Abstract
We studied hypertension-associated changes in prejunctional α2 adrenergic receptor (α2-AR) function using amperometry to monitor in vitro norepinephrine (NE) measured as oxidation currents. Vasoconstriction was measured using video imaging. NE release was induced by electrical stimulation of sympathetic nerves associated with mesenteric arteries (MA) and veins (MV) of sham and DOCA-salt hypertensive rats. NE oxidation currents were larger in DOCA-salt compared to sham MA; there were no differences between currents in sham and DOCA-salt MV. Increases in NE oxidation currents followed a multi-exponential time course in sham MA. In DOCA-salt MA and sham and DOCA-salt MV, the time course was mono-exponential. Yohimbine (α2-AR antagonist, 1 μM), caused a mono-exponential increase in NE oxidation currents in sham and DOCA-salt MA. Yohimbine increased NE oxidation currents and constrictions more in sham compared to DOCA-salt MA and compared to MV. UK 14,304 (α2-AR agonist, 1.0 μM), reduced currents less in DOCA-salt MA and sham and DOCA-salt MV compared to sham MA. Prazosin (α1-AR antagonist, 0.1 μM) did not alter NE oxidation currents. Prazosin inhibited constrictions more in DOCA-salt compared to sham MA and almost completely blocked constrictions in sham and DOCA-salt MV. Prazosin-resistant constrictions in MA were blocked by the P2 receptor antagonist, PPADS (10 μM). Prejunctional α2-ARs modify NE concentrations near neuroeffector junctions in MA and MV. α2-AR function is most prominent in MA and is impaired in DOCA-salt MA but not MV. Purinergic transmission predominates in sham MA. NE is the dominant vasoconstrictor in DOCA-salt MA and sham and DOCA-salt MV.
Keywords: Sympathetic nervous system, DOCA-salt hypertension, Amperometry, Autoreceptors
1. Introduction
Increased resistance to arterial blood flow increases blood pressure control while reductions in venous capacitance shift blood to arteries which increase arterial blood volume and arterial pressure. Therefore, regulation of venous tone contributes to blood pressure control. Arteries and veins in the splanchnic circulation, especially mesenteric arteries (MA) and veins (MV), are particularly important in regulating peripheral resistance and capacitance respectively (Pang, 2001; Rothe, 1983). MA and MV are densely innervated by sympathetic nerves and increases in sympathetic nerve activity increase MA and MV tone causing increased resistance and decreased capacitance respectively (Kreulen, 1986; Nyhof et al., 1983; Pang, 2001). Norepinephrine (NE) and ATP are vasoconstrictor transmitters released by perivascular sympathetic nerves (Gitterman and Evans, 2001; Sneddon, 2000). NE acts at α1-adrenergic receptors (α1-ARs) and ATP acts at P2X and P2Y receptors to cause vasoconstriction (Galligan et al., 2001; Gericke et al., 2007; Gitterman and Evans, 2001; Martínez-Salas et al., 2007; Sneddon, 2000). As MA and MV perform different hemodynamic functions it might be anticipated that different populations of sympathetic neurons would supply MA and MV. Studies using retrograde labeling techniques showed that there are subsets of neurons in guinea pig and rat prevertebral sympathetic ganglia that selectively supply MA or MV (Browning et al., 1999; Hsieh et al., 2000). Therefore, sympathetic neurons selectively supplying MA or MV may have different neuro-chemical or functional properties. Previous work has shown that, in the rat, NE acting at α1-ARs mediates sympathetic neurogenic constriction of MV while ATP acting at P2X receptors mediates neurogenic constriction of MA (Luo et al., 2003; Park et al., 2007). It was also shown that MV are more sensitive to the constrictor effects of sympathetic nerve stimulation than MA (Hottenstein and Kreulen, 1987; Luo et al., 2003; Park et al., 2007). The increased sensitivity of MV to the constrictor effects of sympathetic nerve activity is also partly due to increased NE levels near the venous neuroeffector junction compared to that in the arterial neuroeffector junction during nerve stimulation (Park et al., 2007).
Increased sympathetic nerve activity occurs in human hypertension (de Champlain et al., 1976; Esler et al., 1990a; Wallin and Charkoudian, 2007) and in animal models of hypertension (Bobalova and Mutafova-Yambolieva, 2001; Bouvier and De Champlain, 1985; Rascher et al., 1981). This conclusion is based on studies showing that plasma levels of NE are elevated in hypertensive humans and animals (de Champlain et al., 1976; Drolet et al., 1989). Impaired function of prejunctional a2-adrenergic receptors (α2-ARs) is one mechanism responsible for the increased plasma NE levels in hypertension (Drolet et al., 1989; Esler et al., 1990b; Park et al., 2007; Tsuda et al., 1989).
Plasma NE measurements assess global sympathetic nerve activity but they do not allow selective assessments of the function of sympathetic nerves supplying arteries and veins. This can be accom-plished using artery and vein preparations maintained in vitro. Previous in vitro studies measured NE in overflow samples (Bobalova and Mutafova-Yambolieva, 2001; Cox et al., 1996; Luo et al., 2004; Smyth et al., 2000). These measurements can be used to assess prejunctional mechanisms controlling NE release from perivascular sympathetic nerves but long trains of high frequency stimulation are required to elicit detectable amounts of NE release. In addition, overflow measurements are not made in real time so it is not possible to correlate NE release with vasoconstriction. Finally, prejunctional α2-ARs modulate the amount and rate of sympathetic neurotransmitter release during bursts of nerve activity. As discussed above α2-AR function is known to be impaired in hypertension. Because of the limitations imposed by NE measurements in overflow samples it is not possible to study hypertension-associated changes in release dynamics near the vascular neuroeffector junction.
In this paper, we used continuous amperometry with carbon fiber microelectrodes to detect NE (as an oxidation current) in real time near release sites at the adventitial surface of rat MA and MV maintained in vitro. This technique has been used to measure NE release from periarterial (Brock et al., 2000; Dunn et al., 1999; Gonon et al., 1993; Park et al., 2007) and perivenous (Park et al., 2007) sympathetic nerves. We used tissues from sham normotensive and deoxycorticosterone acetate (DOCA)-salt hypertensive rats (Schenk and McNeill, 1992). Our data show that DOCA-salt hypertension is associated with impaired α2-AR function in MA but these receptors do not influence NE release dynamics in either sham or DOCA-salt MV.
2. Materials and methods
2.1. DOCA-salt and sham rats
The Institutional Animal Care and Use Committee at Michigan State University approved all animal use procedures. Male Sprague–Dawley rats (175–200 g, Charles River Inc., Portage, MI) were used in these studies. Details of surgical procedures, post-operative treatments have been described in detail previously (Fink et al., 2000; Luo et al., 2003). Systolic blood pressure was measured using the tail–cuff method four weeks after surgery. Rats with mean systolic blood pressure ≥150 mm Hg were considered hypertensive.
2.2. Preparation of mesenteric vessels for in vitro electrochemical and diameter measurements
Sham and DOCA-salt rats were euthanized with a lethal pentobarbital injection (50 mg, i.p.). The abdomen was opened and the small intestine was carefully removed and placed in an oxygenated (95% O2, 5% CO2) Krebs’ buffer solution of the following composition (millimolar): 117, NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 glucose. A segment of the ileum segment was placed in a petridish and the mesentery was gently stretched and pinned flat. A section of the mesentery close to the ileal wall was carefully cut free from the intestine and transferred to a small silicone elastomer-lined (Sylgard® 184, Dow Corning) teflon organ bath (4.8 mL volume). Secondary or tertiary arteries and veins (180–330 μm outer diameter) were isolated for in vitro study by carefully clearing away the surrounding adipose and connective tissues using a dissecting microscope and forceps. The bath was mounted on the stage of an inverted microscope (Olympus CKX41) and superfused continuously with Krebs’ solution (37 °C, pH 7.4) at a flow rate of ~6 mL/min. The solution flow was controlled by a peristaltic pump (Masterflex, Cole Parmer).
Preparation of carbon fiber microelectrodes used in these studies has been described in detail previously (Pang, 2001). The carbon fiber microelectrode assemblies were affixed to a micromanipulator (MP-1, Narishige Instruments, Japan), which was used to position the electrode along the side of the blood vessel with some tension. This allowed the microelectrode to move in concert with the constricting vessel, maintaining a constant electrode-release site spacing. This is critical for measuring meaningful NE overflow currents from constricting vessels. The microelectrode was positioned in the center of the bath, equidistant from the inlet and outlet solution ports. A Pt wire counter and a commercial “no leak” Ag/AgCl (3 M KCl, model EE009, Cypress Systems Inc., USA) reference electrode were also mounted in the bath to complete the electrochemical cell. All electrochemical measurements were made with an Omni 90 analog potentiostat (Cypress Systems Inc.), an analog-to-digital converter (Labmaster 125) and a computer running Axoscope software (version 9.0, Molecular Devices, Sunnyvale, CA). Continuous amperometric i-t curves were recorded at 400 mV as at this potential NE was oxidized at a mass transfer limited rate. The analog output from the potentiostat was low-pass-filtered at a time constant of 200 ms (5 Hz). The filtered analog current was then digitized using the A/D converter at a sampling rate of 100 Hz, and the data were stored on a computer for further processing. The Krebs’ buffer flowed over the electrode and the tissue sample for 30 min prior to the start of a series of measurements. Our recent work (Dong et al., 2009) has shown that a high concentration (100 μM), the α2AR agonist UK 14,304, the α2AR antagonist, yohimbine and the α1AR antagonist, prazosin, all interact with carbon fiber electrodes and produce some attenuation of NE oxidation currents. These effects are small particularly at the concentrations of drugs used in these studies (1 μM) and they do not alter the overall conclusion regarding NE release dynamics in mesenteric blood vessels.
2.3. Focal stimulation of perivascular nerves
Perivascular nerves were stimulated using a bipolar focal stimulating electrode positioned on the surface of the blood vessel at a distance of ca. 200 μm from the carbon fiber. This positioning minimized the stimulus artifact in the current recordings. The focal stimulating electrode consisted of two Ag/AgCl wires inserted into double barrel capillary glass (tip diameter=180 μm). The wires were connected to a stimulus isolation unit and a Grass Instruments stimulator (S88, Quincy, MA). Trains of 60 stimuli (0.3 ms duration, 30–70 V) at frequencies ranging between 0.5 and 20 Hz were used. Prior to starting experiments, tetrodotoxin (TTX, 0.3 μM) was tested against constrictions (see below) and NE oxidation currents evoked by a 20 Hz stimulus train. Preparations in which the constriction and oxidation current were not blocked by TTX were discarded. Some studies were also done using guanethidine (1 μM) to block NE oxidation currents. NE oxidation currents and associated constrictions were always blocked completely by guanethidine.
2.4. Video monitoring of vasoconstriction
The output of a black and white video camera (KP-111, Hitachi, Yokohama, Japan) attached to the microscope was fed to a PC Vision Plus frame-grabber board (Imaging Technology, Woburn, MA) mounted in a personal computer. Changes in blood vessel diameter of 0.1 μm could be resolved. Video images were analyzed using Diamtrak software (http://www.diamtrak.com, Adelaide, Australia). The digitized signal was converted to an analog output (DAC-02 board, Keithley Metrabyte, Tauton, MA) for subsequent processing by an analog-to-digital converter (Labmaster 125) and analysis in a second computer running Axotape for a permanent recording of blood vessel diameter as function of time. Analog signals were sampled at 100 Hz and data were stored on the computer’s hard drive for subsequent analysis and display.
2.5. Drug application
All chemicals and drugs were reagent-grade quality, or better, and were used without additional purification. Ultrapure water (distilled, deionized, and filtered over activated carbon, 17–18 MΩ, Barnstead E-Pure System) was used for all solution preparation, and glassware and electrode cleaning. Drugs were added to the superfusing Krebs’ solution and it took ca. 2 min. for the drug concentrations to come to equilibrium in the recording chamber and they were applied for 20 min. prior to assessing their effects on neurogenic responses. All drugs were obtained from Sigma-Aldrich Chemical Company (Saint Louis, MO, USA).
2.6. Data analysis
Analysis of the current-time and diameter-time profiles was performed using Clampfit 9.2 as part of the pCLAMP 9.2 software package (Molecular Devices). The time course of the increase in NE oxidation current caused by a 20 Hz stimulus train s was fitted using the standard exponential function and the Chebyshev method to find the best fit. The time course of the current rise caused by 1, 3 and 7 Hz stimulus trains was highly variable and was not readily fitted with single or multi-exponential models. The rise time of the current was more stable at 20 Hz and we chose responses caused this frequency for detailed study.
Mean values were compared using the Student’s paired t-test or analysis of variance and Student Newman Keuls test for multiple comparisons where appropriate. Proportional data were analyzed using Fisher’s Exact test in GraphPad Instat (GraphPad Software, San Diego, CA). P<0.05 was regarded as statistically significant. Data are mean ±the S.E.M. and “n” values are the number of animals from which the data were obtained. MA and MV were measured either as peak constriction as a percentage of the initial resting diameter (μm) of the blood vessel or as area under the constriction-time curve for individual responses (μm s).
3. Results
3.1. Sham and DOCA-salt rats
Mean systolic blood pressures for sham and DOCA-salt rats were 125±2 mmHg (n = 40) and 194±3 mmHg (n = 35), respectively (P< 0.05). The mean body weights of sham and DOCA-salt rats were 416±5 g and 336±7 g, respectively (P< 0.05).
3.2. Simultaneous measurement of NE oxidation currents and vasoconstriction
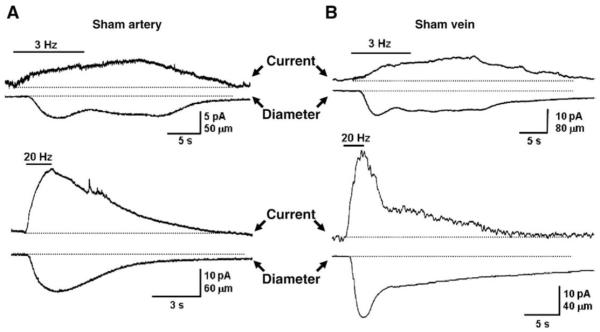
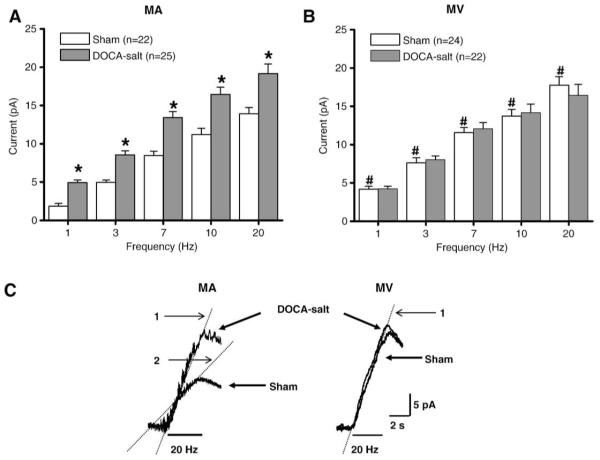
Since there are more nerve fibers associated with MA compared to MV, measurement of NE overflow at MV was critically dependent on the electrode position. Therefore, NE oxidation current measurements were made with MA and MV only after the electrode had been optimally positioned to record the maximum current (Park et al., 2007). The amplitude and time course MA and MV constriction followed the time course of the NE oxidation current and amplitude. Fig. 1 shows representative responses obtained in MV and MA from a sham rat. Qualitatively similar results were obtained in blood vessels from DOCA-salt rats. Previous work has shown that MV are more sensitive to the constrictor effects of sympathetic nerve stimulation than MA (Hottenstein and Kreulen, 1987; Luo et al., 2003), so we focused on comparison of NE oxidation currents in MA and MV from sham and DOCA-salt rats. Focal electrical stimulation produced larger currents in DOCA-salt MA than those in sham MA at all stimulation frequencies (Fig. 2A) but there were no differences in the amplitude of oxidation currents recorded from sham and DOCA-salt MV (Fig. 2B). Oxidation currents recorded in sham MV were larger than those recorded from sham MA (Fig. 2A,B).
Fig. 1.
Representative recordings of NE oxidation currents and constrictions of MA (A) and MV (B) from sham rats. Upper traces in each pair are oxidation currents while lower traces are diameter recordings. In both blood vessels responses were evoked by 60 stimuli applied at frequencies of 3 (upper) and 20 Hz (lower) in both blood vessels.
Fig. 2.
Frequency–response relationship for oxidation currents evoked by focal electric stimulation in MA (A) and MV (B) from sham and DOCA-salt rats. In “A” * indicates significantly different from responses in sham MA. In “B” # indicates that NE oxidation currents recorded from sham MV are significantly greater than those recorded from sham MA (P< 0.05). Data are mean ±S.E.M. C. Representative recordings of the rising phase of the NE oxidation current in a sham and DOCA-salt MA (left) and sham and DOCA-salt MV (right). In the MA traces “1” and “2” indicate the two components to the time course of the current increase in sham MA.
Non-linear curve fitting methods were used to fit the time course of the current increase caused by a 20 Hz stimulus train in MA and MV from sham and DOCA-salt rats (Fig. 2C). We studied the NE current caused by the 20 Hz stimulus train because it yielded the maximum constriction (Luo et al., 2003, see below). The current increase in sham MA was best fitted by a 2 or 3 exponential time course in 17 of 19 tissues (Fig. 2C left; Table 1); in two tissues the time course was fitted best with a single exponential function. In DOCA-salt MA a single exponential provided the best fit in 6 of 11 tissues while a two exponential time course provided the best fit in 5 tissues (Fig. 2C left; Table 1); these proportions were significantly different from those occurring in sham MA (P<0.05). The time course of the current increase was fitted by a single exponential in all 17 sham MV studied and in 12 of 16 DOCA-salt MV. In 4 DOCA-salt MV, the current-time course was best fit by two exponentials; this proportion was significantly different from that in sham MV (P<0.05).
Table 1.
Time constants (t) in seconds for the increase in NE oxidation currents during a 20 Hz stimulus train
| Control |
Yohimbine (1 μM) |
|||
|---|---|---|---|---|
| t1 | t2 | t1 | t2 | |
| Sham MA | 1.1±0.2 (2) | n.d. | 2.1±1.2 (5) | n.d. |
| Sham MA | 1.3±0.2 (16) | 0.4±0.05 | 1.2±0.6 (2) | 0.2±0.1 |
| DOCA-salt MA | 3.1±0.6 (6) | n.d. | 4.7±1.3 (4) | n.d. |
| DOCA-salt MA | 2.1±0.5 (5) | 0.8±0.3 | n.d. | n.d. |
| Sham MV | 2.8±0.7 (17) | n.d. | 1.5±0.2 (6) | n.d. |
| DOCA-salt MV | 3.3±0.9 (12) | n.d. | 3.4±1.2 (10) | n.d. |
| DOCA-salt MV | 1.2±0.6 (4) | 0.3±0.1 (4) | 1.2±0.2 (2) | 0.2±0.1 (2) |
The time course of the rising phase of the oxidation current in individual tissues was fitted to a single or multiple exponential function using a non-linear curve fitting routine. Data are mean±S.E.M. Numbers in parenthesis indicate the number of animals from which tissues were obtained. n.d., not detected.
3.3. Impaired function of prejunctional α2-ARs in MA but not MV
We used the α2-AR receptor antagonist, yohimbine (1 μM), to investigate the contribution of α2-AR function to regulation of the time course of the NE oxidation current increase in MA and MV. Yohimbine did not change the time course of the increase in NE oxidation current in either sham or DOCA-salt MV (Table 1). However, in sham MA in the presence of yohimbine, the time course of the increase in oxidation current in sham MA was best fitted by one exponential in 5 of 7 tissues and by two exponentials in 2 tissues (Table 1). This proportion was significantly different (P<0.05) from that occurring in sham MA in the absence of yohimbine. Finally, yohimbine did not alter the time course of current increase in DOCA-salt MA (Table 1).
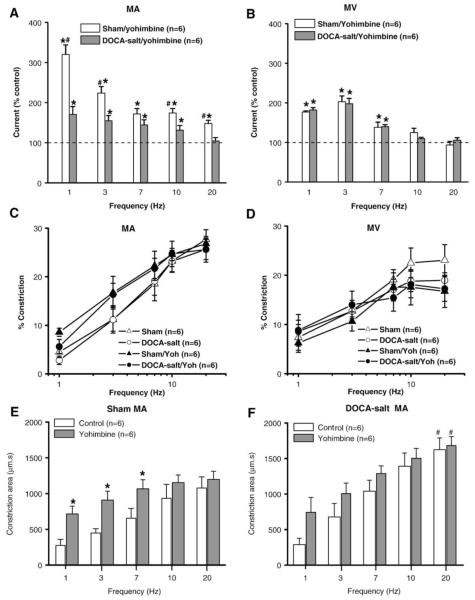
Yohimbine increased peak oxidation currents in sham MA at all stimulation frequencies but this effect was most prominent at 1 and 3 Hz (Fig. 3A). Yohimbine increased oxidation currents in DOCA-salt MA but the facilitation was significantly less than that occurring in sham MA (Fig. 2A). Oxidation currents recorded from sham and DOCA-salt MV were increased to a similar extent by yohimbine but only at stimulation frequencies <7 Hz (Fig. 2B). Yohimbine was less effective at increasing oxidation currents in sham and DOCA-salt veins compared to sham MA (Fig. 3A,B). The increase in NE oxidation currents caused by yohimbine in sham and DOCA-salt MA was associated with a leftward shift in the frequency response curve for constriction (Fig. 3C, Table 2). Constriction area was also measured in the absence and presence of yohimbine in sham and DOCA-salt MA in order to determine if the increased NE oxidation current was associated with an increased duration of arterial constriction (Fig. 3E,F). Yohimbine increased the constriction area in sham MA only at low stimulation frequencies (<7 Hz) but it did not change constriction area in DOCA-salt MA. The constriction area caused by 20 Hz stimulation in DOCA-salt MA was larger than that in sham MA in both in the absence and presence of yohimbine (Fig. 3F). Yohimbine did not change the frequency response curves for constriction in either sham or DOCA-salt MV (Fig. 3D, Table 2).
Fig. 3.
Effects of yohimbine on NE oxidation currents and neurogenic constrictions. (A) NE oxidation currents recorded in the presence of yohimbine (1 μM) in sham and DOCA-salt MA. (B) NE oxidation currents recorded in the presence of yohimbine in sham and DOCA-salt MV. Data are expressed as % of the response obtained in each tissue before the application of yohimbine. * Indicates a significant increase over control levels (100%, P< 0.05). # Indicates significantly different from the levels in DOCA-salt MA (P< 0.05). Frequency–response curves for peak neurogenic constrictions in sham and DOCA-salt MA (B) and sham and DOCA-salt MV (D) in the absence and presence of yohimbine. (E) Frequency-dependent increase in the area of neurogenic constrictions in the absence and presence of yohimbine in sham MA. * Indicates significantly different from sham MA in the absence of yohimbine. (F) Frequency-dependent increase in the area of neurogenic constrictions in the absence and presence of yohimbine in DOCA-salt MA. Yohimbine did not change the duration of constrictions in DOCA-salt MA. # Indicates significantly different from constriction area in sham MA at the 20 Hz stimulation. Data are mean ±S.E.M.
Table 2.
Maximum constriction (Emax) and half maximum stimulation frequency (S50) for MA and MV from sham and DOCA-salt rats in the absence (control) and presence of yohimbine (Yoh, 1.0 μM) and UK 14,304 (UK, 1.0 μM)
| Sham artery |
DOCA-salt artery |
|||
|---|---|---|---|---|
| Control (n=5) | Yoh (n=5) | Control (n=6) | Yoh (n=6) | |
| S50 (Hz) | 5.4±1.1 | 2.4±0.7 | 5.6±0.9 | 2.5±0.3* |
| Emax (%) | 33.9±1.9 | 30.0±2.3 | 33.0±2.7 | 29.4±1.9 |
| Control (n=6) | UK (n=6) | Control (n=6) | UK (n=6) | |
|---|---|---|---|---|
| S50 (Hz) | 5.4±0.4 | 8.0±1.1 | 5.6±1.7 | 7.4±0.7 |
| Emax (%) | 37.4±2.5 | 9.7±2.1& | 32.0±1.5 | 17.2±1.5*# |
| Sham vein |
DOCA-salt vein |
|||
|---|---|---|---|---|
| Control (n=6) | Yoh (n=6) | Control (n=6) | Yoh (n=6) | |
| S50 (Hz) | 3.0±0.7 | 3.4±0.8 | 1.5±0.4 | 1.4±0.7 |
| Emax (%) | 27.3±2.3 | 21.9±2.9 | 21.7±1.9 | 20.0±1.7 |
| Control (n=5) | UK (n=5) | Control (n=5) | UK (n=5) | |
|---|---|---|---|---|
| S50 (Hz) | 1.9±0.8 | 10.1±1.1& | 2.6±0.5 | 8.1±0.6* |
| Emax (%) | 28.3±3.2 | 10.5±1.7& | 24.8±1.5 | 12.8±1.7* |
Data are mean±S.E.M. and “n” value refers to the number of animals from which the data were obtained.
Indicates significantly different from the S50 or Emax in control sham MA or MV(P<0.05).
Indicates significantly different from the S50 or Emax in control DOCA-salt MAor MV (P<0.05).
Indicates significantly different from the S50 or Emax in the UK 14,304 treated sham MA (P<0.05). Data analyzed using analysis of variance and Student Newman Keuls post hoc test.
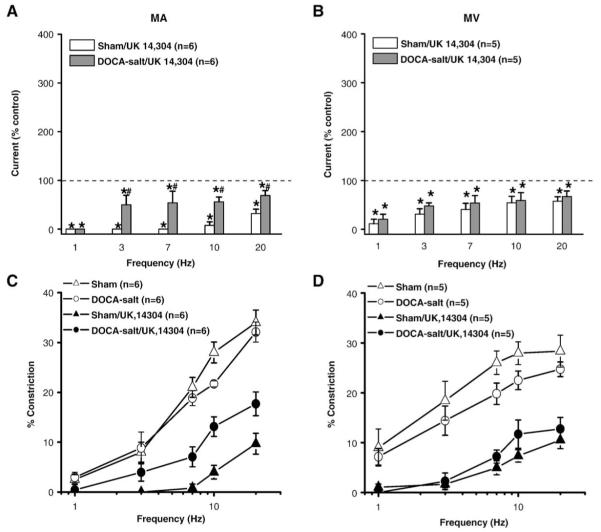
The α2-AR agonist, UK 14,304 (1 μM) decreased oxidation currents in sham and DOCA-salt MA, but more so in sham MA (Fig. 4A). UK 14,304 decreased the oxidation current in sham and DOCA-salt MV to a similar extent (Fig. 4B). UK 14,304 also inhibited neurogenic constrictions in sham and DOCA-salt MA but this effect was significantly greater in sham MA (Fig. 4C, Table 2).
Fig. 4.
Effect of UK 14,304 on NE oxidation currents and neurogenic constrictions. (A) NE oxidation currents recorded in the presence of UK 14,304 (1 μM) in sham and DOCA-salt MA. (B) NE oxidation currents recorded in the presence of UK 14,304 in sham and DOCA-salt MV. Data are expressed as % of the response obtained in each tissue before UK 14,304 application. * Indicates a significant decrease below control levels (100%, P< 0.05). # Indicates significantly different from the levels in DOCA-salt MA (P< 0.05). Frequency–response curves for neurogenic constrictions in sham and DOCA-salt MA (B) and sham and DOCA-salt MV (D) in the absence and presence of UK 14,304. Data are mean ±S.E.M.
3.4. Adrenergic contribution to neurogenic constriction in sham and DOCA-salt MA and MV
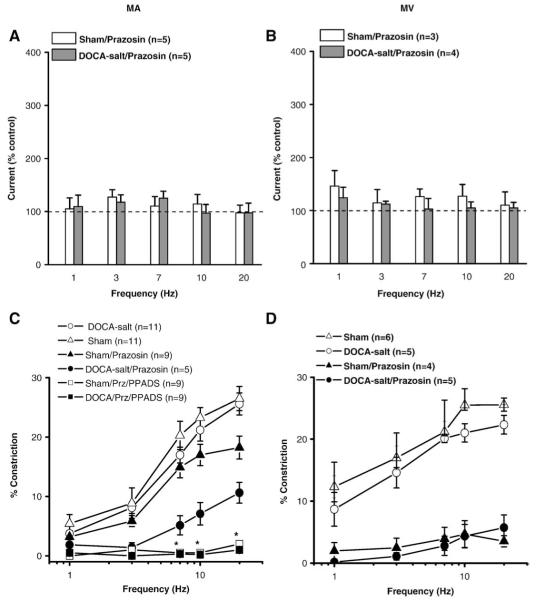
We studied the contribution of nerve released NE to constrictions of sham and DOCA-salt MV by using prazosin (0.1 μM), to block α1-ARs in MA and MV. Prazosin did not alter NE oxidation currents in MA or MV (Fig. 5A,B). Prazosin blocked neurogenic constrictions in sham and DOCA-salt MV (Fig. 5D, Table 3). However, prazosin reduced the neurogenic constriction in sham MA only at 20 Hz without changing the S50 value (Fig. 5C, Table 3). Prazosin produced a greater inhibition of neurogenic constrictions in DOCA-salt compared to sham MA (Fig. 3C, Table 3). Prazosin-resistant constrictions in sham and DOCA-salt MA were blocked by the addition of the P2 receptor antagonist, PPADS (10 μM) (Fig. 5C).
Fig. 5.
Effects of prazosin on NE oxidation currents and neurogenic constrictions in sham and DOCA-salt MA and MV. (A) NE oxidation currents recorded in the presence of prazosin (0.1 μM) in sham and DOCA-salt MA. (B) NE oxidation currents recorded in the presence of prazosin in sham and DOCA-salt MV. Data are expressed as % of the response obtained in each tissue before the application of prazosin. Frequency–response curves for neurogenic constrictions in sham and DOCA-salt MA (C) and sham and DOCA-salt MV (D) in the absence and presence of prazosin or prazosin plus PPADS (10 μM). In “C” *indicates significantly different from constriction in the presence of prazosin alone (P< 0.05). Data are mean ±S.E.M.
Table 3.
Maximum constriction (Emax) and half maximum stimulation frequency (S50) in MA and MV from sham and DOCA-salt rat in the absence (control) and presence of prazosin (0.1 μM) treatment
| Sham artery |
DOCA-salt artery |
|||
|---|---|---|---|---|
| Control (n=5) | Prazosin (n=5) | Control (n=5) | Prazosin (n=5) | |
| S50 (Hz) | 5.7±0.4 | 5.9±1.0 | 5.8±0.9 | 12±2.2*# |
| Emax (%) | 26±1.8 | 18±2.0& | 27±2.0 | 11±2.7*# |
| Sham vein |
DOCA-salt vein |
|||
|---|---|---|---|---|
| Control (n=5) | Prazosin (n=5) | Control (n=5) | Prazosin (n=5) | |
| S50 (Hz) | 1.5±0.6 | - | 1.9±0.5 | - |
| Emax (%) | 26±2.0 | 3.5±1.0& | 23±1.5 | 5.7±2.0* |
Data are mean±S.E.M. and “n” values refer to the number of animals from which the data were obtained.
Indicates significantly different from the S50 or Emax in control sham MA (MV) (P<0.05).
Indicates significantly different from the S50 or Emax in control DOCA-saltMA (MV) (P<0.05).
Indicates significantly different from the S50 or Emax in prazosin treated sham MA (P<0.05). Data analyzed using analysis of variance and Student Newman Keuls post hoc test.
4. Discussion
4.1. Modulation of NE oxidation currents by a2-ARs in MA and MV from sham rats
Real time measurements of NE near its release sites allowed an assessment of the kinetics of the increase in NE levels during nerve stimulation. The time course of the increase in NE oxidation currents near the surface of sham MA was best described by two or three exponents suggesting that multiple factors influenced the increase of NE near the electrode. Prejunctional a2-ARs modulate NE release from sympathetic nerves (Langer, 1970, 1980; Starke) and α2-ARs could control the rate of rise of NE in the neuroeffector junction. This seems to be the case as the time course of NE increase in sham MA was monophasic in the presence of the α2-AR antagonist, yohimbine. Examination of the rising phase of the NE current showed that early in a train of stimulation, the increase was linear but as NE accumulated during the train of stimulation, the rate of rise of the oxidation current declined. This could be due at least in part to activation of α2-ARs which would inhibit further NE release. However, it is also possible that NE diffusion away from release sites is delayed by binding to a low affinity site in the junction that acts to buffer free NE during trains of nerve activity (Starke). If this low affinity binding site is blocked by yohimbine then it would not be available to buffer free NE (Stjärne and Stjärne, 1995). Yohimbine also increased the peak oxidation current throughout the frequency response curve, but yohimbine-induced facilitation was most prominent at low frequencies of nerve stimulation. These data are similar to those obtained for idazoxan-induced facilitation of oxidation currents in rat MA (Dunn et al., 1999). Although yohimbine increased NE oxidation currents significantly, there was relatively little change in the sham MA constriction caused by nerve stimulation. This could be due partly to the fact that in rat small MA neurogenic constrictions are largely mediated by ATP acting at P2X receptors (Gitterman and Evans, 2001; Luo et al., 2003). ATP release from perivascular nerves may be regulated differently than NE release (Stjärne and Stjärne, 1995). Although yohimbine had relatively little effect on the peak amplitude of neurogenic constrictions, the duration of neurogenic constrictions was increased in the presence of sham MA at least at low stimulation frequencies. The increased oxidation current indicates more NE availability and clearance time would be increased as well. Increased NE clearance time would be associated with increased constriction duration. Yohimbine did not change constriction duration in DOCA-salt MA and this result is consistent with our conclusion that α2-AR function in impaired in periarterial sympathetic nerves in DOCA-salt rats. Constriction area was larger in DOCA-salt MA compared to sham MA at the highest stimulation frequency. This result parallels the increase in NE oxidation currents in DOCA-salt MA.
UK 14,304 produced substantial inhibition of neurogenic constrictions in sham MA. We also found that the α2-AR agonist, UK 14,304, markedly inhibited the peak NE oxidation current in sham MA. These data are similar to those published previously showing that α2-AR agonists inhibit NE oxidation currents in the rat vasculature (Brock and Tan, 2004). However, previous work has shown that exogenously applied α2-AR agonists produce equivalent inhibition of NE and ATP release from perivascular sympathetic nerves. Therefore, it is likely that in our studies UK 14,304 inhibited ATP release and this would account for the marked inhibition of neurogenic constrictions of MA caused by this drug.
Anovel finding from the present work is that α2-ARs have little influence on the time course of the increase in NE oxidation currents in MV. The rise time of the oxidation current in MV was mono-exponential and was not changed by yohimbine. These data indicate that as the NE concentration rises in the junctional cleft in MV, there is little influence on the rate of increase in NE concentration by α2-AR function. Similarly, the low affinity binding site which may act as a NE buffer also has little influence on NE diffusion away from the junction (Stjärne and Stjärne, 1995). Yohimbine did increase peak oxidation currents evoked by low frequency nerve stimulation and UK 14,304 reduced peak NE oxidation currents recorded from MV although this effect was less prominent than that occurring in MA. These data indicate that functional α2-ARs are expressed by perivenous sympathetic nerve terminals but these receptors are not efficiently activated by endogenously released NE.
Differences in the effects of yohimbine on NE oxidation currents in sham MA and MV suggest that there are differences in the efficiency of coupling of α2-ARs to NE release from sympathetic nerve terminals supplying these blood vessels. In sham MA, α2-ARs (and the proposed low affinity binding site) must be close to the sites of NE release because the rate of rise of the oxidation current is modulated by α2-AR function. In MV, the α2-AR modulates peak currents without changing the rate of increase. Thus in MV the α2-AR may be localized at some distance from the NE release sites so that they become activated only when NE has diffused away from the release sites. Under these conditions, α2-ARs could still modulate peak currents but these receptors would function less efficiently in modulation of the rate of NE increase than α2-ARs localized close to release sites as occurs in the sympathetic nerve terminals in sham MA.
Our data showing a more prominent role of prejunctional α2-ARs in MA compared to MV differ from data obtained in canine MA and MV (Bobalova and Mutafova-Yambolieva, 2001). In this previous work it was shown that α2-ARs are more tightly coupled to modulation of NE and ATP release from perivenous compared to periarterial sympathetic nerves. These investigators used relatively long trains of stimulation (2 min) to evoke transmitter release which was measured in overflow solutions. Methodological or species differences could account for the different results obtained in our study.
4.2. Impaired α2-AR function in DOCA-salt MA but not MV
In the early stages of hypertension, sympathetic nervous system activity increases (Aalkjaer et al., 1987; de Champlain, 1977; de Champlain et al., 1989; Esler et al., 1990b). This is associated with increased NE overflow from sympathetic nerves in vascular tissues from DOCA-salt rats (Bouvier and De Champlain, 1985; Bouvier and de Champlain, 1986; de Champlain et al., 1989; Luo et al., 2003; Westfall et al., 1986). We have confirmed these results in the present study for DOCA-salt MA using real time NE measurements but we have also found that there is no change in NE release in DOCA-salt MV. This result differs somewhat from our previous in vitro using HPLC techniques where we showed increased NE overflow in DOCA-salt MV nerves associated with multiple blood vessels (Luo et al., 2004). In the present study, NE oxidation currents were measured in real time and very close to the release sites on the surface of a single blood vessel. Methodological differences are likely to account for the differences in results.
Previous studies showed that prejunctional regulation of transmitter release by α2-ARs was impaired in animal models of hypertension (de Champlain, 1990; Luo et al., 2004; Tsuda et al., 1989; Westfall et al., 1986). The data described above indicate that in sham MA, α2-ARs modulate the time course of the increase in the NE oxidation current causing a multi-exponential rate of rise. However, in DOCA-salt MA the time course of the rising phase of the NE oxidation current was mono-exponential and similar to that occurring after α2-AR blockade by yohimbine in sham MA. Furthermore, yohimbine did not alter the time course of the increase in DOCA-salt MA. These data indicate α2-ARs near NE release sites are either downregulated or the signaling mechanism coupled to α2-ARs is impaired in DOCA-salt MA. Our conclusion that either expression or function of α2-AR is reduced in DOCA-salt MA is also supported by the observation that yohimbine is less effective at increasing the peak amplitude of the oxidation current in DOCA-salt MA compared to sham MA. This result is consistent with studies showing a failure of yohimbine to increase plasma NE levels (de Champlain, 1990; Moreau et al., 1995) and nerve stimulation-induced NE overflow in the mesenteric vasculature of DOCA-salt rats (Langer, 1980; Luo et al., 2004; Tsuda et al., 1989). We also showed that UK 14,304 is less effective at reducing the oxidation current DOCA-salt MA compared to sham MA. These data indicate impaired regulation of NE release by α2-ARs is not specific for activation of the receptor by endogenous NE but that exogenously applied agonists are also less able to modulate NE release in DOCA-salt MA.
Although yohimbine did not modify the rise time of oxidation currents in MV, yohimbine and UK 14,304 were equally effective at changing the amplitude of the oxidation current in sham and DOCA-salt MV. These data indicate that the pathophysiological changes responsible for altered regulation of NE release are specific for periarterial nerve endings. These data are also consistent with the proposal that there are subsets of sympathetic nerves that selectively target MA and MV (Browning et al., 1999; Hsieh et al., 2000).
4.3. Neuropharmacological switch in constrictor mechanism in DOCA-salt MA
NE mediates constrictions in MV while constrictions in sham MA are largely resistant to the α1-AR antagonist, prazosin. This is because neurogenic constrictions in sham MA are mediated by ATP acting at P2X receptors (Gitterman and Evans, 2001; Luo et al., 2004). However, in DOCA-salt MA, neurogenic constrictions were reduced substantially by prazosin without a change in NE oxidation currents. This can be attributed in part to the increased amplitude of NE oxidation currents in DOCA-salt MA that may be due to impaired α2-AR function in these tissues. Although there is an increase in apparent NE release from DOCA-salt sympathetic nerves, the amplitude of these currents is not substantially different from those recorded from sham MA. An increase in NE release alone can not account for the neuropharmacological change in MA constrictor mechanisms. PPADS blocked the prazosin-resistant constrictions in sham and DOCA-salt MA indicating that purinergic transmission contributes to neurogenic vasoconstriction in these blood vessels. Recent work has shown that neurotransmitter stores of ATP may be reduced in sympathetic nerves associated with MA of DOCA-salt rats. Excitatory junction potentials (EJPs) mediated by ATP are reduced in amplitude and neuronal stores of ATP are more easily depleted in MA of DOCA-salt rats compared to those in MA of sham rats (Demel and Galligan, 2008). The combined effects of a modest increase in NE release and a more profound reduction ATP (or other nucleotide) release could account for the switch from a predominately purinergic constriction in sham MA to a predominately adrenergic constriction in DOCA-salt MA. Postjunctional changes might also contribute to this change as smooth muscle a1-AR function may be upregulated in arteries from DOCA-salt rats (de Champlain, 1990; Meggs et al., 1988). Finally other vasoconstrictor transmitters released from sympathetic nerves could play a role in hypertension-associated changes in sympathetic transmission to MA. Neuropeptide Y is released from sympathetic nerves supplying rat MA and NPY mediated constriction is enhanced in MA from spontaneously hypertensive rats (Gradin et al., 2006). The contribution of NPY to changes in vascular neuroeffector transmission in the mesentery of DOCA-salt hypertensive rats requires further study.
Prazosin blocked neurogenic constriction in both sham and DOCA-salt MV. Therefore, NE is the predominant sympathetic constrictor transmitter in MV. We found that Emax values were somewhat lower in DOCA-salt MV compared with sham MV, although NE oxidation currents were not significantly different between sham and DOCA-salt MV. Therefore, the reduced contractile response was not due to the decreased NE release in DOCA-salt MV. α1-AR sensitivity to NE may be decreased in DOCA-salt MV due to downregulation of α1-ARs in DOCA-salt veins caused by the increased sympathetic nerve activity (de Champlain, 1990; Fink et al., 2000; Luo et al., 2003).
5. Conclusion
The present study demonstrates that, in normotensive rats, endogenous NE acting at prejunctional α2-ARs alters NE dynamics at the neuroeffector junction in MA but not in MV. This mechanism is impaired in MA from DOCA-salt rats leading to more rapid increase in junctional NE and increased peak NE levels. The elevated NE oxidation current in DOCA-salt MA is likely due to decreased function of α2-ARs. The mechanism responsible for impaired α2-AR function is unknown. Finally, DOCA-salt hypertension is associated with a neuropharmacological switch from purinergic to adrenergic neurogenic constrictions in MA. Overall we conclude that: 1) there are distinct mechanisms of sympathetic control of MA and MV; 2) differential alterations in these mechanisms may play a part in the development of DOCA-salt hypertension; and 3) it may be possible to separately affect sympathetic control of vascular resistance and capacitance in the splanchnic circulation for therapeutic purposes.
References
- Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation–contraction coupling in isolated resistance vessels from essential hypertensives. Circ. Res. 1987;61:181–186. doi: 10.1161/01.res.61.2.181. [DOI] [PubMed] [Google Scholar]
- Bobalova J, Mutafova-Yambolieva VN. Presynaptic alpha2-adrenoceptor-mediated modulation of adenosine 5′ triphosphate and noradrenaline corelease: differences in canine mesenteric artery and vein. J. Auton. Pharmacol. 2001;21:47–55. doi: 10.1046/j.1365-2680.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Bouvier M, De Champlain J. Increased apparent norepinephrine release rate in anesthetized DOCA-salt hypertensive rats. Clin. Exp. Hypertens., Part A Theory Pract. 1985;A7:1629–1645. doi: 10.3109/10641968509073614. [DOI] [PubMed] [Google Scholar]
- Bouvier M, de Champlain J. Increased basal and reactive plasma norepinephrine and epinephrine levels in awake DOCA-salt hypertensive rats. J. Auton. Nerv. Syst. 1986;15:191–195. doi: 10.1016/0165-1838(86)90014-7. [DOI] [PubMed] [Google Scholar]
- Brock JA, Tan JH. Selective modulation of noradrenaline release by alpha2-adrenoceptor blockade in the rat-tail artery in vitro. Br. J. Pharmacol. 2004;142:267–274. doi: 10.1038/sj.bjp.0705779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Dunn WR, Boyd NS, Wong DK. Spontaneous release of large packets of noradrenaline from sympathetic nerve terminals in rat mesenteric arteries in vitro. Br. J. Pharmacol. 2000;131:1507–1511. doi: 10.1038/sj.bjp.0703733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning K, Zheng Z, Kreulen D, Travagli R. Two populations of sympathetic neurons project selectively to mesenteric artery or vein. Am. J. Physiol. 1999;276:H1263–H1272. doi: 10.1152/ajpheart.1999.276.4.H1263. [DOI] [PubMed] [Google Scholar]
- Cox SL, Story DF, Ziogas J. Multiple prejunctional actions of angiotensin II on noradrenergic transmission in the caudal artery of the rat. Br. J. Pharmacol. 1996;119:976–984. doi: 10.1111/j.1476-5381.1996.tb15767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Champlain J. The sympathetic system in hypertension. Clin. Endocrinol. Metab. 1977;6:633–655. doi: 10.1016/s0300-595x(77)80074-1. [DOI] [PubMed] [Google Scholar]
- de Champlain J. Pre- and postsynaptic adrenergic dysfunctions in hypertension. J. Hypertens. 1990;(Suppl. 8):S77–S85. [PubMed] [Google Scholar]
- de Champlain J, Farley L, Cousineau D, van Ameringen MR. Circulating catecholamine levels in human and experimental hypertension. Circ. Res. 1976;38:109–114. doi: 10.1161/01.res.38.2.109. [DOI] [PubMed] [Google Scholar]
- de Champlain J, Eid H, Drolet G, Bouvier M, Foucart S. Peripheral neurogenic mechanisms in deoxycorticosterone acetate-salt hypertension in the rat. Can. J. Physiol. Pharm. 1989;67:1140–1145. doi: 10.1139/y89-181. [DOI] [PubMed] [Google Scholar]
- Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2008;52:322–329. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang SH, Liu AH, Galligan JJ, Swain GM. Drug effects on the electrochemical detection of norepinephrine with carbon fiber and diamond microelectrodes. J. Electroanal. Chem. 2009;632:20–29. [Google Scholar]
- Drolet G, Bouvier M, de Champlain J. Enhanced sympathoadrenal reactivity to haemorrhagic stress in DOCA-salt hypertensive rats. J. Hypertens. 1989;7:237–242. doi: 10.1097/00004872-198903000-00011. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JA, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br. J. Pharmacol. 1999;128:174–180. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J. Hypertens. 1990a;(Suppl. 8):S53–57. [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev. 1990b;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension. 2000;35:464–469. doi: 10.1161/01.hyp.35.1.464. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Hess MC, Miller SB, Fink GD. Differential localization of P2 receptor subtypes in mesenteric arteries and veins of normotensive and hypertensive rats. J. Pharmacol. Exp. Ther. 2001;29:478–485. [PubMed] [Google Scholar]
- Gericke A, Martinka P, Nazarenko I, Persson PB, Patzak A. Impact of alpha1-adrenoceptor expression on contractile properties of vascular smooth muscle cells. Am. J. Physiol. 2007;293:R1215–1221. doi: 10.1152/ajpregu.00076.2007. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br. J. Pharmacol. 2001;16:337–340. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F, Msghina M, Stjarne L. Kinetics of noradrenaline released by sympathetic nerves. Neuroscience. 1993;56:535–538. doi: 10.1016/0306-4522(93)90354-i. [DOI] [PubMed] [Google Scholar]
- Gradin KA, Buus CL, Li JY, Frøbert O, Simonsen U. Neuropeptide Y2 receptors are involved in enhanced neurogenic vasoconstriction in spontaneously hypertensive rats. Br. J. Pharmacol. 2006;148:703–713. doi: 10.1038/sj.bjp.0706774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottenstein OD, Kreulen DL. Comparison of the frequency dependence of venous and arterial responses to sympathetic nerve stimulation in guinea-pigs. J. Physiol. 1987;384:153–167. doi: 10.1113/jphysiol.1987.sp016448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh NK, Liu JC, Chen HI. Localization of sympathetic postganglionic neurons innervating mesenteric artery and vein in rats. J. Auton. Nerv. Syst. 2000;80:1–7. doi: 10.1016/s0165-1838(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Kreulen DL. Activation of mesenteric arteries and veins by preganglionic and postganglionic nerves. Am. J. Physiol. 1986;251:H1267–1275. doi: 10.1152/ajpheart.1986.251.6.H1267. [DOI] [PubMed] [Google Scholar]
- Langer SZ. The metabolism of 3H-noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J. Physiol. 1970;208:515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol. Rev. 1980;32:337–362. [PubMed] [Google Scholar]
- Luo M, Hess MC, Fink GD, Olson LK, Rogers J, Kreulen DL, Dai X, Galligan JJ. Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats. Auton. Neurosci. 2003;104:47–57. doi: 10.1016/S1566-0702(02)00287-4. [DOI] [PubMed] [Google Scholar]
- Luo M, Fink GD, Lookingland KJ, Morris JA, Galligan JJ. Impaired function of alpha2-adrenergic autoreceptors on sympathetic nerves associated with mesenteric arteries and veins in DOCA-salt hypertension. Am. J. Physiol. 2004;286:H1558–1564. doi: 10.1152/ajpheart.00592.2003. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas SG, Campos-Peralta JM, Pares-Hipolito J, Gallardo-Ortíz IA, Ibarra M, Villalobos-Molina R. Alpha1A-adrenoceptors predominate in the control of blood pressure in mouse mesenteric vascular bed. Auton. Autacoid. Pharmacol. 2007;27:137–142. doi: 10.1111/j.1474-8673.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Meggs LG, Stitzel R, Ben-Ari J, Chander P, Gammon D, Goodman AI, Head R. Upregulation of the vascular alpha-1 receptor in malignant DOCA-salt hypertension. Clin. Exp. Hypertens. A. 1988;10:229–247. doi: 10.3109/10641968809103525. [DOI] [PubMed] [Google Scholar]
- Moreau P, Drolet G, Yamaguchi N, de Champlain J. Alteration of prejunctional alpha 2-adrenergic autoinhibition in DOCA-salt hypertension. Am. J. Hypertens. 1995;8:287–293. doi: 10.1016/0895-7061(94)00211-s. [DOI] [PubMed] [Google Scholar]
- Nyhof RA, Laine GA, Meininger GA, Granger HJ. Splanchnic circulation in hypertension. Fed. Proc. 1983;42:1690–1693. [PubMed] [Google Scholar]
- Pang CCY. Autonomic control of the venous system in health and disease effects of drugs. Pharmacol. Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Park J, Galligan JJ, Fink GD, Swain GM. Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging. J. Physiol. 2007;584:819–834. doi: 10.1113/jphysiol.2007.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascher W, Schomig A, Dietz R, Weber J, Gross F. Plasma catecholamines, noradrenaline metabolism and vascular response in desoxycorticosterone acetate hypertension of rats. Eur. J. Pharmacol. 1981;75:255–263. doi: 10.1016/0014-2999(81)90552-5. [DOI] [PubMed] [Google Scholar]
- Rothe CF. Handbook of Physiology; Section 2. The Cardiovascular System. III. American Physiological Society; 1983. Venous system: physiology of capacitance vessels; pp. 397–452. [Google Scholar]
- Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J. Pharmacol. Toxicol. Methods. 1992;27:161–170. doi: 10.1016/1056-8719(92)90036-z. [DOI] [PubMed] [Google Scholar]
- Smyth L, Bobalova J, Ward SM, Keef KD, Mutafova-Yambolieva VN. Co-transmission from sympathetic vasoconstrictor neurons: differences in guinea-pig mesenteric artery and vein. Auton. Neurosci. 2000;86:18–29. doi: 10.1016/S1566-0702(00)00203-4. [DOI] [PubMed] [Google Scholar]
- Sneddon P. Electrophysiology of autonomic neuromuscular transmission involving ATP. J. Auton. Nerv. Syst. 2000;81:218–224. doi: 10.1016/s0165-1838(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J. Neurochem. 78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L, Stjärne E. Geometry, kinetics and plasticity of release and clearance of ATP and noradrenaline as sympathetic cotransmitters: roles for the neurogenic contraction. Prog. Neurobiol. 1995;47:45–94. doi: 10.1016/0301-0082(95)00018-q. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y. Inhibition of norepinephrine release by presynaptic alpha 2-adrenoceptors in mesenteric vasculature preparations from chronic DOCA-salt hypertensive rats. Jpn. Heart J. 1989;30:231–239. doi: 10.1536/ihj.30.231. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Westfall TC, Carpentier S, Naes L, Meldrum MJ. Comparison of norepinephrine release in hypertensive rats: II. Caudal artery and portal vein. Clin. Exp. Hypertens., Part A Theory Pract. 1986;8:221–237. doi: 10.3109/10641968609074773. [DOI] [PubMed] [Google Scholar]
- Further reading King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am. J. Physiol. 2008;294:R1262–1267. doi: 10.1152/ajpregu.00819.2007.
- Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ. Renal sympathetic nerve activity in the development of hypertension. Curr. Hypertens. Rep. 2006;8:242–248. doi: 10.1007/s11906-006-0057-0. [DOI] [PubMed] [Google Scholar]
- Reid JL, Zivin JA, Kopin IJ. Central and peripheral adrenergic mechanisms in the development of deoxycorticosterone-saline hypertension in rats. Circ. Res. 37:569–579. doi: 10.1161/01.res.37.5.569. [DOI] [PubMed] [Google Scholar]