Abstract
Fibronectin fragments (FN-f) that bind to the α5β1 integrin stimulate chondrocyte-mediated cartilage destruction and could play an important role in the progression of arthritis. The objective of this study was to identify potential cytokine mediators of cartilage inflammation and destruction induced by FN-f and to investigate the mechanism of their stimulation. Human articular chondrocytes, isolated from normal ankle cartilage obtained from tissue donors, were treated with a 110-kDa FN-f in serum-free culture, and expression of various cytokine genes was analyzed by cDNA microarray and by a cytokine protein array. Compared with untreated control cultures, stimulation by FN-f resulted in a >2-fold increase in IL-6, IL-8, MCP-1, and growth-related oncogene β (GRO-β). Constitutive and FN-f-inducible expression of GRO-α and GRO-γ were also noted by RT-PCR and confirmed by immunoblotting. Previous reports of IL-1β expression induced by FN-f were also confirmed, while TNF expression was found to be very low. Inhibitor studies revealed that FN-f-induced stimulation of chondrocyte chemokine expression was dependent on NF-κB activity, but independent of IL-1 autocrine signaling. The ability of FN-f to stimulate chondrocyte expression of multiple proinflammatory cytokines and chemokines suggests that damage to the cartilage matrix is capable of inducing a proinflammatory state responsible for further progressive matrix destruction, which also includes the chemoattraction of inflammatory cells. Targeting the signaling pathways activated by FN-f may be an effective means of inhibiting production of multiple mediators of cartilage destruction.
Fibronectin is an extracellular matrix, as well as a circulating protein, that is readily degraded into fragments (FN-f)3 by many different types of proteases. Synovial fluid, as well as the cartilage matrix, of patients with osteoarthritis (OA) and rheumatoid arthritis (RA) contain high levels of FN-f (1–3). FN-f stimulate matrix metalloproteinase (MMP) expression by articular chondrocytes (4–6) as well as synovial fibroblasts (7) and suppress synthesis of cartilage proteoglycan (8), suggesting that FN-f could play a role in the progressive matrix destruction in arthritis.
The signals generated by the 110-kDa FN-f, which contains the Arg-Gly-Asp integrin-binding sequence, are transmitted through the α5β1 integrin receptor (7, 9). Previous studies have shown that FN-f stimulation of the chondrocyte α5β1 integrin results in the activation of a signaling cascade, which includes protein kinase C; proline-rich tyrosine kinase-2; the MAPKs ERK, JNK, and p38; and a subsequent increase in the activity of AP-1 and NF-κB transcription factors (10, 11). Activation of these signaling pathways was shown to be responsible for an increase in expression of MMP-13 (collagenase-3) (6, 10). MMP-13 is a potent degrader of type II collagen, is found in articular cartilage, and may be responsible for collagen degradation in arthritis (12, 13). Furthermore, MMP-13, as well as MMP-3, which is also increased in response to FN-f (7, 14), can degrade fibronectin-producing additional FN-f, resulting in a vicious cycle of progressive matrix destruction, which has been referred to as chondrocytic chondrolysis (4).
Stimulation of chondrocytes with FN-f or with α5β1-activating Abs has also been shown to stimulate production of NO and the proinflammatory cytokines TNF-α, IL-1α, IL-1β, IL-6, and IL-8 (5, 15, 16). These inflammatory mediators, which are present in arthritic cartilage, could also contribute to cartilage destruction through stimulation of MMP expression as well as by their ability to inhibit synthesis of cartilage matrix proteins, including collagen and proteoglycan (reviewed in Ref. 17). Thus, the effects of FN-f on matrix degradation can be both direct, through activation of cell signaling pathways that stimulate MMP expression, and/or indirect through up-regulation of cytokines that act on chondrocytes in an autocrine or paracrine fashion.
The objective of the present study was to identify additional, potentially novel, cytokine mediators of cartilage inflammation and destruction induced by FN-f stimulation of human articular chondrocytes. In a previous study (6), we noted that MMP-13 expression stimulated by FN-f was only partially inhibited by blocking IL-1 activity with the IL-1 receptor antagonist (IL-1ra), indicating either direct activation of MMP-13 expression by integrin-mediated signaling or the involvement of additional cytokines. It was also of interest to determine whether FN-f stimulated expression of factors that could serve as chemoattractants for leukocytes and/or fibroblasts. In the development of RA, and to a lesser extent in OA, cells from the synovium that migrate to the articular cartilage are thought to participate in destruction of the cartilage matrix, but the mechanism responsible for their attraction to cartilage is incompletely understood. Based on initial results that identified FN-f-stimulated expression of several cytokines and chemokines, further studies were performed to determine whether activation of NF-κB could be a common mediator for their expression.
Materials and Methods
Chondrocyte isolation and culture
Normal human ankle (talar) cartilage was obtained from tissue donors (n = 28) through the Gift of Hope Organ and Tissue Donor Network. Donors had no known history of arthritis, and the joint tissues were graded on a scale of 0–4, as previously described (18), with only tissue of grades 0–1 used for chondrocyte isolation. Chondrocytes were isolated by enzymatic digestion using pronase and collagenase, as previously described (18). Cells were routinely plated in high density monolayer culture in DMEMF12 containing 10% FBS and antibiotics (Invitrogen Life Technologies). For some experiments, chondrocytes were cultured in suspension in alginate, as previously described (18), to test the response to FN-f in a three-dimensional culture system that has been found to better maintain the differentiated chondrocyte phenotype. All experiments were performed using primary cells cultured between 5 and 7 days. Due to the limited number of cells available from individual donors, cells from different donors were used for gene array, protein array, RT-PCR, and ELISA experiments. Cultures were switched to serum-free medium overnight before treatment with FN-f or cytokines.
Chondrocyte stimulation with FN-f or cytokines
Confluent cultures in serum-free medium were treated for 6 or 16 h with 0.5 µM 110-kDa FN-f (Upstate Biotechnology), 2 ng/ml IL-1β (R&D Systems), or 10 ng/ml TNF-α (PeproTech). For cells cultured in alginate beads, 10 beads in 0.5 ml of medium in 24-well plates were treated overnight with FN-f. Inhibition of IL-1β was performed using 100 ng/ml IL-1ra (Anakinra, gift from Amgen) added at the time of FN-f. Inhibition of NF-κB was performed using 50 µM hypoestoxide (bicycl(9,3,1)pentadecane; Calbiochem) added 30 min before FN-f. MAPK inhibition was by a 30-min preincubation with 20 µM SB203580 for p38 (Sigma-Aldrich), 25 µMPD98059 for MAP/ERK kinase MEK (Calbiochem), and 20 µM SP600125 for JNK (Calbiochem). Cell survival was determined using the live/dead cell viability assay kit (Molecular Probes), as previously described (18), and apoptosis was assessed by fragmentation analysis of isolated genomic DNA.
Chondrocyte transfection
In selected experiments, inhibition of NF-κB was performed by overexpression of an IκBα mutant (pCMV-IκBαM; BD Clontech). For this, chondrocytes were transfected using the Nucleofection method (Human Chondrocyte Nucleofector Kit; Amaxa). To maximize the transient transfection efficiency and at the same time minimize cell death occurring during the Nucleofection procedure, we modified the manufacturer’s instructions. Briefly, human articular chondrocytes in monolayer were harvested by incubating the cells with collagenase P and pronase solution at a concentration of 1 mg/ml each for 5~10 min at 37°C. After collagenase/pronase treatment, the cells were washed in cold PBS three times, and the washed cells were resuspended in Human Chondrocyte Nucleofector Solution that was prewarmed to room temperature to a final concentration of 2 × 106 cells/100 µl. The cell-Nucleofector solution complex (100 µl) and the plasmid construct DNA (2.5 µg) were then transferred into a cuvette and gently mixed, followed by Nucleofection, using the Nucleofector program setting H20. After stimulation, 500 µl of prewarmed culture medium, containing 20% serum, was added to the cells, mixed, and then the cell suspension was transferred into poly(l-lysine) (Sigma-Aldrich; 1/3 dilution in cold PBS)-precoated six-well plates. The cells were then placed in an incubator, and after 24 h replenished with fresh culture medium containing 10% serum, followed by further incubation for 24 h. The cells were then switched to serum-free conditions for stimulation by FN-f, as described above.
RNA and genomic DNA extraction
Total cellular RNA and genomic DNA were isolated using the RNeasy Mini kit and DNeasy Tissue kit (Qiagen), respectively, according to the manufacturer’s instructions. All samples were stored at −80°C until analyzed.
Gene array experiments and analysis
A cytokine/receptor array was obtained from BD Clontech and used according to manufacturer’s instructions with minor changes. Briefly, 10 µg of total RNA was used for reverse transcription and cDNA probe synthesis using primers supplied and [α-32P]dATP. The arrays were exposed to a Amersham Pharmacia Biotech Typhoon PhosphorImager for 3 days for image collection. The images were then analyzed using BD Clontech’s AtlasImage software. Background normalization was performed using global sum normalization, which is a method in which the values of signal over background for all genes on the arrays are added together to calculate the normalization coefficient to use between the two membranes. Background normalization was also performed using either GAPDH or 40S ribosomal protein with comparable results. The treated samples were matched to their time-matched controls. Comparisons of the two arrays were performed using two different measures: either a fold (ratiometric analysis (x/y)) or a difference (subtractive analysis (x – y)). For ratiometric analysis, initially a 2-fold change was used, but this yielded too few genes. This was then revised to a minimum 1.5-fold change for significance.
RNA from cells isolated from three additional donors was analyzed using Affymetrix arrays. For these experiments, duplicate cultures from each of three donors were treated with FN-f or control medium for 16 h, and then RNA was isolated as above. The integrity of purified RNA was assessed using the Agilent 2100 Bioanalyzer. Biotin-labeled cRNA was prepared from 10 µg of RNA and hybridized to Affymetrix U133A gene chips, according to the manufacturer’s instructions (Affymetrix). The arrays were scanned using the GeneChip 3000 laser scanner, and primary data captured using the MAS5.0 software (Affymetrix). A target intensity of 150 was used to scale the data from each individual array experiment to account for differences in global chip intensities. The .Cel files were imported into the Rosetta Resolver system (Rosetta Biosoftware). Comparison analyses and fold changes between baseline and FN-f-treated samples for each individual duplicate were computed using the Resolver error model and ratio-generating algorithm (19). Genes that showed a fold change of 2-fold or greater were retained for further interrogation.
Reverse transcription and semiquantitative PCR
Reverse transcription was conducted with 1 µg of total cellular RNA using the ThermoScript RT-PCR System (Invitrogen Life Technologies) for first strand cDNA synthesis in 25 µl of reaction volume. The sequences for the primers were as follows: growth-related oncogene-α (GRO-α), 5′-ACT CAAGAATGGGCGAAAG sense and 5′-TGGCATGTTGCAGGCTCCT antisense (468-bp PCR product); GRO-γ, 5′-AAGTGTGAATGTAAGGTCCCC sense and 5′-CTTTCCAGCTGTCCCTAGAA antisense (270-bp PCR product) (20); GRO-β, 5′-GTGGCGCTGCTGCTCCTGCT sense and 5′-CCATGGGCGATGCGGGGTTG antisense (226-bp PCR product); MCP-1, 5′-ATAGCAGCCACCTTCATTCC sense and 5′-TTTCCCCAAGTCTCTGTATCT antisense (480-bp PCR product); IL-6, 5′-GGATGCTTCCAATCTGGATTCAATGAG sense and 5′-CGCAGAATGAGATGAGTTGTCATGTCC antisense (302-bp PCR product); IL-8, 5′-CGTGGCTCTCTTGGCAGCCTTCCTGAT sense and 5′-TCAAAAACTTCTCCACAACCCTCTGCA antisense (270-bp PCR product); and GAPDH, 5′-GGTATCGTGGAAGGACTCAT sense and 5′-ACCACCTGGTGCTCAGTGTA antisense (341-bp PCR product) (11). For all experiments, conditions were determined to be in the linear range for the PCR amplification. Briefly, all the samples were subjected to reverse transcription at the same time, and subsequently, all samples of cDNA amplified by PCR at the same time to avoid any potential experiment to experiment variation in efficiency. GAPDH was used as an internal control. Levels of GAPDH mRNA did not vary with time after addition of FN-f. Genomic DNA was included for the PCR as a negative control to ensure that there was no genomic DNA contamination in the total RNA samples. The cDNA was amplified by PCR using 30 cycles of 95°C for 30 s, 57–65°C for 30 s, and 72°C for 40 s in the presence of Taq polymerase (Invitrogen Life Technologies) and 50 pmol of sense and antisense primers. Annealing temperature was 65°C for GRO-β, 60°C for GRO-γ and GRO-α, and 57°C for GAPDH and MCP-1. IL-6 and IL-8 amplification was conducted, as previously described (11). PCR products were resolved on 1.5% agarose gels and visualized by staining with ethidium bromide and UV transillumination. Integrated density values for the genes in question were normalized to the GAPDH values to yield a semiquantitative assessment.
Human cytokine Ab protein array
Serum-free conditioned medium from chondrocytes stimulated overnight with FN-f or IL-1β was collected and used for human cytokine Ab protein array with the TransSignal Raybio Human Cytokine Ab Array 1.0 (Panomics). The array membrane was pretreated according to the manufacturer’s instruction, incubated with 2 ml of 5-fold diluted conditioned medium for 2 h, washed, incubated with biotin-conjugated anticytokine mix, washed, and then developed with streptavidin-HRP conjugate and subsequent ECL (Amersham). The experiments were repeated in duplicates.
Cytokine immunoblotting and quantitative ELISA analysis
The protein concentration in the medium was quantified by the bicinchoninic acid (BCA) method (Pierce). A total of 50 µg of protein was loaded in each lane for immunoblotting. The samples were run in a 20% reduced SDS-PAGE gel, transferred to nitrocellulose, which, after blocking with 5% BSA for 2 h was incubated for 2 h with primary Abs (1/1000 dilution) for human IL-6, IL-8, total GRO and MCP-1 (R&D Systems), and GRO-β and GRO-γ (PeproTech). The expected proteins were 20.3, 8, 8, 8.7, 7.8, and 7.8 kDa, respectively. The primary Abs were detected with rabbit anti-mouse secondary Ab (1/2000 dilution) (American Qualex), followed by ECL. All experiments were repeated with cells from at least two different tissue donors.
For quantitative ELISA, 150 µl of conditioned medium in a 1/50 dilution in duplicates was used in the QuantiGlo IL-6 and IL-8 immunoassays (R&D Systems), according to the manufacturer’s instructions.
Immunoblotting for phospho-IκB kinase (IKK) and nuclear phospho-p65
After stimulation of cells with FN-f or IL-1β, as described above, cell monolayers were washed with ice-cold PBS. A total cell lysate was prepared with cell lysis buffer (Cell Signaling Technology) to which fresh phosphatase inhibitor mixture and PMSF (Sigma-Aldrich) were added. The soluble lysate protein concentration was determined with BCA reagent, and samples were analyzed by SDS-PAGE and immunoblotting, as above. Blots were probed with phospho-IKKα (Ser180)/IKKβ (Ser181) from Cell Signaling Technology and, for loading control, with a nonphosphospecific Ab to IKKα. For analysis of NF-κB translocation, cells were stimulated with Fn-f for 30 min, and then nuclear fractions were prepared using a nuclear extraction kit (Panomics) following the manufacturer’s instructions. Protein content in the nuclear fractions was measured using the BCA assay, and equal amounts were used for immunoblotting with anti-phospho-NF-κB p65 (Ser536) (Cell Signaling Technology).
Results
Fn-f induced cytokine and chemokine expression by articular chondrocytes
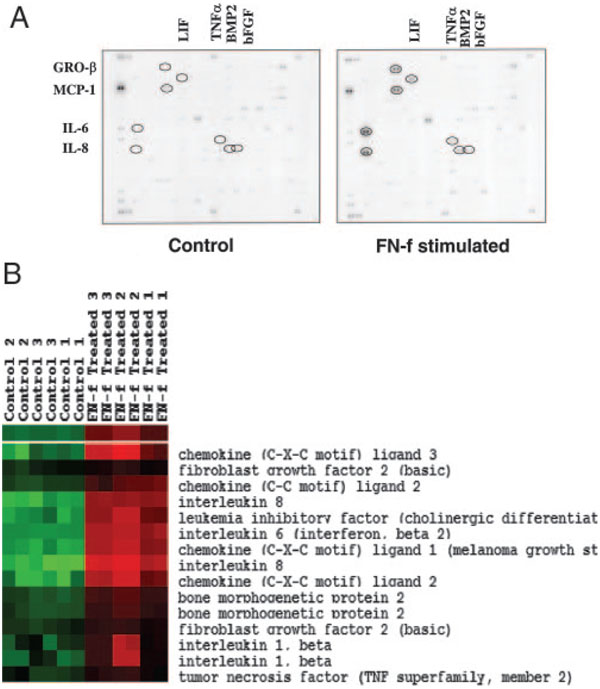
An initial screen was performed on RNA isolated from articular chondrocytes stimulated in serum-free cultures for 6 or 16 h with 0.5 µM FN-f. Compared with untreated control cultures, stimulation by FN-f resulted in up-regulation (>1.5-fold ratio FN-f/control) of 19 of 268 (7.1%) of the chemokine and cytokine genes at 6 h and 22 of 268 (8.2%) at 16 h. Significantly up-regulated genes included IL-6, IL-8, MCP-1, GRO-β, leukemia-inhibitory factor, bone morphogenic protein-2, TNF-α, and basic fibroblast growth factor genes in the order of gene expression level (Fig. 1A). Gene expression ratios were not calculated for each gene, because the expression of some of these genes was not detectable, giving rise to zero values in the unstimulated normal chondrocytes.
FIGURE 1.
FN-f-inducible cytokine and chemokine genes analyzed by cDNA microarray. A, Primary human chondrocytes were stimulated with 0.5 µM FN-f for 6 h in serum-free medium. Total RNA was reverse transcribed, and cDNA was hybridized to the BD Atlas Human Cytokine/Receptor Array containing 268 genes in duplicate. IL-6, IL-8, MCP-1, and GRO-β gene expression was increased after FN-f stimulation compared with unstimulated control chondrocytes. Leukemia-inhibitory factor (LIF), bone morphogenic protein (BMP-2), TNF-α, and basic fibroblast growth factor (bFGF) gene expression were also increased to a lesser extent. B, Cluster analysis of selected cytokine and chemokine genes from Affymetrix arrays performed with primary human chondrocytes from three additional donors stimulated with FN-f in duplicate. CXC ligand 3 = GROγ, CC ligand 2 = MCP-1, CXC ligand 1 = GROα, and CXC ligand 2 = GROβ.
Chondrocyte cultures established from three additional donors were stimulated with FN-f for 16 h, and isolated RNA was analyzed by Affymetrix array. Analysis of the cytokine and chemokine genes on the array confirmed significant up-regulation of the same cytokine and chemokine genes noted on the BD Clontech cytokine array (Fig. 1B). In addition to GRO-β, GRO-α and GRO-γ were present on the Affymetrix array and were significantly up-regulated as well.
The cytokine gene array results were then compared with results using a human cytokine protein array to screen for cytokines released into the medium during 16 h of treatment with FN-f. Of the 23 cytokine pairs on the array, the most highly produced were IL-6, IL-8, MCP-1, and GRO-β in the order of intensity (Fig. 2). Cytokines included on the array that were not detected in cultures stimulated with FN-f were G-CSF, GRO-α, IL-1α, IL-2, IL-3, IL-5, IL-7, IL-10, IL-13, IL-15, IFN-γ, MCP-2, MCP-3, monokine induced by IFN-γ, RANTES, TGF-β1, and TNF-β. Of note, IL-1β was not included on the protein membrane; however, its up-regulation by FN-f is well documented (5, 6). TNF-α was only slightly increased compared with controls. Similar results were obtained using chondrocytes isolated from three different donors. In addition, the protein array experiment was repeated using chondrocytes stimulated with FN-f when cultured in alginate beads instead of in monolayer. The same protein pattern of cytokine and chemokine expression was noted, demonstrating that the response to FN-f could be elicited by chondrocytes stimulated while in a three-dimensional culture system (data not shown).
FIGURE 2.
Cytokine protein array of FN-f-stimulated chondrocytes. Primary human chondrocytes in serum-free medium were treated with 0.5 µM FN-f for 16 h. Cytokine protein array (Raybio) was performed with the conditioned medium. IL-6, IL-8, MCP-1, and the GRO family members of chemokines were detected in the medium after FN-f stimulation compared with medium from untreated (control) chondrocytes.
Expression of the cytokines and chemokines that were identified by both gene and protein arrays was further studied using RT-PCR. Consistent with the array studies, FN-f (0.5 µM) treatment for 16 h stimulated >1.5-fold expression of IL-6, IL-8, GRO-β, and MCP-1 when compared with unstimulated controls (results not shown). Because previous studies had already documented α5β1 integrin-mediated expression of IL-6 and IL-8 (15, 16), but not GRO family members or MCP-1, additional experiments were performed to determine the ability of FN-f to stimulate expression of all three GRO family members (GRO-α, GRO-β, and GRO-γ) as well as MCP-1. FN-f stimulation was compared with IL-1β and TNF-α.
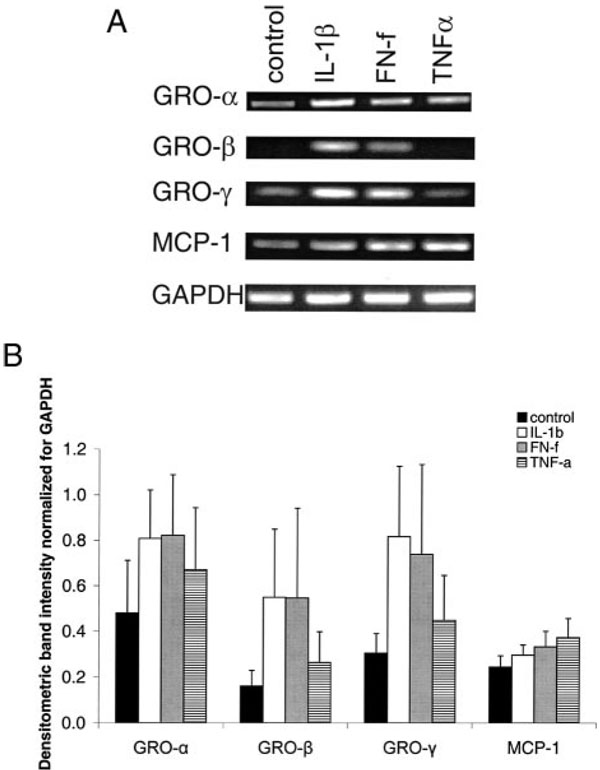
These experiments showed that in addition to GRO-β, GRO-α and GRO-γ were expressed by articular chondrocytes (Fig. 3). The GRO family members appeared to be expressed constitutively at a low level, but were increased in response to FN-f treatment as well as IL-1β, but less so with TNF-α. In response to FN-f, mRNA levels (normalized for the level of GAPDH) for GRO-α increased by 1.7-fold, GRO-β by 3.4-fold, and GRO-γ by 2.4-fold. Stimulation of chondrocytes with IL-1β (2 ng/ml) resulted in similar increases in mRNA levels for GRO family members as 0.5 µM FN-f: GRO-α increased by 1.68-fold, GRO-β by 3.4-fold, and GRO-γ by 2.7-fold. TNF-α (10 ng/ml)-stimulated expression of GRO-α, GRO-β, or GRO-γ was 1.4-, 1.6-, and 1.5-fold, respectively. MCP-1 expression was elevated to a much smaller extent in response to any of the cytokines or FN-f (Fig. 3B). Of possible significance, the SEs noted in the RT-PCR data were high because these experiments were performed using chondrocytes isolated from three donors aged 43, 55, and 73 years old, and the cells from the younger donor had a consistently lower level of basal and stimulated chemokine expression than the other two donors.
FIGURE 3.
Comparison of chemokine expression in response to proinflammatory cytokines and FN-f. A, Primary human chondrocytes were serum starved for 24 h and then were stimulated with 2 ng/ml IL-1β or 0.5 µM FN-f, or 10 ng/ml TNF-α, or were left unstimulated (control). A representative sample for RT-PCR analysis by using total RNA extracted from the cells is shown for GRO family members, MCP-1, and GAPDH. The expected RT-PCR produce sizes are shown in Materials and Methods. B, Densitometric intensity analysis of the RT-PCR experiments. Chemokine expression is illustrated as a ratio of the integrated density values for the genes in question and the integrated density of the GAPDH values to yield a semiquantitative assessment. Unstimulated control (■), IL-1β (□), FN-f ( ), and TNF-α (
), and TNF-α ( ). Data represent the means with SEs of three independent experiments from three different donors.
). Data represent the means with SEs of three independent experiments from three different donors.
FN-f-induced cytokine and chemokine expression is regulated by NF-κB, but independent of IL-1
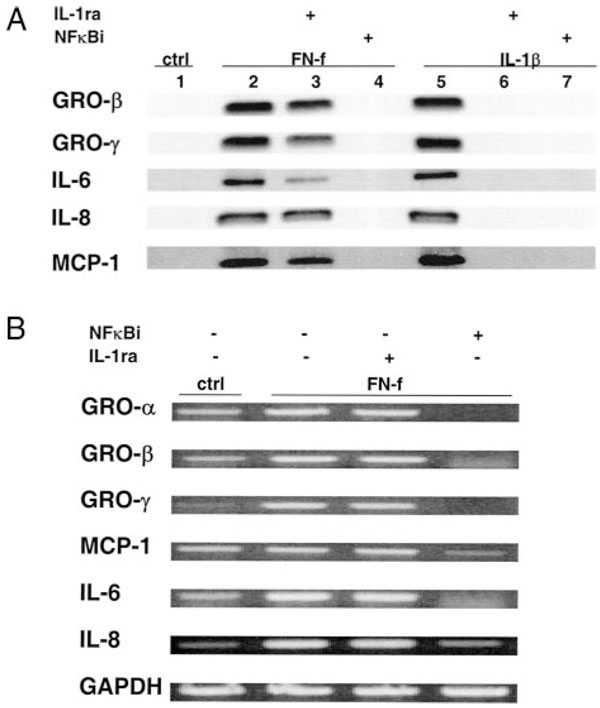
The mechanism by which FN-f stimulated articular chondrocyte cytokine and chemokine gene expression and corresponding protein secretion was further investigated. In previous studies, we had found that FN-f stimulation of chondrocytes resulted in increased expression of IL-1β and increased NF-κB activity (6, 11), either of which or both could be responsible for additional cytokine and chemokine expression. In previous experiments, we found that treatment of chondrocytes with 0.5 µM FN-f produced from 0.2 to 0.4 ng/ml IL-1β measured by ELISA (our unpublished observations). Using the cytokine protein array, pretreatment with excess IL-1ra (100 ng/ml) did not result in an appreciable decrease in cytokine or chemokine production, while treatment with hypoestoxide, which inhibits the IKK (21), significantly reduced FN-f-stimulated production to the level of the unstimulated control chondrocytes (compare Figs. 2 and 4). The concentration of the NF-κB inhibitor used for these experiments did not result in significant cell death, analyzed by the live/dead cell assay, or DNA fragmentation (results not shown).
FIGURE 4.
Effects of IL-1 and NF-κB inhibition on FN-f-induced cytokine and chemokine expression analyzed by cytokine protein array. A, After overnight incubation in serum-free medium, primary human chondrocytes were stimulated with 0.5 µM FN-f for 16 h in serum-free medium in the presence of 100 ng/ml IL-1ra or 50 µM NF-κB inhibitor hypoestoxide. Human cytokine protein array (RayBio) revealed that IL-6, IL-8, MCP-1, and the GRO expression were dramatically reduced in the presence of NF-κB inhibition, but were unchanged with IL-1ra. B, The ability of FN-f to activate IKK and the inhibitory effects of hypoestoxide were determined by immunoblotting for phospho-IKK using cell lysates from chondrocytes stimulated for 10 min in control and FN-f-containing medium.
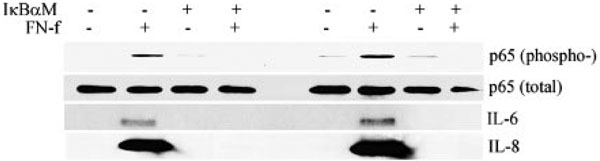
We confirmed that treatment of chondrocytes with FN-f stimulated activation of the IKK by examining IKK phosphorylation, and found that pretreatment with hypoestoxide blocked this phosphorylation (Fig. 4B). In addition, overexpression of a dominant-negative mutant of IκBα, which lacks the normal IKK phosphorylation sites, inhibited FN-f-stimulated phosphorylation of the p65 subunit of NF-κB, and this was associated with inhibition of IL-6 and IL-8 production (Fig. 5).
FIGURE 5.
Transfection of an IκBα dominant-negative expression vector blocks FN-f activation of NF-κB and inhibits IL-6 and IL-8 production. Primary human articular chondrocytes from two different donors were transfected with the dominant-negative construct pCMV-IκBαM by nucleofection, as described in Materials and Methods. Cells were stimulated with 0.5 µM FN-f for 30 min to assess phosphorylation of the p65 NF-κB subunit by immunoblotting of cell lysates with an anti-phospho-p65 Ab or nonphosphospecific (total) Ab as control. Medium collected from parallel cultures incubated for 16 h with FN-f was used for immunoblotting for IL-6 and IL-8.
Additional experiments were performed using immunoblotting and RT-PCR for analysis. IL-1ra appeared to partially inhibit FN-f-stimulated IL-6 production measured by immunoblotting, with little to no effect on GRO-β, GRO-γ, IL-8, or MCP-1, while the NF-κB inhibitor completely blocked production of all the factors studied (Fig. 6A). As a control, IL-1ra was found to inhibit IL-1β-stimulated production of all of the cytokines and chemokines studied. IL-1β-induced chemokine and cytokine production was similarly reduced in the presence of hypoestoxide, demonstrating a similar requirement for NF-κB activation for IL-1β and FN-f (Fig. 6A). Similar results were obtained using chondrocytes cultured in alginate (data not shown) or when cytokine expression was analyzed by RT-PCR (Fig. 6B).
FIGURE 6.
Effects of IL-1 and NF-κB inhibition on FN-f- and IL-1β-stimulated cytokine expression. A, Primary human chondrocytes were serum starved overnight and then were treated for 16 h with FN-f (0.5 µM) or IL-1β (2 ng/ml) in the presence of either IL-1ra (100 ng/ml) or the NF-κB inhibitor hypoestoxide (50 µM) in serum-free medium. Samples of conditioned medium were used for immunoblotting with the indicated primary Abs. B, Chondrocytes were treated with FN-f and the inhibitors, as described in A, and RNA was isolated and used for RT-PCR with the indicated primers.
Fn-f stimulation of IL-6 and IL-8 was also measured by ELISA in cells cultured in either monolayer or alginate (Table I). Both cytokines were present at very low levels in control cultures, while 16 h of treatment with FN-f resulted in levels of IL-6 of 299 ± 11 ng/ml and IL-8 of 172 ± 2.1 ng/ml in monolayer and 89.4 ± 0.4 ng/ml and 91.2 ± 2.3 ng/ml, respectively, in alginate. The lower cytokine levels in alginate cultures are most likely due to the lower cell numbers used in the alginate experiments (10 alginate beads/well in 0.5 ml of medium, which translates to ~8 × 105 cells/ml) compared with monolayer cultures (2 × 106 cells/ml). Consistent with the immunoblotting and RT-PCR results, NF-κB inhibition completely blocked FN-f stimulation of IL-6 and IL-8 in both monolayer and alginate cultures. Inhibition with IL-1ra resulted in a slight decrease in IL-6 levels in monolayer (~16%), which was similar to the immunoblotting and RT-PCR results. Levels of both IL-6 and IL-8 produced in response to 2 ng/ml IL-1β were somewhat higher than levels in FN-f-treated wells. As expected, the IL-1β stimulation was blocked by NF-κB inhibition and, unlike FN-f, was also completely blocked by IL-1ra.
Table 1.
Effects of NF-κB and IL-1 inhibition on IL-6 and IL-8 levels in cultured chondrocytes treated with FN-f or IL-1βa
| IL-6 (ng/ml) | IL-8 (ng/ml) | |||
|---|---|---|---|---|
| Treatment | Monolayer | Alginate | Monolayer | Alginate |
| Control | 0.4 ± 0.2 | 0.5 ± 0.03 | 0.3 ± 0.01 | 7.5 ± 0.9 |
| FN-f | 299 ± 11 | 89.4 ± 0.4 | 172 ± 2.1 | 91.2 ± 2.3 |
| FN-f + NF-κBib | 0.06 ± 2.4 | 0.16 ± 0.04 | 0.22 ± 0.05 | 3.2 ± 0.04 |
| FN-f + IL-1Ra | 250 ± 12.2 | 81.9 ± 4.6 | 165 ± 5.5 | 69.4 ± 8.1 |
| IL-1β | 380 ± 0.9 | 154 ± 7.5 | 207 ± 0.02 | 146 ± 0.86 |
| IL-1β + NF-κBi | 0.26 ± 4.6 | 0.64 ± 0.04 | 0.21 ± 0.02 | 7.7 ± 0.04 |
| IL-1β + IL-1Ra | 0.35 ± 4.3 | 1.51 ± 0.13 | 2.7 ± 0.02 | 9.96 ± 0.19 |
ELISA results shown are mean ± SD for duplicate cultures.
NF-κBi, NF-κB inhibitor.
Stimulation of the p38 and JNK MAPKs is required for Fn-f activation of NF-κB
Activation of NF-κB by FN-f is likely to occur downstream from MAPK activation, but this has not been previously documented. Therefore, chondrocytes were pretreated with standard inhibitors specific for each of the three major MAPK families, and FN-f stimulation of NF-κB was determined by measuring nuclear levels of the phosphorylated p65 subunit of NF-κB. Inhibition of p38 or JNK, but not MEK, dramatically reduced the levels of nuclear phospho-p65 (Fig. 7) and also blocked FN-f stimulation of IL-6 and IL-8 production (data not shown). Consistent with inhibition of IKK, hypoestoxide completely blocked the ability of FN-f to increase levels of nuclear phospho-p65.
FIGURE 7.
Inhibition of FN-f-stimulated NF-κB activation by MAPK and IKK inhibitors. Primary human articular chondrocytes were pretreated with 50 µM hypoestoxide (IκBi), 20 µM SB203580 (p38i), 25 µM PD98059 (MEKi), or 20 µM SP600125 (JNKi), and then stimulated with 0.5 µM FN-f. Controls included unstimulated cells (Cntl), cells stimulated with FN-f without inhibitors (−), and cells stimulated with 2 ng/ml IL-1β. Cell lysates were obtained after 30 min, and nuclear fractions were prepared, as described in Materials and Methods. Samples with equal amounts of protein were immunoblotted with Ab to the phosphorylated form of the p65 NF-κB subunit.
Discussion
In this study, FN-f, a component of the damaged extracellular matrix of arthritic cartilage, was capable of inducing production of multiple inflammatory mediators from normal human articular chondrocytes. The data provide evidence that FN-f stimulates expression of the proinflammatory cytokine IL-6; neutrophil-attracting C-X-C chemokines IL-8 (CXCL8), GRO-α (CXCL1), GRO-β (CXCL2), and GRO-γ (CXCL3); and the monocyte-attracting C-C chemokine MCP-1. Because the FN-f used in these experiments signals through the α5β1 integrin (7, 9), the results demonstrate that FN-f-stimulated integrin signaling can induce not only cytokine expression, but also expression of multiple chemokines. Other cell types may also respond to FN-f stimulation with increased chemokine expression, suggesting a novel mechanism by which damaged matrix components could be capable of initiating recruitment and activation of leukocytes at sites of tissue injury and inflammation.
Consistent with the present findings, induction of IL-6, IL-8, MCP-1, and GRO family members has been previously described after stimulation of chondrocytes with proinflammatory cytokines, including IL-1β and TNF-α, or by using LPS (22–25). Moderately increased expression of chemokines, including the C-C chemokine subfamily member RANTES, as well as IL-8 and GRO-α, has been demonstrated in chondrocytes present in OA and RA cartilage (25–28). Importantly, chemokine receptors, including CCR1, CCR2, CCR3, CCR5, CXCR1, and CXCR2, are also expressed by chondrocytes (29, 30). The highly selective IL-8R (CXCR1) may be moderately increased in OA cartilage (29, 31). Therefore, the potential exists for multiple cytokine and chemokine autocrine or paracrine loops within articular cartilage, which are stimulated during the development of arthritis.
IL-8 and GRO-α have recently been shown to induce articular chondrocyte hypertrophy, including type X collagen and MMP-13 expression, as well as matrix calcification, providing a link between inflammation and altered differentiation of articular chondrocytes (30). Stimulation of IL-8 and GRO-α as well as IL-6, and MCP-1 production by FN-f could act as an amplifier of the molecular inflammation noted in OA cartilage. High levels (500 nM) of GRO-α have also been shown to stimulate chondrocyte apoptosis (32), which may be an important cause of cartilage loss in more advanced stages of arthritis (33).
In addition to OA, FN-f stimulation of chemokine production could play an important role in the progressive cartilage destruction that occurs in RA. Regulation of angiogenesis by the C-X-C chemokines IL-8 and epithelial neutrophil-activating peptide-78 in human synovial tissue has also been demonstrated in patients with RA (34). Formation of pannus tissue containing lymphocytes, macrophages, neutrophils, and fibroblasts and the growth of this tissue, which can invade and destroy the cartilage, could be stimulated in part by release of cytokines and chemokines by the chondrocytes in response to the initial matrix damage. Synovial inflammation and pannus formation have also been noted in patients with advanced OA (35, 36), although usually not to the extent seen in RA. This is most likely due to a greater influx of cells into the joint driven by the early inflammatory activity in the RA synovium, while local generation of chemokines in OA cartilage would attract fewer cells to the joint due to the avascular nature of cartilage.
The present results also add to the growing body of evidence that the NF-κB pathway plays a pivotal role in regulation of chemokine expression. Inhibition of IκB was sufficient to eliminate chemokine release induced by FN-f, indicating a crucial role of this signal transduction pathway. We have shown previously that FN-f stimulation of chondrocytes results in activation of the MAPK pathway through the α5β1 integrin (6). Activated MAPKs can further trigger activation of several nuclear transcription factors, including NF-κB, which regulate the gene expression of proinflammatory cytokines and chemokines. Consistent with this, we found that inhibition of either p38 or JNK inhibited FN-f stimulation of NF-κB, suggesting that activation of both MAPK pathways, but not ERK, is required for FN-f stimulation of NF-κB. Previous studies have also shown that FN-f activates AP-1 in chondrocytes (6, 11), which could also contribute to the stimulation of cytokine and chemokine expression. Although the role of AP-1 was not investigated in this study, the present results, demonstrating a requirement for NF-κB, indicate that AP-1 activation by itself would not be sufficient for stimulation of cytokine and chemokine expression.
Even though FN-f treatment of chondrocytes resulted in release of IL-1β, which also has the potential to stimulate chondrocyte chemokine expression (25), the production and release of IL-1β were not necessary for the initial chemokine expression induced by FN-f. The IL-1ra protein blocked IL-1β, but not FN-f stimulation of cytokine and chemokine expression. In unpublished experiments, we have used Affymetrix arrays to analyze the gene expression profile of human knee articular chondrocytes treated with IL-1β. Although the cytokine and chemokine expression profiles were similar to the present results using ankle chondrocytes treated with FN-f, differences existed in expression of several genes, including TNF family members (e.g., TNFR superfamily member 6) that were regulated by FN-f, and not IL-1β and IL-1 family members (e.g., the IL-1ra) that were regulated by IL-1 and not FN-f. It is possible that IL-1 autocrine signaling contributes to further cytokine and chemokine production by chondrocytes after the initial stimulation by FN-f; however, the finding that IL-1 was not required for FN-f stimulation is consistent with a recent study demonstrating the development of OA in IL-1 knockout mice (37).
The results of this study suggest that targeting the signaling pathways activated by FN-f may be an effective means of inhibiting production of multiple mediators of cartilage destruction (Fig. 8). Direct inhibition of the chondrocyte α5β1 fibronectin receptor with blocking Abs results in significantly reduced cell survival (18), making it unlikely to be a direct therapeutic target. Because NF-κB is involved in normal immune and homeostatic processes, such as prevention of apoptosis in certain tissues, its prolonged inhibition may exert deleterious effects on chondrocytes as well. Other targets include the selective inhibition of upstream molecules such as IKK-2, a kinase previously shown to be essential for IL-1-induced IκBα degradation, NF-κB activation, and cytokine expression (38), and, as shown in this study, is required for FN-f induction of cytokine expression. Alternatives would include inhibition of the p38 or JNK MAPKs, which has recently been shown to be effective in reducing joint destruction in animal models of arthritis (39, 40).
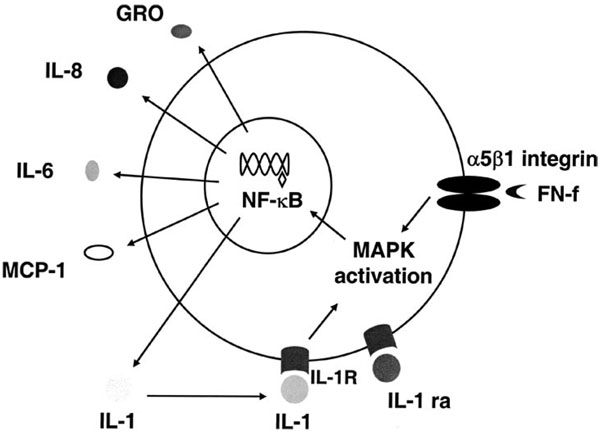
FIGURE 8.
Schematic diagram of the proposed mechanism for FN-f-stimulated cytokine and chemokine production. The 110-kDa FN-f stimulates the α5β1 integrin (fibronectin receptor) and activates MAPK signaling pathways, which result in the downstream activation of the IκB/NF-κB complex. Liberated NF-κB translocates to the nucleus and binds in a sequence- specific manner to target cytokine and chemokine genes, resulting in their transcription, translation, and secretion from the cell. The presence of IL-1β is not required for the initial FN-f stimulation of cytokine and chemokine expression; however, it could participate in an autocrine (or paracrine) loop to stimulate further production.
In summary, this study provides direct in vitro evidence that FN-f are capable of promoting chondrocyte activation and increased secretion of proinflammatory C-X-C and C-C cytokines, specifically IL-8, IL-6, MCP-1, GRO-α, GRO-β, and GRO-γ, through an NF-κB-dependent pathway. Our data suggest that damage to the extracellular matrix, resulting in fragmentation of fibronectin, is able to amplify an inflammatory response that may lead to further progressive matrix destruction and cartilage degradation. Targeting the signaling pathways activated by FN-f may be an effective means of inhibiting production of multiple mediators of cartilage destruction.
Acknowledgments
We gratefully acknowledge the Gift of Hope Organ and Tissue Donor Network and the donor families for providing tissue, and the assistance of Dr. Arkady Margulis for collecting donor tissues. We also thank Ashley Hughes for technical assistance with the Affymetrix arrays and Amgen for providing IL-1Ra (Anakinra).
Footnotes
This work was supported by National Institutes of Health Grant AR49003 and a grant from GlaxoSmithKline.
Abbreviations used in this paper: FN-f, fibronectin fragment; BCA, bicinchoninic acid; GRO, growth-related oncogene; IKK, IκB kinase; IL-1ra, IL-1 receptor antagonist; MEK, MAP/ERK kinase; MMP, matrix metalloproteinase; OA, osteoarthritis; RA, rheumatoid arthritis.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Carnemolla B, Cutolo M, Castellani P, Balza E, Raffanti S, Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984;27:913. doi: 10.1002/art.1780270811. [DOI] [PubMed] [Google Scholar]
- 2.Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J. Rheumatol. 1992;19:1448. [PubMed] [Google Scholar]
- 3.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 4.Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J. Biol. Chem. 1992;267:3597. [PubMed] [Google Scholar]
- 5.Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38:1304. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- 6.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to α2β1 and α5β1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 7.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J. Cell Biol. 1989;109:877. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie D, Hui F, Homandberg GA. Fibronectin fragments alter matrix protein synthesis in cartilage tissue cultured in vitro. Arch. Biochem. Biophys. 1993;307:110. doi: 10.1006/abbi.1993.1568. [DOI] [PubMed] [Google Scholar]
- 9.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 10.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J. Biol. Chem. 2003;278:24577. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J. Biol. Chem. 2003;278:25386. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes: a role in osteoarthritis. J. Clin. Invest. 1996;97:2011. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46:2087. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 14.Xie DL, Hui F, Meyers R, Homandberg GA. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch. Biochem. Biophys. 1994;311:205. doi: 10.1006/abbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- 15.Homandberg GA, Hui F, Wen C, Purple C, Bewsey K, Koepp H, Huch K, Harris A. Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem. J. 1997;321:751. doi: 10.1042/bj3210751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by α5β1 and αVβ3 integrins. J. Immunol. 2000;164:2684. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- 17.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Pulai JI, Del Carlo M, Jr, Loeser RF. The α5β1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 19.Roberts CJ, Nelson B, Marton MJ, Stoughton R, Meyer MR, Bennett HA, He YD, Dai H, Walker WL, Hughes TR, et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- 20.Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 21.Ojo-Amaize EA, Kapahi P, Kakkanaiah VN, Takahashi T, Shalom-Barak T, Cottam HB, Adesomoju AA, Nchekwube EJ, Oyemade OA, Karin M, Okogun JI. Hypoestoxide, a novel anti-inflammatory natural diterpene, inhibits the activity of IκB kinase. Cell. Immunol. 2001;209:149. doi: 10.1006/cimm.2001.1798. [DOI] [PubMed] [Google Scholar]
- 22.Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes: modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 1990;144:499. [PubMed] [Google Scholar]
- 23.Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation: regulation of IL-8 expression by human articular chondrocytes. J. Immunol. 1992;148:466. [PubMed] [Google Scholar]
- 24.Recklies AD, Golds EE. Induction of synthesis and release of interleukin-8 from human articular chondrocytes and cartilage explants. Arthritis Rheum. 1992;35:1510. doi: 10.1002/art.1780351215. [DOI] [PubMed] [Google Scholar]
- 25.Pulsatelli L, Dolzani P, Piacentini A, Silvestri T, Ruggeri R, Gualtieri G, Meliconi R, Facchini A. Chemokine production by human chondrocytes. J. Rheumatol. 1999;26:1992. [PubMed] [Google Scholar]
- 26.Alaaeddine N, Olee T, Hashimoto S, Creighton-Achermann L, Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, Facchini A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 28.Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46:130. doi: 10.1002/1529-0131(200201)46:1<130::aid-art10020>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene α/CXCL1 induce chondrocyte hypertrophic differentiation. J. Immunol. 2003;171:4406. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 31.Silvestri T, Meliconi R, Pulsatelli L, Dolzani P, Zizzi F, Frizziero L, Borzi RM, Facchini A. Down-modulation of chemokine receptor cartilage expression in inflammatory arthritis. Rheumatology. 2003;42:14. doi: 10.1093/rheumatology/keg020. [DOI] [PubMed] [Google Scholar]
- 32.Borzi RM, Mazzetti I, Magagnoli G, Paoletti S, Uguccioni M, Gatti R, Orlandini G, Cattini L, Facchini A. Growth-related oncogene α induction of apoptosis in osteoarthritis chondrocytes. Arthritis Rheum. 2002;46:3201. doi: 10.1002/art.10650. [DOI] [PubMed] [Google Scholar]
- 33.Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999;7:389. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- 34.Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, Woodruff DC, Burdick MD, Strieter RM. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001;44:31. doi: 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Oehler S, Neureiter D, Meyer-Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin. Exp. Rheumatol. 2002;20:633. [PubMed] [Google Scholar]
- 36.Yuan GH, Tanaka M, Masuko-Hongo K, Shibakawa A, Kato T, Nishioka K, Nakamura H. Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. Osteoarthritis Cartilage. 2004;12:38. doi: 10.1016/j.joca.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1β, interleukin-1β-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48:3452. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 38.Andreakos E, Smith C, Kiriakidis S, Monaco C, de Martin R, Brennan FM, Paleolog E, Feldmann M, Foxwell BM. Heterogeneous requirement of IκB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum. 2003;48:1901. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- 39.Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, Stroup GB, Webb E, Rieman DJ, Gowen M, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 2001;108:73. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]