Abstract
Mirror neurons are a distinct class of neurons that discharge both during the execution of a motor act and during observation of the same or similar motor act performed by another individual. However, the extent to which mirror neurons coding a motor act with a specific goal (e.g., grasping) might also respond to the observation of a motor act having the same goal, but achieved with artificial effectors, is not yet established. In the present study, we addressed this issue by recording mirror neurons from the ventral premotor cortex (area F5) of two monkeys trained to grasp objects with pliers. Neuron activity was recorded during the observation and execution of grasping performed with the hand, with pliers and during observation of an experimenter spearing food with a stick. The results showed that virtually all neurons responding to the observation of hand grasping also responded to the observation of grasping with pliers and, many of them to the observation of spearing with a stick. However, the intensity and pattern of the response differed among conditions. Hand grasping observation determined the earliest and the strongest discharge, while pliers grasping and spearing observation triggered weaker responses at longer latencies. We conclude that F5 grasping mirror neurons respond to the observation of a family of stimuli leading to the same goal. However, the response pattern depends upon the similarity between the observed motor act and the one executed by the hand, the natural motor template.
Keywords: Monkey, Mirror neurons, Grasping, Premotor cortex, Tool use, Goal coding
Introduction
A fundamental aspect of social life is the capacity to understand the meaning of others’ actions. Experiments carried out in the last decade have shown that in everyday life, although not in unusual conditions (Brass et al. 2007; De Lange et al. 2008; Liepelt et al. 2008), the capacity to understand others’ actions is mediated by the mirror mechanism (Gallese et al. 1996; Rizzolatti et al. 1996). This mechanism transforms sensory information describing the acts of others into a motor format similar to the one generated by the observer when preparing for or actually performing the observed behavior. The similarity between the motor representation generated in observation and that generated during motor behavior allows the observer to understand others’ actions, without the necessity for inferential processing (Rizzolatti et al. 2001; Rizzolatti and Craighero 2004).
One cortical area that contains neurons endowed with the mirror properties (“mirror neurons”) is F5. This area forms the lateral part of the monkey ventral premotor cortex, and its activity is related to the control of hand and mouth movements (Kakei et al. 2001; Matsumura and Kubota 1979; Rizzolatti et al. 1988; Rizzolatti and Luppino 2001). An important characteristic of F5 neurons, regardless of whether they are mirror neurons or motor neurons devoid of any visual property, is that most of them discharge in association with specific motor acts (e.g., grasping, holding, tearing) rather than with the movements that comprise an act (Rizzolatti et al. 1988; Gallese et al. 1996; Rizzolatti et al. 1996). Thus, many F5 grasping neurons will discharge irrespective of whether grasping is achieved using the right hand, the left hand, or the mouth. Recently, it has been shown that a set of grasping neurons in F5 also fire when the monkey uses tools to grasp objects. Interestingly, these neurons become active both when the monkey uses normal pliers, that is pliers requiring a hand closure to grasp an item, or reverse pliers, that is pliers requiring hand opening for the same purpose (Umiltà et al. 2008). Taken together, these data clearly show that neurons in F5 code the goal of the motor act, regardless of how it is achieved. This finding is consistent with the now accepted concept that the premotor areas and, in part, even the primary motor cortex are organized in terms of the goal of a given motor act (Alexander and Crutcher 1990; Crutcher and Alexander 1990; Kakei et al. 1999; Kakei et al. 2001).
The defining characteristic of F5 mirror neurons is that they fire in response to the presentation of a motor act, which is congruent with the one coded motorically by the same neuron. While a small percentage of mirror neurons in area F5, termed strictly congruent, require that observed and executed motor acts should be similar both in terms of the goal and of the movements that constitute them, the vast majority of F5 mirror neurons, termed broadly congruent respond to different motor acts, provided that they serve the same goal (Gallese et al. 1996). Although a similar distinction has never been explicitly proposed for the visuomotor neurons in the anterior intraparietal area (AIP) and for the neurons that respond to the presentation of 3D objects in F5 (“canonical” neurons), also for these neurons a different degree of congruence could be observed between the optimal visual stimuli triggering a given neuron and the motor properties of the same neuron (Murata et al. 2000; Raos et al. 2006).
Thus, like the visual system, where, as postulated by Shepard (1984), resonating elements (neurons or neuronal assemblies) respond maximally to a set of stimuli, but are also able to respond to similar stimuli when they are incomplete or corrupt, a set of mirror neurons (broadly congruent) appears to resonate to all visual stimuli that have sufficient critical features to describe the goal of a given motor act. This type of stimulus matching is particularly useful for efficient extraction of information from complex stimuli.
These considerations raise some interesting problems concerning the extent of deviations from the preferred stimulus to which mirror neurons resonate. In the earlier studies on mirror neurons, it was noted that while hand (or mouth)-object interactions were effective in triggering F5 mirror neurons, tool–object interactions were typically not (Gallese et al. 1996), except in a few cases after months of visual experience with that tool (Rizzolatti and Arbib 1998). The family of effective stimuli appeared therefore to be essentially limited to natural effectors. The main aim of the present study was to address this issue by assessing whether hand-grasping mirror neurons respond to the observation of grasping with tools in monkeys that have learned to use reverse pliers. If this were to occur, it would show that prolonged visuomotor experience with tools can make tool grasping part of the family of stimuli that are able to trigger grasping mirror neurons.
The second aim of our study was to assess whether the onset and the intensity of F5 mirror neuron discharge during grasping observation is invariant or changes according to the type of effector used to execute the observed motor act. Grasping is a goal-directed motor act which, when performed with natural effectors, develops in time and consists of an opening and closing phase. It takes some amount of time, therefore, to recognize a grasping act and differentiate it from other goal-directed motor acts. Since the onset of the discharge during grasping observation indicates the point at which visual information is sufficient to trigger the neuron, one may assume that this moment also represents the beginning of encoding of the observed motor act.
We addressed these issues by recording hand-grasping mirror neurons from area F5 of monkeys trained to grasp food with a pair of reverse pliers. Observation of the experimenter spearing objects with a stick, a motor act never performed by the monkey, was also tested.
Methods
Experimental procedures
Single-unit activity was recorded from the anterior ventral premotor cortex (area F5) of left (Monkey 1) and right (Monkey 2) hemispheres, contralateral to the moving forelimb of two macaque monkeys (Macaca nemestrina), a male and a female weighing 8 and 5 kg, respectively. The experimental protocols were approved by the Veterinarian Animal Care and Use Committee of the University of Parma and complied with the European law on the humane care and use of laboratory animals.
Before the beginning of recording sessions, the monkeys were habituated to sit on a primate chair and familiarized with the experimental environment. They were then trained to use a pair of reverse pliers to grasp food. Note that unlike standard pliers, reverse pliers require closing of the hand to open the pliers and opening of the hand to close the pliers and thus grasp the food. The total length of the reverse pliers was 14 cm, the length of the plier tips was 2.5 cm. The elastic constant of the pliers was 3.35 Nm. For picture illustrating the functioning of the reverse pliers used in the present study, see also Umiltà et al. (2008).
Food was held on a metallic stick located in front of the monkey at a distance of 20 cm from its body. This stick was attached to the monkey chair in a fixed vertical position with the food fastened to the tip of the stick. The whole experiment was run in full light. Each trial started with the experimenter placing the food on the tip of the stick and covering it with his/her hand. The removal of the hand was the signal for the monkey to grasp the food. Intermixed with tool trials, there were trials in which the monkey grasped the food with its hand. The grip used by the monkeys was congruent with the food size and was typically a “side grip” (opposition of the thumb and the radial surface of the second distal phalanx of the index finger). Each trial was followed by an inter-trial period of variable duration during which the monkey waited for the experimenter instruction and was not holding anything. Before the beginning of each grasping with pliers trial, the experimenter gave the pliers to the monkey. At the end of each grasping with pliers trial, the experimenter always showed his/her hand with the palm open, and this was a signal for the monkey to give the pliers back. In the case of a subsequent grasping with pliers trial, the experimenter returned the pliers to the monkey before the beginning of the trial. After each hand or tool grasping trial, the monkey was allowed to eat the food.
The monkeys were trained for 6–8 months. On completion of the training, i.e., when the monkeys performed at least 80% of the execution trials correctly in both motor conditions (hand and pliers grasping), the head restraint system and titanium recording chamber were implanted. Procedures for the implantation of the head restraint and recording chamber were as described in previous studies (Fogassi et al. 1996). During recording sessions, the monkey was seated on a primate chair with the head fixed.
Monkeys were tested in two experimental protocols. The first consisted of four conditions: grasping execution with the hand and with reverse pliers and observation of grasping by the experimenters using the same two effectors. The second protocol consisted of five conditions: grasping execution with the hand and with reverse pliers; observation of hand grasping, observation of reverse pliers grasping, and observation of spearing with a sharpened stick that the monkey never learned to use. The length of the stick used to spear food was 30 cm, its diameter was 1.5 cm at the graspable top and 0.2 cm at the sharpened tip. During the two execution conditions, the monkey grasped a piece of food using its hand or the reverse pliers. During the three observation conditions, the monkey watched the experimenter grasping food with the hand, with reverse pliers or spearing with the stick. The experimenters performed a period of training in order to perform the hand and tool grasping acts at the same pace and with same proximal movements during each trial. The food was placed on a metallic plate located at a distance beyond the monkey’s reach. The food was of the same size in all conditions and consisted of small pieces of fruit chopped in cubes of 1.5 cm3, using a commercially available device. In the motor trials, the monkey was rewarded after each trial by allowing it to eat the food just grasped; in the observation trials, the experimenter gave to the monkey the food he/she had just grasped. The monkey was not rewarded when it did not pay attention to the experimenter’s behavior. For each condition, neural responses were recorded in 10 trials. Motor and visual trials were completely randomized across all conditions. Not all neurons were tested with the stick spearing observation condition because it was introduced later in the experimental paradigm (second protocol). A contact-detecting device, placed on the vertical metallic stick (execution conditions) or on the metallic plate (observation condition), generated a signal every time the food was touched with a hand or a tool. This signal was fed to a PC and used to trigger recording allowing the alignment of neural response to food grasping. A potentiometer (ALPS 16 mm 50 mW) inserted between the handles of the reverse pliers measured voltage changes (0–2 V), thus giving precise indications of the instantaneous hand position during the opening/closing cycle. The potentiometer voltage changes were fed into the same PC used for recording neural data. The temporal sequence of opening and closing the pliers (obtained from the potentiometer signal) was used to define the different Epochs of grasping (see below) used for statistical analyses.
Recording and electrical stimulation procedures
Single neurons were recorded using tungsten microelectrodes (impedance: 0.5–1.5 MW measured at 1 kHz) inserted through the dura. Individual action potentials were isolated with a time–amplitude voltage discriminator (BAK Electronics, Germantown, MD, USA). The output signal from the voltage discriminator was monitored and fed to a PC for analysis. The same microelectrodes were used also for microstimulation. Intracortical microstimulation (ICMS) consisted of trains of cathodal pulses (train duration: 50 ms, pulse width: 0.2 ms, pulse frequency: 330 Hz) generated by a constant current stimulator. The current intensity used was 3–40 μA. The current intensity was controlled on an oscilloscope by measuring the voltage drop across a 10-kW resistor placed in series with the stimulating electrode. The threshold for each movement evoked by microstimulation was defined as the current intensity at which movements were evoked in 50% of trials. In both monkeys, intracortical microstimulation was performed in the cortical sites where task-related neurons were recorded.
The size of the implanted recording chamber made it possible to access a large cortical area that included the entire ventral premotor cortex, area F1, and the caudal part of the frontal eye fields. The accessible cortical area was functionally explored (single neuron recordings and intracortical microstimulation) in order to assess the location of area F5. The criteria used to functionally characterize area F5 were the following: distal movements evoked by microstimulation at relatively high threshold (>20 μA); neurons discharging in association with hand and mouth motor act execution, neurons discharging to the observation of hand and mouth motor acts and to presentation of 3D objects (Raos et al. 2006). Thus, the recording sites were attributed to area F5 based on topographical and physiological properties. The correct location of the recording sites was confirmed by histological reconstruction. The neurons presented in this study have been recorded from the same two monkeys trained to use tools in a previous study (Umiltà et al. 2008). Note, however, that the database analyzed in the present study is different from that of the previous study.
Neuron selection
Clinical testing preceded the selection of neurons to be tested with the experimental paradigms. The activity of each recorded neuron was tested during the execution of active movements as well as during visual stimulation. Active movements consisted of forelimb movements, such as reaching for and grasping objects of different sizes, shapes and orientations, presented in all space sectors. Neurons were classified as grasp related only when they fired consistently during hand grasping regardless of whether the arm was flexed, extended, adducted or abducted (see Rizzolatti et al. 1988).
Visual properties were tested by presenting food to the monkey and performing a series of motor actions in front of it. These actions were reaching, grasping, manipulating, breaking, holding and placing. These motor acts were performed both with food and other objects and were repeated on the right and on the left of the monkey at various distances (50 cm, 1 and 2 m). Because mirror neurons are by definition those neurons that discharge when the monkey observes a specific hand-object interaction and do not respond to the mere presentation of the food (Gallese et al. 1996; Rizzolatti et al. 1996), only neurons with these characteristics were selected for the study. Furthermore, only neurons that responded to hand grasping in both motor and observation conditions (hand-grasping mirror neurons) and maintained stable responses during the whole testing were selected for further acquisition with the formal experimental paradigm.
Data analysis
In order to assess statistically the neuronal response during the different experimental conditions, the discharge of each neuron was aligned with the moment in which the monkey (execution trials) or the experimenter (observation trials) touched the food. The peri-response time used for the analysis was 2 s before and 2 s after the alignment signal. In the initial experiments, the period taken was 1 s before and 3 s after the signal.
The averaged discharge of each neuron was subdivided into four Epochs. Epoch 1: background activity; Epoch 2: hand or pliers opening (or stick approaching the food); Epoch 3: hand or pliers closure (or stick spearing the food); Epoch 4: food holding.
In all execution and observation conditions, the durations of Epochs 1 and 4 were defined in the same way. Epoch 1 corresponded to the background activity, e.g.: period of rest before the beginning of the task-related motor act. During this period of time, in all observation conditions and in the hand grasping execution condition, the monkey kept its hand still on the surface of a tray fixed on the primate chair. In the reverse pliers execution condition, the monkey held the pliers waiting for the go-signal (see Experimental procedures). The duration of Epoch 1 was determined considering the first 300 ms of acquisition time. This early period of acquisition time was selected in order to avoid any contamination from the beginning of motor preparation or even task-related movements. Epoch 4 (food holding) corresponded to the period of time following the achievement of grasping or spearing. In order to avoid any contamination by subsequent, non-task-related, movements (like bringing food to the mouth), the duration of Epoch 4 was limited to its first 300 ms.
The durations of Epochs 2 and 3 have been determined using two different methods depending on the testing conditions. For the reverse pliers execution and observation conditions, Epochs 2 and 3 were defined using potentiometer data. As mentioned above, a potentiometer was inserted between the reverse plier handles and used to measure voltage changes, due to reduction or increase in the distance between the handles, during manipulation of the pliers. Recorded voltage changes were fed into the same PC used for recording neural data giving an indication of instantaneous hand position during the opening/closing cycle of the pliers. This was done in order to synchronize instantaneous voltage changes with recorded neural activity. For each neuron, potentiometer voltage changes were acquired for each trial, and then averaged across trials in each condition. For each recorded neuron, the temporal limits of Epoch 2 were defined by the first decrease in the voltage values (the point at which the hand started to close, and the distance between the handles began to decrease) until the last decreasing value (corresponding to maximal hand closure and the correspondingly maximal plier tips aperture). Epoch 3 temporal limits were defined by the first increase in the voltage values (when the hand started to open, and the distance between handles began to increase) until the last increasing value (corresponding to the maximal hand aperture and the correspondingly maximal plier tips closure). During the execution condition, Epoch 2 lasted on average 548.33 ms (SD ± 87.87 ms), or 44% of the grasping act. During the observation condition, Epoch 2 lasted on average 636.66 ms (SD ± 59.03 ms), or 52% of the grasp. During the execution condition, Epoch 3 lasted 693.33 ms (SD ± 113.32 ms), i.e., 55.8% of the grasp; in the observation condition, Epoch 3 lasted 565.83 ms (SD ± 71.14 ms), or 47% of the act. The averaged durations of Epoch 2 and 3 were rounded to the nearest ten.
In the hand grasping and stick spearing conditions, food contact was used as the point at which to synchronize neural activity with the different phases of the motor act. As other markers defining the temporal dynamic of the motor act were lacking in these conditions, the temporal limits of the Epochs 2 and 3 were defined off-line. In particular, these Epochs were calculated by means of a frame-by-frame analysis on video recorded with a digital camera (25 frames/s) as explained in detail below.
In order to define the duration of Epochs 2 and 3 of hand grasping execution and observation conditions, 20 trials of hand grasping executed by each monkey and by the experimenters were filmed. The individual grip times and their constituting phases were calculated for each filmed trial and then averaged across trials for each individual. As the grip timing showed little variability across trials, the mean could be used to set the Epoch durations used for subsequent statistical analyses. Epoch 2: hand opening, defined as the phase starting with the beginning of finger opening and finishing when the fingers reached their maximum aperture. During the execution condition, this Epoch lasted on average 337.5 ms (SD ± 31.62 ms) representing 59% of the time course of the whole grasping motor act. During the observation condition, this Epoch lasted on average 382.92 ms (SD ± 44.93 ms), that is, 47.8% of the grasping act. Epoch 3: hand closing, defined as the phase starting with the beginning of finger closing and finishing when the fingers reached their maximum closure. During the execution condition, this Epoch lasted 234.58 ms (SD ± 53.65 ms), that is, 30.7% of the grasp, while during the observation condition, this Epoch lasted 417.5 ms (SD ± 55.51 ms), or 52% of the grasp. The averaged durations of Epoch 2 and 3 were rounded to the nearest ten. For the temporal relation between the beginning and the end of Epochs 2 and 3, we proceeded as following. Maximum finger closure coincided with food contact, which acted as the trigger signal for neural acquisition and alignment. This temporal event was used to define the end of Epoch 3. The end of Epoch 2 (maximum finger aperture) coincided with the beginning of Epoch 3 (beginning of finger closure).
Frame-by-frame video analysis was also used to define the duration of Epochs 2 and 3 in the stick spearing observation condition. Twenty trials of stick spearing executed by the experimenters were filmed. The different phases of the spearing motor act were calculated for each filmed trial and then averaged across all trials. In the food spearing observation condition, Epoch 2 consisted in the stick approach phase, i.e., the period during which the stick started to move toward the food item until 150 ms before contacting it. This 150 ms were considered as the beginning of the spearing phase (Epoch 3) because of the proximity of the tool with the food item, in analogy with hand position in grasping. Epoch 3 was centered on the trigger signal, but for the reason mentioned earlier it was defined as the time window starting 150 ms before food contact and ending 150 ms after this event. Off-line, frame-by-frame analysis revealed that the average duration of the approaching and spearing phases was 839.38 ms (SD ± 60.35 ms).
Statistical analysis
Single neuron analysis
The response of each recorded neuron was statistically assessed by repeated-measures multivariate analysis of variance (MANOVA, P < 0.05) on the firing rate of each neuron. For the majority of recorded cells (N = 16), the MANOVA was performed with two factors: Condition (5 levels: hand and reverse pliers execution, hand, reverse pliers and stick observation) × Epoch (4 levels: Epochs 1–4). For four cells (in which no response to the spearing observation condition was found), the MANOVA was performed with two factors: Condition (4 levels: hand and reverse pliers execution, hand and reverse pliers observation) × Epoch (4 levels: Epochs 1–4).
All neurons displaying a significant interaction Condition × Epoch were further tested with a Newman–Keuls post hoc test in order to compare the neuron discharge during background activity with the activity in subsequent Epochs for all conditions. All neurons displaying statistically significant differences (P < 0.05) between Epoch 1 and one of the three subsequent Epochs in the hand grasping execution and observation conditions were considered to be hand-grasping mirror neurons, and therefore included in the database.
Population analysis
For each neuron, activity was averaged, within each Epoch, across trials for each condition. The maximum level of activity of each neuron was identified across all conditions and Epochs. The activity of each neuron was then normalized for all Epochs and conditions by dividing the mean activity in each Epoch by the maximum observed activity for that neuron and multiplying the resulting value by 100. After normalization, the normalized average discharge frequency of each neuron was used for two different population analyses, using one entry for each neuron.
On all the hand-grasping mirror neurons (N = 20, 10 mirror neurons from Monkey 1; 10 mirror neurons from Monkey 2), a first MANOVA (P < 0.05) was performed with two factors: Condition (4 levels: hand and reverse pliers execution, hand and reverse pliers observation) × Epoch (4 levels: Epochs 1–4). Some of these 20 hand-grasping mirror neurons (N = 16), which were also tested in the spearing observation condition, were included in another MANOVA (P < 0.05) with two factors: Condition (3 levels: hand, reverse pliers and stick observation) × Epoch (4 levels: Epochs 1–4). The significant main factors and interactions obtained from both population analyses were further investigated by comparing the discharge intensity in Epoch 2 and 3 across the conditions (planned comparisons). The comparisons in Epochs 1 and 4 were not performed because they were not considered relevant in the case of population analyses.
Response onset analysis
In order to assess the onset of neuronal response during the observation of grasping performed with different effectors (hand, pliers and stick), the following analyses were carried out (see Bonini et al. 2009). The response of each neuron was expressed in terms of normalized mean activity, calculated as follows. First, the mean activity was calculated for each 20-ms bin in all the recorded trials of the three observation conditions. Then, for each condition, the highest activity value among those of the compared conditions was taken to divide the value of each single bin (normalized mean activity). In order to reliably identify the timing of peak activity timing in each condition, a moving average (period = 60 ms), centered on each 20-ms bin, was applied to the normalized mean activity. To compare the temporal pattern of the discharge during the three observation conditions, an off-set procedure was performed using as an off-set value the mean baseline activity plus its standard deviation multiplied by two (baseline threshold). This allowed identification of the period between the peak of activity and the first preceding negative value as the onset of the neuron discharge. In order to align the discharge onsets of all neurons to a common event, we referred them to the trigger signal (2000 ms). This calculation allowed us to measure the onset of neural activity across all conditions independently of the different Epoch durations. In addition, so as to assess statistically whether the beginning of the neuronal response was modulated by the different observation conditions, we performed a repeated-measures analysis of variance (ANOVA, P < 0.05) on the discharge onset, with the main factor Condition (3 levels: hand; pliers and stick observation). The Newman–Keuls post hoc test was applied on the significant main factor.
Results
Ninety-two neurons were clinically characterized as hand-grasping mirror neurons. In accord with previous findings (Gallese et al. 1996; Rizzolatti et al. 1996), they represented approximately 30% of the total hand-grasping motor neurons recorded in the present study (N = 282). Out of 92 mirror neurons, 27 neurons were recorded for a sufficient time to be tested in the 4 experimental conditions of the first protocol (execution and observation of hand and reverse pliers grasping). The response of each recorded neuron was assessed statistically by performing a MANOVA, P < 0.05 (4 Conditions × 4 Epochs) followed by a Newman–Keuls post hoc test (for details see "Methods").
The results of this analysis showed that out of 27 neurons tested, 20 were statistically confirmed (P < 0.05) as hand-grasping mirror neurons (i.e., they responded both during hand grasping execution and observation) and were therefore included in the database (for the statistical criteria used for the inclusion, see "Methods"). Eighteen (90%) of these 20 neurons responded during grasping execution and observations with reverse pliers. One neuron responded during reverse pliers execution, but not during reverse pliers observation. One neuron responded during reverse pliers observation, but not during reverse pliers execution.
An example of a mirror neuron tested during the execution and observation of hand and ‘reverse’ pliers grasping is given in Fig. 1. The neuron responded vigorously during active grasping by the monkey. The shorter response of the neuron during hand grasping relative to tool grasping was due to the rapidity of hand movements. Strong responses were also present when the monkey observed the experimenter grasping an item by hand and with the reverse pliers. Note that during hand grasping observation, the discharge started earlier than when the monkey observed grasping with the reverse pliers. Statistical analyses (see "Methods") of Epochs 2 and 3 showed that in Epoch 2, the discharge was significantly stronger during hand grasping observation than during observation of grasping with reverse pliers (P = 0.015), while there was no difference in discharge intensity in the third Epoch of the two conditions (P = 0.056).
Fig. 1.
Response of one hand-grasping mirror neuron during the execution and observation of hand and reverse pliers grasping. The upper panels show the rasters and histograms of ten trials recorded during grasping execution with hand (left) and reverse pliers (right). The lower panels illustrate the neuron’s responses during the observation of hand (left) and reverse pliers (right) grasping performed by an experimenter. Reverse pliers grasping was achieved with an inverted sequence of fingers movements with respect to natural hand grasping: the monkey and the experimenter had to first close the hand in order to open the pliers tips and then to open the hand in order to close the pliers tips over the object. The short-lasting high intensity discharge during hand grasping execution was due to the high speed of monkey hand movements. Colored stripes on rasters and histograms illustrate the mean duration of the 4 Epochs. Pink stripes (Epoch 1) delimit the period of rest activity. Note, however, that only the first 300 ms of that period have been taken for analysis. This avoided possible contamination from motor preparation. Violet stripes (Epoch 2) delimit the opening phase of the grasping motor act. Green stripes (Epoch 3) delimit the closure phase of grasping. Yellow stripes (Epoch 4) delimit the holding phase. Black arrows (trigger signal) show the moment in which the effectors contacted the food in all conditions. Rasters and histograms are aligned with the trigger signal. Bin duration was 20 ms
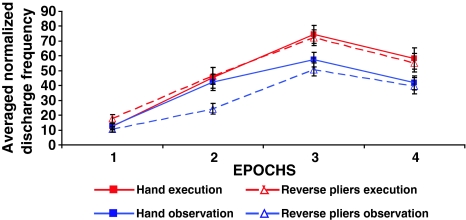
A population analysis was conducted on all 20 statistically confirmed hand-grasping mirror neurons (Fig. 2). Their discharge was subdivided into 4 Epochs (see “Methods”). A 4 × 4 MANOVA (P < 0.05) with main factors Condition and Epoch showed a significant main effect of the two factors (P < 0.0001) and a significant interaction between them (P = 0.0058). Post hoc comparisons performed on Condition showed stronger responses during execution conditions (red lines) than during observation conditions (blue lines) (P = 0.000 and P = 0.003). No differences were found between the two motor conditions (continuous and dotted red lines) (P = 0.925), while significant differences were present between the two observation conditions (P = 0.001) (continuous and dotted blue lines), with stronger responses to hand grasping observation. Planned comparisons performed across Epochs and conditions showed that in all 4 conditions, the peak of discharge occurred in Epoch 3 (P < 0.007). The peaks of discharge were not statistically different between the two motor conditions (red lines, P = 0.803), while they differed from those of the two visual conditions (blue lines, P < 0.04). During Epoch 2, the neural discharge during reverse pliers observation (dotted blue line) was significantly lower than the discharge in the other three conditions (P < 0.000) that did not differ one from another (P > 0.5).
Fig. 2.
Population response of hand-grasping mirror neurons during the execution and observation of hand and reverse pliers grasping. The plots show the averaged normalized discharge frequency of all the recorded F5 hand-grasping mirror neurons (N = 20). Neural discharge was subdivided into 4 Epochs. Epoch 1: Background activity; Epoch 2: Finger or plier tips opening; Epoch 3: Finger or plier tips closing; Epoch 4: Food holding. Statistical analysis showed a maximal discharge frequency during the goal accomplishment (Epoch 3) in all conditions. The execution of hand and reverse pliers grasping (red lines) triggered a significantly stronger response than their observation (blue lines). Black bars SEM
Sixteen out of the 20 hand-grasping mirror neurons were additionally tested when the monkey observed the experimenter spearing a piece of food using a sharpened stick which the monkey had never used (stick observation condition). Twelve neurons significantly responded in this condition, while 4 did not.
Figure 3 illustrates two paradigmatic examples of mirror neurons discharging during hand, reverse pliers grasping and spearing observation. In the upper panels, the responses of the first neuron are illustrated. During hand grasping observation, this neuron started to discharge from the beginning of the observed motor act, i.e., when the fingers were opening (violet stripe) until their closure (green stripe). During reverse pliers observation, the discharge was concentrated on the pliers closure phase (green stripe). Finally, in the stick observation condition, the discharge was concentrated around the moment of spearing (green stripe) and holding (yellow stripe). In other words, for this neuron, the peaks of discharge did not occur in the same phase across conditions but shifted in time according to the different effectors that performed the observed grasping. Statistical analyses performed on Epochs 2, 3 and 4 showed that during the hand observation condition, the peak of discharge occurred in Epoch 2 (P = 0.004); during reverse pliers observation the peak occurred during Epoch 3 (P = 0.006); and finally during spearing observation, peaks occurred in the last two Epochs (Ps < 0.001) with no differences between them. Calculation of the discharge onset showed that this neuron started to respond 620 ms before food contact (trigger signal) when observing hand grasping. When observing reverse pliers grasping, the response started 460 ms before trigger signal. Finally, during stick observation the discharge onset started 140 ms before trigger signal.
Fig. 3.
Response of two hand-grasping mirror neurons during the observation of grasping with hand and reverse pliers and the observation of spearing. Upper panels: Left panel shows the hand-grasping mirror neuron response during the observation of hand grasping. The neural activity starts at the beginning of the grasping motor act, during the finger opening, and continues during finger closure (discharge onset: 620 ms). Middle panel shows the neural discharge during the observation of grasping performed with reverse pliers. The response is more concentrated on the closure of the pliers tips around the food item, i.e., the achievement of the grasping goal (discharge onset: 460 ms). Right panel shows the neural activity during the observation of spearing. The neuron discharge reaches its maximal rate at the end and after goal accomplishment, when the stick penetrates and holds the food item (discharge onset: 140 ms). Lower panels show another example of a neuron tested in the 3 observation conditions. This neuron shows the maximum discharge frequency when the goal is accomplished (green stripes) in all conditions, but the response is prolonged to the holding phase (yellow stripe) only when the monkey observes the food being held by the stick. The discharge onsets of this neuron are 280 ms during hand observation, 320 ms during reverse pliers observation and 60 ms during stick observation. All conventions as in Fig. 1
Another example of a neuron tested in the 3 observation conditions is shown in the lower panels of the same figure. For this neuron, the peak of activity occurred in all conditions in Epoch 3 (green stripe, all Ps < 0.03) but during stick observation where the peak straddled Epoch 3 and Epoch 4 (yellow stripe, P = 0.014). The discharge onset of this neuron was very similar during hand and reverse pliers grasping observation (280 and 320 ms, respectively) while it was largely postponed during stick observation (60 ms before trigger signal).
Figure 4 shows the results of the population analysis conducted on mirror neurons tested with the three observation conditions (n = 16). A 3 × 4 MANOVA (P < 0.05) with main factors Condition and Epoch showed a significant main effect of the two factors (P < 0.01) and a significant interaction between them (P = 0.033). Post hoc comparisons of conditions showed that the neural discharge in the hand observation condition (red line) was significantly higher than that in the reverse pliers observation condition (blue line, P = 0.002) and that the latter was significantly higher than the response in the stick observation condition (green line, P = 0.01). Comparisons across conditions and Epochs showed a peak of activity in Epoch 3 in all three visual conditions (P < 0.02). However, during spearing observation, the neural discharge in Epoch 3 was significantly weaker than during observation of hand and pliers grasping (P < 0.01). No significant differences in the discharge intensity were found between grasping with the hand or the reverse pliers in this Epoch (P = 0.064). Post hoc comparisons of Epoch 2 showed that the discharge during hand grasping observation was significantly higher (all Ps < 0.02) than during the observation of grasping with tools.
Fig. 4.
Population response of hand-grasping mirror neurons during the observation of grasping by hand and with reverse pliers and during the observation of spearing. The plots show the averaged normalized discharge frequency of the F5 hand-grasping mirror neurons (N = 16) tested during the 3 observation conditions. Hand grasping observation (red line) significantly triggers the population discharge during all phases of grasping, e.g., from finger opening to food holding. The response during reverse pliers observation (blue line) reaches its maximum during goal accomplishment (Epoch 3). The normalized discharge frequency during Epoch 3 does not significantly differ in hand and reverse pliers grasping observation. The population discharge in Epoch 3 during spearing observation (green line) is significantly weaker than that during hand and pliers grasping observation. In Epoch 2, the discharge during hand observation is significantly higher than that found during observation of the two tools. All conventions as in Fig. 2
Figure 5 shows the response onset of the population of neurons (n = 12) responding during the three observation conditions. The results of the ANOVA showed a significant main effect of Condition (P = 0.041). Newman–Keuls post hoc test showed that the discharge onset occurred significantly earlier during hand grasping observation than during stick spearing observation (P = 0.014). The comparison of the discharge onset during hand observation with that during pliers observation showed a trend for an earlier onset during hand grasping, although this comparison did not reach significance (P = 0.072).
Fig. 5.
Observation conditions: onset of the neuronal response relative to the contact of the effectors with the food. Response onset of the population of neurons (n = 12) shows a clear pattern that is the earliest onset occurred during hand grasping observation, followed by that during the observation of pliers, while the latest discharge onset occurred during stick spearing observation. Results of the statistical analyses show that differences in discharge onset were significant only when comparing the hand grasping observation condition with that of food spearing
It is worth underlining that the earlier onset times of neural activity was not due to a difference in the duration of the three observation conditions. In fact, the measure of the durations of the three different motor acts showed that the opening–closing phases (Epochs 2 plus Epoch 3) of hand grasping was on the average 800.42 ms, that of the reverse pliers was on the average 1193.49 ms and, finally that of the stick approaching and spearing phases was on the average 839.38 ms. Because the neuronal discharge started 370 ms before food touching in the case of hand grasping, it means that the discharge began about 400 ms after the onset of the hand opening. In the case of pliers, the discharge started about 310 ms before food touching and thus at 890 ms after the onset of the pliers opening phase. These figures indicate that, regardless of the different durations of the two motor acts, it took more time to elicit a neuronal discharge in the case of the pliers than in the case of hand grasping. Thus, it appears that the “recognition” of the motor act, as shown by the discharge onset, was signaled earlier during the observation of natural grasping. The same logic shows that also the “recognition” of stick spearing occurred later than that of hand grasping, regardless of the fact that the duration of spearing (839 ms) was approximately the same as that of the hand grasping. The discharge elicited by the stick spearing observation started 679 ms after the movement onset.
Discussion
Several studies have shown that most F5 motor neurons code the goals of motor acts rather than the movements forming them (Rizzolatti et al. 1988; Kakei et al. 2001; Umiltà et al. 2008). The strongest evidence in favor of this has been achieved by recording the activity of F5 motor neurons in monkeys trained to grasp objects with tools that required opposite hand movements to achieve the same goal (grasping). It was found that F5 motor neurons became active during goal-related phases of tool grasping regardless of whether the hand was opening or closing in that phase (Umiltà et al. 2008).
The first aim of the present experiment was to find out whether F5 hand-grasping mirror neurons respond to the observation of grasping performed in atypical ways, that is, by using tools like reverse pliers or a sharpened stick. The results showed that both these tools were effective in triggering grasping mirror neurons in spite of the fact that they markedly differed one from another (as well as from a hand, the natural grasping effector) both in their visual aspects and in their movement kinematics. Note that all neurons studied in the present experiment were selected after extensive naturalistic testing (see "Methods") and none of them responded during the observation of reaching. Thus, the described response properties could not derive from the mere approach of the effectors to the target. The generalization in recognition of grasping performed by others was greater than that one might predict from the operational correspondence between the hand and the reverse pliers. In fact, the closing of two elements approaching an object, which characterizes grasping in the case of hand and reverse pliers, is not present in the case of stick spearing. Yet most neurons also responded to this type of “grasping”. Thus, what counts in triggering grasping mirror neurons is the identity of the goal (e.g., taking possession of an object) even when achieved with different effectors.
These results also accord with the findings of a recent TMS study on humans in which motor evoked potentials (MEPs) were recorded from the observers’ opponens pollicis muscle during the observation of grasping performed with normal and reverse pliers (Cattaneo et al. 2009). It was found that the amplitude of the recorded MEPs was modulated by the goal of the observed motor act regardless of the movements required to accomplish it.
In earlier studies on mirror neurons, it was reported that mirror neurons do not respond to the observation of actions done by tools (Gallese et al. 1996; Rizzolatti et al. 1996). Exceptions to this were a few mirror neurons that showed a weak response to tool use observations in monkeys tested for a long time with a variety of visual stimuli, including tools (Rizzolatti and Arbib 1998). The present study shows a different pattern. In fact, almost all hand-grasping mirror neurons discharged in response to the observation of grasping with a tool (reverse pliers). Although we did not record the neuronal response prior to the monkeys’ having learned to use this instrument, the strong discrepancy between our results and those of previous experiments is most likely due to the prolonged practice that the monkey’s had with the pliers prior to testing. We cannot state, however, whether this generalization was due to motor practice or to the fact that the monkey had also a rich visual experience with the reverse pliers.
The findings obtained during the observation of spearing with the stick seem to favor the motor practice hypothesis. In fact, from the first experiment in which the stick was used, F5 mirror neurons responded to spearing observation. Since the monkeys had never previously seen such a tool used to take possession of an object, it is likely that their expertise using other tools enabled a generalization from pliers to stick. In other words, it is plausible that, once a general set has been learned, a generalization occurs to other implements, even to those the monkey has never used. Note, however, that a visual generalization from one tool to another cannot be excluded.
It has been previously reported that a set of neurons discharging during grasping with the mouth and/or the hand also responded to tool use observation (Ferrari et al. 2005). This class of neurons, located in a more ventral part of F5 with respect to our recording site and mostly controlling mouth motor acts, was called “tool-responding mirror neurons”. It is important to note that, unlike the present study, these neurons did not respond (or responded very weakly) to the observation of grasping performed with natural effectors (i.e., the hand or mouth). These neurons therefore lacked, in spite of their name, the fundamental characteristic of mirror neurons: that of responding to the observation of motor act performed with natural effectors (hand and mouth). Hence, their classification as mirror neurons does not appear to be fully justified.
The question of why these neurons responded to the observation of tool use remains open. It might be, as suggested by the authors, that they represent a distinct class of visuomotor neurons specifically sensitive to tool action observation. Alternatively, it might be that these neurons, which were recorded only after many experimental sessions, were mouth motor neurons that discharged during tool grasping observation as a consequence of the fact that the monkey had learned that the tool was used to grasp and to bring food items to its mouth (food reward). Thus, unlike mirror neurons of the present study, the neurons recorded by Ferrari et al. (2005) did not perform a visuomotor transformation during tool grasping observation, but rather, expecting reward, prepared mouth aperture.
The second main finding of the present study concerns the intensity and the time course of mirror neuron responses during grasping observation in different experimental conditions. As far as intensity is concerned, the strongest response occurred during the observation of grasping performed by hand, followed by that with pliers, and lastly by stick spearing observation. Note that the number of neurons responding to grasp observation also varied according to the experimental condition. Thus, while almost all recorded neurons responded to the observation of grasping with pliers, 25% of them were unresponsive to the observation of spearing with a stick. The time course of the neural response in the three observation conditions supports this finding. As illustrated in Fig. 5, the pattern of neuronal discharge showed the earliest onset was during hand grasping observation and the latest during the observation of spearing.
Our interpretation of these findings is that the visually driven responses of grasping mirror neurons are based on a “motor template”. Similarly to the visual system, where stimuli that are most similar to a template are also the most effective in eliciting a visual response, the visual mirror responses in F5 were stronger when the effector-object interaction resembled more faithfully that performed by the natural effector (hand grasping that is the motor template). On the contrary, the more dissimilar is the observed motor act from the motor template, the weaker and the more delayed the neural response.
Thus, when the monkey observed hand grasping, i.e., grasping performed in the natural way, the goal of the motor act was recognized earlier and its observation determined the strongest discharge. Observation of grasping with the reverse pliers produced a weaker and later response pattern. This type of grasping, on the one side, resembles hand grasping for the way in which the pliers close around the object to be grasped, while, on the other, it differs from natural grasping in its visual appearance, and, most importantly, for the sequence of movements required to operate the reverse pliers. Finally, spearing an object with a stick—a motor act that radically differs from the motor template—elicited the weakest responses. It is difficult to compare the onset times of neural response during stick spearing observation with those of the other two observation conditions because the movements of stick and those of fingers and pliers are markedly different. However, also in the case of stick spearing observation, the response occurred later than during hand grasping observation.
In conclusion, the present study shows that grasping mirror neurons in area F5 are triggered by the goal of the observed motor act. In addition, it shows that, while the activation of these neurons indicates “grasping” generically, the intensity of their discharge reflects the reliability of this information. Finally, the discharge onset marks the rapidity with which grasping is understood.
Acknowledgments
We thank C. Sinigaglia for his comments and R. Wood for her help in revising the manuscript. This research was supported by Ministero dell’Università della Ricerca [Relevant National Interest Projects (PRIN) and the Italian Fund for Basic Research (FIRB)] and by Information Society Technologies (IST)-Future and Emerging technologies (FET) Neurobotics. L.E. was supported by a Marie Curie Fellowship; I.I. was supported by IST-FET Mirrorbot; F.G. was supported by the Fyssen Foundation and the Cognitique program from the French government; M.J.R. was supported by FIRB; and A.J. was supported by IST-FET Neurobotics and Neuroprobes.
Conflict of interest statement
The authors declare no competing financial interests. The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J Neurophysiol. 1990;64:164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Ugolotti-Serventi F, Simone L, Ferrari PF, Fogassi L (2009) Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. doi:10.1093/cercor/bhp200 [DOI] [PubMed]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Curr Biol. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. J Neurosci. 2009;29(36):11134–11138. doi: 10.1523/JNEUROSCI.2605-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher MD, Alexander GE. Movement-related neuronal activity selectively coding either direction or muscle pattern in three motor areas of the monkey. J Neurophysiol. 1990;64:151–163. doi: 10.1152/jn.1990.64.1.151. [DOI] [PubMed] [Google Scholar]
- De Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17(2):212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex. Nat Neurosci. 2001;4:1020–1025. doi: 10.1038/nn726. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Von Cramon DY, Brass M. How do we infer others’ goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage. 2008;43:784–792. doi: 10.1016/j.neuroimage.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Kubota K. Cortical projection of hand arm motor area from postarcuate area in macaque monkey: a histological study of retrograde transport of horseradish peroxidase. Neurosci Lett. 1979;11:241–246. doi: 10.1016/0304-3940(79)90001-6. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83(5):2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Raos V, Umiltà MA, Murata A, Fogassi L, Gallese V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol. 2006;95(2):709–729. doi: 10.1152/jn.00463.2005. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21(5):188–194. doi: 10.1016/S0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/S0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey: II. Area F5 and the control of distal movements. Exp Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Ecological constraints on internal representation: resonant kinematics of perceiving, imagining, thinking, and dreaming. Psychol Rev. 1984;91:417–447. doi: 10.1037/0033-295X.91.4.417. [DOI] [PubMed] [Google Scholar]
- Umiltà MA, Escola L, Intskirveli I, Grammont F, Rochat MJ, Caruana F, Jezzini A, Gallese V, Rizzolatti G. When pliers become fingers in the monkey motor system. Proc Natl Acad Sci USA. 2008;105(6):2209–2213. doi: 10.1073/pnas.0705985105. [DOI] [PMC free article] [PubMed] [Google Scholar]