Abstract
OBJECTIVES
To describe retention by age and visit type (clinic, home, phone) and to determine characteristics associated with visit types for a longitudinal epidemiologic study in older adults.
DESIGN, SETTING, PARTICIPANTS
Cardiovascular Health Study (CHS) participants (N=5888; aged 65–100 years at 1989–90 or 1992–93 enrollment; 58% women; 16% black) were contacted every 6 months, with annual assessments through 1999 and in 2005–06 for the All Stars Study visit of the CHS cohort (aged 77–102 years; 67% women; 17% black).
INTERVENTION
MEASUREMENTS
All annual contacts through 1999 (N=43,772) and for the 2005–06 visit (N=1942).
RESULTS
From 1989–99, CHS had 79% clinic, 4% home, 10% phone, and 2% other visits. In 2005–06, the All Stars participants of the CHS cohort had 36.6% clinic, 22.3% home, and 41.1% phone visits. Compared to 65–69 year olds, the odds (95% CI) of not attending a CHS clinic visit were 1.82 (1.54–2.13), 2.94 (2.45–3.57), 4.55 (3.70–5.56) and 9.09 (CI: 7.69–11.11) for ages 70–74, 75–79, 80–84 and 85+ years, respectively in sex-adjusted regression. In multivariable regression, participants with a 2005–06 clinic visit were younger, more likely men, in good health, with better cognitive and physical function 7 years prior compared to participants with other visit types. Participants with home, phone and missing visits were similar on characteristics measured 7 years prior.
CONCLUSIONS
Offering home, phone and proxy visits are essential to optimize follow-up of aging cohorts. Home visits increased in-person retention from 37% to 59% and diversified the cohort with respect to age, health and physical functioning.
Keywords: epidemiology, retention, aging, longitudinal cohort
INTRODUCTION
Adults >80 years, are the fastest growing population strata and crucial to study with respect to risk factors and health outcomes. Retention is defined as retaining surviving participants enrolled at baseline for subsequent assessments in a longitudinal cohort study. Overall follow-up in established epidemiologic cohorts is often reported as >90%,1,2,3 cohorts have not published retention by age and visit type. Longitudinal cohort studies cite 70–80% attendance at a clinic visit within the first five years from baseline,1,2,4,5,6 with lower attendance in later years.3 Loss to follow-up for in-person visits is common and largely due to dementia, disability or end stage disease.1,3,7 In the oldest adults, non-participation for in-person exams has also been related to depressive symptoms.8
Retention at repeated in-person visits is important since change for many physiologic indicators is accelerated in the oldest adults and key preventable risk factor associations can be identified. For example, accelerated bone loss at the oldest ages is associated with risk factors of diabetes and weight loss.2,4,6,9 Accelerated decline with age was recently observed for grip strength, gait speed and cognitive processing speed in the 2005–06 follow-up of the Cardiovascular Health Study cohort and participants with cognitive and physical impairments had greater changes in these measures compared to functionally intact participants.10 The magnitude of changes in physical function, body composition,2 strength,1,11 and cognitive function12 are most likely to be affected by potential effects of retention bias, though it has also been observed for lung function and brain white matter grade.7,13 This bias tends to be isolated to the sickest individuals or those with the worst starting values and therefore, retaining these participants may be essential for interpreting physiologic change in the oldest adults.
We evaluated whether older age was associated with returning to a clinic visit in the Cardiovascular Health Study, a longitudinal epidemiologic study. We then determined the extent to which age, in addition to other risk factors measured in 1998–99, differed by the type of visit in 2005–06. We hypothesized that the type of visit would be related to key demographic, lifestyle, health and function characteristics and that the oldest aged participants would have the poorest retention for in-person visits, particularly clinic visits.
METHODS
Study participants
The CHS is a prospective, multicenter, cohort study of risk factors for cardiovascular disease as previously described in detail.14,15 Based on sampling from Medicare eligibility lists, non-institutionalized, ambulatory men and women age 65 and older (mean enrollment age: 73 years, range: 65–100; 58%; 16% black) were enrolled, including 5201 at 4 US field centers (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania) in 1989–90 and an additional 687 African-American participants in 1992–93 at 3 of the 4 sites. Each center’s institutional review committee approved the study and participants gave informed consent prior to exams. Participants underwent an extensive baseline evaluation, including standardized clinical examinations, laboratory assessments, physical and cognitive functioning, and medical history, components of which were repeated at annual clinic visits through 1998–99. All annual contacts with surviving participants from 1989–1999 (N=43,772) were included. In 2005–06, the entire surviving CHS cohort was re-recruited to reevaluate physical and cognitive functioning for the CHS All Stars exam, an ancillary study to reassess functional status (median age: 85, range 77–102; 67% women; 17% black).
Visit types
Annual visit types from 1989–1999 included clinic, home, phone, or other types (e.g., nursing home visit, self-completed mailed forms). In 2005–06, the clinic and home visits were identical in-person physiologic assessments and questionnaires, in contrast to prior years, when the home visit was abbreviated. The exam included psychosocial (health, depression) and medical factors (medications, medical history, hospitalizations), blood pressure, anthropometry, physical activity, physical function, cognitive function and laboratory assays, also collected at previous exams.14,10 The phone visit was limited to questionnaires on medications, functioning, and health status. Split visits were defined as those in which data were collected by two different visit types in order to maximize the amount of data collected and were classified by the highest level contact (e.g., clinic exam with questionnaires collected by phone was classified as clinic visit). The type of visit was determined foremost by the preference of the participant though may have been ultimately determined conditionally (e.g., a phone visit if the participant did not ever attend a clinic visit). Proxy visits, with a proxy specified by the participant, could occur within any visit type.
Demographics, health and functional characteristics, 1998–99 and 2005–06
Demographic and lifestyle characteristics, including education, weight and current smoking, and self-reported health (excellent/very good/good/fair/poor) were collected by questionnaire. Physical function was assessed by activities of daily living (ADL) difficulty (defined as difficulty with transferring, bathing, dressing, eating, or toileting), instrumental activities of daily living (IADL) difficulty (defined as difficulty with heavy housework, light housework, shopping, preparing meals, paying bills, or using the phone), time in seconds to walk 15 feet, and timed chair stands. Cognitive function was assessed with the modified mini-mental status examination (3MSE),16 Digit Symbol Substitution Test (DSST),17 a Telephone Interview for Cognitive Status (TICS)18 and a modified Center for the Study of Depression (CES-D) scale.19 Clinical cardiovascular disease (CVD) measures included coronary heart disease (CHD: angina or myocardial infarction), congestive heart failure, claudication, and stroke, self-reported and adjudicated as previously described.20,21
Statistical analyses
Visit types were described by age group and by visit year. A sex-adjusted generalized estimating equation model was utilized to ascertain the association of age with participation in a clinic visit (yes/no) in order to adjust the standard errors of the estimates to account for intra-person correlation. Separate models were developed for age as a categorical or continuous variable and an interaction by sex was tested in both models.
Participant characteristics at the last prior clinic visit in 1998–99 and in 2005–06 were compared across visit types in 2005–06 using analysis of variance and Chi-Square tests. Pairwise comparisons were adjusted for multiple comparisons using a Bonferroni correction. Multinomial logistic regression for visit type in 2005–06 by characteristics in 1998–99 was performed with the phone visit as the reference group. Variables considered were age, sex, race, clinic site, weight, current smoking, high school graduate, low cognition (3MSE<80), ADL difficulty, self-reported health, and CVD. If the 3MSE was not obtained, it was estimated from the TICS or Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE),22 as previously described.12
RESULTS
CHS had 43,772 total participant contacts from 1989–99, including 34,582 visits in the clinic (79%), 1,811 at home (4%), 4,401 by phone (10%), and 740 other types (2%). Participant refusals accounted for 2,238 contacts (5%). Of the 4182 individuals alive in 1998–99, 1942 (46.4%) participated in the re-examination of the cohort in 2005–06 with visits including 710 (36.6%) at the clinic, 433 (22.3%) at the home, and 534 (41.1%) by phone. At the 2005–06 exam, 1901/4182 had died since 1998–99 (45.5%) and 339 (8.1 %) were alive but did not give consent for the 2005–06 re-examination, although 265 of these 339 individuals had a more limited phone follow-up for CHS outcomes and were classified as phone visits. The participants in the 2005–06 visit had a somewhat better baseline health status than those who were alive but did not participate, but were generally representative of the surviving cohort.10 Proxies occurred within each visit type, accounting for 14.5% of visits in 2005–06, including 9 proxy visits in clinic; 5 in the home; and 268 by phone.
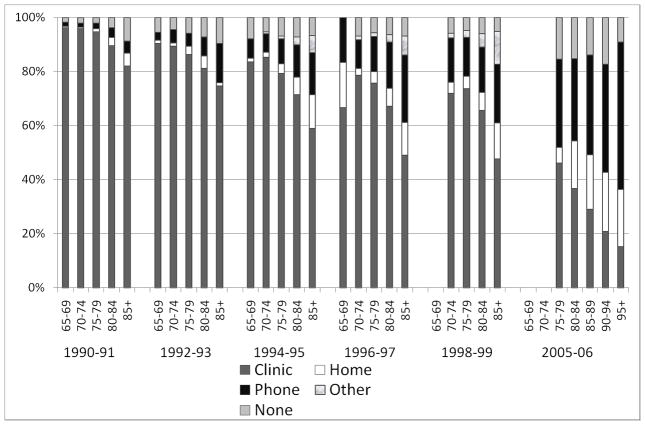
Figure 1 indicates the visit types by age group and by visit year for selected follow-up contacts between 1990–1999 and also for the 2005–06 re-examination of the cohort. The age groups 80 to 84 and 85+ years, had fewer clinic visits and more home and phone visits, compared to those <75 years. These age differences were apparent at each study year, though over time the age effect increased. In-person visits, at either the clinic or the home, for the oldest ages groups were approximately 60% of the overall contacts for the later years of the follow-up period. Overall retention for the 2005–06 in-person visit was lower than for the follow-up in 1998–99 and strongly affected by the larger number of oldest participants. In 2005–06, those aged 75–79 years were more likely to return for a clinic visit (46%) than those aged 80–84 (37%), 85–89 (29%), 90–94 (21%), and 95+ years (15%) (overall p<0.001). Self-reported data, defined as data collected by questionnaire at any visit, were obtained for >80% of participants across all age groups.
Figure 1.
CHS visit type by age and year
Abbreviations: Cardiovascular Health Study, CHS.
Using the age group 65–69 years as the reference group, the odds of failing to attend an annual clinic visit between 1989 and 1999 increased with each increasing 5 year age group. Sex-adjusted odds ratios (95% confidence intervals) were 1.82 (1.54–2.13), 2.94 (2.45–3.57), 4.55 (3.70–5.56) and 9.09 (7.69–11.11) for age groups of 70–74, 75–79, 80–84, and 85+, respectively. When age was entered continuously, a significant linear trend for age was present with no quadratic effect. There was no interaction by sex for either the categorical age variable, (p=0.61) or the continuous age variable (p=0.29).
Demographic, lifestyle, health and function characteristics in 1998–99 are shown in Table 1A, by visit type in 2005–06. Participants with clinic visit were younger; more likely white, men, and high school graduates; and had better self-reported health and cognitive functioning (lower CES-D, fewer scores <80 on the 3MSE, higher DSST) 7 years earlier compared to other visit types. Participants with clinic visit in 2005–06 had better physical functioning (less ADL and IADL difficulty, faster gait speed) 7 years earlier compared to other visit types. Physical function 7 years earlier did not differ between home, phone or missing visit types, but other characteristics did. Participants with home visits had intermediately better characteristics, particularly for cognitive functioning, between those with clinic visits and those with phone or missing a visit. Black participants were less likely to have home visits compared to other visit types. Health and cognitive function 7 years earlier were similar for phone visits and those missing visits. Cardiovascular disease (CVD) did not differ across visits though those with clinic visits had less CVD than those missing a visit. These trends were consistent for risk factor data assessed in 2005–06 (Table 1B), with one difference. Participants with home visits did not differ from those who had a phone visit for impaired cognition.
Table 1, A.
Descriptive characteristics in 1998–99 by type of study visit in 2005–06 (N=2281).
| Type of study visit in 2005–06 | ||||||
|---|---|---|---|---|---|---|
| Descriptive characteristics: 1998–99 | Clinic (a) (N=710) | Home (b) (N=433) | Phone (c) (N=799) | None (d) (N=339) | Overall P-value | Significant pairs |
| Age, years | 78.0 + 3.5 | 79.2 + 3.8 | 79.2 + 4.2 | 78.7 + | <0.0001 | ab, ac |
| Black race, % | 18.9 | 12.2 | 19.4 | 23.0 | 0.001 | ab, bc, bd |
| Male sex, % | 39.6 | 30.0 | 28.4 | 34.5 | <0.001 | ab, ac |
| Good to excellent health, % | 86.8 | 76.1 | 76.9 | 70.8 | <0.001 | ab, ac, ad |
| High School graduate, % | 85.1 | 75.3 | 73.0 | 67.4 | <0.001 | ab, ac, ad |
| Current smoker, % | 4.6 | 6.9 | 6.8 | 6.4 | 0.29 | none |
| Weight, lbs | 162 + 30 | 158 + 31 | 159 + 31 | 159 + 32 | 0.12 | none |
| Mental and cognitive function | ||||||
| CES-D score* | 4.5±4.4 | 5.3±4.1 | 5.2±4.7 | 5.8±5.2 | 0.0001 | ab, ac, ad |
| 3 MSE <80, % | 2.0 | 5.0 | 9.3 | 15.8 | <0.001 | ab, ac, ad, bd |
| 3 MSE score | 94.8 + 5.6 | 93.4 + 6.5 | 91.3 + 9.6 | 89.2 + 10.4 | <0.0001 | all |
| DSST score | 44.3 + 12.3 | 42.0 + 13.1 | 38.5 + 13.2 | 36.1 + 11.4 | <0.001 | ab, ac, ad, bc, bd |
| Physical function | ||||||
| ADL difficulty, % | 13.0 | 22.9 | 23.3 | 22.3 | <0.001 | ab, ac, ad |
| IADL difficulty, % | 21.4 | 35.2 | 35.3 | 30.3 | <0.001 | ab, ac, ad |
| Gait speed (ft/sec) | 3.1 + 0.7 | 2.9 + 0.8 | 2.9 + 0.8 | 2.8 + 0.8 | <0.0001 | ab, ac, ad |
| Unable to do timed chair stands | 3.1 | 6.6 | 6.0 | 8.0 | 0.02 | none |
| Cardiovascular disease | ||||||
| CHD, % | 20.0 | 22.9 | 23.1 | 28.3 | 0.03 | ad |
| Stroke, % | 3.1 | 5.3 | 4.6 | 8.3 | 0.003 | ad |
| Any CVD, % | 26.1 | 31.2 | 29.5 | 35.7 | 0.01 | ad |
CES-D score tested using a square-root transformation
Abbreviations: Center for Epidemiological Studies Depression Scale, CES-D; Modified Mini-Mental State Examination, 3 MSE; Digit Symbol Substitution Test, DSST; Activities of Daily Living, ADL; Instrumental Activities of Daily Living, IADL; Coronary Heart Disease, CHD; Cardiovascular Disease, CVD.
Table 1, B.
Descriptive characteristics in 2005–2006 by type of study visit in 2005–06 (N=2281).
| Type of study visit in 2005–06 | ||||||
|---|---|---|---|---|---|---|
| Descriptive characteristics: 2005–06 | Clinic (a) (N=710) | Home (b) (N=433) | Phone (c) (N=799) | None (d) (N=339) | Overall P-value | Significant pairs |
| Age, years | 84.9±3.5 | 86.2±3.7 | 86.2±4.2 | 85.7±3.9 | <0.001 | ab, ac, ad |
| Good health, % | 81.3 | 70.2 | 59.3 | - | <0.001 | ab, ac, bc |
| ADL difficulty, % | 13.0 | 22.9 | 23.3 | - | <0.001 | ab, ac |
| Low cognition (3MSE<80 or TICS<25), % | 12.0 | 26.4 | 25.3 | - | <0.001 | ab, ac |
Activities of Daily Living, ADL; Modified Mini-Mental State Examination, 3MSE; Telephone Interview for Cognitive Status, TICS.
In multivariable analyses, participants with a clinic visit in 2005–06 had significant differences in demographics and health characteristics 7 years earlier compared to those with a phone visit (Table 2). Analyses were adjusted for clinic site since the visit types differed significantly across the four sites. Participants with a clinic visit were younger, in better overall health, had less cognitive or ADL difficulty and were more likely male compared with those with a phone visit. Participants with a home visit were similar on all measures to those with a phone visit, but were older, and more likely to report poor health and difficulty with ADLs than those who had a clinic visit. Participants missing a visit were similar on all prior measures to those with a phone visit, but were older and more likely to have poor health and poor cognition compared to those with a clinic visit. In general, participants with home visits, phone visits and missing a visit were similar on characteristics measured 7 years prior.
Table 2.
Odds ratio (OR, 99% Confidence intervals)† of a 2005–06 clinic visit, a home visit, or no visit compared to a phone visit (reference) by 1998–99 characteristics.
| 1998–1999 | Clinic Visit | Home Visit | No Visit | p-value |
|---|---|---|---|---|
| Age, years | 0.92 (0.89–0.96) | 1.00* (0.96–1.04) | 0.99* (0.94–1.05) | <0.001 |
| Male sex | 1.45 (1.06–1.98) | 1.03 (0.71–1.48) | 1.47 (0.92–2.34) | <0.01 |
| Good Health | 1.54 (1.02–2.33) | 0.81* (0.53–1.22) | 0.71* (0.42–1.20) | <0.001 |
| 3MSE<80 | 0.27 (0.12–0.61) | 0.67 (0.33–1.34) | 1.52* (0.76–3.03) | >0.01 ns |
| Any ADL difficulty | 0.56 (0.37–0.84) | 0.98* (0.65–1.49) | 0.83 (0.47–1.47) | <0.01 |
p<0.01 pairwise comparison with clinic visit
All variables retained at p<0.10. Model additionally adjusted for clinic site (p<0.001).
Abbreviations: Modified Mini-Mental State Examination, 3MSE; Activities of Daily Living, ADL.
DISCUSSION
Our results in CHS clearly illustrate that retention over a very long follow-up period becomes increasingly challenging at advanced ages. The oldest old (>80 years) adults have the poorest retention compared to other age groups of older adults - particularly for in-person visits. Loss to follow-up is expected in the oldest age group; however retaining these oldest adults for longitudinal studies is critical, as they experience dynamic changes in risk factors and health outcomes. With respect to health status and functioning, our data suggest that one way to diversify the sample returning for physiologic data collection at follow-up is to offer a home visit. A home visit may make results more generalizable to the entire population of community-dwelling older adults. Importantly, self-reported data from questionnaires at the clinic, home or phone visits remained high - at approximately 80% - even in the oldest adults through eighteen years follow-up. Inclusion of a self-reported outcome that is collected by a phone visit in longitudinal studies will increase retention substantially and allow those in poorer health status with more function difficulties to participate. Requiring proxy contacts for participants in longitudinal studies is important, since 15% of visits in our oldest old cohort in 2005–06 were completed by proxies.
Differences in retention by age were more dramatic with study progression, consistent with a healthy participant bias in early years of the study. Our results suggest that the difficulty of obtaining clinic visits in the oldest old is likely due to their poorer health along with greater functional impairments and disability. Even with an option of a home visit, 60% participation at an in-person visit in a longitudinal cohort of the oldest old is likely the reality for later years of the follow-up. The Study of Osteoporotic Fractures had 76.8% participation for an in-person visit, but a younger mean age at enrollment, only white women participants vs. our more diverse cohort, and follow-up at 15 years from baseline vs. our 18 years.3
Previously, epidemiologic cohorts with repeated exams have not published retention of participants by age and visit type. The immense number of contacts over the 18 year follow-up was a strength of our analyses. CHS established methods to maximize retention by providing frequent phone contacts (every 6 months), alternative home and nursing home visits, and pre-identification of proxy contacts. Limitations to our results included use of risk factor data from seven years prior in the analyses for visit types. Although CHS had always included abbreviated home visits, the home visit was identical to the clinic visit in 2005–06.
This eighteen year epidemiologic cohort study shows that the oldest old adults >80 years of age have a very high likelihood of missing clinic visits, with more home and phone visits compared to other age groups of older adults. This was offset somewhat by >80 retention for self-reported questionnaire data. To optimize follow-up for critical longitudinal data characterizing the rapid changes in these oldest adults, essential information needs to be collected in a phone visit with option of a proxy completion of the interview.12 Since key characteristics at an earlier time in the study are related to the type of visit longitudinally, individuals with certain characteristics could be targeted for additional recruitment efforts (e.g., offering a home visit) for the collection of in-person physiologic data. The option of a home visit increased retention for in-person visits for physiologic assessments from 37% to 59% for those >80 years of age and diversified the cohort with respect to age, overall health status and physical function characteristics.
Acknowledgments
This work was funded by the National Heart, Lung, and Blood Institute (N01 HC-35129, N01 HC-45133, N01 HC-75150, N01 HC-85079 through N01 HC-85086, N01 HC-15103, N01 HC-55222, U01 HL-080295 and R01 HL-075366) with additional contribution from the National Institute of Neurological Disorders and Stroke, the National Institute on Aging (R01 AG-023629, R01 AG-15928, R01 AG-20098, and R01 AG-027058), the Intramural Research Program of the NIH, National Institute on Aging, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG-024827).
The authors express their gratitude to the CHS and All Stars participants and the staff, and Ms. Michelle Utz-Kiley for assistance with manuscript preparation.
This work was supported by the National Institute on Aging R01-AG-023629. The CHS was supported from contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke and the Intramural Research Program of the NIH, National Institute on Aging. Additional support was provided through R01-AG-15928, R01-AG-20098, and R01-AG-027058 from the National Institute on Aging, HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827.
Sponsor’s Role:
The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST DISCLOSURE: The authors report no conflict of interest.
Conflict of Interest Checklist: Below is the table for all authors to complete and attach to their papers during submission.
| Elements of Financial/Personal Conflicts | *Author 1 | Author 2 | Author 3 | Author 4 | Author 5 | Author 6 | Author 7 | Author 8 | ||||||||
| ESS | AMA | RMB | DGI | MC | JAR | TBH | ABN | |||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | ||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | ||||||||
| Honoraria | X | X | X | X | X | X | X | X | ||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | ||||||||
| Consultant | X | X | X | X | X | X | X | X | ||||||||
| Stocks | X | X | X | X | X | X | X | X | ||||||||
| Royalties | X | X | X | X | X | X | X | X | ||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | ||||||||
| Board Member | X | X | X | X | X | X | X | X | ||||||||
| Patents | X | X | X | X | X | X | X | X | ||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | ||||||||
*Authors can be listed by abbreviations of their names; Yes = written explanation; No = Check (✓) mark
For “yes”, provide a brief explanation:__________
Author Contributions:
ESS: Participated in the study concept and design, data analysis and interpretation, and preparation of the manuscript.
AMA: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript.
RMB: Participated in the data analysis and interpretation, and preparation of the manuscript.
DGI: Participated in the study concept and design, acquisition of subjects and data, data analysis and interpretation, and preparation of the manuscript.
MC: Participated in the data analysis and interpretation, and preparation of the manuscript.
JAR: Participated in the acquisition of subjects and data, data analysis and interpretation, and preparation of the manuscript.
TBH: Participated in the data analysis and interpretation, and preparation of the manuscript.
ABN: Participated in the study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
References
- 1.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61A:M1059–M1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005;20:596–603. doi: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Hochberg MC, Lui L-Y, et al. Long-term risk of incident vertebral fractures. JAMA. 2007;298:2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Fullman RL, Barett-Connor E, et al. Voluntary weight reduction in older men increases hip bone loss: The Osteoporotic Fractures in Men Study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Arnold AM, Psaty BM, et al. 10-Year Follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the Cardiovascular Health Study. Arch Intern Med. 2006;166:71–78. doi: 10.1001/archinte.166.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Lui L-Y, Stone KL, et al. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American Women. J Am Geriatr Soc. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 7.Griffith KA, Sherrill DL, Siegel EM, et al. Predictors of loss of lung function in the elderly: The Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163:61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 8.Bootsma-van der Wiel A, van Exel E, de Craen AJM, et al. A high response is not essential to prevent selection bias: Results from the Leiden 85-plus study. J Clin Epidemiol. 2002;55:1119–1125. doi: 10.1016/s0895-4356(02)00505-x. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Ewing SK, Stone KL, et al. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Arnold AM, Sachs MC, et al. Long term function in an older cohort - The CHS All Stars Study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. NIHMS ID# 102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of strength and poor muscle quality in older adults with type 2 diabetes. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 12.Arnold AM, Newman AB, Dermond N, et al. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline: The Cardiovascular Health Study. Neuroepidemiology. 2009;33:55–65. doi: 10.1159/000215830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 16.Teng EL, Chui HC. Modified mini mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 17.Salthouse PA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol. 1978;33:232–238. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- 18.Gallo JJ, Breitner JCS. Alzheimer’s disease in the NAS-NRC Registry of Ageing Twin Veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer’s dementia. Psychol Med. 1995;25:1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 19.Orme J, Reis J, Herz E. Factorial and indiscriminate validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 22.Jorme AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psych Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]