Abstract
Aims
Coronary late stent thrombosis, a rare but devastating complication, remains an important concern in particular with the increasing use of drug-eluting stents. Notably, pathological studies have indicated that the proportion of uncovered coronary stent struts represents the best morphometric predictor of late stent thrombosis. Intracoronary optical frequency domain imaging (OFDI), a novel second-generation optical coherence tomography (OCT)-derived imaging method, may allow rapid imaging for the detection of coronary stent strut coverage with a markedly higher precision when compared with intravascular ultrasound, due to a microscopic resolution (axial ∼10–20 µm), and at a substantially increased speed of image acquisition when compared with first-generation time-domain OCT. However, a histological validation of coronary OFDI for the evaluation of stent strut coverage in vivo is urgently needed. Hence, the present study was designed to evaluate the capacity of coronary OFDI by electron (SEM) and light microscopy (LM) analysis to detect and evaluate stent strut coverage in a porcine model.
Methods and results
Twenty stents were implanted into 10 pigs and coronary OFDI was performed after 1, 3, 10, 14, and 28 days. Neointimal thickness as detected by OFDI correlated closely with neointimal thickness as measured by LM (r = 0.90, P < 0.01). The comparison of stent strut coverage as detected by OFDI and SEM analysis revealed an excellent agreement (r = 0.96, P < 0.01). In particular, stents completely covered by OFDI analysis were also completely covered by SEM analysis. All incompletely covered stents by OFDI were also incompletely covered by SEM. Analyses of fibrin-covered stent struts suggested that these may rarely be detected as uncovered stent struts by OFDI. Importantly, optical density measurements revealed a significant difference between fibrin- and neointima-covered coronary stent struts [0.395 (0.35–0.43) vs. 0.53 (0.47–0.57); P < 0.001], suggesting that differences in optical density provide information on the type of stent strut coverage. The sensitivity and specificity for detection of fibrin vs. neointimal coverage was evaluated using receiver-operating characteristic analysis.
Conclusion
The present study demonstrates that OFDI is a highly promising tool for accurate evaluation of coronary stent strut coverage, as supported by a high agreement between OFDI and light and electron microscopic analysis. Furthermore, our data indicate that optical density measurements can provide additional information with respect to the type of stent strut coverage, i.e. fibrin vs. neointimal coverage. Therefore, coronary OFDI analysis will provide important information on the biocompatibility of coronary stents.
Keywords: Optical frequency domain imaging, Stent strut coverage, Histological validation
Introduction
Since their introduction in 2001, drug-eluting stents (DES) have markedly changed the treatment of coronary artery disease due to a substantial reduction of repeat revascularization procedures compared with bare metal stents (BMS).1 However, with the increasing clinical use of DES, evaluation of the safety of these devices has become an important issue. In particular, DES have been associated with an increased risk of late stent thrombosis and its potentially devastating consequences such as myocardial infarction and death.2–6 Recent pathological studies have suggested that the number of uncovered stent struts was associated with a markedly increased risk for late stent thrombosis.7–10 Furthermore, the ratio of uncovered to total stent struts was identified as the morphometric parameter that best correlated with the degree of stent endothelialization.7
While in patients with coronary disease, stent strut coverage cannot be reliably identified by intracoronary intravascular ultrasound (IVUS) analysis, optical coherence tomography (OCT), a novel imaging tool with a substantially higher axial image resolution of about 10–20 µm has been developed as an important advancement for in vivo evaluation of coronary stents.11 In a 2-year follow-up observation by coronary OCT in patients that had received a first-generation sirolimus-eluting stent, a substantial portion of patients had remaining cross-sections with more than 30% uncovered stent struts.12 Moreover, OCT evaluation of coronary DES implanted in patients with ST-elevation myocardial infarction revealed a higher frequency of uncovered stent struts at follow-up when compared with patients with stable coronary disease.13 These observations are in line with recent pathological observations suggesting that the ratio of uncovered to total stent struts is increased in patients with an acute coronary syndrome after DES implantation.14 Moreover, acute coronary syndromes have been identified as predictors of late stent thrombosis after DES implantation.5
After the introduction of first-generation time-domain OCT imaging systems for clinical studies, which frequently required coronary occlusion for reliable high-quality image acquisition, the second generation of OCT imaging systems, i.e. optical frequency domain imaging (OFDI), has provided a substantial advance due to a markedly increased speed of image acquisition.15 Yun et al. have described the development of the prototype of this technology, i.e. the fibre-optic imaging device for OFDI.16 These authors have reported a three-dimensional resolution of approximately 15 × 15 × 10 µm by using the first OFDI-prototype system. Optical frequency domain imaging substantially improves the feasibility of this intracoronary imaging method and reduces patient discomfort during image acquisition; however, the characterization of stent strut coverage by this method remains to be evaluated against light and electron microscopic analysis of stent strut coverage. The present study was therefore designed to validate in an in vivo model the analysis of stent strut coverage using these methods. Furthermore, we examined differences in optical density of stent strut coverage at different time points after stent implantation in order to determine whether there is a difference with respect to optical density between fibrin- and neointima-covered stent struts.
Methods
Animal study protocol
Twenty stents (3.0 × 18 mm) were implanted in a total of 10 farm swine (Landrace Large White Duroc pigs, aged 8–10 weeks) into the left anterior descending and the ramus circumflexus. Animals were medicated with acetylsalicylic acid (ASS, 500 mg daily p.o.) and ticlopidine (200 mg daily p.o.) for 3 days before stent implantation. Immediately before implantation, 200 IU/kg unfractionated heparin was administered via the introducer. Suitable implantation sites within the vessels were identified by angiography (target vessel diameter between 2.5 and 2.75 mm) and stents (10 BMS; Terumo CoCr, and 10 DES; Biolimus A9 eluting Terumo Nobori) were implanted with a vessel overstretch of 10–20%. After operation, the animals were allowed to recover while under continuous medication of ASS (500 mg daily p.o.) and ticlopidine (200 mg daily p.o.) for the duration of the study. Optical frequency domain imaging was performed after stent implantation and at follow-up after 1, 3, 10, 14, and 28 days (four stents each time point). Immediately after follow-up angiography animals were sacrificed by exsanguinations under deep anaesthesia. Hearts were explanted; coronary arteries were perfused with saline solution and 4% formalin for 10 min. The present investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 85-23, revised 1996). The study protocol has been approved by the local veterinary inspection office. Study procedures, including pathologic evaluation, were performed in compliance with good laboratory practices.
Optical frequency domain imaging image acquisition and analysis
The Terumo-OFDI catheter was advanced over a 0.014-inch guide wire and the imaging core was placed distal of the stent. The catheter position was confirmed by the radiopaque markers of the OFDI catheter. This OFDI system provides images at 160 frames/s with a total of 512 radial scans per circular cross-sectional image.17 The Terumo-OFDI system has an axial resolution of <20 µm and a lateral resolution of 30 µm (at 2.5 mm) according to the manufacturer's information (New Technology Development Group, Terumo R&D Center). The acquisition of OFDI images was performed using the automatic pull-back at a speed of 20 mm/s17 after manual injection of contrast (Ultravist-300, Bayer Schering Pharma) at a rate of 2–4 mL/s (based on the runoff of the artery) for approximately 4–6 s under continuous online assessment of OFDI image quality (in particular demonstrating that the blood was completely displaced from the artery during the whole image acquisition time).
Optical frequency domain imaging images were analysed offline in each cross section by two independent investigators (C.T. and M.M.) blinded to the histological examination and all stent struts were classified as either uncovered or covered stent struts. Stent struts were classified as uncovered if any part of the strut was visibly exposed to the lumen and covered if tissue was visible over all of the circumference of the strut. Metallic stent struts typically appear as bright, signal intense structures with dorsal shadowing.
Electron microscopy analysis
For scanning electron microscopy (SEM), tissue fixation was performed as described previously.18 Briefly, the samples were transferred from Formalin 4% to a solution of 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4, 350 mOsm) for 3 days. The vessels were opened by a longitudinal cut, the two parts of the vessel were washed, dehydrated in ascending concentrations of ethanol, and finally critical point dried. Critical point drying was used as a tissue preparation method for SEM as commonly applied for this technique.19,20
Samples were then mounted on aluminium stabs, sputter-coated with gold, and viewed under a Philips XL 30 FEG scanning electron microscope at different magnifications. The extent of surface coverage above stent struts was traced and measured by iTEM morphometry software (iTEM Analyses imaging program; Olympus Soft Imaging Solutions, 48149 Münster, Germany). The results are expressed as a percentage of total surface area above struts. Endothelial cells were identified as sheets of spindle- or polygonal-shaped monolayers in close apposition. In contrast, fibrin coverage was characterized by fibrin aggregates intermixed with red blood cells and inflammatory cells. The percentage of stent strut coverage was determined using computer-assisted planitometry and type of coverage (endothelium/neointima vs. fibrin) was assessed by two experienced pathologists blinded to the stent type. Percent coverage was compared with the corresponding OFDI images. Struts over side-branches were excluded for both the analyses. To determine whether optical density of OFDI images was related to the type of stent strut coverage (fibrin vs. neointima), the optical density was measured as described below.
The comparison of stent strut coverage between OFDI and SEM was performed for 13 stents, where the preparation was possible without clear artefacts destroying stent coverage. Seven stents had substantial loss of coverage clearly attributable to preparation artefacts, and could therefore not be included into the analysis.
Light microscopy analysis
Stents were embedded for light microscopy (LM) analysis in polymethyl methacrylate21 and stained with haematoxylin/eosin as well as Elastica van Gieson. An image analysis system was used for histomorphometric measurements (Olympus Vanox-S). The system was calibrated prior to each measurement session against a standard. Images were captured with a CCD video camera (JVC, KY55) and neointimal thickness was measured with Analysis v.3.0 (Soft Imaging Systems, 48149 Münster, Germany). The correspondence of LM and OFDI images was confirmed using landmarks, such as stent edges and side-branches as described previously (Figure 1).22 Neointimal thickness as determined by OFDI and LM was statistically compared as described below.
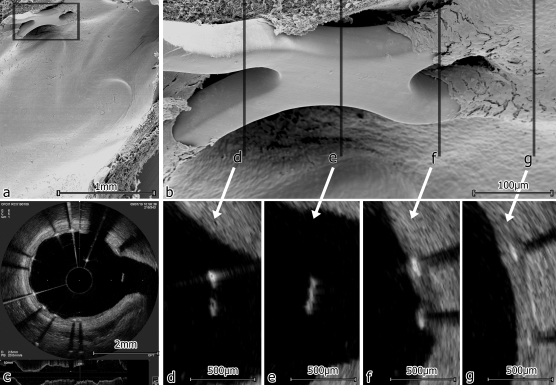
Figure 1.
Representative corresponding images of scanning electron microscopy (SEM) and optical frequency domain imaging (OFDI) analysis of the same stent segment. (A) Overview in SEM; (B) details of the side-branch in SEM; (C) overview in OFDI; (D–G) sequential OFDI images showing covered and uncovered stent struts corresponding to the marked positions in the electron microscopy image in (B); (F) shows one strut covered (upper) and one strut blank (lower) as seen on the SEM image as well.
Optical density analysis of stent strut coverage
Pixel intensity (optical density) of stent strut coverage in OFDI images, normalized for optical density of the stent struts, was evaluated as a potential predictor of the type of stent strut coverage. In particular, optical density was determined for stent struts covered by fibrin vs. neointima. Computer-assisted densitometric analysis was performed as described previously23 to compare the optical intensity of areas of stent struts (is) with the intensity of the surrounding tissue facing the intraluminal side of the strut (it, Figure 2) by using Genetools software (Syngene, Cambridge, UK). The quotient (it/is) was correlated to the morphologic information gathered by the SEM and LM evaluation for stents which were covered with fibrin (Days 1 and 3; 56 SEM and LM spots) and for stents which were covered by neointima (Days 10, 14, and 28; 104 SEM and LM spots). These stents served as references to establish ranges of (it/is) for fibrin and neointimal coverage. Then we evaluated struts in 160 randomly chosen images at the different time points after stent implantation (32 images/time point), calculated the (it/is)-quotient and showed the changes of optical density of stent strut coverage as assessed by OFDI over time.
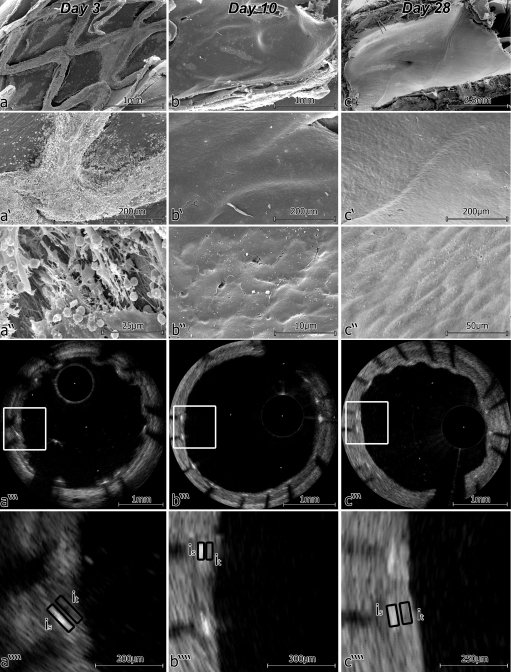
Figure 2.
Different characteristics of stent strut coverage at different time points after stent implantation, i.e. fibrin- vs. neointima-covered stent struts. (A) Fibrin at Day 3 in scanning electron microscopy (SEM) and optical frequency domain imaging (OFDI), where a low intensity of stent strut coverage can be seen (A‴′); (B) neointima at Day 10 in SEM and OFDI, where stent strut coverage with higher intensity when compared with fibrin can be observed; (C) neointima at Day 28 in SEM and OFDI, where a high intensity of stent strut coverage is present (is, intensity of strut; it, intensity of tissue).
Statistics
The rate of covered stent struts in OFDI images was calculated in relation to total stent struts counted in the segments corresponding to electron microscopy (SEM) images. Data are reported as means and standard error for normally distributed continuous data or medians with interquartile ranges for non-normally distributed data. The association between OFDI and SEM analysis was calculated using Pearson's correlation coefficient. Student's t-test (two-sided) was used for the statistical comparison of normally distributed continuous data, Mann–Whitney U-test (two-sided) was used for the statistical comparison of non-normally distributed continuous data. Receiver-operating characteristics (ROC) analysis was used to compare diagnostic accuracy of optical density analysis of OFDI images of stent strut coverage for detecting fibrin- vs. neointima-covered stent struts. Area under the ROC curve (AUC) was calculated to compare the accuracy of the approach. The agreement between neointimal thickness as measured by OFDI and LM analysis was examined by Bland–Altman plots.24 Statistical analyses were performed using SPSS for Windows software (release 18.0; SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered statistically significant.
Results
The OFDI imaging procedure was successfully performed in all animals and a good visualization of the coronary artery wall was obtained. A total number of 7929 stent struts were evaluated in the OFDI analysis. Stent strut apposition was observed in all cases.
Relation of coronary stent strut coverage as detected by in vivo optical frequency domain imaging and scanning electron microscopy analysis
The OFDI measurements of stent strut coverage were highly reproducible, i.e. the interobserver correlation was r = 0.98 (P < 0.01). Notably, the comparison of stent strut coverage as detected by OFDI and SEM analysis revealed an excellent agreement (r = 0.96, P < 0.01, Table 1, Figure 1).
Table 1.
Comparison of stent strut coverage by optical frequency domain imaging and scanning electron microscopy analysis
| Coverage | OFDI (coverage in %) | SEM (coverage in %) |
|---|---|---|
| Completely neointima-covered stents | 100 | 100 |
| 100 | 100 | |
| Mean value | 100 | 100 |
| Incompletely neointima-covered stents | 97 | 96 |
| 99 | 98 | |
| 99 | 96 | |
| 99 | 95 | |
| 99 | 98 | |
| 98 | 99 | |
| Mean value ± SEM | 98.5 ± 0.1 | 97 ± 0.3 |
| Fibrin covered stents | 76 | 69 |
| 70 | 72 | |
| 53 | 58 | |
| 49 | 65 | |
| Mean value ± SEM | 62 ± 3.3 | 66 ± 1.5 |
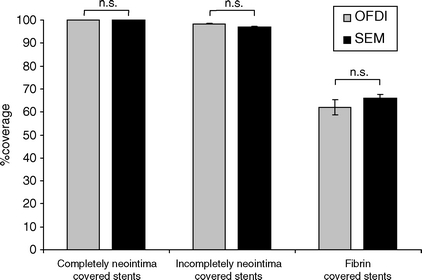 | ||
In particular, stents that were completely covered according to OFDI analyses were also completely covered in the SEM analysis. Stents that had uncovered struts by OFDI analysis had also uncovered struts by the SEM analysis. The mean value of stent strut coverage of fibrin-covered stents, examined early after stent implantation, tended to be lower when stent strut coverage was determined by OFDI when compared with the SEM analysis, although this did not reach statistical significance (Table 1). However, we cannot exclude that fibrin-covered stent struts may rarely appear as uncovered by OFDI (Table 1). Notably, this was not observed for neointima-covered stents (Table 1). The minimal differences in the percentages of uncovered stent struts between OFDI and SEM at later time points may likely relate to the different measurement approaches (OFDI analyses from sections vs. SEM analyses by planimetry).
Evaluation of the optical density of stent strut coverage to distinguish between fibrin and neointimal stent strut coverage
For the analysis of the optical density of stent strut coverage completely fibrin-covered stents (1–3 days after implantation) and stents completely covered by a neointima (10–28 days after implantation) according to the light and electron microscopic analysis were used. These densitometric measurements of the different types of stent strut coverages (i.e. fibrin- vs. neointima-covered stent struts) revealed significant differences in the optical intensity (normalized for the optical density of the stent struts; it/is). The median of normalized optical density of stent strut coverage was significantly lower for fibrin-covered when compared with neointima-covered stent struts [0.395 interquartile range (0.35–0.43) vs. 0.53 interquartile range (0.47–0.57); P < 0.001; n = 56–104; Figures 2 and 3A].
Figure 3.
Assessment of the optical density of the optical frequency domain imaging (OFDI) images of stent strut coverage—differences between fibrin vs. neointimal stent strut coverage. (A) Densitometric analysis of OFDI images with histologically approved fibrin- and neointima-covered stent struts (medians with interquartile ranges); (B) receiver-operating characteristic curve of data presented in (A); (C) densitometric analysis of the development of neointimal coverage of stents at different time points after stent implantation.
The diagnostic accuracy of the optical density measurements was assessed by ROC curve analysis for differentiating fibrin vs. neointimal stent strut coverage. The ROC curve analysis revealed an excellent diagnostic accuracy of the optical density measurements (AUC = 0.859, Figure 3B). When an optical density of 0.44 (it/is) was considered as a cut-off value, the sensitivity of detecting neointimal coverage was 82.7% with a specificity of 83.9%. Fibrin coverage was detected at the same cut-off value with a sensitivity of 83.9% and a specificity of 82.7%. For values of optical density >0.53 (it/is), neointimal coverage was detected with a specificity of >95% (‘safe area’), whereas values of optical density <0.38 (it/is) specify fibrin-covered stent struts with a specificity >95%. Figure 3C shows the values of the normalized optical density of tissue covering stent struts at different time points. The time course shows the development of optical density of stent strut covering tissue from Days 1 to 28, i.e. associated with the change from fibrin to neointimal coverage of stent struts. Therefore, densitometric analysis may represent a promising tool to get further information on the type of stent strut coverage.
Relation of neointimal thickness as detected by optical frequency domain imaging and light microscopic analysis
There was an excellent agreement between coronary OFDI and light microscopic analysis of neointimal thickness (r = 0.90, P < 0.01). The Bland–Altman analysis is shown in Figure 4, demonstrating the agreement between neointimal thickness measurements obtained by LM and OFDI analysis. 89.9% of struts detected by LM were visible in OFDI (Figure 5). The remaining struts were not detectable due to the small segment of the coronary wall that was covered by the guide wire (i.e. guide wire artefact). Measurements of very early time points (i.e. Days 1 and 3) were not included due to the limited presence of neointimal coverage.
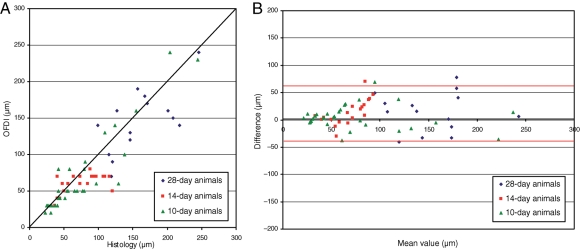
Figure 4.
Relation of measurements of neointimal thickness between light microscopic (LM) and optical frequency domain imaging (OFDI) analysis on Days 10–28 after coronary stent implantation. Dot plot (A) and log transformation of Bland–Altman plot (B; red lines = limit of agreements) are shown demonstrating the agreement between neointimal thickness measurements obtained by light microscopy and OFDI analysis.
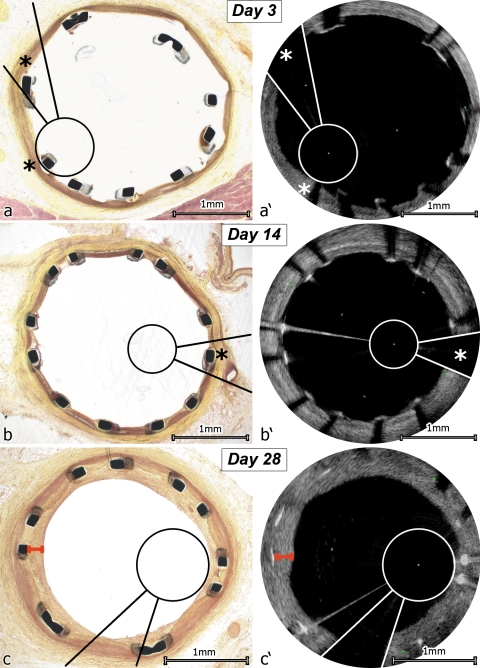
Figure 5.
Corresponding images of light microscopic (LM) and optical frequency domain imaging (OFDI) analysis of neointima measurements. The image wire and the guide wire shadow have been marked in OFDI (white) and on their corresponding sites in LM (black), matching pairs were found as positions of LM images in the stent were known and positions of OFDI images could be calculated from the number of images between start and end of the stent. (A) Day 3, one strut on OFDI is hidden behind the guide wire shadow, one is hidden behind the image wire shadow (indicated by asterisks); (B) Day 14, again, one strut is hidden behind the guide wire shadow in the OFDI image (asterisks); (C) Day 28 LM vs. OFDI, all struts are visible in OFDI and LM images, measurement of neointimal thickness is marked in red.
Discussion
With the increasing use of DES, the evaluation of the safety and biocompatibility of these devices in patients has become an important concern. Pathological studies have suggested that the proportion of uncovered coronary stent struts represents the best morphometric predictor for late DES thrombosis. Recently, coronary OFDI has emerged as a novel, highly rapid coronary imaging method for the assessment of coronary stent strut coverage in patients in vivo; however, the accuracy of this imaging method needs to be further evaluated. The present study demonstrates a high agreement between coronary OFDI and light and electron microscopic analysis for the evaluation of coronary stent strut coverage in an in vivo model. Moreover, our data indicate that optical density measurements provide additional information with respect to the type of stent strut coverage, i.e. fibrin vs. neointimal coverage that may further aid in the assessment of the in vivo biocompatibility of coronary stents. In addition, neointimal thickness as detected by OFDI correlated closely with neointimal thickness as measured by LM, suggesting an excellent agreement between these quantitative measures.
Late coronary DES thrombosis, although a rare complication, remains an important concern owing to its high morbidity and mortality and the increasing use of DES,2–6 due to the reduced necessity of repeated coronary revascularization procedures when compared with BMS.1 Of note in this regard, Finn et al.7 have observed in pathological studies that the percentage of uncovered stent struts represented the best morphometric predictor of late DES thrombosis. In particular, these authors observed a continuum of risk for LST for individuals that increases with the percentage of uncovered stent struts per section, i.e. in a stent with 30% uncovered struts, the odds ratio for thrombosis is 9.0 when compared with a stent with complete stent strut coverage.7 Therefore, there is a great interest in an adequate in vivo evaluation of coronary stent strut coverage.
Notably, OCT has emerged as a promising in vivo coronary imaging technology utilizing infrared light to achieve a substantially higher resolution of cross-sectional images when compared with coronary IVUS.11 Indeed, first-generation OCT has a markedly increased capacity to detect coronary stent strut coverage when compared with coronary IVUS analysis.11 However, first-generation OCT systems are limited by a rather low image acquisition speed. The second-generation OCT imaging system has the advantage of a substantially accelerated coronary image acquisition, thus avoiding the need of the proximal occlusion of the coronary artery for high-quality image acquisition. These developments have markedly improved the feasibility of this novel coronary imaging method. However, an evaluation with respect to the accuracy for detection of stent strut coverage in vivo when compared with light and electron microscopic analysis is urgently needed.
The detection of the presence or absence of neointimal coverage may potentially be limited by the resolution of OFDI. Thus, struts classified as uncovered by OFDI might still have a very thin coverage of tissue of less than 10–20 µm. Although the biological protection offered by such a thin coverage is debatable, struts might in fact be covered by an endothelial layer of less than 10–20 µm thickness. However, the histological evaluation performed in the present in vivo study suggests that neointimal coverage of stents as assessed by OFDI compares very well with this gold standard. In particular, the stent strut coverage as detected by OFDI analysis was not lower when compared with the data obtained by the histological analysis. Only in fibrin-covered stents, very early after stent implantation, it appeared that these may rarely be falsely classified as uncovered struts by OFDI, when fibrin coverage was detected by the SEM analysis. However, fibrin coverage of stent struts has also been observed in conditions associated with an increased risk of stent thrombosis.8,14,25
Indeed, the distinction of fibrin vs. endothelial coverage might be important for proper assessment of novel stents in patients. We therefore examined whether the determination of the optical density of stent strut coverage may aid in distinguishing between fibrin and neointimal coverage of stent struts, as validated in an in vivo model by electron and light microscopic analysis that has not been possible until now.25 Of interest, impaired stent healing with a persistent fibrin deposition has been observed late after coronary implantation of DES,8 likely as a consequence of an incomplete endothelial repair process. In this respect, we and others have recently observed that sirolimus and similar compounds do not only inhibit vascular smooth muscle cell proliferation, but also endothelial cell growth.26 Interestingly, the percentage of fibrin-covered stent struts is increased in patients with DES implantation and ACS and in those receiving overlapping DES.8,14,25 Importantly, both conditions have been associated with an increased risk of late stent thrombosis.8,14,25 In the present analysis, fibrin-covered stent struts had a lower optical density when compared with stent struts covered by a neointima. Safe areas as well as an appropriate cut-off value for distinction of fibrin vs. neointimal stent strut coverage were obtained using histologically validated densitometric measurements. These observations raise the possibility that OFDI may not only allow adequate detection of stent strut coverage in vivo, but may also aid in the characterization of the type of stent strut coverage. Therefore, OFDI analysis provides valuable information on the coronary stent strut coverage not available with previous imaging systems that will aid in the assessment of the biocompatibility of coronary stent systems in vivo.
Limitations of the study
The comparison of OFDI and light and electron microscopic analysis of coronary stent strut coverage was performed after stent implantation into porcine coronary arteries without coronary disease, whereas in the clinical setting stent implantation is performed into coronary arteries with coronary disease, and this should be acknowledged as a potential limitation of the present study. However, coronary arteries in domestic crossbred swine are suitable as their size, access, and injury response are similar to human vessels, and have therefore been considered a particularly valuable model in pre-clinical evaluation of stent healing.27
Conclusions
Based on pathological studies, the proportion of uncovered coronary stent struts has been identified as a potent risk factor for late stent thrombosis of DES. The present study evaluated the accuracy of coronary OFDI for the detection of uncovered stent struts by comparing OFDI with light and electron microscopic analysis in a porcine coronary artery model, and demonstrates a close relation between stent strut coverage as assessed by OFDI and microscopic analysis. Furthermore, our study suggests that the optical density of OFDI-detected stent strut coverage is different for fibrin- vs. neointima-covered stent struts. Therefore, coronary OFDI represents a highly promising technology for the characterization of stent strut coverage and a valuable tool for the assessment of coronary stent biocompatibility.
Funding
The study was supported in part by a grant of the Swiss National Research Foundation ‘Sonderprogramm Universitäre Medizin’ (Nr. 33CM30-124112/1). The work was supported by the Zurich Center of Integrated Human Physiology. Furthermore, this work was supported by an unrestricted educational grant of Terumo Europe Interventional Systems. Funding to pay the Open Access publication charges for this article was provided by Terumo Europe.
Conflict of interest: D.P. is an employee of Terumo Europe Interventional Systems.
Acknowledgements
We thank Isao Mori (Terumo) for his excellent technical support with the Terumo-OFDI system.
References
- 1.Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park SJ, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198–3206. doi: 10.1161/CIRCULATIONAHA.108.826479. doi:10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- 2.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. doi:10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 3.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. doi:10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 4.Moreno R, Fernandez C, Hernandez R, Alfonso F, Angiolillo DJ, Sabate M, Escaned J, Banuelos C, Fernandez-Ortiz A, Macaya C. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–959. doi: 10.1016/j.jacc.2004.11.065. doi:10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 5.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. doi:10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 6.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134–1140. doi: 10.1016/j.jacc.2008.07.006. doi:10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. doi:10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 8.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. doi:10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. doi:10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 10.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. doi:10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, Sawada T, Paredes OL, Hirata K, Yokoyama M. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–967. doi: 10.1093/eurheartj/ehl413. doi:10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 12.Takano M, Yamamoto M, Inami S, Murakami D, Seimiya K, Ohba T, Seino Y, Mizuno K. Long-term follow-up evaluation after sirolimus-eluting stent implantation by optical coherence tomography: do uncovered struts persist? J Am Coll Cardiol. 2008;51:968–969. doi: 10.1016/j.jacc.2007.09.070. doi:10.1016/j.jacc.2007.09.070. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, Regar E. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2:445–452. doi: 10.1016/j.jcin.2009.01.012. doi:10.1016/j.jcin.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–1145. doi: 10.1161/CIRCULATIONAHA.107.762047. doi:10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 15.Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MJ, Freilich MI, Desjardins AE, Oh WY, Bartlett LA, Rosenberg M, Bouma BE. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging. 2008;1:752–761. doi: 10.1016/j.jcmg.2008.06.007. doi:10.1016/j.jcmg.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, Suter MJ, Chan RC, Evans JA, Jang IK, Nishioka NS, de Boer JF, Bouma BE. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429–1433. doi: 10.1038/nm1450. doi:10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamura T, Serruys PW, Regar E. Three-dimensional visualization of intracoronary thrombus during stent implantation using the second generation, Fourier domain optical coherence tomography. Eur Heart J. 2010;31:625. doi: 10.1093/eurheartj/ehp519. doi:10.1093/eurheartj/ehp519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makanya AN, Hlushchuk R, Baum O, Velinov N, Ochs M, Djonov V. Microvascular endowment in the developing chicken embryo lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1136–L1146. doi: 10.1152/ajplung.00371.2006. doi:10.1152/ajplung.00371.2006. [DOI] [PubMed] [Google Scholar]
- 19.Makanya AN, Djonov V. Development and spatial organization of the air conduits in the lung of the domestic fowl, Gallus gallus variant domesticus. Microsc Res Tech. 2008;71:689–702. doi: 10.1002/jemt.20608. doi:10.1002/jemt.20608. [DOI] [PubMed] [Google Scholar]
- 20.Cheneau E, John MC, Fournadjiev J, Chan RC, Kim HS, Leborgne L, Pakala R, Yazdi H, Ajani AE, Virmani R, Waksman R. Time course of stent endothelialization after intravascular radiation therapy in rabbit iliac arteries. Circulation. 2003;107:2153–2158. doi: 10.1161/01.CIR.0000062648.39025.09. doi:10.1161/01.CIR.0000062648.39025.09. [DOI] [PubMed] [Google Scholar]
- 21.Donath K. Preparation of Histologic Sections by the Cutting-Grinding Technique for Hard Tissues and other Material. Norderstedt: 1993. [Google Scholar]
- 22.Prati F, Zimarino M, Stabile E, Pizzicannella G, Fouad T, Rabozzi R, Filippini A, Pizzicannella J, Cera M, De Caterina R. Does optical coherence tomography identify arterial healing after stenting? An in vivo comparison with histology, in a rabbit carotid model. Heart. 2008;94:217–221. doi: 10.1136/hrt.2006.112482. doi:10.1136/hrt.2006.112482. [DOI] [PubMed] [Google Scholar]
- 23.Marano RJ, Rakoczy PE. An improved method using densitometry for evaluating severity of laser photocoagulation induced CNV. Biotech Histochem. 2006;81:59–62. doi: 10.1080/10520290600799131. doi:10.1080/10520290600799131. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 25.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. doi:10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 26.Matter CM, Rozenberg I, Jaschko A, Greutert H, Kurz DJ, Wnendt S, Kuttler B, Joch H, Grunenfelder J, Zund G, Tanner FC, Luscher TF. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2006;48:286–292. doi: 10.1097/01.fjc.0000248233.22570.8b. doi:10.1097/01.fjc.0000248233.22570.8b. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RS, Edelman ER, Carter A, Chronos N, Rogers C, Robinson KA, Waksman R, Weinberger J, Wilensky RL, Jensen DN, Zuckerman BD, Virmani R. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation. 2002;106:1867–1873. doi: 10.1161/01.cir.0000033485.20594.6f. doi:10.1161/01.CIR.0000033485.20594.6F. [DOI] [PubMed] [Google Scholar]