Abstract
Aims
To assess the joint influence of inflammatory biomarkers on the risk of incident atrial fibrillation (AF) in women.
Methods and results
We performed a prospective cohort study among women participating in the Women's Health Study. All women were free of AF at study entry and provided a baseline blood sample assayed for high-sensitivity C-reactive protein, soluble intercellular adhesion molecule-1, and fibrinogen. To evaluate the joint effect of these three biomarkers, an inflammation score was created that ranged from 0 to 3 and reflected the number of biomarkers in the highest tertile per individual. During a median follow-up of 14.4 years, 747 of 24 734 women (3.0%) experienced a first AF event. Assessed individually, all three biomarkers were associated with incident AF, even after adjustment for traditional risk factors. When combined into an inflammation score, a strong and independent relationship between inflammation and incident AF emerged. Across increasing inflammation score categories, there were 1.66, 2.22, 2.73, and 3.25 AF events per 1000 person-years of follow-up. The corresponding hazard ratios (95% confidence intervals) across inflammation score categories were 1.0, 1.22 (1.00–1.49), 1.32 (1.06–1.65), and 1.59 (1.22–2.06) (P for linear trend 0.0006) after multivariable adjustment.
Conclusion
In this large-scale prospective study among women without a history of cardiovascular disease, markers of systemic inflammation were significantly related to AF even after controlling for traditional risk factors.
Keywords: Atrial fibrillation, Inflammation, C-reactive protein, Intercellular adhesion molecule-1, Fibrinogen
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its prevalence in the general population is increasing rapidly.1–3 The importance of AF is further underscored through its association with death, stroke, heart failure, cognitive dysfunction, and a reduced quality of life.4–8 As treatment of established AF is of limited long-term success and carries significant risks,9,10 characterizing potentially modifiable risk factors for AF has substantial clinical relevance.
Although several small, mainly cross-sectional studies found increased levels of high-sensitivity C-reactive protein in patients with prevalent AF,11,12 it remains unclear whether inflammation has a causal role in the development of AF. In one of the first prospective studies, high-sensitivity C-reactive protein levels in the highest quartile were associated with a 31% increased risk of incident AF among elderly individuals.13 This relationship was recently confirmed in an analysis from the Framingham Heart Study.14 Both of these studies included participants with prevalent cardiovascular disease at baseline; and therefore, associations could be due in part to well-established associations between inflammation and prevalent cardiovascular conditions that predispose to AF.15 Currently, prospective data on the association between inflammation and AF in apparently healthy populations is limited,16 as is data on inflammatory biomarkers other than high-sensitivity C-reactive protein.17,18
The ongoing Women's Health Study (WHS) presents a unique opportunity to prospectively examine the joint relationships between three markers of inflammation (high-sensitivity C-reactive protein, soluble intercellular adhesion molecule 1 (sICAM-1), and fibrinogen) and incident AF among 24 734 women free of cardiovascular disease and AF at baseline.
Methods
Participants
All study subjects were participants of the WHS, a completed randomized trial evaluating the risks and benefits of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. Details of the study design have been described previously.19 Briefly, beginning in 1993, 39 876 female health professionals in the United States who were 45 years or older and free of cardiovascular disease, cancer or other major illnesses were randomized to receive 100 mg aspirin every other day, 600 IU vitamin E every other day, both agents or placebo. Randomized treatment ended on March 31, 2004, and women were invited to participate in continued observational follow-up.
Women participating in the observational follow-up represented the target population for this study. Blood samples drawn at randomization were available for 25 883 of these women. Of these, 565 and 3 women were found to have AF and cardiovascular disease at study entry, respectively, and 581 women had incomplete data for high-sensitivity C-reactive protein, sICAM-1 or fibrinogen. Excluding these participants left 24 734 for the present analysis. Follow-up for all women started at randomization and was truncated on 2 March 2009. The study was approved by the institutional review board of Brigham and Women's Hospital, Boston, and was monitored by an external data and safety monitoring board.
Laboratory measurements
Three inflammatory markers were selected based on prior evidence of association with cardiovascular events and availability in the WHS.20,21 Plasma high-sensitivity C-reactive protein was measured with a validated high-sensitivity immunoturbidimetric method (Denka Seiken, Niigata, Japan). sICAM-1 was assayed by quantitative sandwich ELISA (R&D Systems, Minneapolis, MN) with a reproducibility of 8.9 and 6.4% at concentrations of 171.8 and 289.1 ng/mL, respectively. Fibrinogen levels were measured using an immunoturbidimetric assay, which is a mass-based assay with international standards (Kamiya Biomedical, Seattle, WA).22
Study variables
Information on baseline variables was collected using mailed questionnaires. Follow-up questionnaires asking participants about study outcomes and other information were sent every six months during the first year and every 12 months thereafter. Covariates of interest that were assessed at study entry included age, blood pressure, smoking, diabetes, race/ethnicity, body mass index, exercise, and alcohol consumption.
Ascertainment of incident atrial fibrillation
Women were asked to report diagnoses of incident AF at baseline, 48 months, and then annually thereafter.23,24 Beginning on 19 September 2006, women enrolled in the continued observational follow-up who reported an incident AF event on at least one yearly questionnaire were sent an additional questionnaire to confirm the episode and collect additional information. They were also asked for permission to review their medical records, particularly available electrocardiograms, rhythm strips, 24 h electrocardiograms and information on cardiac structure and function. For all deceased participants who reported AF during the trial and extended follow-up period, we contacted family members to obtain consent and additional relevant information. An endpoint committee of physicians reviewed medical records for reported events according to pre-defined criteria. An incident AF event was confirmed if there was electrocardiographic evidence of AF or if a medical report clearly indicated a personal history of AF. The earliest date in the medical records when documentation was believed to have occurred was set as the date of onset of AF. Only confirmed events are included in the present report.
Inflammation score
Simultaneous assessment of multiple inflammatory biomarkers may provide further insights in the relationship between inflammation and incident AF. To evaluate whether a multimarker approach using high-sensitivity C-reactive protein, sICAM-1, and fibrinogen provided additive information on the risk of incident AF, two separate analyses were performed. First, an inflammation score was created, where we a priori specified that one point would be added to the score of an individual woman for every biomarker in the highest tertile. We used tertiles instead of quartiles in order to have enough events in the highest inflammation score category. In a sensitivity analysis, biomarker-specific medians were used to create the score. As inflammatory markers are known to be interrelated, we used the first component of a principal component analysis of logarithmically transformed biomarker levels as a second method to capture common variation between different biomarkers.25 The first principal component is the linear combination of markers, similar to a weighted average that explains the most total variance in the inflammatory markers and thus represents a summary measure of inflammation.
Statistical analysis
Baseline characteristics across inflammation score categories were compared using Kruskal–Wallis tests for continuous variables and χ2 tests for categorical variables. Spearman correlation coefficients were used to assess the correlation between biomarkers. To determine whether there was a gradient of risk between individual biomarkers and incident AF, the study population was categorized into approximate tertiles according to plasma levels of high-sensitivity C-reactive protein, sICAM-1, and fibrinogen. We constructed Cox proportional hazards models to compare hazard ratios (HRs) and 95% confidence intervals (CIs) across biomarker tertiles. We also examined biomarkers as continuous variables. All biomarkers were log-transformed for analysis to improve the normality of their distributions as well as the linearity of the association with AF. We tested for deviation from linearity by including a quadratic term in the multivariable adjusted models.
For each woman, person-years of follow-up were calculated from the date of return of the baseline questionnaire to the date of first endpoint, death, loss to follow-up, or to 2 March 2009, whichever came first. Age-adjusted models were further adjusted for smoking, systolic blood pressure, body mass index, history of diabetes mellitus, HDL-C, LDL-C, exercise, alcohol consumption, and race/ethnicity. Cumulative probabilities of incident AF across inflammation score categories were estimated using the Kaplan–Meier method. Event rates across categories were compared using the log-rank test. Cox proportional hazards models were constructed to evaluate the association between incident AF and inflammation using inflammation score categories or principal component parameters either as continuous variables or categorized into quartiles, respectively.
Even though the population was free of cardiovascular disease at baseline, an association between inflammation and incident AF may be caused by intercurrent cardiovascular events, given the strong relationship between inflammation and cardiovascular disease.15,20,21,26 We therefore refitted all Cox proportional hazards models after censoring women at the date of their first confirmed myocardial infarction, stroke, or coronary revascularization.19
Categorical variables were entered in the Cox models using binary indicator variables. Tests for linear trend were performed by assigning all women the tertile-specific median value for each biomarker. Effect modification was assessed using multiplicative interaction terms and likelihood ratio tests. The proportional hazards assumption was examined for all models by including a biomarker by logarithm of time interaction into the model.27 No violation of this assumption was detected. All analyses were carried out using SAS version 9 (SAS Institute, Inc., Cary, NC). A two-tailed P-value <0.05 was considered to indicate statistical significance.
Results
The median (interquartile range) age of the 24 734 women was 53 (49–59) years. During a median follow-up (interquartile range) of 14.4 (13.8–14.8) years, 747 women experienced a first incident AF event. Median plasma levels of high-sensitivity C-reactive protein, sICAM-1, and fibrinogen were 2.0 (0.8–4.3) mg/L, 342 (300–393) ng/mL, and 350 (307–402) mg/dL. Spearman correlation coefficient was 0.29 for the association between high-sensitivity C-reactive protein and sICAM-1, 0.41 for the association between high-sensitivity C-reactive protein and fibrinogen, and 0.27 for the association between sICAM-1 and fibrinogen, suggesting weak to moderate correlations between the three biomarkers. When the study population was divided into tertiles of the inflammatory parameters, the cut-off values for the highest tertiles were 3.4 mg/L for high-sensitivity C-reactive protein, 373 ng/mL for sICAM-1, and 382 mg/dL for fibrinogen. After summing the number of inflammatory parameters in the highest tertile, 9640 women (39.0%) had a score of zero, 7876 (31.8%) a score of 1, 4909 (19.9%) a score of 2, and 2309 (9.3%) a score of 3. Baseline characteristics of the study population stratified by inflammation score categories are shown in Table 1.
Table 1.
Baseline characteristics according to inflammation score categories
| Characteristic | Inflammation score |
P-value | |||
|---|---|---|---|---|---|
| 0 (n = 9640) | 1 (n = 7876) | 2 (n = 4909) | 3 (n = 2309) | ||
| Age (years) | 51 (48–56) | 54 (49–59) | 55 (50–61) | 55 (50–61) | <0.0001 |
| Body mass index (kg/m2) | 23.3 (21.6–25.7) | 25.0 (22.6–28.2) | 27.1 (24.0–31.1) | 29.3 (25.7–33.7) | <0.0001 |
| Current smoking | 4.9 | 12.2 | 15.9 | 23.5 | <0.0001 |
| History of diabetes | 0.8 | 2.1 | 4.2 | 8.5 | <0.0001 |
| History of hypercholesterolemia | 22.3 | 31.1 | 36.4 | 38.3 | <0.0001 |
| Alcohol consumption | <0.0001 | ||||
| Rarely/never | 36.5 | 43.6 | 51.3 | 55.6 | |
| 1–3 drinks per month | 12.7 | 13.6 | 14 | 13.4 | |
| 1–6 drinks per week | 37.6 | 32.8 | 27.3 | 24.7 | |
| ≥1 drink per day | 13.2 | 10 | 7.5 | 6.3 | |
| Systolic blood pressure | <0.0001 | ||||
| <120 mmHg | 56.9 | 44.7 | 34.1 | 26.2 | |
| 120–139 mmHg | 36.3 | 43.5 | 48.3 | 48.7 | |
| ≥140 mmHg | 6.7 | 11.8 | 17.6 | 25.1 | |
| Highest education level | <0.0001 | ||||
| Less than a bachelor's degree | 47.9 | 55.8 | 60.6 | 63.8 | |
| Bachelor's degree | 27 | 23.5 | 21.3 | 21 | |
| Master's degree or doctorate | 25.1 | 20.7 | 18.1 | 15.2 | |
| Exercise (times/week) | <0.0001 | ||||
| Rarely/never | 30 | 37 | 43.2 | 50.8 | |
| <1 | 18.6 | 19.5 | 21.1 | 20.4 | |
| 1–3 | 36.5 | 32.4 | 27.7 | 22.9 | |
| >3 | 15 | 11.1 | 8 | 5.9 | |
| High-sensitivity C-reactive protein (mg/L) | 0.9 (0.4–1.7) | 2.1 (1.0–3.8) | 4.6 (2.9–7.0) | 7.0 (4.9–14.4) | |
| sICAM-1 (ng/mL) | 307 (277–336) | 351 (310–396) | 386 (342–433) | 433 (401–489) | |
| Fibrinogen (mg/dL) | 314 (281–343) | 354 (315–395) | 405 (368–445) | 446 (412–497) | |
Data are median (interquartile range) or percentages. sICAM-1, soluble intercellular adhesion molecule-1.
Results for the individual biomarkers are shown in Table 2. When we assessed high-sensitivity C-reactive protein, sICAM-1, and fibrinogen as log-transformed continuous variables, all biomarkers were significantly associated with AF in the fully adjusted model (Table 2). HRs (95% CIs) per increase in 1 standard deviation were 1.11 (1.02–1.21), P = 0.02, 1.11 (1.02–1.20), P = 0.02, and 1.10 (1.02–1.20), P = 0.02 for high-sensitivity C-reactive protein, sICAM-1, and fibrinogen, respectively. Models including a quadratic term provided no evidence of a curvilinear relationship between any of the three biomarkers and incident AF. Tertile-specific analyses showed a gradual increase of incidence rates from 1.62 to 2.77 events per 1000 person-years for high-sensitivity C-reactive protein, from 1.84 to 2.67 events for sICAM-1, and from 1.95 to 2.68 events for fibrinogen. After multivariable adjustment, HRs (95% CIs) of incident AF among women in the highest tertile compared with those in the lowest tertile were 1.32 (1.07–1.63) for high-sensitivity C-reactive protein, 1.31 (1.08–1.60) for sICAM-1, and 1.18 (0.97–1.44) for fibrinogen.
Table 2.
Tertiles of inflammatory parameters and risk of incident atrial fibrillation
| Tertile 1 | Tertile 2 | Tertile 3 | Plinear trend | Continuousa | P | |
|---|---|---|---|---|---|---|
| High-sensitivity C-reactive protein (mg/L) | ≤1.1 | 1.1–3.4 | >3.4 | |||
| Number of events | 162 | 258 | 327 | |||
| Age-adjusted incidence rateb | 1.62 | 2.19 | 2.77 | |||
| Age-adjusted HR (95% CI) | Referent | 1.38 (1.13–1.68) | 1.76 (1.45–2.12) | <0.0001 | ||
| Multivariable adjusted HR (95% CI)c | Referent | 1.20 (0.98–1.47) | 1.32 (1.07–1.63) | 0.02 | 1.11 (1.02–1.21) | 0.02 |
| sICAM-1 (ng/mL) | ≤315 | 315–373 | >373 | |||
| Number of events | 179 | 247 | 321 | |||
| Age-adjusted incidence rateb | 1.84 | 2.14 | 2.67 | |||
| Age-adjusted HR (95% CI) | Referent | 1.19 (0.98–1.44) | 1.48 (1.23–1.78) | <0.0001 | ||
| Multivariable adjusted HR (95% CI)c | Referent | 1.10 (0.90–1.31) | 1.31 (1.08–1.60) | 0.005 | 1.11 (1.02–1.20) | 0.02 |
| Fibrinogen (mg/dL) | ≤322 | 322–382 | >382 | |||
| Number of events | 192 | 224 | 331 | |||
| Age-adjusted incidence rateb | 1.95 | 1.99 | 2.68 | |||
| Age-adjusted HR (95% CI) | Referent | 1.02 (0.84–1.24) | 1.33 (1.11–1.60) | 0.0007 | ||
| Multivariable adjusted HR (95% CI)c | Referent | 1.01 (0.83–1.23) | 1.18 (0.97–1.44) | 0.07 | 1.10 (1.02–1.20) | 0.02 |
HR, hazard ratio; CI, confidence interval; sICAM-1, soluble intercellular adhesion molecule-1.
aPer increase in one biomarker-specific standard deviation (log-transformed).
bPer 1000 person-years of follow-up.
cAdjusted for age, smoking, systolic blood pressure, body mass index, history of diabetes mellitus, HDL-C, LDL-C, exercise, alcohol consumption, and race/ethnicity. Due to missing covariates, the multivariable model was based on 722 events in 23 781 women.
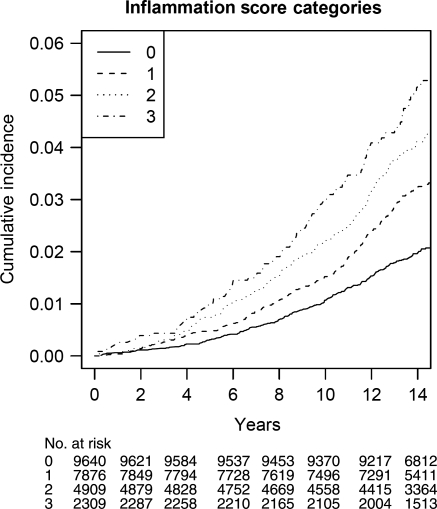
Table 3 displays the results using the combined inflammation score. This score partitioned women into four groups with a gradually increasing risk of incident AF, as shown in Figure 1. Differences in event rates across inflammation score categories were statistically highly significant (P < 0.0001). Similarly, age-adjusted incidences gradually increased from 1.66 to 3.25 events per 1000 person-years from the lowest to the highest inflammation score category (Table 3). This trend was attenuated after controlling for cardiovascular risk factors, but remained statistically significant (P = 0.0006). The multivariable adjusted HR (95% CI) for women with an inflammation score of 3 was 1.59 (1.22–2.06). Among established risk factors, adjustment for body mass index resulted in the greatest attenuation in the association between the inflammation score and AF [HR (95% CI) 1.0, 1.23 (1.01–1.50), 1.31 (1.06–1.63), and 1.57 (1.22–2.03); P-trend 0.0005]. Again, there was no evidence of a curvilinear relationship (Table 3). Similar results were obtained when the inflammation score was based on biomarker-specific median values (2 mg/L for high-sensitivity C-reactive protein, 342 ng/mL for sICAM-1, and 350 mg/dL for fibrinogen). The multivariable adjusted HRs (95% CIs) for women with a score of 0, 1, 2, and 3 were 1.0, 1.37 (1.04–1.79), 1.49 (1.14–1.95), and 1.68 (1.26–2.23), respectively (P for linear trend across categories = 0.0006). Our findings were not significantly altered when 45 women who had a cardiovascular event prior to the occurrence of AF were censored from the analysis [multivariable adjusted HR (95% CI) 1.0, 1.24 (1.01–1.51), 1.30 (1.04–1.63), and 1.52 (1.16–2.01) for women with a score of zero, one, two and three; p linear trend 0.003].
Table 3.
Inflammation score and risk of incident atrial fibrillation
| Inflammation score |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Plinear trend | Pnonlinear trend | |
| Number of events | 190 | 248 | 196 | 113 | ||
| Age-adjusted incidence ratea | 1.66 | 2.22 | 2.73 | 3.25 | ||
| Age-adjusted HR (95% CI) | Referent | 1.34 (1.11–1.63) | 1.58 (1.29–1.93) | 2.04 (1.62–2.59) | <0.0001 | 0.74 |
| Multivariable adjusted HR (95% CI)b | Referent | 1.22 (1.00–1.49) | 1.32 (1.06–1.65) | 1.59 (1.22–2.06) | 0.0006 | 0.84 |
HR, hazard ratio; CI, confidence interval.
aPer 1000 person-years of follow-up.
bAdjusted for age, smoking, systolic blood pressure, body mass index, history of diabetes mellitus, HDL-C, LDL-C, exercise, alcohol consumption, and race/ethnicity. Due to missing covariates, the multivariable model was based on 722 events in 23 781 women.
Figure 1.
Cumulative incidence of incident atrial fibrillation, by inflammation score categories.
Similar findings were also obtained, when we used the first component of a principal components analysis as a summary measure of inflammation. The first principal component explained 55% of the total common variation between the three biomarkers. Similar to the inflammation score, there was a significant relationship between the extent of inflammation as measured by the first principal component and incident AF. The HR (95% CI) per 1 standard deviation increase of the first principal component was 1.29 (1.19–1.39), P < 0.0001 and 1.17 (1.07–1.28), P = 0.0005 after age and multivariable adjustment, respectively. Fully adjusted HRs (95% CIs) across increasing quartiles of the first principal component were 1.0, 1.54 (1.20–1.98), 1.49 (1.15–1.94), and 1.57 (1.20–2.06). Again, we did not find evidence for a curvilinear relationship.
Subgroup analyses revealed consistent results across strata of age, body mass index, systolic blood pressure, diabetes, LDL-C, and HDL-C. Accordingly, none of the inflammation score by subgroup interaction terms was statistically significant (all P for interaction >0.05).
Discussion
This study indicates that inflammation, as jointly measured by plasma levels of high-sensitivity C-reactive protein, sICAM-1, and fibrinogen, is significantly associated with the risk of incident AF in a female population free of cardiovascular disease at baseline, suggesting that inflammation may confer an increased risk of incident AF that is independent of clinically manifest cardiovascular disease. In this large cohort of women free of cardiovascular disease at baseline, a high inflammation score was a significant predictor of incident AF after adjustment for other cardiovascular risk factors. These results were consistent when other measures were used to define inflammation, suggesting that our findings were not dependent on the cut-off chosen to create the score.
Although it has long been recognized that inflammation post-cardiac surgery may lead to post-operative AF,28,29 whether inflammation plays a causal role in AF development in other settings as well is more controversial. Inflammatory infiltrates, myocyte necrosis, and fibrosis have been found in atrial biopsies of patients with both lone and non-valvular AF,30,31 and chronic inflammation may induce electrophysiological and structural changes in the atrial myocardium that may predispose patients with triggering atrial foci to both the development and persistence of AF.13 In agreement with these pathologic findings, high-sensitivity C-reactive protein levels and other markers of inflammation have been found to be higher in patients with AF when compared with those without in retrospective studies.11,12,32 However, as inflammatory markers were measured at the time of or after the diagnosis of AF in these studies, the temporal relationship is unclear, and thus inflammatory marker elevations may be a consequence rather than a cause of AF.
Several prospective cohort studies, which reduce the potential for this type of bias, have reported associations between individual markers of inflammation and AF. Together with our own, three prospective cohort studies involving different populations have found that high-sensitivity C-reactive protein levels were directly associated with AF incidence. The risk estimates observed for high-sensitivity C-reactive protein in our apparently healthy population are similar to those previously reported in the Cardiovascular Health Study [HR (95% CI) 1.31 (1.08–1.58)]13 and the Framingham Study [HR (95% CI) 1.25 (1.07–1.45)],14 where a proportion of participants had prevalent cardiovascular disease. With regard to fibrinogen levels, we found a much more modest association than that previously reported in a Danish study18 using hospitalized AF as the endpoint, which likely included more severe cases of AF. Finally, to our knowledge, our study is the first to have found a significant association between sICAM-1 and AF. In one prior prospective study utilizing a smaller number of AF events, an HR of 1.08 (95% CI 0.91–1.28) was observed,17 which would be consistent with the HR of 1.11 (1.02–1.20) observed in the present analysis with a larger number of events. These findings may suggest that endothelial dysfunction could have a role in the pathogenesis of AF.33
The simultaneous examination of multiple inflammatory biomarkers may provide a more comprehensive picture of the complex inflammatory process34 and allow for more complete estimates of the association between inflammation and AF. Previously, a panel of 12 inflammatory biomarkers was found to be significantly associated with incident AF in another analysis from the Framingham Heart Study utilizing 148 incident AF events.17 However, these relationships were markedly attenuated and became non-significant after adjusting for interim myocardial infarction or heart failure.17 Another prospective study performed exclusively among apparently healthy men and women16 found a strong association with only 99 AF events when a combination of high C-reactive protein and complement levels was examined.16 We now confirm these latter results utilizing a combination of three inflammatory biomarkers in a much larger population of women free of cardiovascular disease at baseline, where the association persists even when women with interim cardiovascular events were censored from the analysis. Taken together, these prospective studies suggest that chronic inflammation may have a pro-arrhythmic effect and lead to the development of AF in susceptible individuals.
Strengths of the present study include its prospective design, sample size, and long-term follow-up with a large number of confirmed events. Potential study limitations that require discussion are the following: First, we included initially healthy, middle-aged female health professionals, most of whom are Caucasian, and generalizability to other populations may be limited. Second, screening electrocardiograms were not performed in this cohort. Therefore, it is possible that asymptomatic AF cases may have gone undetected. However, the number of asymptomatic AF cases in this cohort of health professionals (n = 74, 9.9%) was very similar to the number of asymptomatic cases detected by screening electrocardiograms in other population-based cohort studies.35 Third, our analysis was based on a single baseline determination of each plasma marker, and events occurred over an extended follow-up up to 15.8 years. Our inability to account for changes in these markers over time may have limited our power to detect small-to-moderate relative risks. Fourth, only three inflammatory biomarkers were available for analysis in this study. Although they are among the best evaluated markers in association with cardiovascular disease,15,20,21,26 we might have obtained different results if other or more inflammation markers had been assessed. Fifth, as with any observational study, the significant association between inflammation and AF could be due to residual confounding by other factors, such as undetected subclinical cardiac disease, and may not imply causality.36 Sixth, the 4324 women from the original cohort who opted out of the observational follow-up were excluded from this analysis because their AF could not be reliably confirmed. However, very similar results were obtained when we repeated our analyses using self-reported AF events among all women as the main outcome variable (data not shown). Finally, given that medical records on AF were not obtained until 2006, some of the medical records were obtained retrospectively. However, information on potential endpoints and all exposure variables were collected prospectively, and medical record review was performed without knowledge of exposure status.
Conclusion
In this prospective study, markers of inflammation were independently associated with incident AF in initially healthy, middle-aged women, even after controlling for traditional risk factors. These findings suggest that inflammation may be involved in the pathogenesis of AF.
Funding
This work was supported by grants from the National Heart, Lung and Blood Institute (R21 HL093613) and the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation. The WHS was supported by grants HL-043851, HL-080467, and CA-047988 from the National Heart, Lung and Blood Institute and the National Cancer Institute.
Conflict of interest: Paul M. Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention. The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. doi:10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. doi:10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. doi:10.1016/S0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. doi:10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. doi:10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. doi:10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 10.Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. Am Heart J. 2006;151:771–778. doi: 10.1016/j.ahj.2005.06.014. doi:10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. doi:10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 12.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–380. doi: 10.2143/AC.56.6.2005701. doi:10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 13.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. doi:10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 14.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, Tofler GH, Selhub J, Jacques PF, Wolf PA, Magnani JW, Ellinor PT, Wang TJ, Levy D, Vasan RS, Benjamin EJ. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. doi:10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation aspirin the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. doi:10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 16.Dernellis J, Panaretou M. Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardiol. 2006;97:245–248. doi: 10.1016/j.amjcard.2005.08.027. doi:10.1016/j.amjcard.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, Keaney JF, Wang TJ, Vasan RS, Benjamin EJ. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. doi: 10.1016/j.amjcard.2009.02.053. doi:10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukamal KJ, Tolstrup JS, Friberg J, Gronbaek M, Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the Copenhagen City Heart Study) Am J Cardiol. 2006;98:75–81. doi: 10.1016/j.amjcard.2006.01.067. doi:10.1016/j.amjcard.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. doi:10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 20.Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114:381–387. doi: 10.1161/CIRCULATIONAHA.106.634089. doi:10.1161/CIRCULATIONAHA.106.634089. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. doi:10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 22.Whitton CM, Sands D, Hubbard AR, Gaffney PJ. A collaborative study to establish the Second International Standard for Fibrinogen, Plasma. Thromb Haemost. 2000;84:258–262. [PubMed] [Google Scholar]
- 23.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–2496. doi: 10.1001/jama.2008.755. doi:10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. doi:10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cureton EE, D'Agostino RB. Factor Analysis: An Applied Approach. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 26.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. doi:10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 28.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 29.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann Thorac Surg. 2005;79:1530–1535. doi: 10.1016/j.athoracsur.2004.10.004. doi:10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Nakamura K, Fukushima-Kusano K, Ohta K, Matsubara H, Hamuro T, Yutani C, Ohe T. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thromb Res. 2003;111:137–142. doi: 10.1016/s0049-3848(03)00405-5. doi:10.1016/S0049-3848(03)00405-5. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freestone B, Chong AY, Nuttall S, Blann AD, Lip GY. Soluble E-selectin, von Willebrand factor, soluble thrombomodulin, and total body nitrate/nitrite product as indices of endothelial damage/dysfunction in paroxysmal, persistent, and permanent atrial fibrillation. Chest. 2007;132:1253–1258. doi: 10.1378/chest.07-1185. doi:10.1378/chest.07-1185. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. doi:10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. doi:10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 36.Yap YG. Inflammation and atrial fibrillation: cause or para-phenomenon? Europace. 2009;11:980–981. doi: 10.1093/europace/eup191. doi:10.1093/europace/eup191. [DOI] [PubMed] [Google Scholar]