precursors are also essential for myoblast differentiation and myotube formation (Krauss et al., 2005; Griffin et al., 2009). In this study, we show that Cdo activates Akt through an indirect interaction with APPL1 in C2C12 myoblasts. Cdo and APPL1 association appears to be strongly increased in the confluent culture condition. This association persists in differentiating myoblasts cultured in DM over several days, suggesting that their complex formation is initiated by cell–cell contact. It is noteworthy that both APPL1 and Cdo expression and complex formation between Cdo and APPL1 are each increased in cells cultured in high cell density conditions, regardless of the presence of high serum concentration in the culture medium. These results suggest further that cell–cell contact may be a stimulus for these processes. We have previously shown that Cdo forms higher-order complexes with the related Ig superfamily proteins, Boc and Neogenin, and cell adhesion molecule Cadherin/β-Catenin complexes at sites of cell–cell contact (Krauss et al., 2005), and this complex regulates some of the cell contact–mediated signaling. Because APPL1 forms complexes with both Cdo and Boc in C2C12 myoblasts it may be involved in the cell contact–mediated Akt activation. Consistent with this notion, the high cell density condition enhances Akt activation in C2C12 cells. However this interaction between Cdo and Boc with APPL1 appears to be indirect, and the mediator of this interaction is currently unknown.
Although mutant CdoΔ1035-1160 exhibited the most severe defect in its association capacity to APPL1, the binding affinity of all deletion mutants was affected, suggesting that any changes in the cytoplasmic tail of Cdo decreased their association with APPL1. It also has been shown that changes in the Cdo's cytoplasmic tail affected Cdo's binding to another scaffold protein JLP (Takaesu et al., 2006). It is possible that multiple regions of the Cdo cytoplasmic domain are required to provide the structural integrity sufficient for complex formation with APPL1 or that the Cdo deletion mutants may not be localized to an appropriate subcellular compartment for association. Interestingly, APPL1 has been implicated in regulation of endocytosis via association with the small GTPase Rab5, a key regulator of transport from the plasma membrane to the early endosomes. It has been proposed that endosomes bearing APPL1 are particularly adapted for signaling and that these endosomes mature into EEA1-positive early endosomes (Miaczynska et al., 2004). It is possible that APPL1 forms complexes with Cdo in a specific compartment that might regulate downstream signaling of Cdo to activate Akt. Consistent with this notion, it appears that transiently transfected GFP-tagged Cdo colocalized with a fraction of the APPL1-positive endosomal compartment in the vicinity of cell membrane, but no significant levels of Cdo-GFP were found in the EEA1-positive early endosome compartment. Further studies will examine the role of the subcellular localization of Cdo in regulation of downstream signaling during myogenic differentiation. APPL1 has been previously shown to translocate into nucleus and to interact with components of the nucleosome remodeling and histone deacetylase complex NuRD/MeCP1 to induce gene expression required for EGF-induced proliferation (Miaczynska et al., 2004). It is possible that APPL1 may be activated by Cdo-mediated signaling and regulates directly expression of muscle-specific genes via its function in nucleus. However, we did not observe any significant nuclear localization of APPL1 in differentiating myoblasts. Additional work will be required to examine whether a nuclear function of APPL1 is required for its promyogenic role. However, the study with the BAR domain–deleted APPL1 mutant, which has been shown to localize exclusively in the cytoplasm (Miaczynska et al., 2004), displayed enhanced activities in induction of differentiation and Akt activation, compared with the wild-type APPL1. This result suggests that its function in the nucleus may be dispensable in myoblast differentiation.
Because components of Cdo multiprotein complexes including Boc, Neogenin, and N-cadherin are coexpressed in a variety of cell types undergoing lineage specification and differentiation, this multiprotein complex may regulate differentiation of multiple cell lineages mediated by cell–cell interaction during development. Interaction through these molecules enhances differentiation by activation of multiple cytoplasmic-signaling molecules. Cdo activates p38MAPK via binding to multiple scaffold proteins. Cdo binds directly to Bnip-2 and JLP, scaffold proteins for Cdc42 and p38MAPK, respectively, and the formation of these complexes results in Bnip-2/Cdc42–dependent activation of p38MAPK bound to JLP. In addition, we have recently shown that nonreceptor tyrosine kinase Abl binds both to the intracellular region of Cdo and to JLP during myogenesis and that Abl regulates p38MAPK activity during differentiation (Takaesu et al., 2006; Kang et al., 2008; Bae et al., 2009b). In addition to p38MAPK, Akt pathways are also required for efficient differentiation, and APPL1 is regarded to be a scaffold protein for Akt activation. It appears that Cdo/APPL1/Akt might form different pools of signaling complexes of Cdo/JLP/p38MAPK. The evidences are twofold: first, we failed to observe any interaction between JLP and APPL1 (data not shown), and second, overexpression and knockdown of APPL1 in myoblasts did not affect p38MAPK activities, whereas Cdo-depleted myoblasts exhibited both defective p38MAPK and Akt activation during differentiation. These data suggest that Cdo signals through p38MAPK and Akt via interaction with scaffold proteins, JLP and APPL1, to induce efficient terminal differentiation, which may be defective in rhabdomyosarcomas. In accordance with this hypothesis, we have previously shown that stable overexpression of Cdo in a rhabdomyosarcoma cell line RD enhanced myogenic differentiation of these cells (Wegorzewska et al., 2003). Interestingly, the defective differentiation and p38MAPK activation of Cdo-deficient myoblasts (Takaesu et al., 2006; Kang et al., 2008) can be rescued by overexpression of AKT-DD, suggesting a cross-regulation between these pathways. The exact mechanism of this cross-regulation is currently unknown. Adiponectin may be one of the candidate linking p38MAPK and Akt signaling pathways mediated by Cdo/APPL1 complexes. Adiponectin expression in early stages of differentiation appears to be dependent on Cdo, which is rescued by expression of AKT-DD in Cdo-depleted cells. Currently it is unclear how Cdo regulates adiponectin expression. Recently, it has been shown that p38MAPK activates IGF2 expression, which in turn activates Akt to promote myogenesis (Gonzalez et al., 2004). It is tempting to speculate that Cdo-mediated activation of p38MAPK and Akt may induce IGF2 and Adiponectin, respectively, to stimulate each other thereby constituting autoregulatory networks to induce efficient myogenic differentiation. It is yet unknown whether Cdo/p38MAPK pathway induces IGF2 expression and further study will characterize the detailed mechanism of this cross-talk. Although both Cdo and APPL1 are required for Akt activation, it is also clear that not all Akt pathway activities during myoblast differentiation are Cdo- or APPL1-dependent, as residual Akt activities are detected in cells depleted for Cdo or APPL1. Potential additional mechanisms for activation of Akt in differentiating myoblasts may include signaling by IGFs.
ACKNOWLEDGMENTS
We thank Dr. Ruth Simon for critical reading of the manuscript and Dr. Robert Krauss for helpful comments. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST; 2009-0079748) to J.S.K., the grant from National Research Foundation of Korea Grant funded by the Korean Government (2009-0071220) to G.U.B., and the grant from the 2001 Good Health R&D Project of the Ministry of Health (01-PJ3-PG6-01GN12-0001) to M.J.H.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-12-1011) on May 19, 2010.
REFERENCES
- Bae G. U., Yang Y. J., Jiang G., Hong M., Lee H. J., Tessier-Lavigne M., Kang J. S., Krauss R. S. Neogenin regulates skeletal myofiber size and FAK and ERK activities in vivo and in vitro. Mol. Biol. Cell. 2009a;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae G. U., Kim B. G., Lee H. J., Oh J. E., Lee S. J., Zhang W., Krauss R. S., Kang J. S. Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol. Cell. Biol. 2009b;29:4130–4143. doi: 10.1128/MCB.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom D. A., Penn B. H., Strand A., Perry R. L., Rudnicki M. A., Tapscott S. J. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell. 2002;9:587–600. doi: 10.1016/s1097-2765(02)00481-1. [DOI] [PubMed] [Google Scholar]
- Charge S., Rudnicki M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Cirulli V., Yebra M. Netrins: beyond the brain. Nat. Rev. Mol. Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- Cole F., Zhang W., Geyra A., Kang J. S., Krauss R. S. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Cuenda A., Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- de Angelis L., Zhao J., Andreucci J. J., Olson E. N., Cossu G., McDermott J. C. Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev. Biol. 2005;283:171–179. doi: 10.1016/j.ydbio.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Fitamant J., Guenebeaud C., Coissieux M. M., Guix C., Treilleux I., Scoazec J. Y., Bachelot T., Bernet A., Mehlen P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc. Natl. Acad. Sci. USA. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A. I., Narayanan S. P., Morse E. N., Shick H. E., Yin X., Kidd G., Avila R. L., Kirschner D. A., Macklin W. B. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y., Mitsuuchi Y., Testa J. R., Walsh K. Activation of Akt2 inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 2001;8:1207–1212. doi: 10.1038/sj.cdd.4400919. [DOI] [PubMed] [Google Scholar]
- Gonzalez I., Tripathi G., Carter E. J., Cobb L. J., Salih D. A., Lovett F. A., Holding C., Pell J. M. Akt2, a novel functional link p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol. Cell. Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. A., Kafadar K. A., Pavlath G. K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell. 2009;17:649–661. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B. H., Aoki M., Zheng J. Z., Li J., Vogt P. K. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Mulieri P. J., Hu Y., Taliana L., Krauss R. S. BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 2002;21:114–124. doi: 10.1093/emboj/21.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Feinleib J. L., Knox S., Ketteringham M. A., Krauss R. S. Pro-myogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:3989–3994. doi: 10.1073/pnas.0736565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Yi M. J., Zhang W., Feinleib J. L., Cole F., Krauss R. S. Netrins and neogenin promote myotube formation. J. Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Bae G. U., Yi M. J., Yang Y. J., Oh J. E., Takaesu G., Zhou Y. T., Low B. C., Krauss R. S. A Cdo/Bnip-2/Cdc42 signaling pathway regulates p38α/β MAPK activity and myogenic differentiation. J. Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. S., Cole F., Gaio U., Takaesu G., Zhang W., Kang J. S. Close encounters: regulation of vertebrate skeletal myogenesis by cell–cell contact. J. Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- Lawlor M. A., Rotwein P. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 2000a;20:8983–8995. doi: 10.1128/mcb.20.23.8983-8995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor M. A., Rotwein P. Coorinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways. J. Cell Biol. 2000b;151:1131–1140. doi: 10.1083/jcb.151.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. R., Chang Y. Y., Hahn M. J. Development of a new epitope tag recognized by a monoclonal antibody to Rickettsia typhi. Biotechniques. 2001;31:541–545. doi: 10.2144/01313st08. [DOI] [PubMed] [Google Scholar]
- Li J., Mao X., Dong L. Q., Liu F., Tong L. Crystal structures of the BAR-PH and PTB domains of human APPL 1. Structure. 2007;15:523–533. doi: 10.1016/j.str.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Li Y., Jiang B., Ensign W. Y., Vogt P. K., Han J. Myogenic differentiation requires signaling through both phosphatidylinositol 3-kinase and p38MAP kinase. Cell Signal. 2000;12:751–757. doi: 10.1016/s0898-6568(00)00120-0. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Quevedo C., Brewer N. E., Bell A., Testa J. R., Grimes M. L., Miller F. D., Kaplan D. R. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yao F., Wu R., Morgan M., Thorburn A., Finley R. L., Jr., Chen Y. Q. Mediation of the DCC Apoptotic Signal by DIP13a. J. Biol. Chem. 2002;277:26281–26285. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
- Lluis F., Ballesta E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis F., Perdiguero E., Nebreda A. R., Munoz-Canoves P. Regulation of skeletal muscle gene expression by p38MAP kinases. Trends Cell Biol. 2006;16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Ludolph D. C., Konieczny S. F. Transcription factor families: muscling in on the myogenic program. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- Mao X., et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Mitsuuchi Y., Johnson S. W., Sonoda G., Tanno S., Golemis E. A., Testa J. R. Identification of a chromosome 3p14.3–21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Olson E. N. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Mulieri P. J., Okada A., Sassoon D. A., McConnell S. K., Krauss R. S. Developmental expression pattern of the cdo gene. Dev. Dyn. 2000;219:40–49. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1032>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nechamen C. A., Thomas R. M., Dias J. A. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 2007;260:93–99. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J. E., Bae G. U., Yang Y. J., Yi M. J., Lee H. J., Kim B. G., Krauss R. S., Kang J. S. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated kinase pathway. FASEB J. 2009;23:2088–2099. doi: 10.1096/fj.08-119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E., et al. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38α in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall M. E., Gustafsson M. K., Emerson C. P., Jr Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- Puri P. L., Wu Z., Zhang P., Wood L. D., Bhakta K. S., Han J., Feramisco J. R., Karin M., Wang J. Y. Induction of terminal differentiation by constitutive activation of p38MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- Rommel C., Bodine S. C., Clarke B. A., Rossman R., Nunez L., Stitt T. N., Yancopoulos G. D., Glass D. J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Round J., Stein E. Netrin signaling leading to directed growth cone steering. Curr. Opin. Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Saito T., Jones C. C., Huang S., Czech M. P., Pilch P. F. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J. Biol. Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr. Opin. Genet. Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra C., et al. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol. Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone C., Forcales S. V., Hill D. A., Imbalzano A. N., Latella L., Puri P. L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- Srinivasan K., Strickland P., Valdes A., Shin G. C., Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Takaesu G., Kang J. S., Bae G. U., Yi M. J., Lee C. M., Reddy E. P., Krauss R. S. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir Y., Bengal E. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J. Biol. Chem. 2000;275:34424–34432. doi: 10.1074/jbc.M005815200. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Vandromme M., Rochat A., Meier R., Carnac G., Besser D., Hemmings B. A., Fernandez A., Lamb N. J. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 2001;276:8173–8179. doi: 10.1074/jbc.M005587200. [DOI] [PubMed] [Google Scholar]
- Varsano T., Dong M. Q., Niesman I., Gacula H., Lou X., Ma T., Testa J. R., Yates J. R., 3rd, Farquhar M. G. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol. Cell. Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska M., Krauss R. S., Kang J. S. Overexpression of the immunoglobulin superfamily members CDO and BOC enhances differentiation of the human rhabdomyosarcoma cell line RD. Mol. Carcinogenesis. 2003;37:1–4. doi: 10.1002/mc.10121. [DOI] [PubMed] [Google Scholar]
- Wu Z., Woodring P. J., Bhakta K. S., Tamura K., Wen F., Feramisco J. R., Karin M., Wang J. Y., Puri P. L. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J. Biol. Chem. 2000;275:36750–36757. doi: 10.1074/jbc.M005030200. [DOI] [PubMed] [Google Scholar]